Abstract
Background
The American Heart Association recommends focusing on 7 health factors (Life's Simple 7) for primordial prevention of cardiovascular health. However, whether greater adherence to Life's Simple 7 in midlife improves prognosis after myocardial infarction (MI) in later life is unknown.
Methods and Results
In 1277 participants who developed MI during the ARIC (Atherosclerosis Risk in Communities) Study follow‐up, a 14‐point score of Life's Simple 7 was constructed according to the status (2 points for ideal, 1 point for intermediate, and 0 points for poor) of each of 7 factors (smoking, adiposity, physical activity, diet, total cholesterol, blood pressure, and fasting glucose) at baseline (1987–1989). Hazard ratios for composite and individual adverse outcomes of all‐cause mortality, cardiovascular mortality, recurrent MI, heart failure, and stroke were calculated according to Life's Simple 7 score. During a median follow‐up of 3.3 years, 918 participants (72%) had subsequent adverse outcomes after MI. Life's Simple 7 score at middle age was inversely associated with adverse outcomes after MI (adjusted hazard ratios of composite outcome, 0.57 [95% confidence interval, 0.39–0.84] if score is ≥10, 0.78 [95% confidence interval, 0.57–1.07] if score is 7–9, and 0.82 [95% confidence interval, 0.60–1.11] if score is 4–6 versus ≤3). The association was largely independent of access to care and MI severity. Individual factors related to better prognosis after MI were ideal nonsmoking, body mass index, blood pressure, and fasting glucose.
Conclusions
Optimal Life's Simple 7 at middle age was associated with better prognosis after MI in later life. Our findings suggest a secondary prevention benefit of having better cardiovascular health status in midlife.
Keywords: cardiovascular disease risk factors, Life's Simple 7, myocardial infarction, secondary prevention
Subject Categories: Epidemiology, Risk Factors, Secondary Prevention
Clinical Perspective
What Is New?
In addition to its association with incident myocardial infarction, higher score on 7 health factors (indicating better lifestyle and health status) at middle age was independently associated with better prognosis after incident myocardial infarction in later life.
Among the individual 7 health factors, ideal status of smoking, body mass index, blood pressure, and fasting glucose in midlife were robust predictors of better prognosis after myocardial infarction in later life.
What Are the Clinical Implications?
Our data provide new insights about the importance of maintaining or achieving optimal lifestyle and health status in midlife for cardiovascular health in later life.
Our data also suggest the potential usefulness of individual historical information for predicting future prognosis after the development of cardiovascular disease, such as myocardial infarction.
Introduction
In 2010, the American Heart Association (AHA) announced the following strategic goal: “By 2020, to improve the cardiovascular health of all Americans by 20% while reducing death from cardiovascular disease and stroke by 20%.”1 To achieve this goal, the AHA recommended focusing on 7 cardiovascular health factors (smoking, body mass index [BMI], physical activity, diet, total cholesterol, blood pressure, and fasting blood glucose) for primordial or primary prevention of cardiovascular disease and defined them as Life's Simple 7.1, 2 The selection of these 7 factors is based on their contributions to incident cardiovascular disease.1
However, it is unknown whether better achievement of Life's Simple 7 at middle age is associated with better prognosis after incident cardiovascular disease in later life. To complicate this question, a few studies have reported that a higher number of traditional risk factors at admission was counterintuitively associated with lower in‐hospital mortality in patients with myocardial infarction (MI).3, 4, 5 However, these studies retrospectively collected information on traditional risk factors at or before MI diagnosis, mainly relying on medication use for defining hypertension, diabetes mellitus, and dyslipidemia. Thus, as the authors of these studies acknowledged, these studies are subject to potential bias (ie, the presence of risk factors reflecting better detection and management of these risk factors and leading to a better prognosis compared with those without diagnosed or treated risk factors).
Therefore, to quantify the potential importance of optimal Life's Simple 7 in secondary prevention setting and overcome the methodological caveat in those previous studies with retrospective data collection at hospital admission, we explored data from a prospective community‐based cohort, the ARIC (Atherosclerosis Risk in Communities) Study, with long‐term follow‐up for the assessment of outcomes. We primarily quantified the association of Life's Simple 7, incorporating measured blood pressure and levels of fasting glucose and total cholesterol in addition to medication use and health behaviors at middle age with the risk of adverse outcomes after incident MI in later life. To provide a complete picture, we secondarily assessed the contribution of Life's Simple 7 to incident MI as well, although ARIC Study data have previously reported the relationship with cardiovascular disease incidence.6, 7
Methods
Study Population and Design
The ARIC Study is a community‐based cohort of 15 792 middle‐aged men and women (45–64 years old) at baseline (1987–1989).8 Detailed policies for accessing ARIC Study data are available at the ARIC Study website.9 It is also possible to obtain ARIC Study data from the National Heart, Lung, and Blood Institute BioLINCC repository.10 Study participants were predominantly whites and blacks and were recruited from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. An institutional review board at each site approved the study, and study participants provided documentation of informed consent at each study visit.
This study has 2 sets of survival analysis: (1) the associations of Life's Simple 7 at middle age with incident MI in the entire study population (secondary analysis) and (2) the associations of Life's Simple 7 at middle age with adverse outcomes after incident MI among those who developed MI during follow‐up (primary analysis) (Figure S1). For the former, of 15 792 ARIC Study participants, we excluded participants who had a history of coronary heart disease, stroke, or heart failure at baseline (n=1595) or who were neither whites nor blacks (n=33). We further excluded 632 participants who did not have complete information on Life's Simple 7 or 421 participants with nonfasting glucose or cholesterol levels, leaving a sample of 13 079 participants. Of these 13 079 participants, 1277 developed incident MI during a median follow‐up of 24.2 years; they constituted the study sample for the latter main analysis in patients with MI (Figure S2).
Data Collection and Life's Simple 7 at ARIC Study Baseline Visit 1
At visit 1 (1987–1989), trained interviewers administered a questionnaire to collect information on demographic characteristics, medical history, medication use, and health behaviors (smoking status, physical activity, and diet). Use of antihypertensive, cholesterol‐lowering, and glucose‐lowering medications within the past 2 weeks of baseline interview were self‐reported and confirmed by the inspection of medication containers. Physical activity was reported with the Baecke questionnaire,11 and diet was assessed by modified 66‐item Harvard food frequency questionnaire.12 Information on education level, total household income for the past 12 months, and insurance status at baseline was based on questionnaire. BMI was calculated as weight (in kilograms) divided by the square of height (in meters). After 5 minutes of rest, 3 seated measurements of blood pressure were taken by a certified technician using a random‐zero sphygmomanometer. The mean of the second and third measurements was used for analysis. Fasting glucose levels were measured by the modified hexokinase/glucose‐6‐phosphate dehydrogenase method. Fasting plasma total cholesterol concentration was assessed by enzymatic procedures.
Seven health factors were each categorized as ideal, intermediate, or poor, according to the AHA Life's Simple 7 criteria. Specifically, as previously done,6 ideal levels of the Life's Simple 7 factors were defined as follows: nonsmoker or quit >1 year ago; BMI of <25 kg/m2; ≥150 min/wk of moderate+vigorous physical activity; 4 to 5 components of a healthy diet pattern; untreated total cholesterol of <5.2 mmol/L; untreated blood pressure of <120/80 mm Hg; and untreated fasting glucose of <5.6 mmol/L (Table S1). Poor Life's Simple 7 factors included the following: Current smoker; BMI of ≥30 kg/m2; no moderate or vigorous physical activity; 0 to 1 components of a healthy diet pattern; total cholesterol of ≥6.2 mmol/L; blood pressure of ≥140/90 mm Hg; and fasting glucose of ≥7.0 mmol/L regardless of medication use. Any conditions between ideal and poor were defined as intermediate. The score of Life's Simple 7 was calculated by providing 2 points for ideal, 1 point for intermediate, and 0 points for poor status of each of the 7 individual factors. Thus, the Life's Simple 7 summary score ranged from 0 to a maximum of 14 points, with a higher score indicating healthier status.
Ascertainment of Incident MI and Data Collection at Incident MI
Participants were followed up through December 31, 2013. Cardiovascular events in the ARIC Study were ascertained by contacting participants annually, identifying hospitalizations and deaths during the previous year, and surveying discharge lists from local hospitals and death certificates from state vital statistics offices for potential cardiovascular events. Incident MI was defined as definite or probable nonfatal MI cases adjudicated by the ARIC Study physician panel.13
We collected records for hospital discharges for MI, which were reviewed by trained abstractors for recording insurance status, medical history, and inpatient pharmacologic treatment. Because those with better Life's Simple 7 status may have less severe MIs compared with those with poorer Life's Simple 7 status, we determined MI severity, using a modified score of the Predicting Risk of Death in Cardiac Disease Tool that uses several clinical variables (cardiogenic shock; history of MI, stroke, or angina; age; severity of electrocardiographic changes; congestive heart failure; and Charlson Comorbidity Index) for a maximum score of 21 points, with a higher score indicating a more severe case.14, 15
Adverse Outcomes After Incident MI
Adverse outcomes after incident MI of interest were all‐cause mortality, cardiovascular mortality, recurrent MI, incident heart failure, and incident stroke. We also investigated the composite of those outcomes. Mortality was identified through active surveillance in the ARIC Study, and cardiovascular mortality was defined as death attributable to coronary heart disease, heart failure, or stroke. Recurrent MI was defined as adjudicated definite and probable MI cases after incident MI. Incident heart failure was defined as occurrence after MI of either a hospitalization or death having in any position an International Classification of Diseases, Ninth Revision (ICD‐9) code 428 or an ICD‐10 code I50 for heart failure diagnosis. For stroke, using criteria adopted from the National Survey of Stroke,16 incident definite or probable strokes were identified by computer algorithm and reviewed by a physician, with disagreements resolved by a second physician.
Statistical Analysis
Analyses were conducted using Stata statistical software version 14 (StataCorp LC, College Station, TX). Baseline characteristics are presented as means and SDs for continuous variables and proportions for categorical variables across categories of Life's Simple 7 summary score of 0 to 3, 4 to 6, 7 to 9, and ≥10.17, 18
In terms of longitudinal analysis, we first examined the associations of Life's Simple 7 score and each individual health factor with incident MI in the entire study population. Survival free of incident MI was estimated across categories of Life's Simple 7 score using the Kaplan‐Meier method. Hazard ratios were estimated using Cox proportional hazards models adjusting for demographic characteristics (age, sex, and race).
Then, as the main analysis, we quantified the associations of Life's Simple 7 score and each individual health factor at study baseline with adverse outcomes after incident MI among those who experienced incident MI during follow‐up. Survival free of adverse outcomes was estimated across categories of Life's Simple 7 score using the Kaplan‐Meier method. Subsequently, we quantified the associations of the Life's Simple 7 score and each health factor with those adverse outcomes using multivariable Cox proportional hazards models. P values for trend were based on Cox proportional hazards models with the Life's Simple 7 score using number 1 through 4 for the categories as discrete variables. We implemented 3 main models to evaluate the impact of potential confounders. Model 1 was adjusted for the following: age at MI, sex, race, calendar year of incident MI (<1995, 1995–2004, and ≥2005), clinical predictors at admission for poor prognosis in patients with MI19 (a history of heart failure, stroke, coronary artery bypass graft, peripheral artery disease, and kidney dysfunction (estimated glomerular filtration rate of <60 mL/min per 1.73 m2 within 1 year before MI or diagnosed chronic kidney disease), hypertension, diabetes mellitus, and current smoking at admission. We also further adjusted for health insurance at baseline (model 2a) and admission (model 2b).
We conducted several sensitivity analyses. We adjusted for year of school completed (≤12 and >12 years) and family income for the past 12 months (<$35 000 and ≥$35 000) at baseline as measures of socioeconomic status instead of health insurance (model 3). We also evaluated whether the adjustment for MI severity (represented by the Predicting Risk of Death in Cardiac Disease Tool score) (model 4) and aspirin use (model 5) altered results. We also evaluated whether the exclusion of MI cases who died within 14 days after incident MI changed results. In addition, we repeated the analysis after restricting the follow‐up to 1, 3, and 5 years after incident MI because participants with poor Life's Simple 7 might have incident MI earlier than those with better Life's Simple 7 and, thus, would have higher chance of developing adverse outcomes with longer follow‐up after incident MI. We also explored potential interaction by sex (female versus male), race (blacks versus whites), and center (Forsyth County versus Jackson versus suburbs of Minneapolis versus Washington County).
Results
Life's Simple 7 at Baseline and Incident MI in the Entire Study Population
In the entire study population, the mean age at baseline was 55 years old, 56% were women, and 24% were black. Those with a higher Life's Simple 7 score were more likely to be younger and white and have lower levels of BMI, blood pressure, fasting glucose, and total cholesterol compared with those with a lower Life's Simple 7 score (Table S2). Over a median follow‐up of 24.2 years, there were 1277 incident MI cases. As anticipated, a higher Life's Simple 7 score was significantly associated with lower risk of incident MI in a graded manner (Table 1 and Figure S3). Compared with participants with Life's Simple 7 score of 0 to 3, those with a score of ≥10 and 7 to 9 had 84% and 67% lower risk of incident MI, respectively (hazard ratio, 0.16 [95% confidence interval, 0.12–0.22] and 0.33 [95% confidence interval, 0.26–0.43]) (Table 1). For individual Life's Simple 7 factors, the risk of incident MI was lower, with ideal levels of health factors compared with poor levels with the exception of diet (Table S3).
Table 1.
Crude Incidence Rate (per 1000 Person‐Years) and Adjusted Hazard Ratio (95% CI) of Incident MI According to Life's Simple 7 Score at Baseline in the Entire Study Population (N=13 079), ARIC Study, 1987 to 2013
| Variable | Score of Life's Simple 7 | ||||
|---|---|---|---|---|---|
| 0–3 (N=374) | 4–6 (N=3137) | 7–9 (N=6042) | ≥10 (N=3526) | P Value for Trend | |
| MI cases | 87 | 472 | 614 | 168 | |
| Incidence rate | 12.1 | 7.4 | 4.7 | 2.0 | |
| Hazard ratio (95% CI) | 1 (Reference) | 0.53 (0.42–0.69) | 0.33 (0.26–0.43) | 0.16 (0.12–0.22) | <0.001 |
ARIC indicates the Atherosclerosis Risk in Communities Study; CI, confidence interval; and MI, myocardial infarction.
*The hazard ratio (95% CI) is adjusted for age at baseline, sex, and race.
Life's Simple 7 at Middle Age and Adverse Outcomes After Incident MI
Among participants with incident MI, the mean age at MI was 69 years old, 42% were women, and 24% were black. Of those participants, 59% had a Life's Simple 7 score of ≥7 at middle age (13% had a Life's Simple 7 score of ≥10) (Table 2 and Figure). Those with higher Life's Simple 7 scores tended to be older, female, and white, and have health insurance, higher family income, and higher education level, and lower levels of BMI, blood pressure, fasting glucose, and total cholesterol compared with those with lower Life's Simple 7 scores (Table 2).
Table 2.
Basic Characteristics of the Participants With Incident MI (N=1277), According to Life's Simple 7 Score
| Characteristics | Total (N=1277) | Score of Life's Simple 7 | |||
|---|---|---|---|---|---|
| 0–3 (n=77) | 4–6 (n=445) | 7–9 (n=593) | ≥10 (n=162) | ||
| Demographic | |||||
| Age, y | 55.9±5.6 | 54.7±5.4 | 55.7±5.5 | 56.3±5.7 | 55.6±5.4 |
| Female sex | 42.4 | 63.6 | 43.8 | 38.1 | 43.8 |
| Black race | 24.3 | 58.4 | 34.2 | 17.4 | 6.2 |
| At study baseline | |||||
| Health insurance (yes) | 89.7 | 76.6 | 84.3 | 93.4 | 97.5 |
| Family income for the past 12 mo | |||||
| ≥$25 000 | 57.0 | 20.8 | 49.2 | 60.2 | 74.7 |
| Highest education | |||||
| ≥12 y | 70.5 | 42.9 | 62.9 | 74.9 | 88.3 |
| Body mass index, kg/m2 | 28.5±5.1 | 32.8±5.4 | 29.8±5.1 | 27.2±4.5 | 24.9±3.6 |
| Systolic blood pressure, mm Hg | 125.9±19.1 | 139.5±21.2 | 131.2±20.2 | 123.5±17.3 | 113.6±11.2 |
| Diastolic blood pressure, mm Hg | 75.3±12.2 | 82.0±13.3 | 78.6±13.3 | 73.7±10.8 | 69.0±8.9 |
| Fasting glucose, mmol/L | 6.4±2.5 | 8.4±3.3 | 7.0±3.1 | 6.0±2.0 | 5.4±1.1 |
| Total cholesterol, mmol/L | 5.8±1.1 | 6.6±1.1 | 6.2±1.2 | 5.6±1.0 | 5.3±1.0 |
| HDL cholesterol, mmol/L | 1.2±0.4 | 1.2±0.4 | 1.2±0.3 | 1.2±0.4 | 1.3±0.5 |
| LDL cholesterol, mmol/L | 3.9±1.1 | 4.4±1.0 | 4.2±1.1 | 3.7±1.0 | 3.4±1.0 |
| Life's Simple 7 categories | |||||
| Smoking status | |||||
| Intermediate | 32.6 | 20.8 | 32.1 | 34.6 | 32.1 |
| Ideal | 33.5 | 15.6 | 27.2 | 34.7 | 54.9 |
| Body mass index | |||||
| Intermediate | 43.2 | 15.6 | 43.6 | 48.4 | 35.8 |
| Ideal | 26.7 | 3.9 | 13.5 | 30.9 | 58.6 |
| Physical activity | |||||
| Intermediate | 4.2 | 3.9 | 4.0 | 4.6 | 3.1 |
| Ideal | 55.8 | 2.6 | 32.8 | 69.5 | 94.4 |
| Healthy diet | |||||
| Intermediate | 43.9 | 22.1 | 34.8 | 49.6 | 58.0 |
| Ideal | 5.3 | 0.0 | 1.8 | 6.1 | 14.8 |
| Total cholesterol | |||||
| Intermediate | 37.4 | 31.2 | 33.7 | 42.0 | 34.0 |
| Ideal | 29.0 | 6.5 | 18.2 | 33.4 | 53.1 |
| Blood pressure | |||||
| Intermediate | 47.6 | 40.3 | 49.4 | 51.3 | 32.7 |
| Ideal | 28.1 | 3.9 | 14.6 | 31.5 | 64.2 |
| Glucose | |||||
| Intermediate | 37.7 | 36.4 | 46.3 | 37.3 | 16.7 |
| Ideal | 46.1 | 7.8 | 29.2 | 53.8 | 82.1 |
| At admission of MI | |||||
| Age at incident MI, y | 68.7±8.8 | 65.1±8.5 | 68.0±8.5 | 69.3±9.0 | 70.4±8.6 |
| Calendar year | |||||
| <1995 | 22.5 | 36.4 | 23.4 | 23.8 | 8.6 |
| 1996–2004 | 43.5 | 37.7 | 47.0 | 40.3 | 48.2 |
| ≥2005 | 34.1 | 26.0 | 29.7 | 35.9 | 43.2 |
| MI severity score | 7.4±3.4 | 7.8±3.2 | 7.5±3.6 | 7.3±3.3 | 7.0±3.6 |
| Health insurancea | 93.1 | 70.1 | 80.1 | 79.6 | 90.5 |
| Medicare/Medicaid | 61.3 | 52.0 | 52.6 | 51.6 | 59.3 |
| Private | 75.4 | 41.6 | 59.8 | 68.1 | 79.6 |
Data are given as mean±SD or percentage. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; and MI, myocardial infarction.
Of 1277 patients with MI, 1102 have insurance data at admission.
Figure 1.
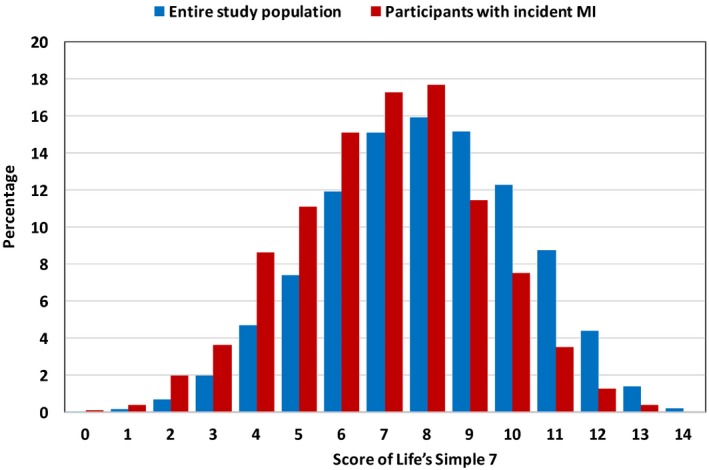
Distribution of Life's Simple 7 score in the entire study population (blue) and participants with incident myocardial infarction (MI) during follow‐up (red).
Of 1277 participants with incident MI, 918 had adverse outcomes (661 all‐cause deaths, including 258 cardiovascular deaths, 292 recurrent MI cases, 571 heart failure cases, and 129 stroke cases) during a median follow‐up of 3.3 years (maximum follow‐up, 26.2 years). For all outcomes tested, there was a sharp initial decrease for the composite outcome because ≈20% of patients with MI presented with heart failure within 1 month after incident MI (Figure S4). Overall, participants with higher Life's Simple 7 scores at middle age had a lower risk compared with those with lower scores. The pattern was generally consistent after adjusting for demographic variables and clinical comorbidities at MI admission, with significant associations for all outcomes except recurrent MI and stroke (model 1 in Table 3). The adjustment for health insurance (particularly at admission) to some extent attenuated the associations, although the associations for all‐cause mortality, cardiovascular mortality, and the composite outcome remained consistently significant (models 2a and 2b in Table 3).
Table 3.
Adjusted Hazard Ratio (95% CI) of Adverse Outcomes After Incident MI According to Life's Simple 7 Score Among Patients With MI
| Variable | Score of Life's Simple 7 | ||||
|---|---|---|---|---|---|
| 0–3 (N=77) | 4–6 (N=445) | 7–9 (N=593) | ≥10 (N=162) | P Value for Trend | |
| Composite outcome | |||||
| Cases | 71 | 345 | 421 | 81 | |
| Model 1 | 1 (Reference) | 0.80 (0.61–1.04) | 0.73 (0.56–0.96) | 0.52 (0.37–0.75) | <0.001 |
| Model 2a | 1 (Reference) | 0.80 (0.61–1.04) | 0.73 (0.56–0.96) | 0.53 (0.37–0.75) | 0.001 |
| Model 2b (N=1102) | 1 (Reference) | 0.82 (0.60–1.11) | 0.78 (0.57–1.07) | 0.57 (0.39–0.84) | 0.007 |
| All‐cause mortality | |||||
| Cases | 57 | 273 | 287 | 44 | |
| Model 1 | 1 (Reference) | 0.78 (0.58–1.05) | 0.51 (0.38–0.70) | 0.32 (0.21–0.49) | <0.001 |
| Model 2a | 1 (Reference) | 0.78 (0.58–1.05) | 0.52 (0.38–0.70) | 0.32 (0.21–0.49) | <0.001 |
| Model 2b (N=1102) | 1 (Reference) | 0.84 (0.59–1.19) | 0.60 (0.41–0.86) | 0.34 (0.21–0.56) | <0.001 |
| Cardiovascular mortality | |||||
| Cases | 32 | 112 | 104 | 10 | |
| Model 1 | 1 (Reference) | 0.79 (0.52–1.20) | 0.57 (0.36–0.88) | 0.28 (0.13–0.61) | <0.001 |
| Model 2a | 1 (Reference) | 0.79 (0.52–1.20) | 0.57 (0.36–0.88) | 0.28 (0.13–0.61) | <0.001 |
| Model 2b (N=1102) | 1 (Reference) | 0.83 (0.50–1.39) | 0.68 (0.40–1.18) | 0.32 (0.14–0.74) | 0.007 |
| Recurrent MI | |||||
| Cases | 24 | 113 | 131 | 24 | |
| Model 1 | 1 (Reference) | 0.92 (0.58–1.45) | 0.79 (0.49–1.26) | 0.59 (0.32–1.10) | 0.048 |
| Model 2a | 1 (Reference) | 0.93 (0.59–1.46) | 0.81 (0.51–1.30) | 0.62 (0.33–1.15) | 0.075 |
| Model 2b (N=1102) | 1 (Reference) | 1.04 (0.60–1.82) | 0.91 (0.51–1.62) | 0.74 (0.36–1.51) | 0.213 |
| Heart failure | |||||
| Cases | 53 | 211 | 254 | 53 | |
| Model 1 | 1 (Reference) | 0.72 (0.52–0.98) | 0.68 (0.49–0.95) | 0.60 (0.39–0.92) | 0.041 |
| Model 2a | 1 (Reference) | 0.72 (0.52–0.98) | 0.69 (0.50–0.95) | 0.61 (0.40–0.93) | 0.050 |
| Model 2b (N=1102) | 1 (Reference) | 0.77 (0.54–1.10) | 0.78 (0.54–1.13) | 0.71 (0.45–1.12) | 0.320 |
| Stroke | |||||
| Cases | 7 | 55 | 58 | 9 | |
| Model 1 | 1 (Reference) | 1.74 (0.78–3.89) | 1.46 (0.64–3.31) | 1.06 (0.37–3.05) | 0.606 |
| Model 2a | 1 (Reference) | 1.76 (0.79–3.93) | 1.42 (0.62–3.22) | 1.03 (0.36–2.95) | 0.505 |
| Model 2b (N=1102) | 1 (Reference) | 1.72 (0.67–4.40) | 1.70 (0.65–4.44) | 1.27 (0.40–4.07) | 0.914 |
Model 1: adjusted for age at MI; sex; race; calendar year of incident MI (<1995, 1995–2004, and ≥2005); history of heart failure, stroke, coronary artery bypass graft, peripheral artery disease, kidney dysfunction, hypertension, and diabetes mellitus; and current smoking at admission. Model 2a: adjusted for model 1+health insurance at baseline. Model 2b: adjusted for model 1+health insurance at admission. CI indicates confidence interval; and MI, myocardial infarction.
We observed similar results when we did the following: (1) adjusted for education level and family income over the past 12 months at baseline, instead of health insurance status; (2) accounted for MI severity; and (3) adjusted for aspirin use (Table S4). Similarly, the exclusion of those who died within 14 days after MI did not alter the results (Table S5). When restricting the follow‐up after MI to 1, 3, or 5 years, the associations were largely consistent across different durations of follow‐up, although fewer outcomes reached statistical significance, given restricted number of MI cases and subsequent outcomes (Table S6). We did not observe any significant interactions by sex, race, and center in models 1 to 3 (data not shown).
We subsequently examined the associations of individual Life's Simple 7 factors with adverse outcomes after incident MI (Table S7). A better status of smoking, BMI, blood pressure, and fasting glucose at baseline was significantly associated with lower risk of adverse outcomes after incident MI, even in model 3, accounting for health insurance.
Discussion
In this prospective community‐based study, a higher Life's Simple 7 score in midlife was associated with lower risk of not only incident MI but also adverse outcomes after incident MI in later life. The association of Life's Simple 7 score with adverse outcomes after incident MI persisted even after accounting for clinical conditions at incident MI admission, health insurance status, and severity of MI. In terms of individual factors, ideal categories for nonsmoking, BMI, blood pressure, and fasting glucose in middle age were especially associated with better prognosis after incident MI at later life.
To our knowledge, our study is the first to report the association of Life's Simple 7 at middle age with adverse outcomes after MI in later life. More important, those with Life's Simple 7 score of ≥7 at the study baseline had ≈40% to 60% lower risk of total mortality after MI compared with those who had Life's Simple 7 score of ≤3. Our results indicate a secondary prevention benefit of ideal cardiovascular health status in midlife, which further supports the promotion of cardiovascular health on the basis of Life's Simple 7. Our results seem critically important because previous studies focusing on health factors at MI admission reported, counterintuitively, that “better” health status led to poorer MI prognosis.3, 4, 5 Although our data do not have information on Life's Simple 7 factors at the time of MI admission, there may be a few explanations for this counterintuitive relationship. For example, some investigators suggested that patients with MI with better health status are older than patients with poor health status and, thus, have worse prognosis. This perspective is in line with our observation of participants with a higher score of Life's Simple 7 having incident MI at an older age than those with a lower score. Also, misclassification of health factors at admission is possible, because the evaluation of health factors during MI hospitalization can be challenging. This may be particularly the case for blood pressure, because a decrease in blood pressure after MI is common and may reflect unstable hemodynamic state.20 Potentially most importantly, apparently “better” health status at admission of MI may result from undiagnosed or untreated risk factors attributable to low awareness or worse healthcare access.21
For individual health and lifestyle factors, we found ideal levels of nonsmoking, BMI, blood pressure, and fasting glucose at middle age were associated with lower risk of adverse outcome after incident MI in later life. Those factors are well known to predict incident cardiovascular disease,22, 23, 24 and our study uniquely demonstrated that the status of these factors at middle age would have prognostic value for prognosis after incident MI as well. In our study, healthy diet and physical activity at baseline were not individually associated with adverse outcomes after incident MI. However, this may be attributable to quality of questionnaire data for these 2 factors. For dietary patterns in Life's Simple 7, some investigators raised a concern that adherence to the 5 AHA dietary recommendations is challenging.25 In fact, in our study as well as previous ones, the prevalence of ideal diet patterns is low (<1%–6%).17, 18, 26, 27, 28 This might partially explain no significant association, even with incident MI, an analysis with much higher statistical power than the analysis after incident MI.
Despite arguments for the so‐called obesity paradox (ie, obesity increasing the risk of incident MI but decreasing the risk of adverse outcomes after MI),29, 30, 31 ideal BMI at middle age was robustly associated with better prognosis after incident MI at later life in our study. Unfortunately, we did not have data on BMI at MI admission, precluding us from assessing obesity status at the time of MI. However, about the obesity paradox, a recent study demonstrated that obese individuals develop cardiovascular disease at a younger age than nonobese individuals and this age difference is a key driver behind the obesity paradox.32 The same study reported that when periods both before and after the cardiovascular event were taken into account, obesity significantly shortened life expectancy. In this context, a lower risk of adverse outcomes after MI related to ideal BMI at baseline in our study may be explained by the fact that the key driver of the obesity paradox (ie, age difference at MI onset) was minimized in the ARIC Study by the narrow age range at baseline.
Contrasting the patterns of associations with incident MI versus adverse outcome after incident MI, smoking, BMI, blood pressure, and fasting glucose demonstrated consistently positive associations, whereas diet was consistently neutral. On the other hand, physical activity and total cholesterol were only significantly associated with incident MI. It is not surprising to see some factors only associated with incident MI, with higher statistical power than adverse outcomes after MI. In addition, physical activity is based on self‐report and, thus, may have similar methodological caveats previously described for diet evaluation. For total cholesterol, the results might be influenced by lipid‐lowering therapy. Specifically, it may be possible that individuals with poor cholesterol category might receive statins, which are shown to substantially reduce the risk of adverse outcomes among patients with MI.33 Indeed, 79% of participants who were categorized in the poor cholesterol category at baseline were receiving lipid‐lowering therapy at the time of MI admission (21% in the optimal category).
Our results have a few clinical and research implications. First, our study provides evidence for a secondary prevention benefit of maintaining better health status at middle age. Our finding demonstrates that adherence to Life's Simple 7, proposed by the AHA, could be effective in the public to prevent both primary and secondary cardiovascular risk. Second, our study suggests the potential usefulness of individual historical information for predicting future risk. Although it is probably not currently practical to obtain risk factor information from 10 to 20 years ago, with a broad implementation of electronical medical records, eventually, we will be able to refer to patients’ historical data.
We also need to mention several limitations in our study. First, as previously noted, we relied on self‐report for smoking, physical activity, and diet, and thus some misclassification is probably unavoidable for those behavioral variables. Second, use of a single measure of health factors might lead to underestimation of the true biological associations between Life's Simple 7 and adverse outcomes because of regression dilution bias.34 Third, we could not include patients with MI who did not reach the hospital. Finally, we had no complete information on treatment of MI after discharge. Patients with better health factors or high socioeconomic status might get better treatment.
In conclusion, optimal (compared with poor) Life's Simple 7 at middle age was associated with not only lower incidence of hospitalized MI but also lower adverse outcomes after MI. Our findings suggest a secondary prevention benefit of ideal cardiovascular health status in midlife.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contracts (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, and HHSN2682017000021). Dr. Rebholz is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant K01 DK107782.
Disclosures
None.
Supporting information
Table S1. Definition of Life's Simple 7 Health Factors at Baseline (1987–1989)
Table S2. Baseline Characteristics for Study Population According to Score of Life's Simple 7, N=13 079
Table S3. Risk of Incident Myocardial Infarction by Life's Simple 7 Health Factors, N=13 079
Table S4. Hazard Ratio (95% CI) of Adverse Outcomes After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients After Adjusting Socioeconomic Status and Myocardial Infarction Severity
Table S5. Adjusted Hazard Ratio (95% CI)* of Adverse Outcomes After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients After Excluding Those Who Died Within 14 Days After Myocardial Infarction (N=1245)
Table S6. Adjusted Hazard Ratio (95% CI)* of Adverse Outcomes After Restricting Follow‐Up Time After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients
Table S7. Hazard Ratios (95% CI)* for Composite Outcome According to the Status of Each Individual Life's Simple 7 Factor Among Those Who Had Incident Myocardial Infarction During Follow‐Up, N=1277
Figure S1. Study design.
Figure S2. Flow chart of study population.
Figure S3. Kaplan–Meier curves for incident myocardial infarction by the score of Life's Simple 7 in the entire study population.
Figure S4. Kaplan–Meier curves for cardiovascular mortality, recurrent myocardial infarction, heart failure, and stroke by Life's simple 7 score among myocardial infarction patients.
Acknowledgments
We thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) Study for their important contributions.
(J Am Heart Assoc. 2018;7:e007658 DOI: 10.1161/JAHA.117.007658.)29455158
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Sacco RL. The new American Heart Association 2020 goal: achieving ideal cardiovascular health. J Cardiovasc Med (Hagerstown). 2011;12:255–257. [DOI] [PubMed] [Google Scholar]
- 3. Roe MT, Halabi AR, Mehta RH, Chen AY, Newby LK, Harrington RA, Smith SC Jr, Ohman EM, Gibler WB, Peterson ED. Documented traditional cardiovascular risk factors and mortality in non‐ST‐segment elevation myocardial infarction. Am Heart J. 2007;153:507–514. [DOI] [PubMed] [Google Scholar]
- 4. Canto JG, Kiefe CI, Rogers WJ, Peterson ED, Frederick PD, French WJ, Gibson CM, Pollack CV Jr, Ornato JP, Zalenski RJ, Penney J, Tiefenbrunn AJ, Greenland P; NRMI Investigators . Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306:2120–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nauta ST, Deckers JW, van der Boon RM, Akkerhuis KM, van Domburg RT. Risk factors for coronary heart disease and survival after myocardial infarction. Eur J Prev Cardiol. 2014;21:576–583. [DOI] [PubMed] [Google Scholar]
- 6. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD; ARIC Study Investigators . Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Folsom AR, Shah AM, Lutsey PL, Roetker NS, Alonso A, Avery CL, Miedema MD, Konety S, Chang PP, Solomon SD. American Heart Association's Life's Simple 7: avoiding heart failure and preserving cardiac structure and function. Am J Med. 2015;128:970–976.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 9. Atherosclerosis Risk in Communities Study. https://www2.cscc.unc.edu/aric/pubs-policies-and-forms-pg. Accessed December 14, 2017.
- 10. NHLBI Biologic Specimen and Data Repository Information Coordinating Center. https://biolincc.nhlbi.nih.gov/home. Accessed December 14, 2017.
- 11. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 12. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 13. Myerson M, Coady S, Taylor H, Rosamond WD, Goff DC Jr; Investigators A . Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119:503–514. [DOI] [PubMed] [Google Scholar]
- 14. Jacobs DR Jr, Kroenke C, Crow R, Deshpande M, Gu DF, Gatewood L, Blackburn H. PREDICT: a simple risk score for clinical severity and long‐term prognosis after hospitalization for acute myocardial infarction or unstable angina: the Minnesota heart survey. Circulation. 1999;100:599–607. [DOI] [PubMed] [Google Scholar]
- 15. Watkins S, Thiemann D, Coresh J, Powe N, Folsom AR, Rosamond W. Fourteen‐year (1987 to 2000) trends in the attack rates of, therapy for, and mortality from non‐ST‐elevation acute coronary syndromes in four United States communities. Am J Cardiol. 2005;96:1349–1355. [DOI] [PubMed] [Google Scholar]
- 16. The National Survey of Stroke : National Institute of Neurological and Communicative Disorders and Stroke. Stroke. 1981;12:I1–I91. [PubMed] [Google Scholar]
- 17. Ogunmoroti O, Oni E, Michos ED, Spatz ES, Allen NB, Rana JS, Virani SS, Blankstein R, Aronis KN, Blumenthal RS, Veledar E, Szklo M, Blaha MJ, Nasir K. Life's Simple 7 and incident heart failure: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2017;6:e005180 DOI: 10.1161/JAHA.116.005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olson NC, Cushman M, Judd SE, McClure LA, Lakoski SG, Folsom AR, Safford MM, Zakai NA. American Heart Association's Life's Simple 7 and risk of venous thromboembolism: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2015;4:e001494 DOI: 10.1161/JAHA.114.001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, He P, Lewis BS, Merlini PA, Murphy SA, Sabatine MS, Scirica BM, Morrow DA. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134:304–313. [DOI] [PubMed] [Google Scholar]
- 20. Astrup J, Bisgaard Frantzen HO, Nielsen SL, Rossing N. Blood‐pressure‐lowering effect of acute myocardial infarction. Lancet. 1976;2:903. [DOI] [PubMed] [Google Scholar]
- 21. Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk‐standardized mortality rates from 1995‐2006. JAMA. 2009;302:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26‐year follow‐up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. [DOI] [PubMed] [Google Scholar]
- 23. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 24. Rabkin SW, Mathewson FA, Hsu PH. Relation of body weight to development of ischemic heart disease in a cohort of young North American men after a 26 year observation period: the Manitoba Study. Am J Cardiol. 1977;39:452–458. [DOI] [PubMed] [Google Scholar]
- 25. Denke MA. Changing dietary habits and improving the healthiness of diets in the United States. JAMA. 2016;315:2527–2529. [DOI] [PubMed] [Google Scholar]
- 26. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rebholz CM, Anderson CA, Grams ME, Bazzano LA, Crews DC, Chang AR, Coresh J, Appel LJ. Relationship of the American Heart Association's Impact Goals (Life's Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. J Am Heart Assoc. 2016;5:e003192 DOI: 10.1161/JAHA.116.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehm CD, Drewnowski A. Dietary and economic effects of eliminating shortfall in fruit intake on nutrient intakes and diet cost. BMC Pediatr. 2016;16:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien EC, Fosbol EL, Peng SA, Alexander KP, Roe MT, Peterson ED. Association of body mass index and long‐term outcomes in older patients with non‐ST‐segment‐elevation myocardial infarction: results from the CRUSADE Registry. Circ Cardiovasc Qual Outcomes. 2014;7:102–109. [DOI] [PubMed] [Google Scholar]
- 30. Lazzeri C, Valente S, Chiostri M, Attana P, Picariello C, Sorini Dini C, Gensini GF. Impact of age on the prognostic value of body mass index in ST‐elevation myocardial infarction. Nutr Metab Cardiovasc Dis. 2013;23:205–211. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Liu W, He X, Chen Y, Lu J, Liu K, Cao K, Yin P. Association of overweight and obesity with patient mortality after acute myocardial infarction: a meta‐analysis of prospective studies. Int J Obes (Lond). 2016;40:220–228. [DOI] [PubMed] [Google Scholar]
- 32. Khan SS, Ning H, Wilkins JT, Allen NB, Carnethon M, Berry JD, Sweis RN, Lloyd‐Jones DM. Excess weight, cardiovascular events, and healthy longevity: the lifetime risk pooling project. Circulation. 2017;135:A09. [Google Scholar]
- 33. Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL‐C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta‐analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 34. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long‐term follow‐up of prospective studies. Am J Epidemiol. 1999;150:341–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of Life's Simple 7 Health Factors at Baseline (1987–1989)
Table S2. Baseline Characteristics for Study Population According to Score of Life's Simple 7, N=13 079
Table S3. Risk of Incident Myocardial Infarction by Life's Simple 7 Health Factors, N=13 079
Table S4. Hazard Ratio (95% CI) of Adverse Outcomes After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients After Adjusting Socioeconomic Status and Myocardial Infarction Severity
Table S5. Adjusted Hazard Ratio (95% CI)* of Adverse Outcomes After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients After Excluding Those Who Died Within 14 Days After Myocardial Infarction (N=1245)
Table S6. Adjusted Hazard Ratio (95% CI)* of Adverse Outcomes After Restricting Follow‐Up Time After Incident Myocardial Infarction According to Score of Life's Simple 7 Among Myocardial Infarction Patients
Table S7. Hazard Ratios (95% CI)* for Composite Outcome According to the Status of Each Individual Life's Simple 7 Factor Among Those Who Had Incident Myocardial Infarction During Follow‐Up, N=1277
Figure S1. Study design.
Figure S2. Flow chart of study population.
Figure S3. Kaplan–Meier curves for incident myocardial infarction by the score of Life's Simple 7 in the entire study population.
Figure S4. Kaplan–Meier curves for cardiovascular mortality, recurrent myocardial infarction, heart failure, and stroke by Life's simple 7 score among myocardial infarction patients.


