Abstract
Background
Global longitudinal strain (GLS), reflecting total shortening of the myocardium during the cardiac cycle, has emerged as a more precise myocardial function measure than left ventricular ejection fraction (LVEF). Longitudinal strain may be selectively affected in subclinical heart disease, even in the presence of normal LVEF. This study examines subclinical cardiac dysfunction, assessed by GLS and LVEF, and cognition among older adults.
Methods and Results
Vanderbilt Memory and Aging Project participants who were free of clinical dementia, stroke, and heart failure (n=318, 73±7 years, 58% male) completed neuropsychological assessment and cardiac magnetic resonance to quantify GLS and LVEF. Linear regression models related GLS and LVEF to neuropsychological performances, adjusting for age, sex, race/ethnicity, education, Framingham Stroke Risk Profile, cognitive diagnosis, and APOE*ε4 status. Models were repeated with a cardiac×cognitive diagnosis interaction term. Compromised GLS (reflected by higher values) related to worse naming (β=−0.07, P=0.04), visuospatial immediate recall (β=−0.83, P=0.03), visuospatial delayed recall (β=−0.22, P=0.03), and verbal delayed recall (β=−0.11, P=0.007). LVEF did not relate to worse performance on any measure (P>0.18). No diagnostic interactions were observed.
Conclusions
Our study results are among the first to suggest that compromised GLS relates to worse episodic memory and language performance among older adults who are free of clinical dementia, stroke, and heart failure. Subclinical cardiac dysfunction may correlate with cognitive health in late life, even when LVEF remains normal. The results add to growing evidence that GLS may be a more sensitive and preferred method for quantifying subclinical changes in cardiac function.
Keywords: brain, cardiac magnetic resonance imaging, cognition, global longitudinal strain, vascular risk factors
Subject Categories: Heart Failure, Magnetic Resonance Imaging (MRI), Contractile function, Aging, Physiology
Clinical Perspective
What Is New?
Our study results suggest impaired global longitudinal strain relates to worse episodic memory and language performance among older adults who are free of clinical dementia, stroke, and heart failure, and such subclinical cardiac dysfunction may affect cerebral blood flow homeostasis, resulting in oligemia and compromised cognitive functions mediated by the temporal lobes in the brain.
What Are the Clinical Implications?
Understanding the association between subclinical cardiac dysfunction and cognitive impairment may allow for earlier detection and intervention to prevent more serious cognitive changes associated with compromised cardiovascular health.
Severe cardiac dysfunction, as measured by left ventricular ejection fraction (LVEF), is associated with cognitive impairment.1, 2 However, reduced LVEF represents a mid to late feature of impaired myocardial contractility3 and is normal in 50% of individuals with clinical heart failure.4 Emerging evidence suggests an association between subclinical heart dysfunction and worse brain health in older adults,5 but LVEF may not detect subclinical dysfunction6 or relate to cognitive changes in the absence of severe cardiac dysfunction.
In recent years, cardiac strain has emerged as a more sensitive measure of myocardial function. Global longitudinal strain (GLS) assesses the total deformation or shortening during the cardiac cycle of longitudinal myocardial fibers located predominantly in the subendocardium.7 Because the subendocardium is the layer most susceptible to ischemia,7 GLS may provide a more accurate measure of cardiac contractility6 or subclinical heart disease8 even when LVEF is normal. GLS has also been shown to have prognostic value in identifying cardiac dysfunction independent9 of and additive10 to LVEF. Thus, GLS may be more sensitive in detecting an association between subclinical cardiac dysfunction and cognition in comparison to LVEF. In a prior study, worse GLS was associated with increased cerebral small vessel disease among older adults without prevalent cardiovascular disease (CVD).6 However, cardiac strain has not been thoroughly evaluated in relation to cognition among aging adults who endure decades of vascular risk factor exposure.11, 12
The purpose of this cross‐sectional study was to examine subclinical cardiac dysfunction, assessed by GLS and LVEF, in relation to neuropsychological performance among older adults without clinical dementia, stroke, or heart failure. We hypothesized that GLS would relate to cognition, especially memory13, 14 and executive function,15, 16 known to be most affected by compromised cardiovascular function. We also hypothesized that GLS would have stronger associations with cognition than LVEF.
Methods
Study Cohort
The Vanderbilt Memory and Aging Project is a longitudinal study investigating vascular health and brain aging, enriched for mild cognitive impairment (MCI). Inclusion required participants be aged ≥60 years, speak English, have adequate auditory and visual acuity, and have a reliable study partner. At eligibility, participants underwent medical history and record review, a clinical interview (including a functional questionnaire and Clinical Dementia Rating17 with the informant), and neuropsychological assessment. Participants were excluded for a cognitive diagnosis other than normal cognition (NC), early MCI,18 or MCI19; contraindication to magnetic resonance imaging; history of neurological disease (eg, stroke); prevalent heart failure; major psychiatric illness; head injury with loss of consciousness >5 minutes; and systemic or terminal illness affecting follow‐up examination participation. At enrollment, participants completed a comprehensive evaluation, including (but not limited to) fasting blood draw, physical examination, a clinical interview, medication review, neuropsychological assessment, and cardiac magnetic resonance (CMR) imaging. Participants were excluded from the current study for missing CMR, covariate, or neuropsychological data (see Figure 1 for inclusion and exclusion details).
Figure 1.
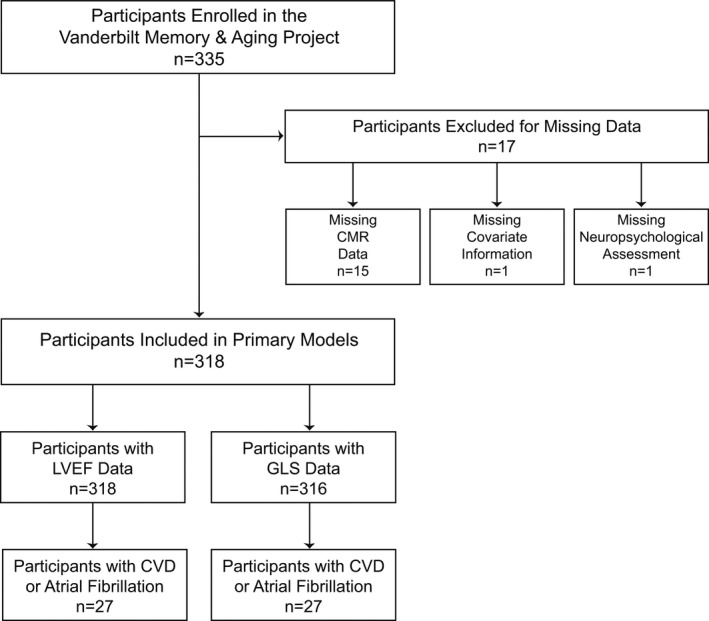
Participant inclusion and exclusion details. Missing data categories are mutually exclusive. In secondary models, sensitivity analyses excluded participants with CVD or atrial fibrillation. CMR indicates cardiac magnetic resonance; CVD, cardiovascular disease; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
Standard Protocol Approvals, Registrations, and Participant Consent
The protocol was approved by the Vanderbilt University Medical Center institutional review board. Written informed consent was obtained before data collection. The data, analytic methods, and study materials are unavailable to other researchers for purposes of reproducing the results or replicating procedures because of participant consent restrictions on data sharing.
CMR Imaging
CMR imaging was acquired at Vanderbilt University Medical Center using a 1.5‐T Siemens Avanto system with a phased‐array torso receiver coil. Left and right ventricular volumes and function were assessed using a breath‐hold, ECG‐synchronized, cine steady‐state free precession sequence with the following parameters: repetition time, 180 ms; echo time, 1.1 ms; flip angle, 80°; field of view, 300 to 340 mm; and 156×192 matrix. Short‐ and long‐axis imaging planes were acquired. Under the supervision of a board‐certified radiologist (J.J.C.), trained analysts blinded to all clinical information (J.G.T., S.N.) drew endocardial and epicardial contours at end systole and end diastole to quantify left ventricular (LV) volume and function using QMass MR 7.6 Enterprise Solution (Medis). Ejection fraction (percentage) was calculated as stroke volume divided by end‐diastolic volume multiplied by 100. Longitudinal strain was assessed for systole using QStrain RE 2.0 (Medis). A trained analyst drew contours in a semiautomated manner in the 4‐ and 2‐chamber long‐axis images in QMass (Medis). These contours, along with LV epicardial and endocardial contours drawn in QMass were imported into QStrain. Base, middle, and apical slices were iteratively selected in a manual fashion for automated analyses. At each step, the analyst reviewed image clips generated in QStrain to ensure that tracking curves and vectors were correct and that selected images yielded accurate results. Strain results were determined per cardiac segment using the 17‐segment model and averaged to yield a global value for the longitudinal strain orientation. GLS is represented by a negative value to measure the shortening of the myocardium during the cardiac cycle as a percentage of its original length. Lower (ie, more negative) values indicate increased systolic shortening and thus better function.
Neuropsychological Assessment
Participants completed a common, comprehensive neuropsychological protocol assessing language, information processing speed, executive functioning, visuospatial skills, and episodic memory (see Table 1 for details). Measures were carefully selected to preclude floor or ceiling effects and were not used to screen or select participants into the study.
Table 1.
Participant Characteristics
| Total (n=318) | NC (n=166) | eMCI (n=27) | MCI (n=125) | |
|---|---|---|---|---|
| Demographic and health characteristics | ||||
| Age, y | 73±7 | 72±7 | 73±6 | 73±8 |
| Sex, % male | 58 | 58 | 74 | 55 |
| Race, % non‐Hispanic white | 87 | 88 | 85 | 86 |
| Education, y | 16±3 | 16±2 | 16±3 | 15±3 |
| Montreal Cognitive Assessment, total | 25±3 | 27±2 | 25±2 | 23±3 |
| APOE*ε4 % carrier | 34 | 29 | 22 | 44 |
| Framingham Stroke Risk Profile, totala | 12.3±4.2 | 11.7±4.1 | 13.4±3.2 | 12.8±4.3 |
| Systolic blood pressure, mm Hg | 142±18 | 140±17 | 150±18 | 145±19 |
| Antihypertensive medication usage, % | 53 | 52 | 56 | 54 |
| Diabetes mellitus, % | 18 | 14 | 22 | 22 |
| Cigarette smoking, % current | 2 | 1 | 4 | 2 |
| Prevalent CVD, % | 3 | 4 | 0 | 3 |
| Atrial fibrillation, % | 5 | 5 | 11 | 5 |
| LV hypertrophy, % | 4 | 2 | 4 | 6 |
| Global longitudinal strain, % | −21.6±4.5 | −21.9±4.7 | −21.0±4.9 | −21.4±4.2 |
| LVEF, % | 63.5±7.8 | 64.0±7.4 | 63.0±10.0 | 63.0±7.9 |
| Neuropsychological performances | ||||
| Boston Naming Test | 26.8±3.1 | 27.9±2.0 | 26.6±2.4 | 25.3±3.8 |
| Animal Naming | 18.9±5.4 | 20.9±4.8 | 19.4±3.4 | 16.20±5.2 |
| WAIS‐IV Coding | 52.7±12.8 | 57.5±11.5 | 53.4±11.2 | 46.3±12.1 |
| D‐KEFS Number Sequencing Test, sb | 42.0±18.9 | 35.9±12.6 | 42.0±13.2 | 50.3±23.4 |
| Executive Function composite score | 0.01±0.90 | 0.43±0.62 | 0.17±0.42 | −0.59±0.96 |
| D‐KEFS Number–Letter Switching Test, sb | 107.3±48.3 | 86.5±34.1 | 93.0±22.1 | 138.3±52.3 |
| D‐KEFS Tower Test | 14.97±4.66 | 16.13±4.34 | 16.19±3.53 | 13.16±4.74 |
| D‐KEFS Color–Word Inhibition Test, sb | 69.2±23.5 | 60.0±13.5 | 74.6±15.5 | 80.0±29.6 |
| Letter Fluency (FAS) Test | 38.7±11.6 | 42.9±11.4 | 37.9±11.1 | 33.3±9.7 |
| Hooper Visual Organization Test | 24.5±3.1 | 25.4±2.5 | 24.7±2.2 | 23.3±3.6 |
| Memory composite score | 0.01±0.95 | 0.57±0.70 | −0.06±0.76 | −0.72±0.75 |
| CVLT‐II Total Immediate Recall | 40.6±11.8 | 47.1±9.3 | 40.1±9.7 | 32.2±9.6 |
| CVLT‐II Delayed Recall | 8.1±4.2 | 10.5±3.3 | 7.6±3.5 | 5.1±3.4 |
| CVLT‐II Recognition | 2.4±1.0 | 3.0±0.7 | 2.3±0.8 | 1.7±0.9 |
| BFLT Total Immediate Recall | 112.7±40.7 | 136.1±29.5 | 110.0±28.0 | 82.0±35.0 |
| BFLT Delayed Recall | 27.0±10.4 | 32.6±7.5 | 28.0±6.6 | 19.4±9.7 |
| BLFT Recognition | 0.7±0.2 | 0.8±0.2 | 0.7±0.2 | 0.6±0.2 |
Values denoted as mean±SD or frequency; all neuropsychological performance values are total correct excluding timed tasks measured in seconds. The Boston Naming Test was the 30‐item odd version. APOE*ε4 indicates apolipoprotein E ε4 allele; BFLT, Biber Figure Learning Test; CVD, cardiovascular disease; CVLT‐II, California Verbal Learning Test, Second Edition; D‐KEFS, Delis–Kaplan Executive Function System; eMCI, early mild cognitive impairment; LV, left ventricular; LVEF, left ventricular ejection fraction; MCI, mild cognitive impairment; NC, normal cognition; WAIS‐IV, Wechsler Adult Intelligence Scale, Fourth Edition.
A modified Framingham Stroke Risk Profile Score was included in statistical models, which excluded points assigned to age (total 6.4±3.0, NC 6.0±2.9, eMCI 7.4±2.6, MCI 6.8±3.3).
Higher values in speeded test results indicate worse performance.
Statistical Analyses
Systolic blood pressure was the mean of 2 measurements. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL, hemoglobin A1c ≥6.5%, or oral hypoglycemic or insulin medication usage. Medication review determined antihypertensive medication use. LV hypertrophy was defined on echocardiogram (LV mass index >115 g/m2 in men, >95 g/m2 in women). Self‐report atrial fibrillation was corroborated by any one of the following sources: echocardiogram, documented prior procedure or ablation for atrial fibrillation, CMR, or medication usage for atrial fibrillation. Current cigarette smoking (yes or no within the year before baseline) was ascertained by self‐report. Self‐reported prevalent CVD with supporting medical record evidence included coronary heart disease, angina, or myocardial infarction (note, heart failure was a parent‐study exclusion). Framingham Stroke Risk Profile (FSRP) score was calculated by applying points by sex for age, systolic blood pressure accounting for antihypertensive medication usage, diabetes mellitus, current cigarette smoking, LV hypertrophy, CVD, and atrial fibrillation. APOE (apolipoprotein E) genotyping was performed on whole blood samples. APOE ε4 (APOE*ε4) carrier status was defined as positive (ε2/ε4, ε3/ε4, ε4/ε4) or negative (ε2/ε2, ε2/ε3, ε3/ε3).20
To minimize multiple comparisons, 2 cognitive composites were calculated using previously published methodology.21 First, a memory composite was calculated leveraging item‐level data from the California Verbal Learning Test, Second Edition (CVLT‐II) and the Biber Figure Learning Test (BFLT). Briefly, a bifactor latent variable model was calculated in Mplus (version 7.4, https://www.statmodel.com/) in which each indicator was treated as a continuous variable and loaded on both a general factor and a test factor (CVLT‐II or BFLT to remove nuisance test effects). For both tests, the model included Trials 1 to 5 Total Learning, Immediate Recall, Delayed Recall, and Recognition. The memory composite model fit the data well (ie, root mean square error of approximation: 0.09; comparative fit index: 0.95; Tucker‐Lewis Index: 0.94) and is graphically summarized in Figure S1. Next, an executive function composite was calculated leveraging item‐level data from the Delis–Kaplan Executive Function System (D‐KEFS) Color–Word Inhibition Test, D‐KEFS Tower Test, Letter Fluency (FAS) Test, and D‐KEFS Letter–Number Switching Test in which each indicator was treated as a continuous variable. The executive function composite model also fit the data well (ie, root mean square error of approximation: 0.03; comparative fit index: 0.99; Tucker‐Lewis Index: 0.99) and is graphically summarized in Figure S2. The final composite scores represent z scores.
For hypothesis testing, linear regression models with ordinary least square estimates related GLS to cross‐sectional neuropsychological test performances (1 outcome per model). Models were repeated using LVEF as the predictor. All models were adjusted for age, sex, race/ethnicity, education, modified FSRP (excluding points assigned for age), APOE*ε4, and cognitive diagnosis. Sensitivity analyses were performed excluding participants with atrial fibrillation and prevalent CVD. Models were repeated with cognitive diagnosis (NC, MCI) as an interaction term (excluding participants with early MCI because of small sample size). To formally test whether GLS had stronger associations with cognition than LVEF, a bootstrapping approach was applied to statistically compare the variance in each statistically significant outcome explained by GLS with the variance explained by LVEF using Spearman semipartial correlation. Significance was set a priori at P<0.05. Analyses were conducted using R 3.3.2 (https://www.r-project.org). Post hoc power calculations were conducted using an F distribution based on increased R 2 in a main effect model that included the predictor plus covariates for each outcome (ie, squared semipartial correlation between the predictor and outcome adjusting for covariates). Based on the available sample size (n=318) and type I error of 0.05, the power to detect the increased R 2 for each predictor–outcome pair consistently exceeded 90, suggesting models were sufficiently powered to detect effects between GLS or LVEF and each cognitive outcome of interest.
Results
Participant Characteristics
Participants included 318 adults aged 60 to 92 years (73±7 years), 58% were men, and 87% self‐identified as non‐Hispanic white. GLS ranged from −35.5% to −10.7%. LVEF ranged from 27.0% to 83.1%. See Table 1 for sample characteristics.
GLS and Neuropsychological Performances
Compromised GLS (reflected by higher values) related to worse Boston Naming Test performance (β=−0.07, P=0.04; see Figure 2A) and episodic memory composite score (β=−0.02, P=0.04, see Figure 2B). In secondary models examining the individual indices comprising the memory composite, compromised GLS related to worse BFLT Immediate Recall (β=−0.83, P=0.03; see Figure 2C), BFLT Delayed Recall (β=−0.22, P=0.03; see Figure 2D), and CVLT‐II Delayed Recall performances (β=−0.11, P=0.007; see Figure 2E). GLS was unrelated to all remaining measures (P>0.05; Table 2). In sensitivity analyses excluding participants with prevalent CVD or atrial fibrillation, GLS remained associated with Boston Naming Test (β=−0.08, P=0.046), CVLT‐II Delayed Recall (β=−0.10, P=0.02), and BFLT Immediate Recall (β=−0.81, P=0.046) performance. All remaining associations were attenuated (P>0.05), including the episodic memory composite (P=0.10). Among the entire sample, the GLS×cognitive diagnosis interaction term was unrelated to all neuropsychological performances (P>0.19).
Figure 2.
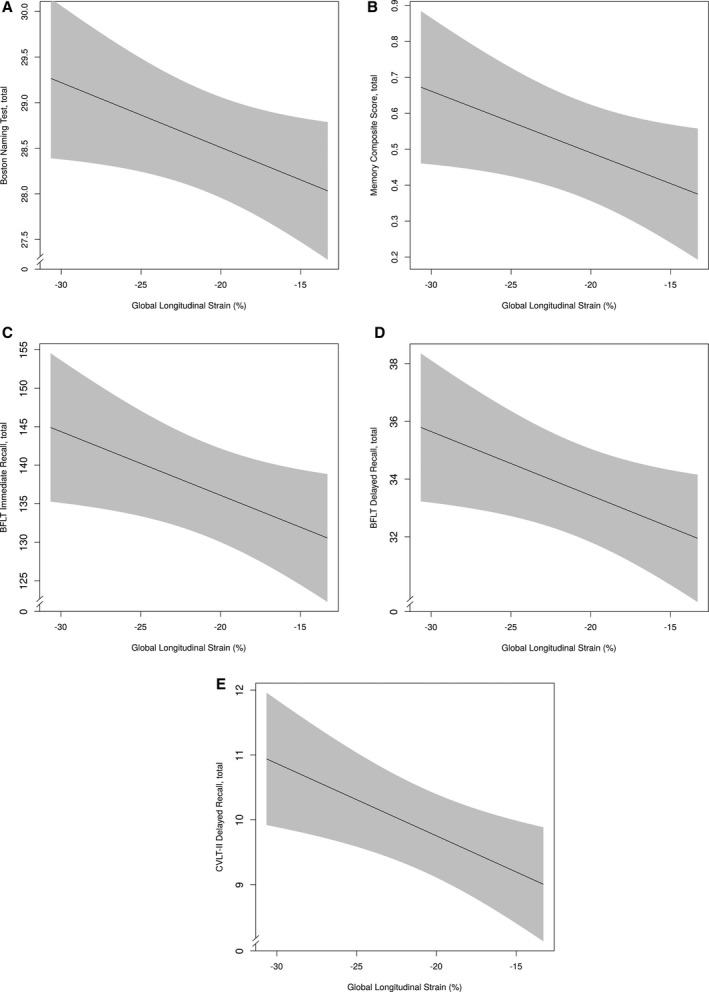
GLS and neuropsychological outcomes. Solid black line reflects fitted (predicted) values of cognitive outcomes (y‐axis) corresponding to cardiac strain (x‐axis) for a given participant profile (using prevalent level for categorical variables and median for continuous variables). Shading reflects 95% confidence interval. A, GLS and Boston Naming Test. B, GLS and memory composite. C, GLS and BFLT Immediate Recall, total. D, GLS and BFLT Delayed Recall, total. E, GLS and CVLT‐II Delayed Recall, total. BFLT indicates Biber Figure Learning Test; CVLT‐II, California Verbal Learning Test, Second Edition; GLS, global longitudinal strain.
Table 2.
Cardiac Dysfunction and Neuropsychological Performance
| GLS | LVEF | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | |
| Boston Naming Test | −0.07 | −0.14, −0.002 | 0.04 | −0.005 | −0.04, 0.03 | 0.82 |
| Animal naming | −0.06 | −0.17, 0.06 | 0.33 | −0.008 | −0.07, 0.06 | 0.81 |
| WAIS‐IV coding | 0.003 | −0.27, 0.28 | 0.98 | −0.02 | −0.17, 0.14 | 0.84 |
| D‐KEFS Number Sequencing Test, sa | −0.38 | −0.80, 0.04 | 0.08 | 0.27 | 0.03, 0.50 | 0.02 |
| Executive composite | −0.004 | −0.02, 0.01 | 0.64 | −0.003 | −0.01, 0.007 | 0.57 |
| D‐KEFS Letter–Number Switching Test, sa | −0.006 | −0.97, 0.96 | 0.99 | 0.30 | −0.24, 0.83 | 0.27 |
| D‐KEFS Tower Test | −0.05 | −0.16, 0.06 | 0.37 | 0.006 | −0.05, 0.07 | 0.85 |
| D‐KEFS Color–Word Inhibition Test, sa | 0.23 | −0.30, 0.76 | 0.39 | −0.07 | −0.37, 0.23 | 0.63 |
| Letter Fluency (FAS) Test | −0.08 | −0.34, 0.18 | 0.52 | 0.03 | −0.11, 0.18 | 0.65 |
| Hooper Visual Organization Test | −0.01 | −0.09, 0.06 | 0.69 | 0.02 | −0.02, 0.06 | 0.34 |
| Memory composite | −0.02 | −0.03, −0.0005 | 0.04 | 0.006 | −0.004, 0.02 | 0.26 |
| CVLT‐II total immediate recall | −0.12 | −0.35, 0.10 | 0.28 | 0.08 | −0.04, 0.21 | 0.19 |
| CVLT‐II delayed recall | −0.11 | −0.19, 0.03 | 0.007 | 0.02 | −0.03, 0.07 | 0.40 |
| CVLT‐II recognition | −0.02 | −0.04, 0.002 | 0.07 | 0.004 | −0.007, 0.02 | 0.43 |
| BFLT total immediate recall | −0.83 | −1.59, −0.07 | 0.03 | −0.04 | −0.47, 0.39 | 0.86 |
| BFLT delayed recall | −0.22 | −0.42, −0.02 | 0.03 | 0.01 | −0.10, 0.12 | 0.86 |
| BFLT recognition | −0.004 | −0.009, 0.00 | 0.05 | 0.001 | −0.001, 0.004 | 0.29 |
Analyses performed on n=318 participants. Models were adjusted for age, race/ethnicity, education, Framingham Stroke Risk Profile minus age, cognitive diagnosis, and APOE*ε4 status; β indicates the change in outcome as a function of 1‐U increase in the raw value of the predictor; neuropsychological performance values are total correct excluding timed tasks measured in seconds. The Boston Naming Test was the 30‐item odd version. BFLT indicates Biber Figure Learning Test; CI, confidence interval; CVLT‐II, California Verbal Learning Test, Second Edition; D‐KEFS, Delis–Kaplan Executive Function System; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; WAIS‐IV, Wechsler Adult Intelligence Scale, Fourth Edition.
Higher values in speeded test results indicate worse performance.
LVEF and Neuropsychological Performances
LVEF was unrelated to worse performance on any neuropsychological measure (P>0.19; Table 2). However, higher (healthier) LVEF was related to slower (worse) Number Sequencing Performance (β=0.27, P=0.02), an observation that persisted in a model unadjusted for covariates (β=0.32, P=0.02) and in a fully adjusted model when participants with prevalent CVD or atrial fibrillation were excluded (β=0.26, P=0.051). Among the entire sample, the LVEF×cognitive diagnosis interaction term was not associated with any neuropsychological performances (P>0.08).
Given the counterintuitive finding between LVEF and Number Sequencing performance, post hoc analyses explored potential interactions, all of which yielded null results, including LVEF×age (P=0.93), LVEF×sex (P=0.32), LVEF×cognitive diagnosis (P=0.66), LVEF×race/ethnicity (P=0.13), LVEF×education (P=0.25), LVEF×FSRP (P=0.46), and LVEF×APOE*ε4 (P=0.63). Despite null interaction models, stratified results suggested the counterintuitive finding was present in participants who were APOE*ε4 negative (β=0.23, P=0.05), were male (β=0.36, P=0.02), were younger (β=0.28, P=0.09), had less education (β=0.39, P=0.01), and had lower FSRP scores (excluding points assigned to age, β=0.35, P=0.03). However, all 3‐way interaction combinations were null (data not shown).
Statistical Comparisons of Effect Size Estimates for GLS and LVEF
Among the 6 significant cognitive outcomes, GLS was more strongly related to CVLT‐II Delayed Recall (P=0.04), BFLT Total Immediate Recall (P=0.008), and BFLT Delayed Recall (P=0.03) than LVEF. There was no difference in variance explained by GLS and LVEF for the remaining 3 measures. See Table 3 for full semipartial correlation results.
Table 3.
Statistical Comparison of Effect Size Estimates
| Semipartial Correlationa | P Value | ||
|---|---|---|---|
| GLS | LVEF | ||
| Boston Naming Test | −0.06 | −0.02 | 0.25 |
| D‐KEFS Number Sequencing Test | −0.01 | 0.07 | 0.87 |
| Memory composite | −0.09 | 0.05 | 0.15 |
| CVLT‐II Delayed Recall | −0.12 | 0.04 | 0.04 |
| BFLT Total Immediate Recall | −0.12 | −0.003 | 0.008 |
| BFLT Delayed Recall | −0.10 | 0.008 | 0.03 |
Analyses performed on n=318 participants. The Boston Naming Test was the 30‐item odd version. BFLT indicates Biber Figure Learning Test; CVLT‐II, California Verbal Learning Test, Second Edition; D‐KEFS, Delis–Kaplan Executive Function System; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction.
Spearman semipartial correlations with models adjusted for age, race/ethnicity, education, Framingham Stroke Risk Profile minus age, and cognitive diagnosis.
Discussion
Among community‐dwelling older adults free of clinical dementia, stroke, and prevalent heart failure, compromised GLS related to worse episodic memory and language performance. Using comprehensive and sensitive CMR methods for quantifying cardiac function, associations were statistically independent of key covariates, including vascular risk factors, prevalent CVD, and atrial fibrillation. In contrast, LVEF was not related to worse performance on any cognitive measure.
Our study is among the first to use GLS as a measure of cardiac dysfunction in relation to cognition. Although LVEF is the most commonly used measure of cardiac function, it is load dependent, influenced by heart rate, and may not accurately reflect cardiac contractility as long as ventricular–vascular coupling is maintained22 or in the presence of hypertrophy.23, 24 GLS, however, has been shown to be a more sensitive measure of cardiac dysfunction.25 Because GLS measures the shortening of the longitudinal myocardial fibers located predominantly in the subendocardium,23 the wall layer most susceptible to ischemia,7 subclinical cardiac dysfunction can be detected earlier. Given extensive and consistent evidence linking clinical cardiac dysfunction to abnormal brain health,13, 14, 26, 27, 28, 29 future research examining cardiac function in relation to cognitive impairment and dementia risk should utilize GLS rather than or in addition to LVEF. By doing so, prevention research can be advanced by understanding how the earliest changes in cardiac dysfunction captured by GLS affect the aging brain.
The mechanisms accounting for associations between subclinical cardiac dysfunction and neuropsychological performances remain elusive but are likely multifactorial. Previous research has demonstrated an association between compromised GLS and altered cardiac hemodynamics, such as decreased stroke volume.30, 31 Despite autoregulatory mechanisms in place to preserve cerebral blood flow (CBF) in the presence of systemic blood flow reductions, animal research suggests that manipulating cardiac output (ie, stroke volume×heart rate) can have a direct impact on CBF.32 Furthermore, among macaque monkeys, blood flow in brain regions with tissue damage is vulnerable to the effects of systemic blood flow changes.33 Autoregulatory mechanisms have also been shown to be less effective in clinical samples with end‐stage heart failure,29 an observation that was recently reported for the first time in older adults without heart failure.34 Such decreases in CBF could affect brain health through multiple pathways. First, subclinical reductions in CBF can result in oligemia or mild reductions in regional CBF. Oligemia can affect protein synthesis required for synaptic plasticity associated with learning and memory.35, 36 In addition, the temporal lobes appear especially vulnerable to CBF reductions possibly due to a less extensive network of collateral blood flow sources.37 Second, as CBF decreases, adherens and tight junctions in the blood–brain barrier are disrupted, resulting in increased diffusion of lipid‐soluble proteins across capillary walls. Subsequently, blood‐derived neurotoxic proteins accumulate in the brain resulting in suppressed capillary blood flow and neurodegeneration.38, 39 Such changes can be exacerbated in older adults as blood–brain barrier breakdown has been reported in the hippocampus with normal aging.38 The increased vulnerability of the hippocampus aligns with the memory and learning results observed in this study, not only with the memory composite but also with the individual verbal and nonverbal visuospatial learning and retention measures. The present findings support such anatomical vulnerability given that only learning, memory, and language performances were affected, all of which are cognitive functions localized to the temporal lobe.40, 41 Furthermore, the anatomical vulnerability of the temporal lobes may explain why cognitive associations reported in this study did not include executive functions (as initially hypothesized), such as planning, sequencing, generation, and inhibition behaviors facilitated by the frontal lobes and frontal–subcortical circuitry.
An alternative mechanism underlying the current findings may be related to the geometry of the left ventricle. Increased LV mass is associated with compromised GLS,42 worse cognition,43 and increased stroke risk.44 Furthermore, longitudinal shortening can be impaired in hypertensive remodeling even without overt LV hypertrophy.45 In response to pressure overload, as seen in aging with increased prevalence of hypertension,46 the ventricular wall thickens to normalize wall stress.45 As this process occurs, LVEF remains unaffected while myocardial shortening decreases.47 Before an increase in ventricular volume and onset of LV hypertrophy, the chamber volume can decrease, resulting in lower stroke volume47 and subsequently decreased CBF, as described earlier. Future research is needed to better understand this potential pathway.
An observation from the current study that warrants brief discussion is the counterintuitive association between LVEF and D‐KEFS Number Sequencing observed in both fully adjusted and raw unadjusted models. To better understand this finding, we examined interaction terms between LVEF and age, sex, education, race/ethnicity, FSRP minus age, and APOE*ε4, all of which were not significant. Exploratory stratified analyses suggested the counterintuitive finding may be most prominent in participants who were APOE*ε4 negative, were male, were younger, were less educated, and had lower FSRP scores. It is plausible that the ambiguous observation may be due to a more complex multivariable interaction or confound not captured by our methods. Replication is needed to better understand this unexpected result.
Our study has several strengths. First, we used CMR, a reliable imaging technique for quantifying both LVEF and GLS. Second, because GLS is unaffected by preload and diastolic abnormalities, it provides a more reliable measure of subclinical cardiac dysfunction in comparison to LVEF. Additional strengths include utilization of a comprehensive neuropsychological protocol capturing a diverse range of cognitive outcomes across domains, comprehensive ascertainment of potential confounders, stringent quality control procedures, and utilization of a core laboratory for processing CMR measurements in which technicians were clinically blinded.
Despite these strengths, a few limitations should be considered when interpreting the current observations. First, the results are cross‐sectional and cannot speak to causality. Longitudinal studies are needed to understand the temporal nature of the associations reported and to rule out potential confounding or concomitant variables unaccounted for in our analytical models. Second, our sample included predominantly white, well‐educated, and healthy older adults (with only 3% prevalent CVD compared with 24–35% in the general population of older adults).48 Generalizability to other races, ethnicities, ages, or adults with poorer health is unknown. Multiple comparisons were made, raising the possibility of a false‐positive finding. We speculate that in a less healthy cohort with more vascular risk factor burden or more compromised cardiac function, the associations reported in this study would likely be stronger. Nevertheless, our results require replication, including testing associations in a less healthy cohort, because we cannot rule out the possibility of a false‐positive finding. We also cannot rule out the possibility of residual confounding.
Among older adults free of clinical stroke, dementia, and heart failure, subtle cardiac dysfunction as captured by strain relates to worse episodic memory and language performance. Using comprehensive and sensitive CMR methods to quantify cardiac function, these associations were statistically independent of key covariates, including vascular risk factors, prevalent CVD, and atrial fibrillation. Further investigation into mechanisms linking cardiac strain and abnormal brain changes with age are warranted to identify primary prevention targets or therapeutic interventions.
Sources of Funding
This research was supported by Alzheimer's Association IIRG‐08‐88733 (Jefferson), R01‐AG034962 (Jefferson), R01‐NS100980 (Jefferson), K24‐AG046373 (Jefferson), Paul B. Beeson Career Development Award in Aging K23‐AG045966 (Gifford), Paul B. Beeson Career Development Award in Aging K23‐AG048347 (Bell), K12‐HD043483 (Gifford, Bell, Hohman), K01‐AG049164 (Hohman), T32‐MH064913 (Cambronero), Cardiovascular Research Grid Project R24‐HL085343 (Carr), UL1‐TR000445 (Vanderbilt Clinical Translational Science Award), S10‐OD023680 (Vanderbilt's High‐Performance Computer Cluster for Biomedical Research), and the Vanderbilt Memory and Alzheimer's Center.
Disclosures
None.
Supporting information
Figure S1. Memory composite model diagram.
Figure S2. Executive composite model diagram.
Acknowledgments
The authors would like to thank the dedicated Vanderbilt Memory and Aging Project participants, their loved ones, and our devoted staff and trainees who contributed to recruitment, screening, and enrollment of the baseline cohort.
(J Am Heart Assoc. 2018;7:e007562 DOI: 10.1161/JAHA.117.007562.)29440034
This article was handled independently by Christopher M. Kramer, MD as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
References
- 1. Hoth KF, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol. 2008;21:65–72. [DOI] [PubMed] [Google Scholar]
- 2. Graham S, Ye S, Qian M, Sanford AR, Di Tullio MR, Sacco RL, Mann DL, Levin B, Pullicino PM, Freudenberger RS, Teerlink JR, Mohr JP, Labovitz AJ, Lip GY, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Thompson JL, Homma S. Cognitive function in ambulatory patients with systolic heart failure: insights from the warfarin versus aspirin in reduced cardiac ejection fraction (WARCEF) trial. PLoS One. 2014;9:e113447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. [DOI] [PubMed] [Google Scholar]
- 4. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 5. Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O'Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging. 2009;25(suppl 1):9–22. [DOI] [PubMed] [Google Scholar]
- 8. Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Relationship of multidirectional myocardial strain with radial thickening and ejection fraction and impact of left ventricular hypertrophy: a study in a community‐based cohort. Echocardiography. 2013;30:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2081. [DOI] [PubMed] [Google Scholar]
- 10. Stanton T, Leano R, Marwick TH. Prediction of all‐cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. [DOI] [PubMed] [Google Scholar]
- 11. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 12. Mathus‐Vliegen EM. Obesity and the elderly. J Clin Gastroenterol. 2012;46:533–544. [DOI] [PubMed] [Google Scholar]
- 13. Pressler SJ, Kim J, Riley P, Ronis DL, Gradus‐Pizlo I. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Card Fail. 2010;16:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hjelm C, Dahl A, Brostrom A, Martensson J, Johansson B, Stromberg A. The influence of heart failure on longitudinal changes in cognition among individuals 80 years of age and older. J Clin Nurs. 2012;21:994–1003. [DOI] [PubMed] [Google Scholar]
- 15. Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggermont LH, de Boer K, Muller M, Jaschke AC, Kamp O, Scherder EJ. Cardiac disease and cognitive impairment: a systematic review. Heart. 2012;98:1334–1340. [DOI] [PubMed] [Google Scholar]
- 17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 18. Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR Jr, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW; Alzheimer's Disease Neuroimaging I . Clinical core of the Alzheimer's Disease Neuroimaging Initiative: progress and plans. Alzheimers Dement. 2010;6:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. [DOI] [PubMed] [Google Scholar]
- 21. Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaasch WH, Little WC. Assessment of left ventricular diastolic function and recognition of diastolic heart failure. Circulation. 2007;116:591–593. [DOI] [PubMed] [Google Scholar]
- 23. Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol. 2012;17:5–11. [PMC free article] [PubMed] [Google Scholar]
- 25. Krishnasamy R, Isbel NM, Hawley CM, Pascoe EM, Burrage M, Leano R, Haluska BA, Marwick TH, Stanton T. Left ventricular global longitudinal strain (GLS) is a superior predictor of all‐cause and cardiovascular mortality when compared to ejection fraction in advanced chronic kidney disease. PLoS One. 2015;10:e0127044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population‐based cohort study. Arch Intern Med. 2006;166:1003–1008. [DOI] [PubMed] [Google Scholar]
- 27. Almeida OP, Tamai S. Congestive heart failure and cognitive functioning amongst older adults. Arq Neuropsiquiatr. 2001;59:324–329. [DOI] [PubMed] [Google Scholar]
- 28. Leto L, Feola M. Cognitive impairment in heart failure patients. J Geriatr Cardiol. 2014;11:316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Massaro AR, Dutra AP, Almeida DR, Diniz RV, Malheiros SM. Transcranial Doppler assessment of cerebral blood flow: effect of cardiac transplantation. Neurology. 2006;66:124–126. [DOI] [PubMed] [Google Scholar]
- 30. Krzesinski P, Uzieblo‐Zyczkowska B, Gielerak G, Stanczyk A, Kurpaska M, Piotrowicz K. Global longitudinal two‐dimensional systolic strain is associated with hemodynamic alterations in arterial hypertension. J Am Soc Hypertens. 2015;9:680–689. [DOI] [PubMed] [Google Scholar]
- 31. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Bigio E, Marchetti L, Biagioli B. Prediction of stroke volume by global left ventricular longitudinal strain in patients undergoing assessment for cardiac transplantation. J Crit Care. 2011;26:433. [DOI] [PubMed] [Google Scholar]
- 32. Wanless RB, Anand IS, Gurden J, Harris P, Poole‐Wilson PA. Regional blood flow and hemodynamics in the rabbit with adriamycin cardiomyopathy: effects of isosorbide dinitrate, dobutamine and captopril. J Pharmacol Exp Ther. 1987;243:1101–1106. [PubMed] [Google Scholar]
- 33. Tranmer BI, Keller TS, Kindt GW, Archer D. Loss of cerebral regulation during cardiac output variations in focal cerebral ischemia. J Neurosurg. 1992;77:253–259. [DOI] [PubMed] [Google Scholar]
- 34. Jefferson AL, Liu D, Gupta DK, Pechman KR, Watchmaker JM, Gordon EA, Rane S, Bell SP, Mendes LA, Davis LT, Gifford KA, Hohman TJ, Wang TJ, Donahue MJ. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology. 2017;89:2327–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bliss TV, Collingridge GL. A synaptic model of memory: long‐term potentiation in the hippocampus. Nature. 1993;361:31–39. [DOI] [PubMed] [Google Scholar]
- 36. Mies G, Ishimaru S, Xie Y, Seo K, Hossmann KA. Ischemic thresholds of cerebral protein synthesis and energy state following middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 1991;11:753–761. [DOI] [PubMed] [Google Scholar]
- 37. Liebeskind DS. Collateral circulation. Stroke. 2003;34:2279–2284. [DOI] [PubMed] [Google Scholar]
- 38. Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood‐brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi YH, Park HK, Paik NJ. Role of the posterior temporal lobe during language tasks: a virtual lesion study using repetitive transcranial magnetic stimulation. Neuroreport. 2015;26:314–319. [DOI] [PubMed] [Google Scholar]
- 41. Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dinh W, Nickl W, Smettan J, Kramer F, Krahn T, Scheffold T, Barroso MC, Brinkmann H, Koehler T, Lankisch M, Futh R. Reduced global longitudinal strain in association to increased left ventricular mass in patients with aortic valve stenosis and normal ejection fraction: a hybrid study combining echocardiography and magnetic resonance imaging. Cardiovasc Ultrasound. 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scuteri A, Coluccia R, Castello L, Nevola E, Brancati AM, Volpe M. Left ventricular mass increase is associated with cognitive decline and dementia in the elderly independently of blood pressure. Eur Heart J. 2009;30:1525–1529. [DOI] [PubMed] [Google Scholar]
- 44. Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke. 2003;34:2380–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lai YH, Lo CI, Wu YJ, Hung CL, Yeh HI. Cardiac remodeling, adaptations and associated myocardial mechanics in hypertensive heart diseases. Acta Cardiol Sin. 2013;29:64–70. [PMC free article] [PubMed] [Google Scholar]
- 46. Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World J Cardiol. 2012;4:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aurigemma GP, Gaasch WH, McLaughlin M, McGinn R, Sweeney A, Meyer TE. Reduced left ventricular systolic pump performance and depressed myocardial contractile function in patients > 65 years of age with normal ejection fraction and a high relative wall thickness. Am J Cardiol. 1995;76:702–705. [DOI] [PubMed] [Google Scholar]
- 48. Lloyd‐Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Memory composite model diagram.
Figure S2. Executive composite model diagram.


