Abstract
Background
We have recently found that 3 repeated doses (12×106 each) of c‐kitPOS cardiac progenitor cells (CPCs) were markedly more effective than a single dose of 12×106 cells in alleviating postinfarction left ventricular dysfunction and remodeling. However, since the single‐dose group received only one third of the total number of CPCs given to the multiple‐dose group, it is unknown whether the superior therapeutic efficacy was caused by repeated treatments per se or by administration of a higher total number of CPCs. This issue has major clinical implications because multiple cell injections in patients pose significant challenges, which would be obviated by using 1 large injection. Accordingly, we determined whether the beneficial effects of 3 repeated CPC doses can be recapitulated by 1 large dose containing the same total number of cells.
Methods and Results
Rats with a 30‐day‐old myocardial infarction received 3 echo‐guided intraventricular infusions, 35 days apart, of vehicle‐vehicle‐vehicle, 36×106 CPCs‐vehicle‐vehicle, or 3 equal doses of 12×106 CPCs. Infusion of a single, large dose of CPCs (36×106 cells) produced an initial improvement in left ventricular function, but no further improvement was observed after the second and third infusions (both vehicle). In contrast, each of the 3 doses of CPCs (12×106) caused a progressive improvement in left ventricular function, the cumulative magnitude of which was greater than with a single dose. Unlike the single dose, repeated doses reduced collagen content and immune cell infiltration.
Conclusions
Three repeated doses of CPCs are superior to 1 dose even though the total number of cells infused is the same, possibly because of greater antifibrotic and anti‐inflammatory actions.
Keywords: cardiac function, cardiac progenitor cells, cell transplantation, ischemic cardiomyopathy, myocardial infarction, repeated doses
Subject Categories: Cell Therapy, Translational Studies, Basic Science Research, Ischemia
Clinical Perspective
What Is New?
Repeated doses of cardiac progenitor cells are therapeutically superior to 1 dose even though the total number of cells infused is the same.
What Are the Clinical Implications?
Splitting a given number of cells into multiple doses appears to be more efficacious than giving 1 dose that contains that number of cells.
Although subjecting patients to multiple treatments and cardiac catheterizations entails additional risks, costs, and logistical challenges, the results achieved with multiple doses cannot be achieved by simply giving 1 larger dose of cells.
The beneficial effects of cell therapy appear to be more repetition dependent than dose dependent.
It is becoming increasingly apparent that poor engraftment of transplanted cells and their rapid clearance from the host myocardium are major factors limiting the therapeutic efficacy of virtually all cell types tested thus far, and may contribute to the small or undetectable beneficial effects observed in clinical trials.1, 2 We have recently shown that repeated cell administrations can partially overcome the failure of cells to engraft and significantly enhance their salubrious actions by augmenting the exposure of diseased myocardium to transplanted cells. Specifically, we have found that, in the setting of ischemic cardiomyopathy, 3 doses of cells, given a few weeks apart, result in greater improvement in left ventricular (LV) function than a single dose; this was observed in 2 different species (mice and rats) and with 2 different cell types (c‐kitPOS cardiac progenitor cells [CPCs]3 and cardiac mesenchymal cells4), suggesting that the superiority of multiple doses is not dependent on species or cell type.1, 2 These results support the concept that the use of multiple doses is an important strategy to augment the efficacy of cell therapy.
The mechanism for the greater efficacy of repeated cell administrations remains unclear, but likely involves a higher average density of transplanted cells over time.1, 2 The myocardial content of transplanted cells declines rapidly after injection.5, 6 In the setting of multiple doses, each injection results in a transient spike in the number of transplanted cells in the tissue, such that the average density of these cells over time is higher than after a single injection; this situation prolongs the exposure of the host tissue to the paracrine actions of the cells.1, 2 However, it remains possible that a similar effect could be achieved simply by increasing the number of cells administered in 1 injection. Since in our previous studies3, 4 the total amount of transplanted cells in the multiple‐dose group was 3 times greater than in the single‐dose group, it is not clear whether the observed therapeutic superiority was caused by the greater total number of cells given or by the use of repeated injections. This issue has major clinical implications because repeated cell administrations in patients (either by the intracoronary or the intramyocardial route) pose significant challenges in terms of safety, cost, and practicality—challenges that could be obviated if the greater efficacy of multiple doses can be achieved by using 1 equivalent large dose. Determining whether this is the case would affect the design of future clinical trials of cell therapy.
The present study was undertaken to answer this question. Specifically, using a well‐characterized rat model of ischemic cardiomyopathy associated with an old myocardial infarction (MI),3, 7 we determined whether the cumulative beneficial effects of 3 repeated CPC doses (12×106 cells each) given 35 days apart can be recapitulated by 1 large dose containing the same number of cells (36×106 CPCs).
Methods
The data, analytic methods, and study materials will be made available upon request to other researchers for purposes of reproducing the results or replicating the procedure.
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Eighth Edition, Revised 2010) and with the guidelines of the Animal Care and Use Committee of the University of Louisville, School of Medicine (Louisville, KY).
Preparation of c‐kitPOS CPCs
The isolation, characterization, and expansion of CPCs have been previously described.3, 8, 9 Briefly, cardiac cells were isolated from adult male Fischer 344 rats (4–6 months of age). c‐kitpos CPCs were sorted with magnetic beads and phenotyped by fluorescence‐activated cell sorting analysis of fixed cells. CPCs expanded in vitro at passages 6 to 8 were used for this study.
Surgical Procedures and Treatment Protocol
The rat model of old MI has been described previously.3, 7 Adult female Fischer 344 rats (age, 4 months; weight 175±2 g) underwent a 2‐hour occlusion of the left anterior descending coronary artery followed by reperfusion. Thirty days after surgery, the animals were randomly allocated to 3 treatment groups: vehicle (PBS control), repeated‐doses, or combined‐dose group (Figure 1A). Randomization was performed using the MS Excel random group generator.
Figure 1.
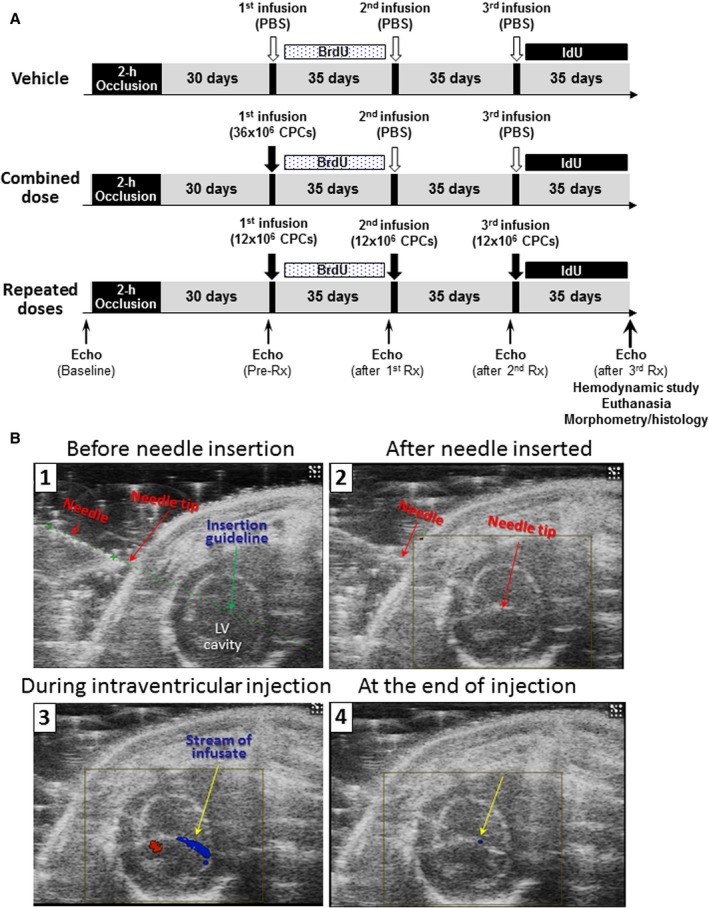
A, Experimental protocol. BrdU indicates 5‐bromo‐2′‐deoxyuridine; CPCs, c‐kitPOS cardiac progenitor cells; Echo, echocardiogram; first, second, third Rx, first, second, and third treatment; IdU, 5‐iodo‐2′‐deoxyuridine; PBS, Dulbecco's phosphate‐buffered saline; Pre‐Rx, pretreatment (30 days after MI). B, Representative images of echo‐guided injection into the LV cavity. 1, B‐mode short‐axis image showing the LV cavity, the injection needle, the needle tip, and the needle insertion guideline; 2, B‐mode color Doppler image showing the needle tip in the center of the LV cavity before start of injection; 3, B‐mode color Doppler image showing a blue stream of infusate during injection; and 4, B‐mode color Doppler image at the end of the infusion. LV indicates left ventricular.
All rats received 3 echo‐guided intraventricular injections, 35 days apart, as described previously.3 Briefly, animals were anesthetized lightly with isoflurane, placed on the imaging table in the right lateral decubitus position, and prepared for imaging using the Vevo 2100 Imaging System (VisualSonics) equipped with a 20‐MHz transducer to determine the optimal site for needle insertion, a site that did not include the infarct scar or a coronary artery. Under the guidance of a real‐time B‐mode view, a 30‐G injection needle (1″ length) was then carefully inserted from the left lateral side (Figure 1B1) using the micromanipulation controls and advanced into the center of the LV cavity (Figure 1B2). Injections were performed with a Chemyx NanoJet Stereotaxic syringe infusion pump (Chemyx Inc, TX). Successful intraventricular injection is documented by a blue stream of infusate during infusion (Figure 1B3) that ceases at the end of the infusion (Figure 1B4).
The vehicle group received 3 injections of Dulbecco's PBS, the repeated‐doses group received 3 injections of 12×106 CPCs each, and the combined‐dose group received 36×106 CPCs on the first injection and PBS on the second and third injections. Injections were 5 mL in volume and were performed at a rate of 1.25 mL/min for 4 minutes.
To discern formation of new cells, in all groups 5‐bromo‐2′‐deoxyuridine (BrdU, Sigma) was given for 35 days after the first treatment and 5‐iodo‐2′‐deoxyuridine (IdU, Sigma) for 35 days after the third treatment (both were given in the drinking water at a final concentration of 0.1%). At 35 days after the third treatment, rats were subjected to hemodynamic studies and euthanized for histological studies (Figure 1).
Echocardiographic Studies
All echocardiographic analyses were performed by investigators who were blinded to treatment allocation. Serial echocardiograms were obtained at 5 time points: baseline (3 days before coronary artery occlusion), 30 days after MI (before the first treatment), 35 days after the first treatment (before the second treatment), 35 days after the second treatment (before the third treatment), and 35 days after the third treatment (before euthanasia; Figure 1). The echocardiographic studies were performed as described3, 7, 8, 9, 10 using a Vevo 2100 Imaging System equipped with a 20‐MHz transducer.
Hemodynamic Studies
All hemodynamic analyses were performed by investigators who were blinded to treatment allocation. The hemodynamic studies were conducted 35 days after the third treatment, just before euthanasia (Figure 1). The protocol has been described previously.3, 7, 8, 9, 10
Histological Studies
Histological studies were performed in formalin‐fixed, paraffin‐embedded, 4‐μm‐thick heart sections. The procedures for Masson's trichrome, picrosirius red, and immunohistochemistry staining and analyses have been described.3, 7, 8, 9, 11, 12 Immunohistochemistry staining was performed with specific antibodies against CD45, wheat germ agglutinin, α‐sarcomeric actin, BrdU , and IdU (Sigma). To assess the fate of the transplanted male CPCs, Y chromosome was detected by fluorescence in situ hybridization according to the modified manufacturer's protocol (ID Labs, London, ON).3, 8, 13
Statistical Analysis
All data are expressed as means±SEM except for cardiomyocyte area, which is expressed as median and interquartile (first and third quartile) range (25th and 75th percentile) because these data are not normally distributed. All parametric data including morphometric, histological, immunohistochemical, and hemodynamic data were analyzed by 1‐way ANOVA followed by Student t tests with Bonferroni correction for intergroup comparisons.14, 15, 16, 17, 18, 19 Echocardiographic data were analyzed with 2‐way repeated‐measures ANOVA (time and group) followed by Student t tests with Bonferroni correction for intra‐ and intergroup comparisons, as appropriate. Cardiomyocyte area was analyzed by Kruskal–Wallis 1‐way ANOVA on ranks with the Dunn's test.7 All analyses were conducted with SigmaStat 3.5 (StatCon, Witzenhausen, Germany). P values <0.05 were considered significant.
Results
Exclusions and Gross Measurements
A total of 30 rats (10 rats/group) underwent coronary artery occlusion/reperfusion. Four rats (1 in the vehicle, 1 in the repeated‐dose, and 2 in the combined‐dose group) died after the MI procedure, and 2 died after the injection procedure (1 in the vehicle group after the second injection and 1 in the combined‐dose group after the first injection). Three rats were excluded because of prespecified criteria (1 in the repeated‐dose group because of small ejection fraction [EF] drop [<15 units] after MI [before treatment], as shown by echocardiography, and 2 in the combined‐dose group because of very small scar [<10% of risk region], as shown by trichrome stain analysis). Therefore, a total of 21 rats (8 in the vehicle, 8 in the repeated‐dose, and 5 in the combined‐dose group) were included in the final analysis.
The 2 rats excluded in the combined‐dose group because of small scar had a small decrease in LV EF before treatment (after MI) of 15.5 and 16.2 EF units, which is close to the threshold for exclusion, indicating that the relatively well‐preserved LV function in these animals was related to an initial small infarct rather than a subsequent therapeutic effect of CPCs. The 3 exclusions did not have a significant impact on the outcome of the study. When the 3 excluded rats were included in the analysis, the differences in LV EF, end‐systolic volume (ESV), end‐diastolic volume (EDV), and scar size at the end of the study were similar to those observed after the rats were excluded (Figure S1A through S1G and Table S1).
There were no significant differences in body weight among the 3 groups throughout the experimental protocol (Table). Postmortem gross measurements revealed that LV longitudinal length was significantly reduced in the repeated‐dose group compared with the vehicle group (P<0.01), but LV weight and LV volume were not significantly different among the groups, although a tendency toward lower LV volumes was observed in the repeated‐dose group (Table).
Table 1.
Body Weight and Postmortem Gross Measurements
| Initial Body Weight, g | Final Body Weight, g | LV Weight, mg | LV Longitudinal Length, mm | LV Chamber Volume, mm3 | |
|---|---|---|---|---|---|
| Vehicle (n=8) | 169.6±6.1 | 216.5±2.6 | 659.5±21.5 | 13.3±0.4 | 207.9±39.8 |
| Combined dose (n=5) | 172.2±6.9 | 210.6±4.7 | 715.2±27.8 | 12.9±0.4 | 207.4±28.8 |
| Repeated doses (n=8) | 177.7±5.0 | 220.7±4.5 | 681.8±46.6 | 11.3±0.5a | 134.1±34.3 |
Data are mean±SEM. LV indicates left ventricular.
P<0.01 vs vehicle.
LV Function Assessed by Echocardiography
Representative echocardiographic recordings are illustrated in Figure 2A, and comprehensive quantitative analyses of echocardiographic parameters are presented in Figure 2B through 2I. As shown in Figure 2B, LV EF was markedly decreased from baseline at 30 days after MI (before treatment [Pre‐Rx]), and was not significantly different among rats assigned to vehicle, combined dose, and repeated doses, indicating that before the first treatment, the severity of post‐MI LV dysfunction was comparable in the 3 groups.
Figure 2.
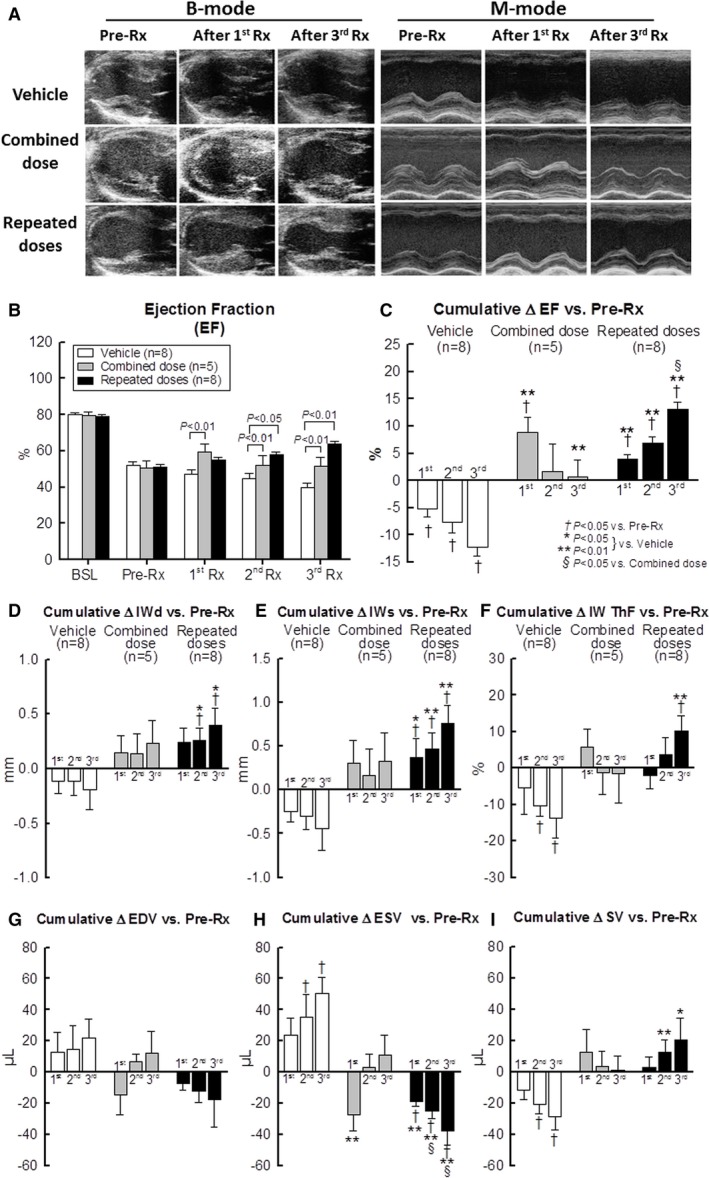
Echocardiographic assessment of LV function. A, Representative B‐mode long‐axis images in systole (left) and M‐mode images (right) obtained before the first treatment (Pre‐Rx) (30 days after MI) and at 35 days after the first (first Rx) and third treatment (third Rx). B, Bar graph illustrating LV EF at baseline (BSL), before the first treatment (Pre‐Rx) (ie, 30 days after myocardial infarction, 35 days after the first treatment (first Rx), 35 days after second treatment (second Rx), and 35 days after the third treatment (third Rx). C through I, Cumulative changes in echocardiographic parameters (absolute units) at 35 days after the first, second, and third treatment vs pretreatment values (Pre‐Rx) values. EF, ejection fraction (C); IWd, infarcted LV wall thickness in diastole (D); IWs, infarcted LV wall thickness in systole (E); IW ThF, infarcted LV wall thickening fraction (F); EDV, end‐diastolic volume (G); ESV, end‐systolic volume (H); SV, stroke volume (I). *P<0.05, **P<0.01 vs vehicle; † P<0.05 vs Pre‐Rx; and § P<0.05 vs Combined dose. Data are means±SEM.
After the first treatment, however, the 3 groups exhibited a different course. As expected,3, 4 the vehicle‐treated (control) group continued to show progressive deterioration of both regional and global LV function (Figure 2B through 2I). Thus, left ventricular ejection fraction, infarcted wall thickness in diastole and in systole, infarcted wall thickening fraction, and stroke volume decreased progressively, whereas LV EDV and ESV increased progressively compared with pretreatment values (at 30 days after MI) (Figure 2B through 2I).
In contrast to the progressive deterioration of LV function in the vehicle group, the combined‐dose group exhibited a robust improvement in global LV function after the first infusion (which consisted of CPCs). Thus, administration of CPCs resulted, 35 days later, in a significant decrease in LV ESV (Figure 2H) and increase in LV EF (Figure 2C) compared with the vehicle group. However, the improvement in these variables was short‐lived and not sustained after the second and third infusions (which consisted of vehicle) (Figure 2C and 2I), although the first infusion (CPCs) prevented the progressive deterioration of LV function observed in the vehicle group. Indices of regional LV function (infarcted wall thickness in systole, infarcted wall thickness in diastole, infarcted wall thickening fraction) were nominally improved but the changes were not statistically significant (Figure 2D through 2F).
In contrast to the combined‐dose group, the repeated‐dose group exhibited a progressive improvement in regional and global LV function after each CPC infusion (Figure 2B through 2I), although the magnitude of LV functional improvement after the first infusion was not as robust as that seen in the combined‐dose group. As found previously,3 each additional treatment produced additional benefit, and the magnitude of the improvement observed after the second and third infusions was roughly comparable to that observed after the first infusion (Figure 2C through 2I). Thus, compared with pretreatment values (at 30 days after MI), LV EF increased by 4.0±0.8%, 6.9±1.2%, and 13.1±1.3% after the first, second, and third infusion of CPCs, respectively (P<0.05 versus the combined‐dose group) (Figure 2C). As a result of this stepwise improvement, the beneficial effects of cell therapy after 3 repeated CPC infusions were significantly greater than the effects seen after a single equivalent dose in the combined‐dose group. The greater therapeutic efficacy of repeated CPC infusions is demonstrated by the fact that, at the end of the protocol (105 days after the first treatment), there were statistically significant differences between the 2 groups in the change in LV EF (+0.8±3.1% in the combined‐dose group versus +13.1±1.3% in the repeated‐dose group, P<0.01) (Figure 2C) and LV ESV (+11.3±12.4 μL versus −37.6±9.2 μL, respectively, P<0.01) (Figure 2H).
In summary, administration of a large number (36×106) of CPCs had different functional effects, depending on whether the cells were given in a single dose (combined‐dose group) or in 3 repeated doses. The combined‐dose regimen improved indices of global LV function only after the first administration; in contrast, the repeated‐dose regimen improved LV function cumulatively and by a similar amount after each administration, so that the total beneficial effects were greater than in the combined‐dose group.
LV Function Assessed by Hemodynamic Measurements
The hemodynamic studies, which were performed just before euthanasia at 105 days after the first treatment, also demonstrated superior LV function in the repeated‐dose group compared with the combined‐dose group as well as the vehicle group (Figure 3A and 3B). A single large dose of CPCs improved some, but not all, hemodynamic parameters (ie, minimal dP/dt [P<0.05], dP/dtmax−EDV [P<0.05], and Ees [P<0.05]) compared with the vehicle group (Figure 3B). In contrast, 3 repeated doses of CPCs improved all parameters of LV function compared with vehicle‐treated rats, including LV end‐diastolic pressure (P<0.05), EDV (P<0.01), ESV (P<0.01), EF (P<0.01), maximal and minimal dP/dt (P<0.01 for both), preload adjusted maximal power (P<0.01), dP/dtmax−EDP (P<0.01), and Ees (P<0.01) (Figure 3B). Furthermore, ESV was significantly smaller (P<0.05) and preload adjusted maximal power and dP/dtmax−EDV significantly greater (P<0.05 each) in rats given repeated treatments versus those given a single combined treatment (Figure 3B). Thus, 2 independent methods of functional assessment (echocardiography and hemodynamic studies with a conductance catheter) demonstrated that administration of repeated doses of CPCs produced a greater improvement in LV function compared with a single combined dose, even though the total number of cells administered was the same.
Figure 3.
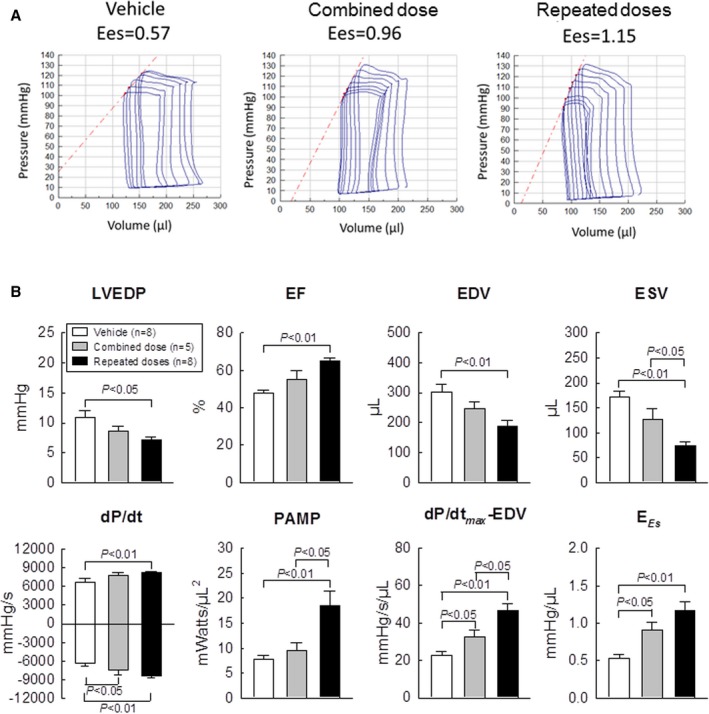
Hemodynamic assessment of LV function. Hemodynamic studies were performed with a Millar conductance catheter at 35 days after the third treatment, just before euthanasia. A, Representative pressure‐volume loops recorded during preload manipulation by brief inferior vena cava occlusions. B, Quantitative analysis of hemodynamic variables. EF indicates ejection fraction; EDV, end‐diastolic volume; ESV, end‐systolic volume; EES end‐systolic elastance; LVEDP, left ventricular end‐diastolic pressure; PAMP, preload adjusted maximal power. Data are means±SEM.
LV Morphology
Figure 4A shows representative trichrome‐stained LV sections from the vehicle, combined‐dose, and repeated‐dose groups. The vehicle group exhibited LV dilatation and an extremely thin infarcted wall with a confluent scar. In the combined‐dose and repeated‐dose groups, the infarcted wall was thicker and the amount of viable tissue within the risk region was greater compared with the vehicle group (Figure 4A). In each heart, a detailed quantitative analysis of trichrome images was performed on 2 sections (1 from each of 2 midventricular slices); the results are summarized in Figure 4B. The size of the risk region was similar among the 3 groups. In the combined‐dose group, morphometric parameters were nominally improved compared with the vehicle group, but only scar size was significantly different (45.9±1.3% versus 38.0±2.1% of the risk region, P<0.05) (Figure 4B). In contrast, 3 repeated doses of CPCs produced a significant improvement in several morphologic parameters compared with the vehicle group (ie, a thicker infarcted LV wall [P<0.05], a smaller scar size [45.9±1.3% versus 32.1±1.9% of the risk region, P<0.01], a greater proportion of viable myocardium in the risk region [P<0.01], and a reduced LV expansion index [P<0.05] [Figure 4B]). There was a trend for the infarcted LV wall to be thicker, the percentage of scarred tissue to be smaller, and the percentage of viable myocardium to be greater in the repeated‐dose compared with the combined‐dose group, but the differences were not statistically significant (Figure 4B). Taken together, the morphometric data indicate that the improvement in LV structure (infarcted wall thickness, scar size, amount of viable myocardium, and expansion index) was more robust after repeated treatments than after a combined treatment, which is consistent with the echocardiographic and hemodynamic findings.
Figure 4.
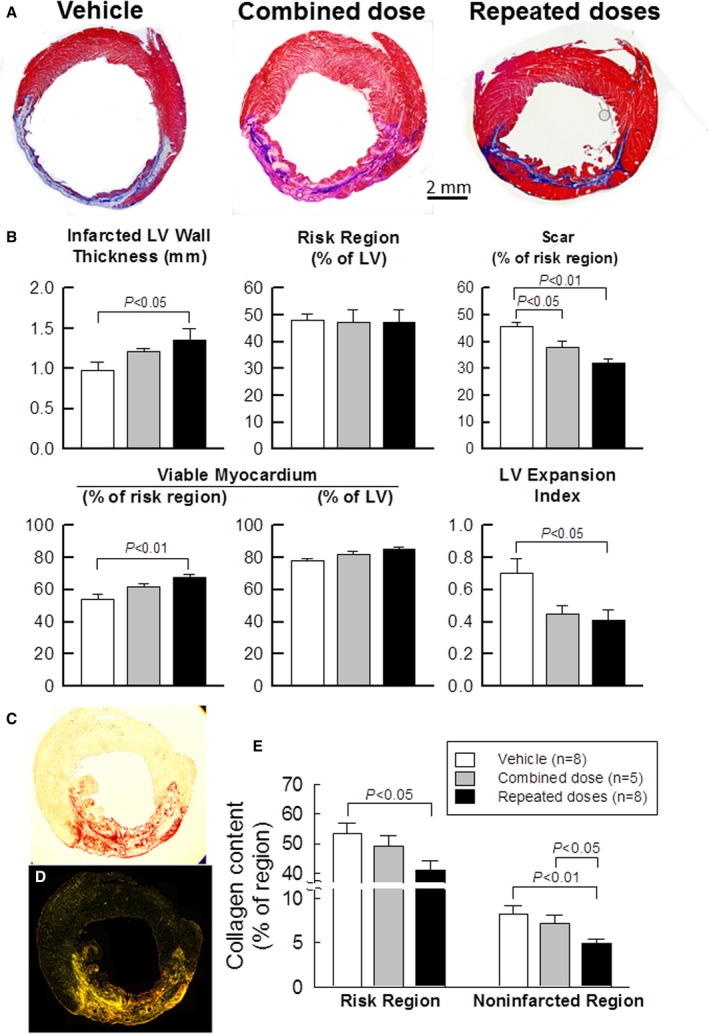
Morphometric analysis (A and B) and myocardial collagen content (C through E). A, Representative Masson trichrome‐stained myocardial sections. Scar tissue and viable myocardium are identified in blue and red, respectively. B, Quantitative analysis of LV morphometric parameters. The risk region comprises both the border zones and the scarred region. C through E, Representative transmission light (C) and polarized light (D) images of an LV section stained with picrosirius red; note the presence of a transmural infarction. E, Quantitative analysis of polarized light microscopic images showing total collagen content as a percent of the risk and noninfarcted regions. Data are means±SEM. LV indicates left ventricular.
Myocardial fibrosis plays a key role in the pathology of LV remodeling after MI.16 We evaluated collagen deposition in LV sections stained with picrosirius red and imaged with polarized light (Figure 4C and 4D). Collagen content was evaluated with pixel density analysis and expressed as a percentage of total myocardial area. As shown in Figure 4E, a single combined dose failed to reduce collagen content significantly in either the risk or the noninfarcted region compared with the vehicle group. Three repeated doses of CPCs, however, significantly reduced collagen content both in the risk (P<0.05) and in the noninfarcted region (P<0.01) compared with the vehicle group (Figure 4E). Furthermore, collagen content in the noninfarcted region was significantly less in the repeated‐dose compared with the combined‐dose group (P<0.05) (Figure 4E). This reduced collagen deposition in the myocardium may have contributed, at least in part, to the functional benefits of repeated CPC treatments.
Myocardial Content of Inflammatory Cells
Because the immune system plays an important role not only in infarct healing but also in adverse remodeling and progression of heart failure,20, 21 we evaluated the myocardial content of immune cells with CD45 staining (Figure 5A). The number of CD45POS cells in the risk and noninfarcted regions was quantitated and expressed as a percent of total cells counted in the corresponding region (Figure 5B). In all groups, the risk region exhibited a higher content of CD45POS cells than the noninfarcted region (Figure 5B). A single combined dose of CPCs failed to alter the content of CD45POS cells. In contrast, 3 repeated administrations of CPCs significantly reduced the content of immune cells both in the risk and noninfarcted regions compared with the combined‐dose (P<0.05 and P<0.01, respectively) as well as the vehicle group (all P<0.05) (Figure 5B).
Figure 5.
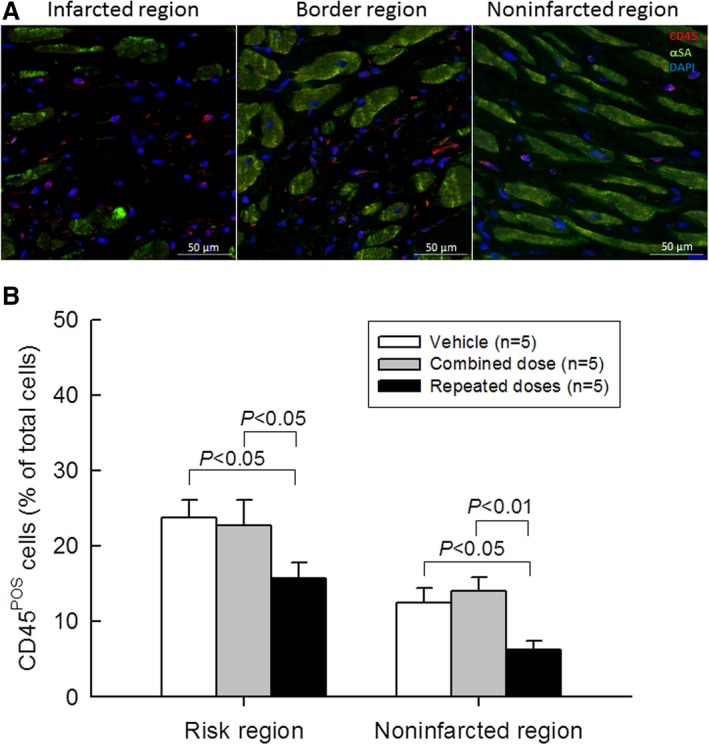
Myocardial content of inflammatory cells. A, Representative microscopic images of LV sections stained for CD45 and deoxyribonucleic acid (with 4′,6‐diamidino‐2‐phenylindole [DAPI]; αSA, α‐sarcomeric actin). B, Quantitative analysis of CD45‐positive cells. Values are means±SEM.
Analysis of Cardiomyocyte Size
Myocyte size was assessed by staining myocytes with an anti‐α‐sarcomeric actin antibody and myocyte membranes with FITC‐conjugated wheat germ agglutinin to facilitate identification of individual myocytes.3, 7, 8 A single combined dose significantly reduced myocyte size in both the risk and the noninfarcted region, whereas repeated doses reduced myocyte size only in the risk region (Figure 6). Interestingly, a single combined dose resulted in a smaller myocyte size than repeated doses (Figure 6) despite the fact that the latter produced greater functional benefits. We do not have a plausible explanation for this finding. It is unlikely that the smaller myocyte size reflected greater formation of new myocytes because the number of BrdU+ or IdU+ myocytes was minuscule (<1% of all myocytes) in all groups (Figure 7H and 7I).
Figure 6.
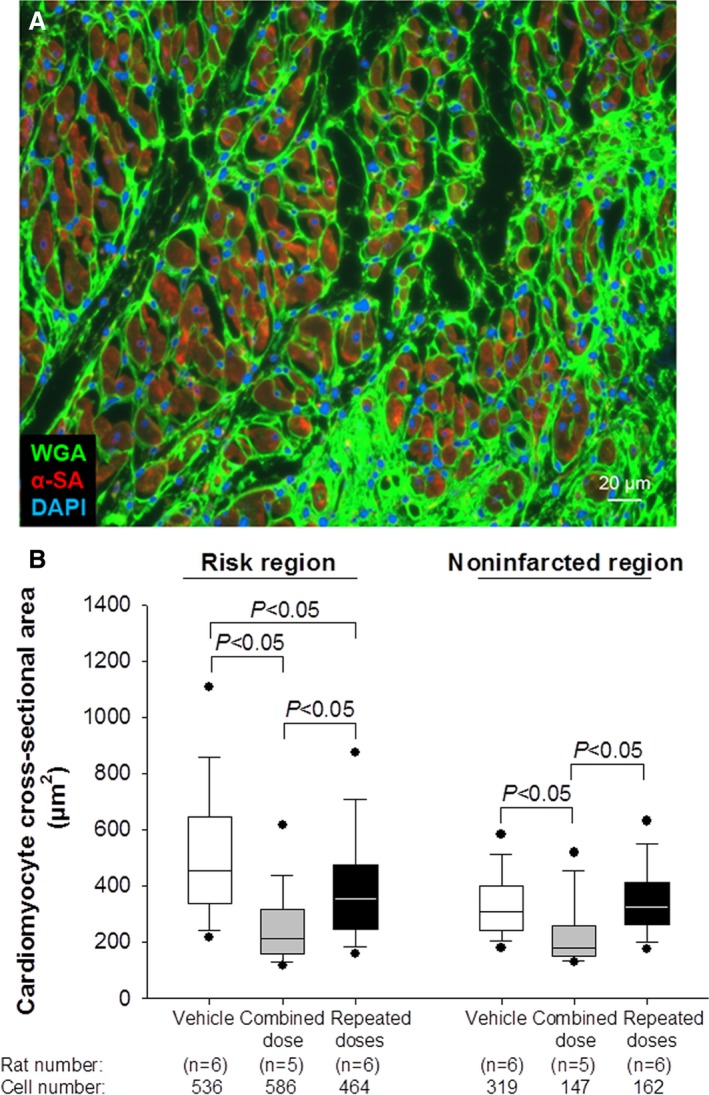
Analysis of myocyte cross‐sectional area. Myocyte cross‐sectional area and myocyte density were determined in rat hearts stained with wheat germ agglutinin (WGA) (green) and α‐sarcomeric actin (red).; A, Representative epifluorescent microscopic image, sequentially acquired from 18 fields of the infarcted region of a vehicle‐treated rat at a magnification of ×300. Myocytes with round nuclei and clearly defined sarcolemmal borders were selected for analysis of cross‐section area. DAPI indicates 4′,6‐diamidino‐2‐phenylindole. B, Box‐and‐whisker plots showing the quantitative analysis of cardiomyocyte cross‐sectional area in the risk and noninfarcted LV regions of hearts treated with vehicle (n=6, with a total of 536 and 319 cells counted in the risk and noninfarcted region, respectively), combined dose (n=5, 586 and 147 cells, respectively), or repeated doses (n=6, 464 and 162 cells, respectively). Boxes show the median and the first and third quartile (25th and 75th percentile); whiskers show the first and last decile (10th and 90th percentile); dots show the smallest and largest observations. LV indicates left ventricular.
Figure 7.
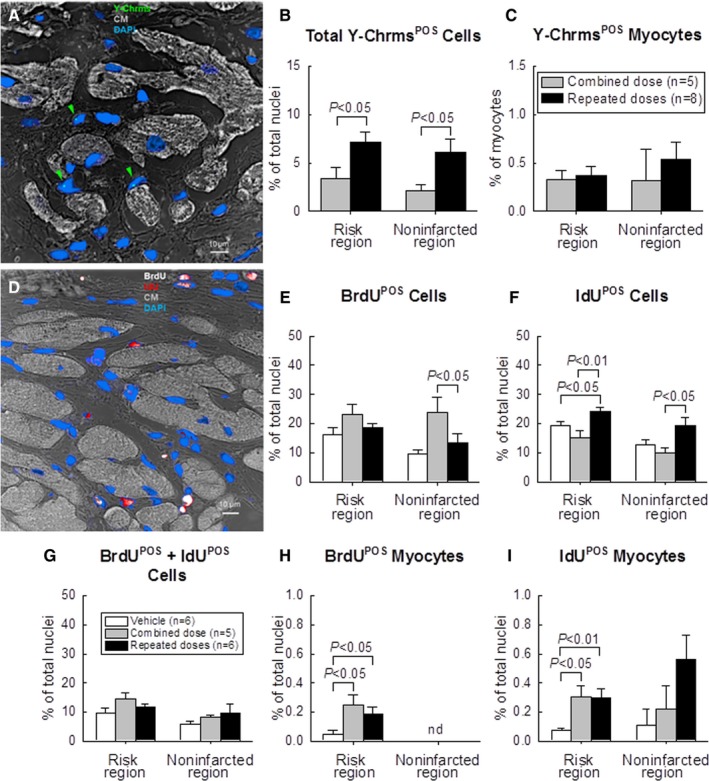
Analysis of Y‐chromosomePOS cells (A through C) and proliferation of CPCs (D through G). A, Representative confocal microscopic image acquired from the infarcted region. Green arrowheads indicate Y‐chromosome fluorescent signals (green/cyan) in nuclei. Nuclei are stained with DAPI in blue. Cardiomyocyte (CM) morphology was examined with a confocal transmitted light channel's detector (ChD) in which the pseudocolor selected for myocardial background in the ChD channel was gray white. B, Quantitative analysis of the number of total Y‐chromosomePOS cells and (C) Y‐chromosomePOS matured myocytes at 105 days after CPC transplantation. The risk region comprises both the border zones and the infarcted region. D, Representative confocal microscopic image acquired from the border zone. BrdU (given in the first 35 days after start of treatment) is shown in white and IdU (given on days 70 to 105 after start of treatment) in red. Nuclei are stained with DAPI. Cardiomyocyte (CM) morphology was examined with the confocal transmitted light ChD; the pseudocolor selected for myocardial background in the ChD channel was gray white. E through G, Quantitative analysis of the number of BrdUPOS, IdUPOS, and BrdUPOS/IdUPOS nonmyocytes at 105 days after start of CPC administration. H through J, Quantitative analysis of the number of BrdUPOS and IdUPOS myocytes at 105 days after start of CPC administration. (“Myocytes” were defined as α‐sarcomeric actin positive cells, but these cells were small and did not resemble mature myocytes.) The risk region comprises both the border zones and the infarcted region. Data are means±SEM. BrdU indicates 5‐bromo‐2′‐deoxyuridine; CPC, cardiac progenitor cells; DAPI, 4′,6‐diamidino‐2‐phenylindole; IdU, 5‐iodo‐2′‐deoxyuridine.
Fate of Transplanted CPCs
To assess the fate of the transplanted male CPCs, the number of Y‐chromosomePOS cells was measured by fluorescence in situ hybridization (Figure 7A). Y‐chromosomePOS cells were detected both in the combined‐dose and in the repeated‐dose groups. Despite the fact that the total number of CPCs administered in both groups was the same, the number of Y‐chromosomePOS cells, expressed as a percentage of total nuclei, was significantly greater in the repeated‐dose group, both in the risk and in the noninfarcted region (Figure 7B). However, the number of Y‐chromosomePOS myocytes (cells positive for both Y‐chromosome and α‐sarcomeric actin) was minuscule in both groups (<1% of total myocytes [α‐sarcomeric actin positive cells]) (Figure 7C). Furthermore, the α‐sarcomeric actin positive cells were small and did not resemble mature cardiomyocytes. These data indicate that repeated CPC infusions increase the number of CPCs that engraft in the heart; however, almost all of these cells are nonmyocytes. The number of CPCs that differentiate into mature cardiomyocytes is negligible.
Analysis of Cell Proliferation
To determine the effect of CPCs on cell proliferation, rats were given BrdU for 35 days after the first treatment (days 1–35 after start of therapy) and IdU for 35 days after the third treatment (days 71–105 after start of therapy) (Figure 1). Formation of new cells was assessed by measuring the number of BrdUPOS or IdUPOS cells (Figure 7D) As seen in Figure 7E and 7F, in all groups the number of newly formed (BrdUPOS and/or IdUPOS) cells was higher in the risk region compared with the noninfarcted region, possibly reflecting a higher density of proliferating inflammatory cells and fibroblasts. In rats that received a single large dose of CPCs at the time of the first treatment, a greater number of BrdUPOS cells was found in the noninfarcted region compared with the vehicle and repeated‐dose groups (Figure 7E), indicating that the large dose of CPCs promoted cellular proliferation over the ensuing 35 days (days 1–35 after start of therapy). This proliferative effect, however, was no longer observed on days 71 to 105, when IdU was infused (Figure 7F). In the repeated‐dose group, no increase in BrdUPOS cells was observed (Figure 7E), suggesting that the expected increase in BrdUPOS cells on days 1 to 35 abated and was no longer apparent on day 105 (day of euthanasia). However, the repeated‐dose group did exhibit a significantly greater number of IdUPOS cells both in the risk and noninfarcted regions compared with the combined‐dose and vehicle groups (Figure 7F), indicating that the third treatment on day 70 promoted cellular proliferation over the ensuing 35 days. There were no significant differences among the 3 groups with respect to BrdUPOS/IdUPOS (double positive) cells (ie, cells that resulted from proliferative activity during both days 1–35 and days 71–105) (Figure 7G).
In the risk region, the number of BrdUPOS or IdUPOS myocytes (where “myocytes” are defined as α‐sarcomeric actin positive cells) was significantly greater in the 2 cell‐treated groups compared with the vehicle group; however, no significant differences were observed in the noninfarcted region (Figure 7H and 7I). Importantly, the number of BrdUPOS or IdUPOS myocytes was minuscule (<1% of total nuclei) both in the risk region and in the noninfarcted region (Figure 7H and 7I), indicating that the beneficial effects of CPC transplantation were not accounted for by cardiomyogenesis (ie, formation of new myocytes either from CPCs or from endogenous cells).
Taken together, these data indicate that each CPC infusion stimulated cellular proliferation over the ensuing 35 days. This proliferative response may have been greater with the single large dose than with the repeated doses, but any such difference disappeared after 35 days. Although repeated doses resulted in greater persistence of transplanted CPCs (or their progeny) in the myocardium compared with a single large dose, formation of new cardiomyocytes was negligible in all groups.
Discussion
In our previous study using a constant dose of 12×106 CPCs per infusion in rats with old MI,3 we compared the beneficial effects of 3 doses of CPCs versus a single dose. We found that 3 repeated CPC infusions of 12×106 cells each were markedly more effective than a single infusion of 12.0×106 cells. However, the rats in the single‐dose group received only one third of the total number of CPCs given to the multiple‐dose group; therefore, it is unknown whether the superior therapeutic efficacy was caused by the use of repeated treatments or by the administration of a higher total number of CPCs. In the present study, we sought to answer this question by keeping the total number of CPCs constant (36×106 cells) and comparing a single administration of the entire dose (combined‐dose group) with 3 administrations, each consisting of one third of the dose (repeated‐dose group).
The salient findings of this study can be summarized as follows: (1) in rats with an old MI, percutaneous infusion of a single, large dose of CPCs (36×106 cells) into the LV cavity produced an initial robust improvement in LV function, as demonstrated by a marked increase in LV EF and decrease in ESV at 35 days after the infusion; however, no further improvement was observed after the second and third infusions (which consisted of vehicle); (2) in contrast, when the total number of 36×106 CPCs was split into 3 doses (12×106 each) given at 35‐day intervals, the improvement in LV function became progressively greater after each injection, as demonstrated by stepwise increases in LV EF, infarcted wall thickness in diastole, infarcted wall thickness in systole, infarcted wall thickening fraction, and stroke volume and decreases in ESV, such that the total changes in echocardiographic and hemodynamic parameters were greater than with a single dose; furthermore, when the 2 treated groups were compared with the vehicle group, a number of parameters of LV function that were not ameliorated in the combined‐dose group (infarcted wall thickening fraction, ESV, stroke volume, LV end‐diastolic pressure, EF measured by hemodynamic studies, LV dP/dtmax, and preload adjusted maximal power) were significantly ameliorated in the repeated‐dose group; (3) unlike rats treated with a combined dose of CPCs, those treated with repeated doses exhibited a significant decrease in expansion index, a significant increase in wall thickness and viable myocardium in the infarcted region (as determined by trichrome staining), and a significant reduction in collagen content both in the risk and noninfarcted regions (as determined by picrosirius red staining); (4) unlike rats treated with a single combined dose of CPCs, those treated with repeated doses exhibited a significant decrease in the number of immune cells in both the risk and the noninfarcted regions; and (5) although the repeated‐dose protocol resulted in greater myocardial content of transplanted CPCs (or their progeny), formation of new myocytes, either from transplanted CPCs or from endogenous sources, was negligible in all groups.
Taken together, these results demonstrate that, in this rat model of ischemic cardiomyopathy, the beneficial effects of 3 repeated doses of CPCs are superior to those of 1 combined dose even though the total number of cells infused is the same. The data further suggest that the superiority of the repeated‐dose protocol may be related to greater antifibrotic and anti‐inflammatory actions.
The present study demonstrates that splitting a dose of CPCs into 3 smaller doses given a few weeks apart is more effective than giving the same number of cells as a single dose. Initially, LV function improved to a greater extent after the combined dose than after the first of the 3 split doses (Figure 2B and 2C), presumably reflecting more intense paracrine actions associated with the larger number of cells (36 million versus 12 million). By the end of the study, however, the cumulative improvements observed after each of the 3 split doses resulted in a greater augmentation of LV function compared with the combined dose (Figure 2B and 2C). The mechanism(s) responsible for this difference is unknown. Although the 3 repeated doses increased myocardial engraftment of transplanted CPCs versus a single dose, as demonstrated by significantly more abundant Y‐chromosomePOS cells both in the risk and in the noninfarcted region (Figure 7B), the superior efficacy of repeated doses cannot be explained by greater engraftment and differentiation of CPCs because <1% of all myocytes were derived from transplanted cells (Y‐chromosomePOS myocytes) regardless of the treatment protocol (Figure 7C). One important difference between the repeated‐dose and combined‐dose groups was the intensity of inflammatory cell infiltration. Recent studies have shown that inflammation plays a crucial role in regulating a wide range of cellular processes involved in injury, repair, and remodeling of the infarcted heart.22, 23, 24 In the infarcted myocardium, necrotic cardiomyocytes release danger signals, activating an intense inflammatory and innate immune response23; immune cells accumulate in the noninfarcted regions of the failing heart21, 25 and may be a target for new therapies.24 Our finding that repeated injections of CPCs markedly reduced CD45POS cells both in the risk and in the noninfarcted region whereas a single injection did not (Figure 5B) is consistent with our previous findings in mice receiving cardiac mesenchymal cells,26 and suggests that the superiority of repeated doses may be related to more powerful anti‐inflammatory actions. Alternatively, or in addition, repeated doses may have a stronger antifibrotic effect, as suggested by the reduced collagen deposition in the noninfarcted region vis‐a‐vis the combined‐dose protocol (Figure 4E).
How repeated administrations of cells result in stronger anti‐inflammatory and antifibrotic effects remains speculative. Clearly, given the lack of significant new myocyte formation (Figure 7C), CPCs exerted their salubrious actions via paracrine mechanisms, as previously reported.3, 8 Such paracrine actions may have been exerted not only by CPCs residing in the myocardium, but also by CPCs retained in other organs such as the lungs, since a large number of CPCs delivered intracoronally are found outside of the heart, primarily in the lungs.5, 6 Regardless of the exact source (cardiac versus extracardiac) of paracrine factors, our data indicate that the duration of myocardial exposure to paracrine factors may be more important than the intensity of the exposure. This hypothesis is illustrated in Figure 8. As proposed previously,1 administration of a single dose of 36×106 CPCs results in a “spike” in myocardial (and extracardiac) CPC numbers, which then fall precipitously as the cells are cleared from the tissue (Figure 8A). These changes in CPC numbers are paralleled by similar changes in the myocardial content of paracrine factors secreted by the cells (Figure 8B). When the 36×106 cells are split into 3 smaller doses of 12×106 each, the peak levels of cells and paracrine factors are lower than with a single dose, but the total period of time during which the tissue is exposed to paracrine factors is much greater (Figure 8B). According to this paradigm, a longer release of paracrine factors at lower levels is superior to a shorter release at higher levels.
Figure 8.
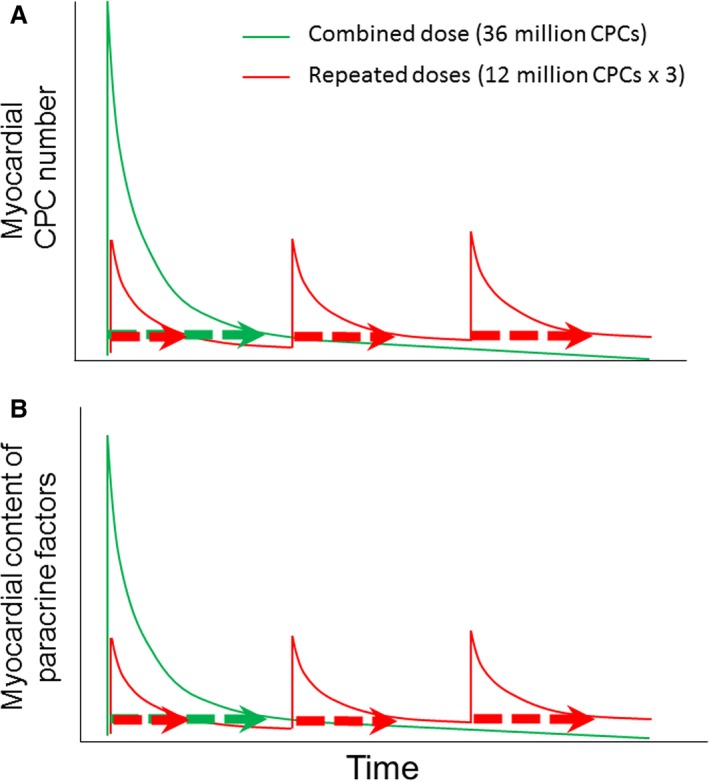
Schematic illustration of different duration of paracrine factor release with single vs multiple cell injections. As proposed previously,1 administration of a single dose of 36×106 CPCs results in a “spike” in myocardial CPC numbers, which then fall precipitously as the cells are cleared from the tissue (A, green line). These changes in CPC numbers are paralleled by similar changes in the myocardial content of paracrine factors secreted by the cells (B, green line). When the 36×106 cells are split into 3 doses of 12×106 each, the peak levels of cells and paracrine factors are lower than with a single dose, but the total period of time during which the tissue is exposed to cells and paracrine factors is much greater (A and B, respectively, 3 green dotted bars vs red dotted bar). According to this paradigm, a longer release of paracrine factors at lower levels is superior to a shorter release at higher levels. A similar paradigm would apply if the factors responsible for the beneficial effects of CPCs are released systemically by CPCs residing in extracardiac tissues rather than by CPCs residing in the heart. CPC indicates cardiac progenitor cells.
Limitations of this study include the relatively small sample sizes and the unclear mechanism of action of repeated treatments, as discussed above. Much more work will be necessary to address these issues. The secretome of transplanted cells is complex, and there are many potential mechanisms whereby it could conceivably affect cardiac structure and function. There are also many possible protocols for repeated treatments that could have different therapeutic efficacy, including variations in the number, frequency, and magnitude of cell doses.
The present study has important implications for the design of future clinical trials of cell therapy. Administration of cells is more efficacious when it is repeated.3, 4 However, subjecting patients to multiple cardiac catheterizations entails additional risks, costs, and logistical challenges that could be avoided if the same results could be achieved with 1 larger dose of cells. Our data suggest that this is not the case: the 2 additional injections in the repeated‐dose group could not be replaced simply by tripling the number of cells in the combined‐dose group. An interesting corollary of these findings is that the beneficial effects of cell therapy may be more repetition dependent than dose dependent.
In conclusion, this is the first study to compare the effects of a constant amount of cells given as a single treatment versus repeated treatments. Despite relatively small sample sizes, our results demonstrate that the salubrious effects of 36×106 CPCs on LV function are significantly greater when the cells are divided into 3 doses of 12×106 given 35 days apart than when they are given as a single dose, thereby supporting the concept that repeated treatments are necessary to achieve the full potential of cell therapy.
Sources of Funding
This study was supported in part by NIH grants HL113530 and HL‐78825.
Disclosures
None.
Supporting information
Table S1. LV Ejection Fraction, LV Volume, and Scar Size Before and After Exclusions (Mean±SEM)
Figure S1. A and B, Time course of LV ejection fraction (EF) in individual rats (dotted line with open symbols) and group means±SEM (solid line with solid symbols) in the 3 groups. The 2 dotted reference lines indicate baseline values (upper) and the exclusion cutoff (lower line), which is 15 EF units below baseline. A, All animals that completed the study protocol (with no exclusions). Blue lines denote 2 rats in the combined‐dose group that were excluded because of scar <10% of LV (these rats had a drop in EF=15.5 and 16.2 units) and 1 rat in the repeate‐doses group that was excluded because of an EF drop <15 units after MI (Pre‐Rx) (specifically, 13.9 units). B, Animals analyzed after exclusion. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). EF was assessed using 2D long‐axis images. Note that the difference in EF between the combined dose and the multiple doses groups at the end of the study is similar with and without exclusions. C and D, Time course of LV end‐diastolic volume (EDV) in individual rats (dotted line with open symbols) and in group mean±SEM (solid line with solid symbols) of the 3 groups. C, All animals that completed the entire study protocol before exclusion. As seen in blue, 3 rats (2 in the combined dose and 1 in the repeated doses) were excluded as detailed in the previous supplementary figure. D, Animals analyzed after exclusions. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). EDV was assessed using 2D long‐axis images. E and F, Time course of LV end‐systolic volume (ESV) in individual rats (dotted line with open symbols) and in group mean±SEM (solid line with solid symbols) of the 3 groups. E, All animals that completed the entire study protocol before exclusions. As seen in blue, 3 rats (2 in the combined dose and 1 in the repeated doses) were excluded as detailed in the previous figures. F, Animals analyzed after exclusions. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). ESV was assessed using 2D long axis images. G, Scar size (% of LV) in individual rats (open symbols) and group mean±SEM (solid symbols) in the 3 groups. Scar size was determined after LV sections were stained with Masson's trichrome. The solid blue diamond represents the group mean before exclusions (n=7). As seen in the blue symbols, 2 rats in the combined‐dose group were excluded because of small scar size (<10% of LV; 7.2 and 8.5% of LV). These 2 rats also had a small decrease in EF before the first treatment (15.5 and 16.2 EF units). No pathology analysis was performed in the rat excluded in the repeated‐doses group because this animal was excluded because of an EF drop <15 EF units.
(J Am Heart Assoc. 2018;7:e007400 DOI: 10.1161/JAHA.117.007400.)29440036
References
- 1. Bolli R. Repeated cell therapy: a paradigm shift whose time has come. Circ Res. 2017;120:1072–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolli R, Ghafghazi S. Stem cells: cell therapy for cardiac repair: what is needed to move forward? Nat Rev Cardiol. 2017;14:257–258. [DOI] [PubMed] [Google Scholar]
- 3. Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res. 2016;119:635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guo Y, Wysoczynski M, Nong Y, Tomlin A, Zhu X, Gumpert AM, Nasr M, Muthusamy S, Li H, Book M, Khan A, Hong KU, Li Q, Bolli R. Repeated doses of cardiac mesenchymal cells are therapeutically superior to a single dose in mice with old myocardial infarction. Basic Res Cardiol. 2017;112:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong KU, Guo Y, Li QH, Cao P, Al‐Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. C‐kit+ cardiac stem cells alleviate post‐myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c‐kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30‐day‐old infarction. Circulation. 2010;121:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang XL, Li Q, Rokosh G, Sanganalmath S, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long‐term outcome of administration of c‐kitPOS cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang XL, Rokosh G, Sanganalmath SK, Tokita Y, Keith MC, Shirk G, Stowers H, Hunt GN, Wu W, Dawn B, Bolli R. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ Heart Fail. 2015;8:757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O'Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ernst C, Christie BR. Isolectin‐IB 4 as a vascular stain for the study of adult neurogenesis. J Neurosci Methods. 2006;150:138–142. [DOI] [PubMed] [Google Scholar]
- 13. Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi‐organ, multi‐lineage engraftment by a single bone marrow‐derived stem cell. Cell. 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 14. Li XY, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open‐chest dogs. J Clin Invest. 1993;92:1025–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bolli R, Zughaib M, Li XY, Tang XL, Sun JZ, Triana JF, McCay PB. Recurrent ischemia in the canine heart causes recurrent bursts of free radical production that have a cumulative effect on contractile function. A pathophysiological basis for chronic myocardial “stunning”. J Clin Invest. 1995;96:1066–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101:2981–2988. [DOI] [PubMed] [Google Scholar]
- 17. Takano H, Bolli R, Black RG Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A(1) or a(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. [DOI] [PubMed] [Google Scholar]
- 18. Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. Pkcepsilon modulates NF‐kappaB and AP‐1 via mitogen‐activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–H1689. [DOI] [PubMed] [Google Scholar]
- 19. Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C‐epsilon‐Raf‐1‐MEK‐1/2‐p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase‐2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Wang H, Li J. Inflammation and inflammatory cells in myocardial infarction and reperfusion injury: a double‐edged sword. Clin Med Insights Cardiol. 2016;10:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res. 2012;94:276–283. [DOI] [PubMed] [Google Scholar]
- 24. Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 2016;167:152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wysoczynski M, Guo Y, Moore JBt, Muthusamy S, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin AA, Zhu X, Hunt G, Gumpert AM, Book MJ, Khan A, Tang XL, Bolli R. Myocardial reparative properties of cardiac mesenchymal cells isolated on the basis of adherence. J Am Coll Cardiol. 2017;69:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. LV Ejection Fraction, LV Volume, and Scar Size Before and After Exclusions (Mean±SEM)
Figure S1. A and B, Time course of LV ejection fraction (EF) in individual rats (dotted line with open symbols) and group means±SEM (solid line with solid symbols) in the 3 groups. The 2 dotted reference lines indicate baseline values (upper) and the exclusion cutoff (lower line), which is 15 EF units below baseline. A, All animals that completed the study protocol (with no exclusions). Blue lines denote 2 rats in the combined‐dose group that were excluded because of scar <10% of LV (these rats had a drop in EF=15.5 and 16.2 units) and 1 rat in the repeate‐doses group that was excluded because of an EF drop <15 units after MI (Pre‐Rx) (specifically, 13.9 units). B, Animals analyzed after exclusion. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). EF was assessed using 2D long‐axis images. Note that the difference in EF between the combined dose and the multiple doses groups at the end of the study is similar with and without exclusions. C and D, Time course of LV end‐diastolic volume (EDV) in individual rats (dotted line with open symbols) and in group mean±SEM (solid line with solid symbols) of the 3 groups. C, All animals that completed the entire study protocol before exclusion. As seen in blue, 3 rats (2 in the combined dose and 1 in the repeated doses) were excluded as detailed in the previous supplementary figure. D, Animals analyzed after exclusions. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). EDV was assessed using 2D long‐axis images. E and F, Time course of LV end‐systolic volume (ESV) in individual rats (dotted line with open symbols) and in group mean±SEM (solid line with solid symbols) of the 3 groups. E, All animals that completed the entire study protocol before exclusions. As seen in blue, 3 rats (2 in the combined dose and 1 in the repeated doses) were excluded as detailed in the previous figures. F, Animals analyzed after exclusions. BSL, baseline (before MI); Pre‐Rx, pretreatment (30 days after coronary occlusion/reperfusion); first, second, and third, 35 days after the first, second, and third treatment with vehicle, combined dose (1 dose of 36×106 CPCs) or repeated doses (3 doses of 12×106 CPCs repeated at a 35‐day interval). ESV was assessed using 2D long axis images. G, Scar size (% of LV) in individual rats (open symbols) and group mean±SEM (solid symbols) in the 3 groups. Scar size was determined after LV sections were stained with Masson's trichrome. The solid blue diamond represents the group mean before exclusions (n=7). As seen in the blue symbols, 2 rats in the combined‐dose group were excluded because of small scar size (<10% of LV; 7.2 and 8.5% of LV). These 2 rats also had a small decrease in EF before the first treatment (15.5 and 16.2 EF units). No pathology analysis was performed in the rat excluded in the repeated‐doses group because this animal was excluded because of an EF drop <15 EF units.


