Abstract
Background
Non–vitamin K antagonist oral anticoagulants (NOACs) are indicated for stroke prevention in atrial fibrillation (AF) but require lower doses in certain patients. We sought to describe the frequency, appropriateness (according to Food and Drug Administration labeling), and outcomes of patients prescribed reduced doses of NOACs in community practice.
Methods and Results
We analyzed data from the ORBIT‐AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II) registry, a prospective, national, observational registry of AF patients. Among 7925 AF patients receiving NOACs, we assessed patterns of use of reduced NOAC doses and associated cardiovascular and bleeding outcomes at median follow‐up of 1 year. Overall, 6636 patients (84%) received a NOAC at standard dose, which was consistent with US Food and Drug Administration labeling in 6376 (96%). Reduced NOAC dose was prescribed to 1289 (16% overall), which was consistent with Food and Drug Administration labeling in only 555 patients (43%). Compared with those whose NOAC dose was appropriately reduced, patients receiving inappropriate dose reductions were younger (median age 79 versus 84, P<0.0001) and had lower ORBIT bleeding risk scores (26% ≥4 versus 45%, P<0.0001). Compared with those appropriately receiving standard dosing, patients receiving inappropriately reduced‐dose NOACs had higher unadjusted rates of thromboembolic events (2.11 versus 1.35 events per 100 patient years, hazard ratio 1.56, 95% confidence interval 0.92‐2.67) and death (6.77 versus 2.60, hazard ratio 2.61, 95% confidence interval 1.86‐3.67). After adjustment, outcomes were not significantly different but tended to favor patients dosed appropriately.
Conclusions
The majority of dose reductions of NOACs in AF are inconsistent with US Food and Drug Administration recommendations. There appear to be opportunities to improve current NOAC dosing in community practice.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01701817.
Keywords: atrial fibrillation, dosing, non–vitamin K antagonist oral anticoagulant, outcome
Subject Categories: Atrial Fibrillation, Quality and Outcomes, Cerebrovascular Disease/Stroke, Health Services
Clinical Perspective
What Is New?
More than 1 in 10 non–vitamin K antagonist oral anticoagulant patients receives reduced doses of the drugs.
The majority of dose reductions of non–vitamin K antagonist oral anticoagulants in atrial fibrillation are inconsistent with US Food and Drug Administration recommendations.
What Are the Clinical Implications?
Inappropriate dose reductions of non–vitamin K antagonist oral anticoagulants for stroke prevention in atrial fibrillation may impact clinical outcomes in these patients.
There appear to be opportunities to improve current non–vitamin K antagonist oral anticoagulant dosing in community practice.
Introduction
The development of non–vitamin K antagonist oral anticoagulants (NOACs) for the prevention of stroke in patients with atrial fibrillation (AF) provided an alternative to vitamin K antagonists, and these NOACs do not require as frequent dose adjustment. However, NOAC dose does need to be modified based on certain clinical features such as renal function, weight, age, and concomitant medications.1, 2, 3, 4 The US Food and Drug Administration (FDA) approved prescribing guidance includes dose recommendations based on the clinical trial results and pharmacokinetic/pharmacodynamic data.5, 6, 7, 8
Previously we described the frequency and outcomes associated with off‐label dosing of these agents in AF.9 Yet a significant proportion of patients are on reduced doses of NOACs, and prescribers often underdose medications in an effort to “do no harm.” The FDA previously expressed concerns regarding off‐label underdosing of anticoagulants.9, 10 In the present analysis we sought to describe the contemporary practice of NOAC dose reductions in the community and subsequent outcomes. The primary objectives of our study were (1) to describe the proportion and characteristics of AF patients who received reduced NOAC dosing; (2) to identify what proportion of dose reductions was appropriate based on FDA‐approved dosing recommendations; and (3) to describe clinical events in patients receiving appropriate dosing versus those inappropriately prescribed reduced doses of NOACs.
Methods
Patient Cohort and Data Collection
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. We used data from ORBIT‐AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II), a nationwide AF registry conducted from 2013 to 2016. Patients with AF treated by primary care physicians, cardiologists, neurologists, and/or electrophysiologists were enrolled from a nationally representative sample of sites. Enrollment was geared toward capturing a contemporary cohort of AF patients treated with NOACs: only patients with either (1) new‐onset AF within the previous 6 months and/or (2) AF patients recently switched to a NOAC within 3 months were eligible. Additionally, patients had to be 21 years or older and have electrocardiographically documented AF that was not due to a reversible cause (eg, hyperthyroidism, cardiac surgery). Consecutive eligible patients were enrolled at each site and followed clinically every 6 months out to at least 2 years. Baseline data are collected at enrollment, which is by inclusion criteria within 6 months of AF diagnosis or 3 months of NOAC initiation.
Data elements were entered in a web‐based case report form and were derived primarily from the patients’ medical records and the treating physicians. They included demographics, medical history, vital signs, electrocardiography, laboratory and imaging data, and AF history (including symptoms). Incident procedures, medications, and adverse events were captured during follow‐up. Outcomes included stroke or systemic embolism, which was adjudicated using primary source documentation (deidentified medical records). Major bleeding was defined according to the International Society on Thrombosis and Haemostasis definition, which include ≥1 of the following criteria11: (1) fall in hemoglobin ≥2 g/dL, (2) transfusion of ≥2 units of packed red blood cells or whole blood, (3) any bleeding in a critical site (intracranial, intraspinal, intraocular, intra‐articular, pericardial, retroperitoneal, or intramuscular with compartment syndrome), or (4) fatal bleeding. Additional outcomes included cause‐specific hospitalization (cardiovascular, bleeding, or noncardiovascular, nonbleeding), and mortality. Full details of the design and methods of the ORBIT‐AF II registry have been published previously.12
Study Population
The current study included patients treated with NOACs who had follow‐up data available. Patients without NOAC dosing available, those with dosing other than standard or reduced dose (eg, NOAC every other day), and patients on warfarin were excluded. The group was then stratified by NOAC dosing—standard or reduced (Table S1): for dabigatran, 150 mg (standard) and 75 mg (reduced) twice daily; rivaroxaban 20 mg (standard) and 15 mg (reduced) daily; apixaban 5 mg (standard) and 2.5 mg (reduced) twice daily; and edoxaban 60 mg (standard) and 30 mg (reduced) daily.
In addition, dosing at the patient level (for standard and reduced groups) was categorized as appropriate or inappropriate based on the approved FDA labeling for each agent (Table S1).1, 2, 3, 4, 9 This included assessments of renal function, as well as weight, age, and chronic concomitant medications (where applicable). Patients for whom the selected NOAC was contraindicated were classified as off‐label (eg, patients with end‐stage renal disease on dialysis or those with preserved renal function receiving edoxaban [contraindicated in patients with creatinine clearance >95 mL/min]).1
Raw and adjusted clinical event rates were calculated and compared across dosing strata. These included thromboembolic events (stroke, non–central nervous system embolism, transient ischemic attack), myocardial infarction, major bleeding, bleeding hospitalization, death, and the composite of major adverse cardiovascular and neurological events (ie, transient ischemic attack, stroke, non–central nervous system embolism, myocardial infarction, or cardiovascular death).
Statistical Analyses
Baseline characteristics are presented as frequencies and percentages for categorical variables and medians (interquartile range) or means (standard deviation) for continuous variables. Univariate comparisons between groups were made using the chi‐squared test for categorical variables and the Wilcoxon rank‐sum test for continuous variables.
Raw frequencies are presented for dosing categories, appropriate versus inappropriate, as defined by US labeling. The dosing categorization was calculated using all relevant data (creatinine, creatinine clearance using the Cockroft‐Gault estimation,13 weight, age, and/or concomitant medications), as previously reported.9 This included both doses prescribed that were higher than recommended (or contraindicated) and those that were lower than recommended.
The incidence rate of outcomes per 100 patient‐years of follow‐up and corresponding 95% confidence intervals are presented first for those treated with reduced NOAC doses and those on the standard NOAC dose. All available follow‐up was used in the calculation of these rates. These incidence rates were calculated only among those who were receiving NOAC doses consistent with US labeling (on‐label).
Last, unadjusted and adjusted analyses of all patients recommended to receive standard dose, stratified by what they actually received (standard versus inappropriately reduced) were performed for patients on rivaroxaban and apixaban. Only these 2 drugs were considered because of limited power in patients receiving other agents. Overlap propensity weighting was performed for adjustment of these outcomes.14 Overlap weights produce exact covariate balance and greater precision by more heavily weighting those with a reasonable chance of receiving either treatment compared with those with extreme propensities who are very likely to receive a particular treatment. A list of covariates included in the logistic regression model to determine appropriate propensity weights can be found in Table S2. Missing data were accounted for using the first imputed data set from multiply imputed data. Linearity was assessed for all continuous covariates, and any nonlinear associations were accounted for using linear splines.
Analyses of dosing were then performed using Cox proportional hazards models, applying the propensity weights for the adjusted results. Both the unadjusted and propensity‐weighted hazard ratio (HR) and corresponding 95% confidence interval (CI) are presented.
Sensitivity Analyses
Due to the dynamic nature of renal function, reevaluation of dosing distributions was performed after dosing categorization had been liberalized to allow patients with borderline renal function (ie, within 5 mL/min [≈10%] of the dosing cutoff) to be recategorized as “appropriately” dose reduced.
An additional, propensity‐matched adjustment was performed to further assess outcomes between patients receiving standard dosing and those inappropriately dose reduced. Propensity score matching was performed in order to match subjects receiving the reduced dosage inappropriately with those appropriately receiving the standard dosage. Matching was performed separately among those on rivaroxaban and those on apixaban, with a 1‐to‐1 match obtained for each drug. A table comparing the standardized differences of covariates after matching can be found in Table S3. The HRs of those inappropriately receiving the reduced dosage relative to those appropriately receiving the standard dosage are presented. The corresponding 95% CIs and P‐values are also included.
Study Management
The study was performed and coordinated by the Duke Clinical Research Institute. The ORBIT‐AF II registry was approved by the Duke University Institutional Review Board as well as all local authorities pursuant to local site regulations. All patients provided written, informed consent. All analyses of the aggregate, deidentified data were performed by the Duke Clinical Research Institute using SAS software (version 9.3, SAS Institute, Cary, NC).
Results
The overall ORBIT‐AF II population included 13 375 patients from 244 sites enrolled from February 2013 to July 2016. After exclusion of patients on warfarin at baseline (n=1808), patients not on a NOAC at baseline (n=1557), those with missing or nonstandard NOAC dosing (n=82), patients in whom appropriateness of recommended dosing could not be determined (n=425), and patients without follow‐up yet available (n=1578), the analysis population was 7925 from 244 sites—6636 patients (84%) receiving standard‐dosed NOACs and 1289 (16%) receiving reduced doses, with median follow‐up of 1 year.
Appropriateness of dose adjustments, stratified by dose level (standard and reduced), for the overall population and by agent is shown in Figure 1. Overall, 96% of patients receiving standard NOAC doses were consistent with the package insert, whereas only 43% of patients receiving reduced‐dose NOACs fulfilled FDA‐recommended criteria for this dose. Patterns of dose reduction appeared to vary across agents.
Figure 1.
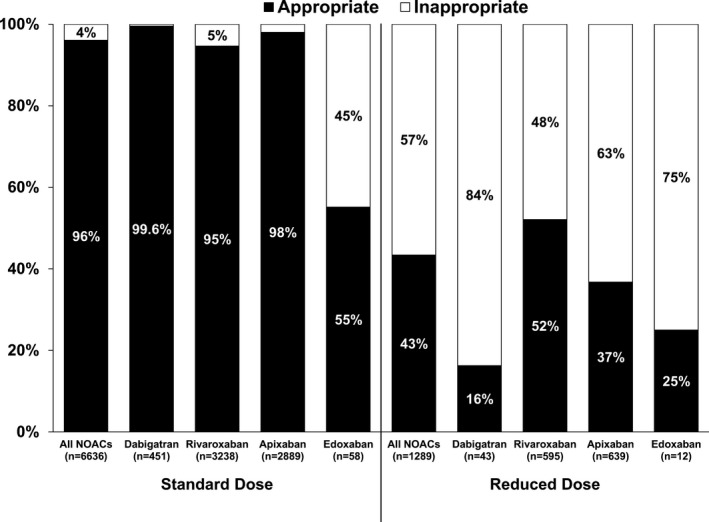
Dosing of NOACs according the US package labeling (“Appropriate” vs “Inappropriate”) for patients receiving standard and reduced dosing. NOAC indicates non–vitamin K antagonist oral anticoagulant.
Patient characteristics, stratified by dose level and dose appropriateness, are shown in Table 1. Compared with appropriately dose‐reduced patients, those receiving reduced NOAC doses inappropriately were younger (median age 79 versus 84, P<0.0001), more likely male (51% versus 36%, P<0.0001), and had lower ORBIT bleeding scores (26% ≥4 versus 45%, P<0.0001). Distribution of dose reduction, stratified by ORBIT bleeding score, is shown in Figure 2. Use of inappropriate dose reductions appeared to be highest among patients with the lowest bleeding risk (64% for ORBIT bleeding score 0‐2 versus 43% for ≥4).
Table 1.
Baseline Characteristics of Patients With Atrial Fibrillation Stratified by Standard Versus Reduced Dosing of NOAC and Whether the Dose Received was Appropriate (According to FDA Labeling)
| Overall (7925) | Received Standard Dose | Received Reduced Dose | 4‐Way P Value | |||
|---|---|---|---|---|---|---|
| Appropriate (n=6376) | Inappropriate (n=260) | Appropriate (n=555) | Inappropriate (n=734) | |||
| Age, y | 71.00 (64.00, 78.00) | 69.00 (62.00, 75.00) | 80.00 (75.00, 83.00) | 84.00 (81.00, 88.00) | 79.00 (72.00, 85.00) | <0.0001 |
| Female | 3274 (41.31%) | 2401 (37.66%) | 163 (62.69%) | 353 (63.60%) | 357 (48.64%) | <0.0001 |
| Race | 0.02 | |||||
| White | 6957 (87.79%) | 5614 (88.05%) | 219 (84.23%) | 488 (87.93%) | 636 (86.65%) | |
| Black | 332 (4.19%) | 274 (4.30%) | 12 (4.62%) | 13 (2.34%) | 33 (4.50%) | |
| Hispanic | 363 (4.58%) | 270 (4.23%) | 16 (6.15%) | 32 (5.77%) | 45 (6.13%) | |
| American Indian/Alaska Native | 13 (0.16%) | 13 (0.20%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Asian | 129 (1.63%) | 91 (1.43%) | 9 (3.46%) | 15 (2.70%) | 14 (1.91%) | |
| Health insurance status | <0.0001 | |||||
| Private health insurance | 4105 (51.80%) | 3484 (54.64%) | 97 (37.31%) | 212 (38.20%) | 312 (42.51%) | |
| Medicaid | 297 (3.75%) | 238 (3.73%) | 10 (3.85%) | 15 (2.70%) | 34 (4.63%) | |
| Medicare | 3127 (39.46%) | 2326 (36.48%) | 139 (53.46%) | 301 (54.23%) | 361 (49.18%) | |
| Other | 310 (3.91%) | 254 (3.98%) | 9 (3.46%) | 25 (4.50%) | 22 (3.00%) | |
| Prior stroke/TIA | 880 (11.10%) | 642 (10.07%) | 35 (13.46%) | 98 (17.66%) | 105 (14.31%) | <0.0001 |
| Prior gastrointestinal bleeding | 321 (4.05%) | 223 (3.50%) | 13 (5.00%) | 43 (7.75%) | 42 (5.72%) | <0.0001 |
| Frailty | 254 (3.21%) | 108 (1.69%) | 11 (4.23%) | 86 (15.50%) | 49 (6.68%) | <0.0001 |
| CHA2DS2‐VASc Stroke Score | <0.0001 | |||||
| 0 | 264 (3.33%) | 257 (4.03%) | 3 (1.15%) | 0 (0.00%) | 4 (0.54%) | |
| 1 | 749 (9.45%) | 721 (11.31%) | 8 (3.08%) | 2 (0.36%) | 18 (2.45%) | |
| ≥2 | 6912 (87.22%) | 5398 (84.66%) | 249 (95.77%) | 553 (99.64%) | 712 (97.00%) | |
| ORBIT Bleeding Score | <0.0001 | |||||
| 0 to 2 (low) | 5529 (72.97%) | 4817 (79.19%) | 137 (53.94%) | 206 (38.22%) | 369 (52.64%) | |
| 3 (medium) | 1034 (13.65%) | 748 (12.30%) | 45 (17.72%) | 92 (17.07%) | 149 (21.26%) | |
| ≥4 (high) | 1014 (13.38%) | 518 (8.52%) | 72 (28.45%) | 241 (44.71%) | 183 (26.11%) | |
| Concurrent aspirin | 2037 (25.70%) | 1659 (26.02%) | 66 (25.38%) | 125 (22.52%) | 187 (25.48%) | 0.3 |
| Concurrent clopidogrel | 242 (3.05%) | 185 (2.90%) | 5 (1.92%) | 26 (4.68%) | 26 (3.54%) | 0.07 |
| LVEF | 58.00 (50.00, 61.00) | 57.50 (50.00, 60.00) | 60.00 (55.00, 64.00) | 57.00 (50.00, 62.00) | 59.00 (51.00, 61.00) | 0.06 |
| Calculated creatinine clearance,a mL/min | 81.69 (59.36, 109.69) | 89.65 (69.67, 116.62) | 44.09 (37.41, 48.51) | 37.39 (29.79, 44.00) | 60.19 (50.35, 75.00) | <0.0001 |
| Physician specialty | <0.0001 | |||||
| Internal medicine/primary care | 374 (4.72%) | 260 (4.08%) | 21 (8.08%) | 38 (6.85%) | 55 (7.49%) | |
| Cardiology | 5521 (69.67%) | 4340 (68.07%) | 195 (75.00%) | 425 (76.58%) | 561 (76.43%) | |
| Electrophysiology | 2026 (25.56%) | 1773 (27.81%) | 44 (16.92%) | 92 (16.58%) | 117 (15.94%) | |
| Neurology | 4 (0.05%) | 3 (0.05%) | 0 (0.00%) | 0 (0.00%) | 1 (0.14%) | |
Values are presented as percentage or median (interquartile range), unless noted otherwise. CHA2DS2VASc is a rating of risk for stroke in patients with atrial fibrillation; FDA, Food and Drug Administration; LVEF, left‐ventricular ejection fraction; NOAC, non–vitamin K antagonist oral anticoagulant; ORBIT, Outcomes Registry for Better Informed Treatment; TIA, transient ischemic attack.
As calculated by the Cockcroft‐Gault formula.13
Figure 2.
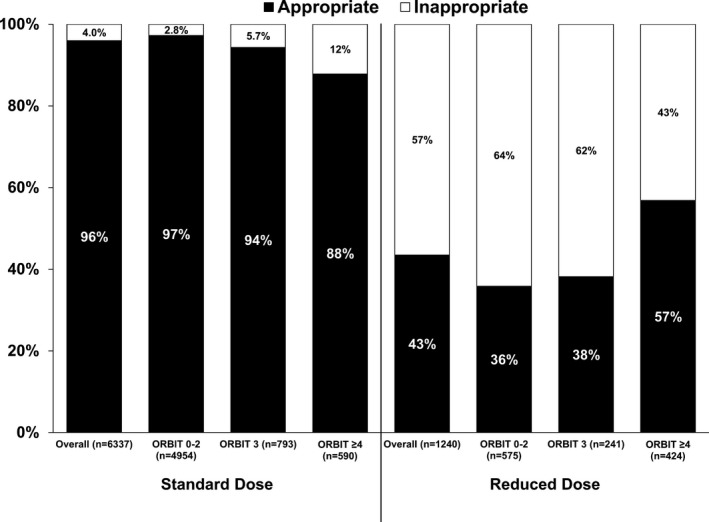
Dosing of NOACs according the US package labeling (“Appropriate” vs “Inappropriate”) for patients receiving standard and reduced dosing, across ORBIT (The Outcomes Registry for Better Informed Treatment) bleeding score levels (in the 7577 patients with bleeding score available). NOAC indicates non–vitamin K antagonist oral anticoagulant.
Crude event numbers, rates, and 95% CIs for patients on standard and reduced NOAC doses are shown in Table 2 (only among patients receiving doses according to the FDA package labeling). Across event types, including thromboembolic, bleeding, and mortality end points, patients treated with reduced NOAC doses were observed to have higher event rates than those receiving standard doses.
Table 2.
Event Rates by NOAC Dosing Stratum Among Patients Dosed Appropriately, According to the Package Labeling (n=6931)
| Overall (N=6931) | Standard Dose (N=6376) | Reduced Dose (N=555) | ||||
|---|---|---|---|---|---|---|
| Number of Events | Rate Per 100 Patient Years (95% CI) | Number of Events | Rate Per 100 Patient Years (95% CI) | Number of Events | Rate Per 100 Patient Years (95% CI) | |
| Thromboembolic outcomes | ||||||
| Stroke, non‐CNS embolism, or TIA | 111 | 1.38 (1.15‐1.67) | 97 | 1.32 (1.08‐1.61) | 14 | 2.17 (1.29‐3.66) |
| MI | 60 | 0.73 (0.57‐0.94) | 46 | 0.61 (0.45‐0.81) | 14 | 2.17 (1.28‐3.66) |
| Death | 249 | 3.05 (2.69‐3.46) | 193 | 2.57 (2.23‐2.96) | 56 | 8.57 (6.60‐11.14) |
| Bleeding outcomes | ||||||
| Major bleeding | 240 | 3.01 (2.65‐3.42) | 207 | 2.83 (2.47‐3.24) | 33 | 5.13 (3.63‐7.25) |
| Bleeding hospitalization | 213 | 2.68 (2.34‐3.06) | 174 | 2.37 (2.04‐2.75) | 39 | 6.32 (4.62‐8.65) |
Event rates are presented per 100 patient‐years. CI indicates confidence interval; CNS, central nervous system; MI, myocardial infarction; NOAC, non‐vitamin K antagonist oral anticoagulant; TIA, transient ischemic attack.
Among 6584 patients treated with rivaroxaban and apixaban, who were recommended to receive standard NOAC doses by FDA labeling, outcomes stratified by actual dose received are shown in Table 3. Patients who were inappropriately dose reduced had numerically higher unadjusted rates of thromboembolic events (HR 1.56, 95% CI 0.92‐2.67), major bleeding events (HR 1.49, 95% CI 1.02‐2.18), and mortality (HR 2.61, 95% CI 1.86‐3.67). Following adjustment, there were no significant differences in outcomes, although HRs consistently trended toward higher risk in patients inappropriately dose reduced (adjusted HR for mortality 1.40, 95% CI 0.97‐2.00, P=0.07).
Table 3.
Unadjusted and Adjusted Event Rates by NOAC Dose Received (Rivaroxaban or Apixaban) Among Patients Recommended for Standard NOAC Dosing (n=6584)
| Overall (n=6584) | Unadjusted (n=6584) | Overlap Propensity Weighted | |||||
|---|---|---|---|---|---|---|---|
| Events (Rate Per 100 Patient‐Years) | Appropriate Standard (n=5895) | Inappropriately Reduced (n=689) | HRa (95% CI) | P Value | HRa (95% CI) | P Value | |
| Thromboembolic outcomes | |||||||
| Stroke, non‐CNS embolism, or TIA | 107 (1.43) | 91 (1.35) | 16 (2.11) | 1.56 (0.92‐2.67) | 0.1 | 1.11 (0.61‐2.02) | 0.7 |
| MI | 47 (0.62) | 41 (0.60) | 6 (0.78) | 1.29 (0.62‐2.69) | 0.5 | 1.27 (0.50‐3.18) | 0.6 |
| Death | 229 (3.03) | 177 (2.60) | 52 (6.77) | 2.61 (1.86‐3.67) | <0.0001 | 1.40 (0.97‐2.00) | 0.07 |
| MACNE | 217 (2.91) | 181 (2.70) | 36 (4.78) | 1.77 (1.28‐2.46) | 0.0006 | 1.40 (0.94‐2.10) | 0.1 |
| Bleeding outcomes | |||||||
| Major bleeding | 221 (2.98) | 189 (2.84) | 32 (4.28) | 1.49 (1.02‐2.18) | 0.04 | 1.15 (0.76‐1.73) | 0.5 |
| Bleeding hospitalization | 186 (2.50) | 159 (2.38) | 27 (3.60) | 1.49 (0.98‐2.27) | 0.06 | 1.04 (0.66‐1.63) | 0.9 |
CI indicates confidence interval; CNS, central nervous system; HR, hazard ratio; MACNE, major adverse cardiovascular and neurological events, including a composite of TIA, stroke, non‐CNS embolism, MI, or cardiovascular death; MI, myocardial infarction; NOAC, non–vitamin K antagonist oral anticoagulant; TIA, transient ischemic attack.
HR for inappropriately reduced‐dose subjects relative to appropriately standard‐dose subjects.
Sensitivity Analyses
When patients with renal function that was borderline for dose adjustment were recategorized as appropriate for dose reduction, there was no significant, qualitative difference in the rates of inappropriate dose reduction across NOACs compared with the primary analysis (Figure S1). In the limited, propensity‐matched adjustment of 651 matched pairs, there were no statistically significant differences in outcomes (Table S4).
Discussion
Our analysis of nearly 8000 AF patients in community practice is the first to report exclusively on the use of NOAC dose reductions in US community practice. We found that ≈1 in 7 patients treated with a NOAC were treated with a reduced NOAC dose. Notably, more than half of NOAC reductions were inconsistent with FDA labeling and appeared to be paradoxically in patients of lower bleeding risk. In unadjusted analyses, patients receiving reduced NOAC doses had high crude adverse event rates, particularly among those who should have received standard NOAC dosing. Although these trends were consistent in adjusted analyses, the differences were not statistically significant.
We have previously reported on the frequency and outcomes of off‐label NOAC dosing in this cohort and found relatively high rates of overdosing and underdosing, relative to the doses recommended by the package inserts.9 Those data also suggested an overall high rate of prescriptions for the lower doses of these medications, and the present analysis explores that phenomenon in depth. The only previous studies of reduced NOAC dosing have been described across 3 centers in Australia and in a single‐center report from the United States.15, 16 Both studies suggested a high rate of inappropriate dosing for patients with AF receiving NOACs but were limited to fewer than 300 patients and not specifically designed to address inappropriate dose reductions. A Medicare analysis of dabigatran use found that among patients receiving reduced‐dose dabigatran, only a minority carried a diagnosis of significant kidney disease.17 However, the prescribers did not have detailed data on renal function and therefore could not determine true expected versus observed rates of dose reductions.
Our nationwide data among all approved regimens of NOACs extended the findings of these earlier studies and found that 16% of patients taking NOACs received a reduced dose but that only 43% of these patients fulfilled FDA dosing recommendations for such dosing. Among those patients inappropriately receiving lower NOAC doses, few objective patient characteristics appeared to drive the practice. These patients were not dramatically older, had paradoxically lower bleeding risk scores, and were not frequently receiving concomitant antiplatelet therapy. Together these data suggest that a sizable proportion of patients are receiving reduced‐dose NOACs based on other characteristics (including prescriber preference) and not based on either FDA‐recommended labeling or classic markers of bleeding risk.
Physician judgment may be a strong influence for reduced NOAC dosing; however, there may also be drug‐specific factors. For example, dosing guidelines for apixaban are dramatically different from those of other agents and include weight and age as well as serum creatinine (versus creatinine clearance). In contrast to the single assessment of creatinine clearance, multiple parameters may represent a barrier to determining the appropriate dose. For other agents, dosing is guided primarily by estimated creatinine clearance, as calculated by the Cockroft‐Gault formula; however, electronic medical records and lab reports routinely report renal function according to the MDRD (Modification of Diet for Renal Disease) estimation. Although these methods are similar, there are well‐described differences between them, and they could lead to inappropriate dosing in some circumstances.
In the largest study of NOAC dosing and outcomes, a nationwide Danish analysis suggested differential outcomes among different reduced‐dose NOACs compared with warfarin, but none of the comparisons was statistically significant.18 Furthermore, it is not clear from that analysis what proportion of patients in each drug group qualified for the reduced dose based on regulatory recommendations—our US data suggest that more than half of these patients may be inappropriately underdosed, and this could certainly account for a difference in clinical outcomes across very large populations.
In our analysis of outcomes, patients receiving lower doses of NOACs were observed to have a notably increased risk of adverse events, including thromboembolic events, bleeding events, and death. When we accounted for differences in patient characteristics with adjustment, these increased rates were not significant. However, we caution that our analysis had limited power to detect significant differences. Biologic plausibility suggests that lower doses could lead to worse thromboembolic outcomes (including death). The numerically higher rate of bleeding in these patients is more likely a marker of underlying risk—prior data from this program would not support the idea that providers’ assessment of bleeding risk superseded that of objective bleeding scores (which were lower in the dose‐reduced cohort).19, 20 Finally, the randomized controlled trials testing these agents for stroke prevention in AF tested the reduced doses in only a minority of patients, if at all.
Limitations
This analysis is observational, and neither NOAC drug nor dose was randomized. Although ORBIT‐AF II was designed to capture a broad selection of sites and patients, selection bias cannot be excluded. Comparisons between these data and the clinical trial populations, or other cohorts, are limited by differences in underlying cohorts. Last, the categorization of appropriate versus inappropriate dosing may be dynamic and influenced by the intermittent use of interacting medications (eg, antibiotics, antifungals, antiarrhythmics). However, our sensitivity analysis suggested that patients with borderline renal dysfunction do not account for the substantial use of reduced doses.
Conclusions
A significant proportion of AF patients receiving NOACs are receiving the reduced dose; however, the majority of reduced‐dose NOAC use is not consistent with FDA recommendations. Moreover, selection of reduced‐dose NOAC therapy does not appear to be associated with bleeding risk. Patients receiving lower NOAC doses are an at‐risk group and have high rates of adverse events. These data support the need for ongoing education of clinicians in community practice on the appropriate dosing of NOACs for AF and further study of the outcomes associated with reduced NOAC doses.
Sources of Funding
The ORBIT‐AF II registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ.
Disclosures
Dr Steinberg reports significant research support from Janssen and consulting from BMS‐Pfizer. Mr Shrader, Dr Pieper, Dr Thomas, and Dr Singer report no disclosures. Dr Allen has received grants from the American Heart Association, the National Institutes of Health, and the Patient‐Centered Outcomes Research Institute; and has received consultancy fees from Janssen, Novartis, ZS Pharma, and St. Jude Medical. Dr Ansell reports modest Consultant/Advisory Board support from Bristol Myers Squibb, Pfizer, Janssen, Daiichi, Boehringer Ingelheim, and Alere. Dr Chan has received research support from the National Heart, Lung, and Blood Institute. Dr Ezekowitz reports serving as a consultant for AstraZeneca, Eisai, Pozen Inc, Boehringer Ingelheim, ARYx Therapeutics, Pfizer, Sanofi, Bristol‐Myers Squibb, Portola, Daiichi Sanko, Medtronic, Merck, Johnson & Johnson, Gilead, Janssen Scientific Affairs, and Armetheon; and received grants from Boehringer Ingelheim, Bayer, Daiichi Sanko, Pfizer, and Bristol‐Myers Squibb. Dr Freeman has acted as a consultant and advisory board member for Janssen Scientific; he also reports personal fees from Janssen Pharmaceuticals and the American College of Cardiology National Cardiovascular Data Registry outside the submitted work. Dr Fonarow reports modest Consultant/Advisory Board support from Ortho McNeil. Dr Gersh reports modest DSMB/Advisory Board support from Medtronic, Baxter Healthcare Corporation, InspireMD, Cardiovascular Research Foundation, PPD Development, LP, Boston Scientific, and St. Jude. Dr Kowey reports modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol‐Myers Squibb, Johnson & Johnson, Portola, Merck, Sanofi, and Daiichi Sankyo. Dr Mahaffey reports research support from Afferent, Amgen, Apple, AstraZeneca, Cardiva Medical, Inc., Daiichi, Ferrings, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, Novartis, Sanofi, St. Jude, and Tenas; consulting to Ablynx, AstraZeneca, Boehringer Ingelheim, Bristol Meyers Squibb, Cardiometabolic Health Congress, Elsevier, Glaxo Smith Kline, Johnson & Johnson, Mederg, Medscape, Merck, Mitsubishi, Myokaria, Novartis, Oculeve, Portola, Radiometer, Springer Publishing, Theravance, UCSF, and WebMD; and equity in BioPrint Fitness. Dr Naccarelli reports research support from Janssen and service as consultant to Glaxo‐Smith‐Kline, Janssen, and Daiichi‐Sankyo. Dr Reiffel reports research support from Janssen and Medtronic; was a consultant to Medtronic, Janssen, In Cardia Therapeutics, Acesion, Portola; and speaker's bureau activities with Janssen and Boehringer Ingelheim. Dr Peterson reports significant research grant support from Eli Lilly & Company, Janssen Pharmaceuticals, Inc, and the American Heart Association; modest Consultant/Advisory Board support from Boehringer Ingelheim, Bristol‐Myers Squibb, Janssen Pharmaceuticals, Inc, Pfizer, and Genentech Inc. Dr Piccini reports significant research grant support from Johnson & Johnson/Janssen Pharmaceuticals; significant other research support from Bayer HealthCare Pharmaceuticals Inc (formerly Berlex Labs), Boston Scientific Corporation, Johnson & Johnson Pharmaceutical Research & Development; modest Consultant/Advisory Board support from Forest Laboratories, Inc and Medtronic, Inc; and significant Consultant/Advisory Board support from Johnson & Johnson/Janssen Pharmaceuticals.
Supporting information
Appendix S1. ORBIT‐AF II Investigators
Table S1. Dosing Criteria
Table S2. List of Covariates for Propensity Score
Table S3. Standardized Differences Between Propensity‐Matched Subjects (N=1302)
Table S4. Event Rates by NOAC Dose Received (Rivaroxaban or Apixaban) Among Patients Recommended for Standard NOAC Dosing (n=6584) and in Propensity‐Matched, Adjusted Cohort (n=651)
Figure S1. Distribution and appropriateness of dosing, recategorizing patients with borderline creatinine clearance as appropriate for reduced dosing.
(J Am Heart Assoc. 2018;7:e007633 DOI: 10.1161/JAHA.117.007633.)29453305
References
- 1. Daiichi Sankyo Inc . Edoxaban prescribing information. 2015. Available at: http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed February 8, 2018.
- 2. Janssen Pharmaceuticals . Rivaroxaban prescribing information. 2011. Available at: https://www.xareltohcp.com/shared/product/xarelto/prescribing-information.pdf. Accessed February 8, 2018.
- 3. Boehringer‐Ingelheim . Dabigatran prescribing information. 2010. Available at: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed February 8, 2018.
- 4. Bristol‐Myers Squibb . Apixaban prescribing information. 2012. Available at: http://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed February 8, 2018.
- 5. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 7. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 8. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 9. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Singer DE, Peterson ED, Piccini JP; ORBIT‐AF Investigators and Patients . Off‐label dosing of non‐vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT‐AF II Registry. J Am Coll Cardiol. 2016;68:2597–2604. [DOI] [PubMed] [Google Scholar]
- 10. Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011;364:1788–1790. [DOI] [PubMed] [Google Scholar]
- 11. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 12. Steinberg BA, Blanco RG, Ollis D, Kim S, Holmes DN, Kowey PR, Fonarow GC, Ansell J, Gersh B, Go AS, Hylek E, Mahaffey KW, Thomas L, Chang P, Peterson ED, Piccini JP; ORBIT‐AF Steering Committee Investigators . Outcomes registry for better informed treatment of atrial fibrillation II: rationale and design of the ORBIT‐AF II registry. Am Heart J. 2014;168:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 14. Li F, Zaslavsky AM, Landrum MB. Propensity score weighting with multilevel data. Stat Med. 2013;32:3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barra ME, Fanikos J, Connors JM, Sylvester KW, Piazza G, Goldhaber SZ. Evaluation of dose‐reduced direct oral anticoagulant therapy. Am J Med. 2016;129:1198–1204. [DOI] [PubMed] [Google Scholar]
- 16. Pattullo CS, Barras M, Tai B, McKean M, Donovan P. New oral anticoagulants: appropriateness of prescribing in real‐world setting. Intern Med J. 2016;46:812–818. [DOI] [PubMed] [Google Scholar]
- 17. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, Sheu TC, Mott K, Goulding MR, Houstoun M, MaCurdy TE, Worrall C, Kelman JA. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation. 2015;131:157–164. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen PB, Skjoth F, Sogaard M, Kjaeldgaard JN, Lip GY, Larsen TB. Effectiveness and safety of reduced dose non‐vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steinberg BA, Kim S, Thomas L, Fonarow GC, Hylek E, Ansell J, Go AS, Chang P, Kowey P, Gersh BJ, Mahaffey KW, Singer DE, Piccini JP, Peterson ED; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation I, Patients . Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) registry. Circulation. 2014;129:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinberg BA, Shrader P, Kim S, Thomas L, Fonarow GC, Ansell J, Kowey PR, Singer DE, Gersh BJ, Mahaffey KW, Peterson ED, Piccini JP; ORBIT‐AF Investigators and Patients . How well does physician risk assessment predict stroke and bleeding in atrial fibrillation? Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Am Heart J. 2016;181:145–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. ORBIT‐AF II Investigators
Table S1. Dosing Criteria
Table S2. List of Covariates for Propensity Score
Table S3. Standardized Differences Between Propensity‐Matched Subjects (N=1302)
Table S4. Event Rates by NOAC Dose Received (Rivaroxaban or Apixaban) Among Patients Recommended for Standard NOAC Dosing (n=6584) and in Propensity‐Matched, Adjusted Cohort (n=651)
Figure S1. Distribution and appropriateness of dosing, recategorizing patients with borderline creatinine clearance as appropriate for reduced dosing.


