Abstract
Background
There are limited data regarding the prognostic impact of angiographic complete revascularization (CR) in patients with chronic kidney disease (CKD). We sought to investigate the differential prognostic impact of angiographic CR over incomplete revascularization (IR), according to the presence of CKD in the drug‐eluting stent era.
Methods and Results
Between 2003 and 2011 at Samsung Medical Center, consecutive patients with multivessel disease were stratified by the presence of CKD (estimated glomerular filtration rate <60 mL/min per 1.73 m2) and classified according to angiographic CR (residual SYNTAX score=0) or IR. Clinical outcomes were compared between angiographic CR and IR, stratified by the presence of CKD. Primary outcome was patient‐oriented composite outcomes (POCO, a composite of all‐cause death, myocardial infarction, any revascularization) at 3 years. Inverse probability weighting was performed between the CR and IR groups. A total of 3224 patients were eligible for analysis: 2295 without CKD; 929 with CKD. Among non‐CKD patients, angiographic CR showed a significantly lower risk of POCO than IR (17.2% versus 21.7%, adjusted hazard ratio 0.76, 95% confidence interval, 0.62–0.95, P=0.014), mainly driven by a significantly lower risk of any revascularization. Among CKD patients, however, angiographic CR was associated with a significantly higher risk of POCO than IR (37.7% versus 28.4%, adjusted hazard ratio 1.42, 95% confidence interval, 1.08%–1.85%, P=0.011), mainly driven by a significantly higher risk of nonfatal target vessel myocardial infarction.
Conclusions
Angiographic CR was associated with reduced risk of POCO than IR in patients without CKD; however, it was associated with a significantly higher risk of POCO and nonfatal myocardial infarction in CKD patients.
Keywords: chronic kidney disease, complete revascularization, outcome, percutaneous coronary intervention
Subject Categories: Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
Angiographic complete revascularization was associated with a lower risk of patient‐oriented outcome than angiographic incomplete revascularization in patients without chronic kidney disease.
However, angiographic complete revascularization was associated with a significantly higher risk of patient‐oriented outcome, all‐cause mortality, and nonfatal target vessel myocardial infarction in patients with chronic kidney disease.
What Are the Clinical Implications?
Our findings suggest that an angiography‐only based complete coronary revascularization may have limited efficacy in the chronic kidney disease population and underscore the importance of appropriate selection of revascularization methods, reasonable deferral of lesions not related with inducible ischemia, and meticulous secondary prevention after percutaneous coronary intervention even in the drug‐eluting stent era.
Percutaneous coronary intervention (PCI) has become an important option in treating patients with coronary artery disease (CAD). However, PCI may be accompanied by inevitable complications such as stent thrombosis and restenosis, especially in patients with high‐risk comorbidities, even in the drug‐eluting stent (DES) era. Among the high‐risk comorbidities, chronic kidney disease (CKD) is an increasing major health problem and a significant prognostic factor in patients with CAD.1, 2 Previous studies have shown that patients with CKD showed worse clinical outcomes after PCI than those without CKD, even with the use of DES.1 Moreover, patients with CKD are often excluded from randomized controlled trials evaluating treatment of CAD, resulting in limited data regarding this patient subset.
Despite the development of physiologic indices to guide treatment decision‐making, the majority of PCI is often performed based on angiography‐only‐based decisions, and the clinical relevance of angiographic completeness of revascularization in patients with multivessel coronary artery disease remains a long‐standing issue. Although previous studies suggested that angiographic complete revascularization (CR) was more advantageous than incomplete revascularization (IR) and residual disease was associated with adverse clinical outcome after PCI,3, 4, 5, 6, 7, 8 the evidence regarding the benefit of angiographic CR over IR has been limited in CKD patients with multivessel CAD. Given the increasing prevalence of CKD, it would be an important issue to clarify the safety and efficacy of angiographic CR in this high‐risk population.1, 9 Therefore, we sought to evaluate the prognostic impact of angiographic CR on clinical outcomes according to the presence of CKD in the DES era.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request.
Study Population
A total of 6129 consecutive patients who underwent PCI with DES implantation were prospectively enrolled at Samsung Medical Center between 2003 and 2011 as a prospective institutional registry without exclusions. Patients were eligible for the present analysis if they had multivessel CAD with information of serum creatinine and measurable Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score. After excluding patients without multivessel CAD, available SYNTAX score and serum creatinine values, 3224 patients were enrolled in the current analysis (Figure 1). The study protocol was approved by the ethics committee and all patients signed a consent form before enrollment.
Figure 1.
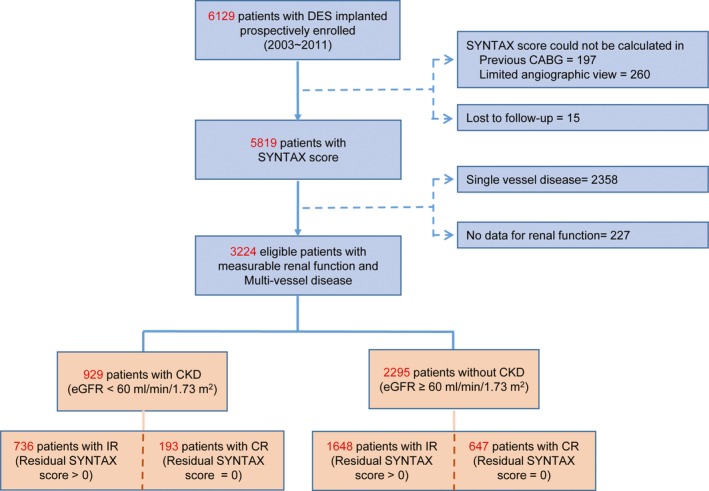
Study flow chart. Flow chart of the current study is presented. CABG indicates coronary artery bypass graft; CKD, chronic kidney disease; CR, complete revascularization; DES, drug‐eluting stent; eGFR, estimated glomerular filtration rate; IR, incomplete revascularization; SYNTAX, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
Data Collection and Management
Clinical, angiographic, and procedural data were recorded prospectively at the index procedure by an independent research panel. Follow‐up information was obtained by clinic visit, telephone interview yearly from index PCI, and medical records of other hospitals, if necessary. Confirmation of death was also collected from the National Insurance data by the Korean National Statistical Office. Follow‐up was considered complete if the mortality was confirmed from the National Population Registry of the Korean National Statistical Office, using a unique personal identification number or if the patient was successfully contacted at the scheduled follow‐up interval. Only 15 patients did not have follow‐up information in this institutional registry, and were therefore excluded from the analysis.
Definition of Complete Revascularization
SYNTAX score and residual SYNTAX score, widely used standard scoring systems to describe the complexity and extent of CAD,10 were used to define angiographic CR. The scores were measured by 2 independent experienced personnel. Angiographic CR was defined when residual SYNTAX score was equal to 0. All other cases of residual SYNTAX >0 were defined as angiographic IR. The interobserver variability of SYNTAX score was 0.81 (0.73–0.87) and intraobserver variability was 0.97 (0.97–0.98).
Definition and Classification of CKD
CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2, calculated by the 4‐component Modification of Diet in Renal Disease equation incorporating age, ethnicity, sex, and serum creatinine.11 The serum creatinine measured at index admission was used to calculate eGFR. CKD was further classified into 4 groups as mild (45≤ eGFR <60), moderate (30≤ eGFR <45), severe (eGFR <30, not on dialysis), and end‐stage renal disease on dialysis.1
Definition of Clinical Outcomes
The primary outcome was POCO, which was a composite of all‐cause death, any nonfatal myocardial infarction (MI), and any revascularization at 3 years from the index procedure. The secondary outcome was stent‐oriented composite outcomes (SOCO), which included cardiac death, nonfatal target vessel MI (not clearly attributed to nontarget vessel), and target lesion revascularization at 3 years after index PCI. All individual components of POCO and SOCO were also defined as secondary outcomes. Nonfatal target vessel MI was further adjudicated into MI occurring in the stented segment at index procedure, or MI‐related with de novo lesions at the index procedure. All deaths without undisputed noncardiac cause were considered cardiac. MI was defined as elevated cardiac enzymes, including troponin and myocardial band fraction of creatine kinase, with ischemic symptoms or electrocardiographic findings indicative of ischemia not related to index PCI. Periprocedural MI was not included as a clinical event. Revascularization was considered clinically indicated in the presence of stenosis with ≥50% diameter stenosis and if 1 of the following occurred: (1) Recurrence of angina symptoms; (2) Positive noninvasive test; (3) Positive invasive physiologic test; or (4) presence of stenosis with ≥70% diameter stenosis, even in the absence of other criteria. Staged PCI was not considered as an outcome of any revascularization. All clinical outcomes were defined in keeping with the Academic Research Consortium criteria,12 and an independent clinical events committee, unaware of clinical and angiographic data, adjudicated all events.
Statistical Analysis
Continuous variables were presented as mean±SD and analyzed using Student t test or Mann–Whitney U test as appropriate. Categorical variables were analyzed using the χ2 test. The clinical outcomes were compared between angiographic CR versus IR groups, stratified according to the presence of CKD. Event rates were calculated based on Kaplan–Meier censoring estimates and log‐rank test was used to compare survival curves between groups.
In order to adjust for differences in baseline characteristics and potential confounding variables, adjusted analysis with inverse probability of treatment weighting (IPTW) was performed. Propensity score was the probability that any patient would be selected for angiographic CR or IR, and was estimated by multivariate logistic regression analysis using entire baseline covariables, except procedural profiles (number, diameter, and total length of used stent). Each patient was weighted by the inverse of the propensity score, then weighting was stabilized by multiplying the marginal probability for receiving angiographic CR or IR. Variables were considered as balanced between angiographic CR and IR groups after stabilized IPTW if the standardized mean difference of variables was < ±10%.13 Outcome analyses were performed with weighted sample.
Comparison of clinical outcomes between angiographic CR and IR was performed with IPTW adjusted multivariate Cox proportional hazard model with further incorporation of clinically relevant covariables including age, sex, hypertension, diabetes mellitus, clinical diagnosis, and type of stent inserted. IPTW adjusted multivariate Cox proportional hazard model was also used for exploratory subgroup analysis, estimating the effects of interaction terms between groups and treatment effects on clinical outcomes, and defining independent predictor of POCO. All probability values were 2‐sided and P<0.05 was considered statistically significant. R software version 3.4.0 (R Foundation for Statistical Computing) was used for statistical analysis.
Results
Patients and Lesion Characteristics
A total of 3224 patients with multivessel CAD were eligible for analysis and 95.3% of total patients completed their 3‐year follow‐up (median follow‐up 1095 days). Of the 3224 patients, 929 had CKD (28.8%) and 2295 (71.2%) had preserved renal function (non‐CKD). Among the total population, 43.5% of patients presented with acute coronary syndrome, and the remaining 56.5% of patients presented with stable angina. Angiographic CR (residual SYNTAX score=0) was achieved in 20.8% of CKD patients (193 of 929 patients), and 28.2% of non‐CKD patients (647 of 2295 patients) (Figure 1). Among the total population, 2.3% of patients underwent intended staged PCI during the same hospitalization or different hospitalization, maximally within 1 month from the index PCI. The CKD population showed significantly lower rates of achieving angiographic CR compared with the non‐CKD population (P<0.001).
Table 1 presents the comparison of baseline characteristics between the angiographic CR and IR groups, according to the presence of CKD. Regardless of the presence of CKD, the angiographic IR group showed a higher proportion of cardiovascular comorbidities and a lower proportion of patients with low SYNTAX score (<12). All patients underwent PCI using DES and 46.0% of them (1482 of 3224 patients) received second‐generation DES only. After IPTW adjustment, most baseline characteristics were well balanced between the angiographic CR and IR groups (Table S1). For the procedural profiles, the angiographic CR group showed a significantly higher number of stents used and longer total stent length compared with the IR group, in both the CKD and non‐CKD populations (Table 2).
Table 1.
Baseline Characteristics According to Completeness of Revascularization, Stratified by the Presence of CKD
| CKD Group (929 Patients) | Non‐CKD Group (2295 Patients) | |||||||
|---|---|---|---|---|---|---|---|---|
| IR (N=736) | CR (N=193) | P Value | SMD (%) | IR (N=1648) | CR (N=647) | P Value | SMD (%) | |
| Demographics | ||||||||
| Age, y | 68.9±9.8 | 68.5±9.8 | 0.640 | −3.8 | 62.3±10.5 | 61.7±10.4 | 0.251 | −5.3 |
| Male | 64.7% | 65.3% | 0.941 | 1.3 | 77.5% | 78.5% | 0.633 | 2.5 |
| Coexisting condition | ||||||||
| Diabetes mellitus | 21.3% | 26.4% | 0.157 | 12.0 | 19.7% | 22.3% | 0.194 | 6.2 |
| Hypertension | 76.5% | 76.7% | 1.000 | 0.4 | 55.3% | 54.9% | 0.896 | −0.8 |
| Dyslipidemia | 28.8% | 28.5% | 1.000 | −0.7 | 28.2% | 31.2% | 0.169 | 6.6 |
| Peripheral vascular disease | 4.3% | 5.7% | 0.546 | 6.2 | 1.4% | 0.8% | 0.312 | −6.0 |
| Renal function | ||||||||
| Creatinine, mg/dL | 2.1±1.9 | 2.1±2.2 | 0.758 | 2.6 | 0.9±0.2 | 0.9±0.2 | 0.562 | −2.7 |
| eGFR, mL/min per 1.73 m2 | 41.7±15.9 | 43.7±16.7 | 0.125 | 12.3 | 81.3±16.6 | 82.6±19.7 | 0.144 | 7.0 |
| CKD stagea | 0.005 | 29.9 | NA | NA | ||||
| Mild | 54.1% | 65.3% | NA | NA | ||||
| Moderate | 23.0% | 13.5% | NA | NA | ||||
| Severe | 19.3% | 15.5% | NA | NA | ||||
| ESRD | 3.7% | 5.7% | NA | NA | ||||
| Cardiac risk factors | ||||||||
| Current smoker | 14.1% | 10.9% | 0.290 | −9.8 | 19.3% | 23.2% | 0.043 | 9.5 |
| Previous CVA | 10.3% | 9.8% | 0.950 | −1.6 | 4.2% | 4.8% | 0.647 | 2.6 |
| Previous MI | 28.4% | 18.7% | 0.008 | −23.1 | 21.7% | 19.8% | 0.334 | −4.8 |
| Previous PCI | 17.5% | 15.0% | 0.474 | −6.8 | 12.6% | 9.9% | 0.087 | −8.5 |
| LVEFb, % | 55.1±14.0 | 57.2±13.1 | 0.061 | 15.9 | 59.3±11.1 | 60.4±10.2 | 0.089 | 10.1 |
| Clinical diagnosis | 0.070 | 19.2 | 0.232 | 8.0 | ||||
| AMI | 29.5% | 21.2% | 24.0% | 21.0% | ||||
| Unstable angina | 17.1% | 20.2% | 19.7% | 19.2% | ||||
| Stable angina | 53.4% | 58.5% | 56.2% | 59.8% | ||||
| Complexity of CAD | ||||||||
| SYNTAX score <12 | 18.2% | 54.9% | <0.001 | 82.3 | 18.8% | 58.6% | <0.001 | 89.6 |
| Treatment of CAD | ||||||||
| Left main coronary artery | 6.5% | 12.4% | 0.010 | 20.3 | 6.3% | 13.1% | <0.001 | 23.2 |
| At least 1 bifurcation lesion | 21.9% | 29.5% | 0.032 | 17.6 | 25.8% | 32.8% | 0.001 | 15.4 |
| At least 1 ostial lesion | 8.8% | 12.4% | 0.169 | 11.7 | 9.8% | 17.8% | <0.001 | 23.2 |
| At least 1 CTO lesion | 14.4% | 10.9% | 0.250 | −10.6 | 17.7% | 14.1% | 0.044 | −9.8 |
| At least 1 type B2/C lesion | 64.7% | 74.1% | 0.017 | 20.5 | 66.6% | 69.6% | 0.186 | 6.4 |
| Type of inserted stentc | 0.325 | 12.0 | 0.001 | 17.5 | ||||
| First‐generation stent only | 61.7% | 56.5% | 47.4% | 38.8% | ||||
| Second‐generation stent only | 32.6% | 38.3% | 48.5% | 56.9% | ||||
| Other | 5.7% | 5.2% | 4.1% | 4.3% | ||||
Values are mean±SD or n/N%. AMI indicates acute myocardial infarction; CAD, coronary artery disease; CKD, chronic kidney disease; CR, complete revascularization; CTO, chronic total occlusion; CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; IR, incomplete revascularization; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention; SMD, standardized mean difference; SYNTAX, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
CKD was divided into 4 stages using MDRD (Modification of Diet in Renal Disease) study equation; mild (45≤ eGFR <60); moderate (30≤ eGFR <45); severe (eGFR <30, not on dialysis); ESRD, on dialysis.
Echocardiographic data were available in 2863 patients (88.8%).
Type of inserted stent included first‐generation (paclitaxel‐eluting stent, sirolimus‐eluting stent), second‐generation (everolimus‐eluting stent, zotarolimus‐eluting stent, biolimus‐eluting stent), and other (simultaneous use of first‐generation stent, second‐generation stent, or bare‐metal stent).
Table 2.
Comparison of Procedural Profiles According to Completeness of Revascularization, Stratified by the Presence of CKD
| CKD Group (929 Patients) | Non‐CKD Group (2295 Patients) | |||||||
|---|---|---|---|---|---|---|---|---|
| IR (N=736) | CR (N=193) | P Value | SMD | IR (N=1648) | CR (N=647) | P Value | SMD | |
| Residual SYNTAX score | 10.98±8.8 | 0 | <0.001 | −177.0 | 9.51±7.5 | 0 | <0.001 | −175.6 |
| Number of inserted stents | 1.78±1.0 | 2.04±1.0 | 0.004 | 29.9 | 1.80±1.0 | 2.11±1.0 | <0.001 | 31.0 |
| Mean stent diameter, cm | 3.11±0.4 | 3.07±0.4 | 0.246 | −11.4 | 3.06±0.4 | 3.08±0.4 | 0.211 | 6.7 |
| Total stent length, cm | 43.00±26.0 | 50.78±27.9 | 0.002 | 32.2 | 43.39±25.7 | 50.38±26.5 | <0.001 | 26.7 |
Values are mean±SD. SMD ≥ ±10% represents significant between group difference. CKD indicates chronic kidney disease; CR, complete revascularization; IR, incomplete revascularization; SMD, standardized mean difference; SYNTAX, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
Clinical Outcomes Between Angiographic CR and IR Groups, According to the Presence of CKD
Figure 2 presents the cumulative incidence of POCO and SOCO between the angiographic CR and IR groups according to the presence of CKD. Among the non‐CKD population, the angiographic CR group showed a significantly lower incidence of POCO than the IR group (17.2% versus 21.7%, adjusted hazard ratio [HR] 0.76, 95% confidence interval [CI], 0.62%–0.95%, P=0.014), mainly driven by a significantly lower risk of any revascularization (12.0% versus 16.8%, adjusted HR 0.69, 95% CI, 0.54%–0.89%, P=0.005). The incidence of SOCO was comparable between the angiographic CR and IR groups (Figure 2 and Table 3).
Figure 2.
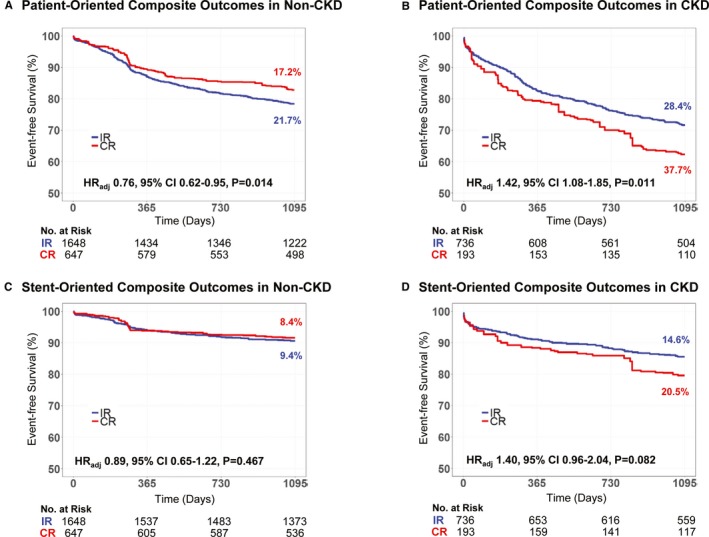
Clinical outcomes according to completeness of revascularization, stratified by the presence of chronic kidney disease. The comparison of POCO and SOCO are presented stratified by the presence of CKD, as (A) POCO in non‐CKD, (B) POCO in CKD, (C) SOCO in non‐CKD, and (D) SOCO in CKD. Multivariable adjusted hazard ratio (HR) with 95% confidence intervals (95% CI) and P values are presented. CKD indicates chronic kidney disease; CR, complete revascularization; IR, incomplete revascularization; POCO, patient‐oriented composite outcomes; SOCO, stent‐oriented composite outcomes.
Table 3.
Comparison of Clinical Outcomes at 3 Years, According to Completeness of Revascularization, Stratified by the Presence of CKD
| (A) Non‐CKD Group | Total (N=2295) | CR (N=647) | IR (N=1648) | Unadjusted HR | Adjusted HRa | P Value |
|---|---|---|---|---|---|---|
| POCO | 465 (20.4%) | 110 (17.2%) | 355 (21.7%) | 0.77 (0.63–0.96) | 0.76 (0.62–0.95) | 0.014 |
| All‐cause death | 123 (5.5%) | 32 (5.1%) | 91 (5.6%) | 0.90 (0.61–1.34) | 0.92 (0.62–1.38) | 0.694 |
| Nonfatal MI | 32 (1.4%) | 9 (1.5%) | 23 (1.4%) | 1.05 (0.49–2.25) | 1.04 (0.48–2.22) | 0.927 |
| Any revascularization | 345 (15.4%) | 76 (12.0%) | 269 (16.8%) | 0.70 (0.55–0.91) | 0.69 (0.54–0.89) | 0.005 |
| SOCO | 205 (9.1%) | 53 (8.4%) | 152 (9.4%) | 0.90 (0.66–1.22) | 0.89 (0.65–1.22) | 0.467 |
| Cardiac death | 60 (2.7%) | 14 (2.4%) | 46 (2.9%) | 0.83 (0.47–1.48) | 0.84 (0.47–1.50) | 0.562 |
| Nonfatal target vessel MI | 25 (1.1%) | 8 (1.3%) | 17 (1.1%) | 1.23 (0.54–2.80) | 1.22 (0.53–2.77) | 0.640 |
| TLR | 137 (6.1%) | 36 (5.7%) | 101 (6.3%) | 0.91 (0.62–1.33) | 0.90 (0.62–1.32) | 0.605 |
| (B) CKD Group | Total (N=929) | CR (N=193) | IR (N=736) | Unadjusted HR | Adjusted HRa | P Value |
|---|---|---|---|---|---|---|
| POCO | 277 (30.4%) | 72 (37.7%) | 205 (28.4%) | 1.40 (1.07–1.83) | 1.42 (1.08–1.85) | 0.011 |
| All‐cause death | 172 (19.1%) | 56 (29.0%) | 116 (16.5%) | 1.87 (1.36–2.56) | 1.88 (1.37–2.59) | <0.001 |
| Nonfatal MI | 20 (2.4%) | 8 (4.5%) | 12 (1.8%) | 2.56 (1.04–6.31) | 2.75 (1.10–6.87) | 0.030 |
| Any revascularization | 118 (14.0%) | 22 (13.0%) | 96 (14.2%) | 0.93 (0.58–1.47) | 0.94 (0.59–1.50) | 0.801 |
| SOCO | 137 (15.7%) | 37 (20.5%) | 100 (14.6%) | 1.42 (0.98–2.08) | 1.40 (0.96–2.04) | 0.082 |
| Cardiac death | 95 (11.1%) | 27 (15.4%) | 68 (10.1%) | 1.51 (0.97–2.35) | 1.47 (0.95–2.29) | 0.086 |
| Nonfatal target vessel MI | 17 (2.0%) | 7 (4.2%) | 10 (1.4%) | 3.02 (1.15–7.93) | 3.08 (1.16–8.17) | 0.024 |
| TLR | 33 (3.9%) | 6 (3.7%) | 27 (4.0%) | 0.98 (0.42–2.32) | 0.94 (0.40–2.22) | 0.884 |
Values are n/N% or hazard ratio (95% confidence interval). The cumulative incidence of clinical outcomes is presented as Kaplan–Meier estimates at 3 years with IPTW adjusted sample. The P values are for adjusted HR and 95% confidence interval. CKD indicates chronic kidney disease; CR, complete revascularization; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IR, incomplete revascularization; MI, myocardial infarction; POCO, patient‐oriented composite outcomes; SOCO, stent‐oriented composite outcomes; TLR, target lesion revascularization.
Adjusted HR was calculated by additional multivariate Cox regression analyses with clinically relevant covariables including age, sex, hypertension, diabetes mellitus, type of inserted stent, and clinical diagnosis.
Conversely, among the CKD population, the angiographic CR group showed a significantly higher incidence of POCO (37.7% versus 28.4%, adjusted HR 1.42, 95% CI, 1.08%–1.85%, P=0.011), mainly driven by a significantly higher risk of all‐cause death (29.0% versus 16.5%, adjusted HR 1.88, 95% CI, 1.37%–2.59%, P<0.001) and nonfatal MI (4.5% versus 1.8%, adjusted HR 2.75, 95% CI, 1.10%–6.87%, P=0.030). Although the risk of SOCO was not significantly different between the angiographic CR and IR groups, the risk of nonfatal target vessel MI was significantly higher in the angiographic CR group than in the IR group (4.2% versus 1.4%, adjusted HR 3.08, 95% CI, 1.16%–8.17%, P=0.024) in the CKD population (Figure 2 and Table 3). Most cases of nonfatal target vessel MI in the CKD population (70.6%, 12 of 17 events) occurred in the stented segment implanted at the index procedure (Table S2). Figure 3 summarizes the differential prognostic impact of angiographic CR, according to the presence of CKD. There was significant interaction between angiographic CR and the presence of CKD for the risk of POCO (interaction P<0.001).
Figure 3.
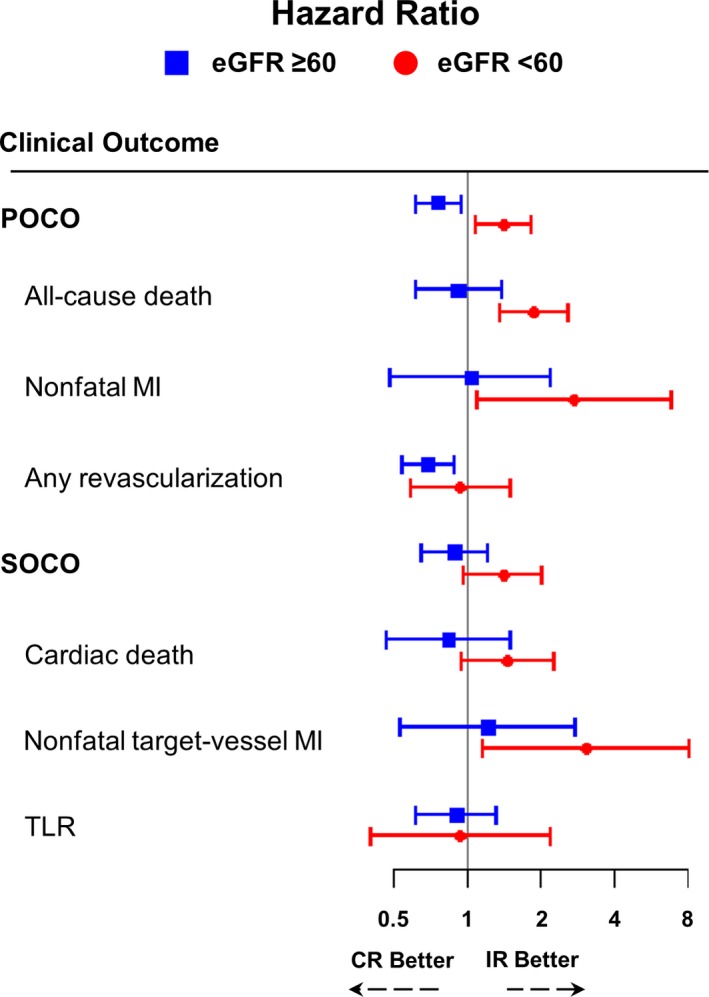
Differential prognostic impact of angiographic complete revascularization on clinical outcomes according to the presence of chronic kidney disease. The composite outcomes and their individual components were compared between angiographic CR vs IR, according to the presence of CKD. Hazard ratio (HR) and 95% confidence intervals (95% CI) were adjusted for clinically relevant variables including age, sex, hypertension, diabetes mellitus, clinical diagnosis, and type of stent inserted. CI indicates confidence interval; CKD, chronic kidney disease; CR, complete revascularization; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); IR, incomplete revascularization; MI, myocardial infarction; POCO, patient‐oriented composite outcomes; SOCO, stent‐oriented composite outcomes; TLR, target lesion revascularization.
Of the 463 events of any revascularization among the total study population, 30.3% were because of acute coronary syndrome, and others with stable ischemic heart disease were because of intractable symptoms with the findings of noninvasive stress tests (40.7%) or angiographic progression of de novo disease (59.3%) during 3‐year follow‐up. The mean percent diameter stenosis of the target lesions at the time of any revascularization events was 86.6±10.4%, implying the progression of epicardial coronary stenosis as the main cause of recurring anginal symptoms and revascularization events.
Among the population with moderate to severe CKD (eGFR <45 mL/min per 1.73 m2), the angiographic CR group showed further increased risk of POCO, all‐cause mortality, and nonfatal target vessel MI compared with the IR group (Figure S1 and Table S3). The sensitivity analysis among patients who were revascularized using only second‐generation DES showed trends similar to the original one; however, statistical significance was not reached (Figure S2 and Table S4).
Exploratory Subgroup Analysis
Figure 4 presents the results of exploratory subgroup analysis. The lower incidence of POCO in the angiographic CR group than in the IR group among non‐CKD population was consistently observed in various subgroups without significant interaction P value. Similarly, the significantly higher risk of POCO in the angiographic CR group among the CKD population was also consistent (Figure 4).
Figure 4.
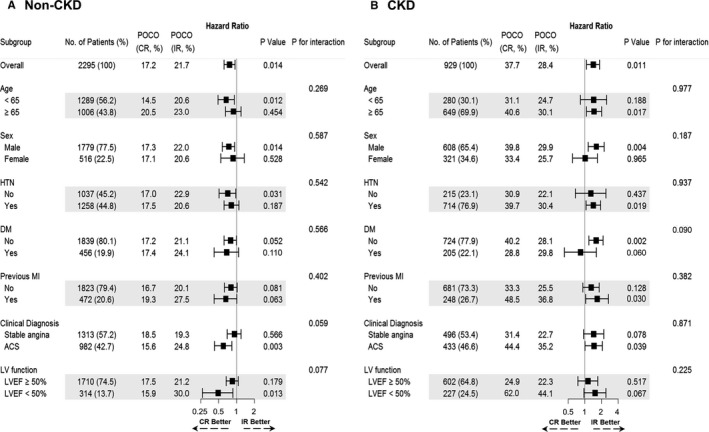
Exploratory subgroup analysis for patient‐oriented composite outcomes. Exploratory subgroup analyses for comparison between angiographic CR vs IR, according to the presence of CKD are presented, as (A) Non‐CKD, (B) CKD. Hazard ratio (HR) with 95% confidence intervals (95% CI) and P values were adjusted for clinically relevant variables including age, sex, hypertension, diabetes mellitus, clinical diagnosis, and type of stent inserted. ACS indicates acute coronary syndrome; CKD, chronic kidney disease; CR, complete revascularization; DM, diabetes mellitus; HTN, hypertension; IR, incomplete revascularization; LV, left ventricular; LVEF, left ventricular ejection fraction; MI, myocardial infarction; POCO, patient‐oriented composite outcomes.
Independent Predictors of POCO
Table 4 presents the independent predictors of POCO by IPTW adjusted multivariable Cox regression analysis. Among non‐CKD patients, angiographic CR and the use of second‐generation DES were independently associated with a lower risk of POCO. Conversely, angiographic CR was independently associated with the occurrence of POCO and the use of second‐generation DES was the only protective factor for POCO in the CKD population.
Table 4.
Independent Predictors of POCO in Weighted Sample
| Adjusted HRa | 95% CI | P Value | |
|---|---|---|---|
| Non‐CKD group | |||
| Complete revascularization | 0.77 | 0.62 to 0.95 | 0.015 |
| Age (each 1 y) | 1.01 | 1.01 to 1.02 | 0.003 |
| Previous CVA | 1.87 | 1.27 to 2.76 | 0.002 |
| Previous PCI | 1.32 | 1.01 to 1.73 | 0.044 |
| Acute MI | 1.35 | 1.06 to 1.71 | 0.015 |
| Second‐generation stent | 0.69 | 0.57 to 0.85 | <0.001 |
| CKD group | |||
| Complete revascularization | 1.39 | 1.06 to 1.82 | 0.018 |
| Male | 1.41 | 1.08 to 1.84 | 0.013 |
| Hypertension | 1.48 | 1.08 to 2.03 | 0.014 |
| Acute MI | 2.80 | 2.09 to 3.76 | <0.001 |
| PCI to CTO lesion | 1.46 | 1.04 to 2.06 | 0.031 |
| Second‐generation stent | 0.68 | 0.51 to 0.92 | 0.011 |
The C‐index of the models were 0.618 (95% CI, 0.605%–0.631%) and 0.655 (95% CI, 0.638%–0.672%) for non‐CKD and CKD population, respectively. CI indicates confidence interval; CKD, chronic kidney disease; CTO, chronic total occlusion; CVA, cerebrovascular accident; HR, hazard ratio; IPTW, inverse probability of treatment weighting; MI, myocardial infarction; PCI, percutaneous coronary intervention; POCO, patient‐oriented composite outcomes; SYNTAX, Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
Adjusted HR was calculated using IPTW adjusted multivariate Cox regression analyses. The included covariables were complete revascularization, age, sex, hypertension, diabetes mellitus, smoking, previous CVA, previous peripheral vascular disease, dyslipidemia, previous MI, previous PCI, clinical diagnosis, type of inserted stent, SYNTAX score, PCI for left main coronary artery, bifurcation, ostial, CTO, and type B2/C lesion.
Discussion
In the present study, we analyzed 3224 patients who had multivessel CAD and who underwent PCI using DES to evaluate the differential prognostic impact of angiographic CR, according to the presence of CKD. The main findings were as follows. First, among the non‐CKD population, the angiographic CR group showed a significantly lower risk of POCO than the IR group, mainly driven by a significantly lower risk of any revascularization. Second, angiographic CR and the use of second‐generation DES were independently associated with a lower risk of POCO among the non‐CKD population. Third, in contrast with the non‐CKD population, there was no clinical benefit of angiographic CR compared with IR among the CKD population. Rather, the risk of POCO was significantly higher in the angiographic CR group compared with the IR group. Fourth, the excess risk of POCO in the angiographic CR group was mainly driven by a higher risk of all‐cause mortality and nonfatal target vessel MI, which occurred mostly in the stented segment implanted at the index procedure. Furthermore, angiographic CR was independently associated with the occurrence of POCO among the CKD population.
Debate on Angiographic CR for Future Clinical Outcome
In the treatment of patients with CAD, PCI has been an important option to relieve angina and reduce the extent of myocardial ischemia.14 Along with selection of the appropriate candidate, determining the extent of revascularization has been a major concern when performing PCI. Regarding the clinical relevance of angiographic CR, previous studies have shown conflicting results. Some reports in the pre‐DES era showed the clear benefit of angiographic CR rather than IR, and adverse clinical events related to the residual disease after PCI.3, 4, 5, 6, 7, 8 However, other studies reported unclear benefit of angiographic CR over IR.15, 16, 17 Along with the constant refinement of revascularization techniques and devices, especially DES, which showed improved clinical outcomes over bare‐metal stents,18 later studies indicated that angiographic CR with DES implantation showed better clinical outcomes than IR.19, 20, 21 However, even in the second‐generation DES era, with enhanced safety and efficacy, high‐risk patients, such as those with diabetes mellitus or CKD, have shown significantly worse clinical outcomes than patients without high‐risk comorbidities.1, 22 Furthermore, there are limited data focused on the prognostic implications of angiographic CR for patients with high‐risk comorbidities, especially CKD. In this regard, the current study evaluated the differential prognostic impact of angiographic CR, according to the presence of CKD.
Angiographic CR in Patients Without CKD
In the current study, angiographic CR was associated with a significantly lower risk of POCO than the IR group in the non‐CKD population, mainly driven by reduced rates of any revascularization in the angiographic CR group. These results are in line with the substudy of the SYNTAX trial, which demonstrated that higher residual SYNTAX score was associated with higher rates of any revascularization.7 Interestingly, the risk of target lesion revascularization was comparable between the angiographic CR and IR groups, and most revascularization events in the IR group were because of progression of residual disease. In addition, the better outcomes seen in the angiographic CR group were similarly observed in various subgroups, especially in patients with younger age (<65 years), acute coronary syndrome, and left ventricular dysfunction. These results are also in line with previous studies that evaluated the prognostic implication of angiographic CR in patients with acute ST‐segment elevation MI or left ventricular dysfunction.23, 24, 25
Angiographic CR in Patients With CKD
Unlike the non‐CKD population, angiographic CR in the CKD population consistently showed a significantly higher risk of POCO, all‐cause mortality, and nonfatal MI, especially in the initially stented segment. Even with the extensive adjustment for baseline differences using IPTW, angiographic CR showed a differential prognostic impact, according to the presence of CKD, with significant interaction. Considering that CKD has an intrinsic nature including accelerated atherosclerosis, impaired platelet function profiles during antiplatelet agent therapy, and multiple comorbidities other than the cardiovascular system,1, 26, 27 some explanations might be possible for these results.
First, CKD accompanies accelerated and extensive atherosclerosis.28 Extensive subclinical coronary atherosclerosis in CKD patients might cause unpredictable progression of lesions that were not detected on angiography alone at the index procedure, and may affect the incidence of any revascularization, irrespective of angiographic CR and IR. In the current results, the CKD population showed a higher risk of non–target lesion‐related events than stent‐related or target lesion–related events, suggesting the intimate association between impaired renal function and progression of atherosclerosis.29 Recent intravascular imaging studies demonstrated higher plaque vulnerability and possibility of lesion progression in CKD patients.30, 31 Kato et al compared the plaque characteristics of non–target vessel stenosis measured by optical coherence tomography between CKD and non‐CKD patients. They showed that non–target vessel plaques in CKD patients showed a significantly higher lipid index (mean lipid arc × lipid length), a higher prevalence of calcification, cholesterol crystal, and plaque disruption, compared with non‐CKD patients.30 Kashiyama et al also presented serial changes of coronary plaques using integrated backscatter intravascular ultrasound, according to the severity of CKD. In their results, patients with CKD stages 3 to 5 showed the serial increases of plaque burden, plaque volume, and lipid and fibrous plaque volumes, despite optimal medical treatment.31 Consequently, this suggests that DES implantation, based on angiography alone, would neither predict nor prevent repeat revascularization in this population and emphasizes the importance of secondary prevention for systemic atherosclerosis and comorbidities in CKD patients.
Second, higher mortality of CKD patients (19.1% versus 5.5% of patients without CKD) might offset the benefit of angiographic CR. Considering the higher incidence of multiple comorbidities in the CKD population, a large portion of the patients had died earlier because of underlying disease associated with CKD, before realizing the potential benefit of CR.
Third, the angiographic CR group showed a higher incidence of nonfatal target vessel MI than the IR group. The recently published post hoc analysis of the ADAPT‐DES trial found a significantly higher prevalence of high platelet reactivity in CKD patients who were treated with clopidogrel.32 The higher prevalence of high platelet reactivity in the CKD population might be another potential explanation for the higher incidence of nonfatal target vessel MI in the CR group than in the IR group among CKD patients. This finding suggests that excessive PCI with DES implantation might be associated with procedure‐related or stent‐related long‐term adverse outcomes rather than the benefit of revascularization as previous studies have suggested.1, 33, 34
Considering the intrinsic nature of CKD as a high‐risk comorbidity of CAD, appropriate selection of revascularization methods (coronary artery bypass grafting versus PCI), careful selection of target lesion for PCI with reasonable deferral of lesions not related to inducible ischemia, and meticulous secondary prevention for noncardiovascular comorbidities might be crucial for this high‐risk population.
Limitations
The present study has several limitations. First, it has an inherent limitation of not being a randomized study. Although baseline variables were balanced between the CR and IR groups after stabilized IPTW adjustment, unmeasured confounders should be considered. Second, the decision of angiographic CR and IR was mainly based on the operator's discretion, according to clinical judgment. Among patients with stable angina, about 38.0% were revascularized based on the findings of noninvasive stress tests findings, while the remaining patients were revascularized based on symptoms and angiographically significant stenosis. However, low diagnostic yield and limited use of noninvasive stress test before elective PCI was not much different from practice in other countries.35, 36 Third, coronary physiologic assessment was not documented in the present study and the definition of angiographic CR was solely based on the residual SYNTAX score, without accounting for post‐PCI fractional flow reserve or instantaneous wave‐free ratio. Therefore, the possibility of residual flow limitation, even after successful angiographic CR, could not be completely excluded. In this regard, future study evaluating the prognostic impact of CR is warranted to use the concept of functionally complete revascularization. Nevertheless, angiographic completeness of coronary revascularization might also carry a certain clinical importance, considering the limited adoption rates of invasive physiologic assessment globally.37 Fourth, as the current study mainly focused on the comparison of clinical outcomes between angiographic CR and IR groups, the lack of an appropriate comparator, such as medical treatment or coronary artery bypass grafting, makes inferences incomplete. Fifth, the proportion of acute kidney injury after the index procedure could not be evaluated. Last, the proportion of patients treated only with second‐generation DES was relatively small. Although there was no significant difference in clinical outcomes between the CR and IR groups, even among the CKD population, any confirmatory statement cannot be made because of the limited sample size of patients treated with second‐generation DES. Further study is warranted to clarify the clinical relevance of CR using only second‐generation DES among the CKD population.
Conclusion
The prognostic impact of angiographic CR was significantly different in patients with multivessel disease, according to the presence of CKD. Angiographic CR was associated with reduced risk of POCO compared with IR in patients without CKD; however, it was associated with a significantly higher risk of POCO and nonfatal MI in the CKD population.
Disclosures
Dr Lee received a research grant from St. Jude Medical (Abbott Vascular) and Philips Volcano. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics According to Complete Revascularization and Chronic Kidney Disease, After Adjustment With Using Stabilized Inverse Probability of Treatment Weighting
Table S2. Details of Nonfatal Target‐Vessel Myocardial Infarction at 3 Years According to Complete Revascularization and Chronic Kidney Disease
Tables S3. Clinical Outcomes at 3 Years, According to Completeness of Revascularization in Patients With eGFR <45 mL/min per 1.73 m2
Table S4. Clinical Outcomes at 3 Years, According to Completeness of Revascularization in Patients With Chronic Kidney Disease and Treated Using Only Second‐Generation Drug‐Eluting Stents
Figure S1. Clinical outcomes according to completeness of revascularization in patients with eGFR (mL/min per 1.73 m2) <45. Among the patients with more advanced CKD, defined as eGFR (mL/min per 1.73 m2) <45, the significant hazard of angiographic CR was similar to the total CKD population. Multivariate adjusted hazard ratio (HR) with 95% confidence intervals (95% CI) and P values are presented. CKD indicates chronic kidney disease; CR, complete revascularization; eGFR, estimated glomerular filtration rate; IR, incomplete revascularization.
Figure S2. According to completeness of revascularization in patients with CKD and treated using only second‐generation DES. The sensitivity analysis among patients who were revascularized using only second‐generation DES showed trends similar to the original one; however, statistical significance was not reached, probably because of limited sample size. Multivariable adjusted hazard ratio (HR) with 95% confidence intervals (95% CI) and P values are presented. CKD indicates chronic kidney disease; CR, complete revascularization; IR, incomplete revascularization DES, drug‐eluting stents.
(J Am Heart Assoc. 2018;7:e007962 DOI: 10.1161/JAHA.117.007962.)29449272
Contributor Information
Joo Myung Lee, Email: drone80@hanmail.net, Email: joomyung.lee@samsung.com.
Seung‐Hyuk Choi, Email: sh1214.choi@samsung.com.
References
- 1. Lee JM, Kang J, Lee E, Hwang D, Rhee TM, Park J, Kim HL, Lee SE, Han JK, Yang HM, Park KW, Na SH, Kang HJ, Koo BK, Kim HS. Chronic kidney disease in the second‐generation drug‐eluting stent era: pooled analysis of the Korean multicenter drug‐eluting stent registry. JACC Cardiovasc Interv. 2016;9:2097–2109. [DOI] [PubMed] [Google Scholar]
- 2. Lima EG, Hueb W, Gersh BJ, Rezende PC, Garzillo CL, Favarato D, Hueb AC, Rahmi Garcia RM, Franchini Ramires JA, Filho RK. Impact of chronic kidney disease on long‐term outcomes in type 2 diabetic patients with coronary artery disease on surgical, angioplasty, or medical treatment. Ann Thorac Surg. 2016;101:1735–1744. [DOI] [PubMed] [Google Scholar]
- 3. Lawrie GM, Morris GC Jr, Silvers A, Wagner WF, Baron AE, Beltangady SS, Glaeser DH, Chapman DW. The influence of residual disease after coronary bypass on the 5‐year survival rate of 1274 men with coronary artery disease. Circulation. 1982;66:717–723. [DOI] [PubMed] [Google Scholar]
- 4. Jones EL, Craver JM, Guyton RA, Bone DK, Hatcher CR Jr, Riechwald N. Importance of complete revascularization in performance of the coronary bypass operation. Am J Cardiol. 1983;51:7–12. [DOI] [PubMed] [Google Scholar]
- 5. Lehmann R, Fichtlscherer S, Schachinger V, Held L, Hobler C, Baier G, Zeiher AM, Spyridopoulos I. Complete revascularization in patients undergoing multivessel PCI is an independent predictor of improved long‐term survival. J Interv Cardiol. 2010;23:256–263. [DOI] [PubMed] [Google Scholar]
- 6. Hannan EL, Racz M, Holmes DR, King SB III, Walford G, Ambrose JA, Sharma S, Katz S, Clark LT, Jones RH. Impact of completeness of percutaneous coronary intervention revascularization on long‐term outcomes in the stent era. Circulation. 2006;113:2406–2412. [DOI] [PubMed] [Google Scholar]
- 7. Farooq V, Serruys PW, Bourantas CV, Zhang Y, Muramatsu T, Feldman T, Holmes DR, Mack M, Morice MC, Stahle E, Colombo A, de Vries T, Morel MA, Dawkins KD, Kappetein AP, Mohr FW. Quantification of incomplete revascularization and its association with five‐year mortality in the synergy between percutaneous coronary intervention with Taxus and cardiac surgery (SYNTAX) trial validation of the residual SYNTAX Score. Circulation. 2013;128:141–151. [DOI] [PubMed] [Google Scholar]
- 8. Sarno G, Garg S, Onuma Y, Gutierrez‐Chico JL, van den Brand MJ, Rensing BJ, Morel MA, Serruys PW. Impact of completeness of revascularization on the five‐year outcome in percutaneous coronary intervention and coronary artery bypass graft patients (from the ARTS‐II study). Am J Cardiol. 2010;106:1369–1375. [DOI] [PubMed] [Google Scholar]
- 9. Baber U, Giustino G, Sartori S, Aquino M, Stefanini GG, Steg PG, Windecker S, Leon MB, Wijns W, Serruys PW, Valgimigli M, Stone GW, Dangas GD, Morice MC, Camenzind E, Weisz G, Smits PC, Kandzari D, Von Birgelen C, Mastoris I, Galatius S, Jeger RV, Kimura T, Mikhail GW, Itchhaporia D, Mehta L, Ortega R, Kim HS, Kastrati A, Chieffo A, Mehran R. Effect of chronic kidney disease in women undergoing percutaneous coronary intervention with drug‐eluting stents: a patient‐level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv. 2016;9:28–38. [DOI] [PubMed] [Google Scholar]
- 10. Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 11. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713–735. [DOI] [PubMed] [Google Scholar]
- 12. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 13. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. [DOI] [PubMed] [Google Scholar]
- 15. Bourassa MG, Yeh W, Holubkov R, Sopko G, Detre KM. Long‐term outcome of patients with incomplete vs complete revascularization after multivessel PTCA. A report from the NHLBI PTCA Registry. Eur Heart J. 1998;19:103–111. [DOI] [PubMed] [Google Scholar]
- 16. Bell MR, Bailey KR, Reeder GS, Lapeyre AC III, Holmes DR Jr. Percutaneous transluminal angioplasty in patients with multivessel coronary disease: how important is complete revascularization for cardiac event‐free survival? J Am Coll Cardiol. 1990;16:553–562. [DOI] [PubMed] [Google Scholar]
- 17. Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T, van der Wieken R, Tangelder G, Serruys PW, Laarman G. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. Am Heart J. 2004;148:467–474. [DOI] [PubMed] [Google Scholar]
- 18. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, Pfisterer ME, Stone GW, Leon MB, de Lezo JS, Goy JJ, Park SJ, Sabate M, Suttorp MJ, Kelbaek H, Spaulding C, Menichelli M, Vermeersch P, Dirksen MT, Cervinka P, Petronio AS, Nordmann AJ, Diem P, Meier B, Zwahlen M, Reichenbach S, Trelle S, Windecker S, Juni P. Outcomes associated with drug‐eluting and bare‐metal stents: a collaborative network meta‐analysis. Lancet. 2007;370:937–948. [DOI] [PubMed] [Google Scholar]
- 19. Hannan EL, Wu C, Walford G, Holmes DR, Jones RH, Sharma S, King SB III. Incomplete revascularization in the era of drug‐eluting stents: impact on adverse outcomes. JACC Cardiovasc Interv. 2009;2:17–25. [DOI] [PubMed] [Google Scholar]
- 20. Valenti R, Migliorini A, Signorini U, Vergara R, Parodi G, Carrabba N, Cerisano G, Antoniucci D. Impact of complete revascularization with percutaneous coronary intervention on survival in patients with at least one chronic total occlusion. Eur Heart J. 2008;29:2336–2342. [DOI] [PubMed] [Google Scholar]
- 21. Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, Xu K, Parise H, Mehran R, Serruys PW, Stone GW. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (Synergy Between PCI with Taxus and Cardiac Surgery) score. J Am Coll Cardiol. 2012;59:2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park KW, Lee JM, Kang SH, Ahn HS, Kang HJ, Koo BK, Rhew JY, Hwang SH, Lee SY, Kang TS, Kwak CH, Hong BK, Yu CW, Seong IW, Ahn T, Lee HC, Lim SW, Kim HS. Everolimus‐eluting Xience v/Promus versus zotarolimus‐eluting resolute stents in patients with diabetes mellitus. JACC Cardiovasc Interv. 2014;7:471–481. [DOI] [PubMed] [Google Scholar]
- 23. Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, Berry C, Oldroyd KG; Investigators P . Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369:1115–1123. [DOI] [PubMed] [Google Scholar]
- 24. Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, Blackman DJ, Dalby M, Fairbrother KL, Banya W, Wang D, Flather M, Hetherington SL, Kelion AD, Talwar S, Gunning M, Hall R, Swanton H, McCann GP. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sohn GH, Yang JH, Choi SH, Song YB, Hahn JY, Choi JH, Gwon HC, Lee SH. Long‐term outcomes of complete versus incomplete revascularization for patients with multivessel coronary artery disease and left ventricular systolic dysfunction in drug‐eluting stent era. J Korean Med Sci. 2014;29:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Angiolillo DJ, Bernardo E, Capodanno D, Vivas D, Sabate M, Ferreiro JL, Ueno M, Jimenez‐Quevedo P, Alfonso F, Bass TA, Macaya C, Fernandez‐Ortiz A. Impact of chronic kidney disease on platelet function profiles in diabetes mellitus patients with coronary artery disease taking dual antiplatelet therapy. J Am Coll Cardiol. 2010;55:1139–1146. [DOI] [PubMed] [Google Scholar]
- 27. Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, Caillard S, Campia U, Moulin B, Gachet C, Ohlmann P. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011;57:399–408. [DOI] [PubMed] [Google Scholar]
- 28. Baber U, Stone GW, Weisz G, Moreno P, Dangas G, Maehara A, Mintz GS, Cristea E, Fahy M, Xu K, Lansky AJ, Wennerblom B, Mathey DG, Templin B, Zhang Z, Serruys PW, Mehran R. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S53–S61. [DOI] [PubMed] [Google Scholar]
- 29. Kaneko H, Yajima J, Oikawa Y, Matsuno S, Funada R, Tanaka S, Fukamachi D, Suzuki S, Aizawa T, Yamashita T. Role of arterial stiffness and impaired renal function in the progression of new coronary lesions after percutaneous coronary intervention. Cardiovasc Interv Ther. 2013;28:56–62. [DOI] [PubMed] [Google Scholar]
- 30. Kato K, Yonetsu T, Jia H, Abtahian F, Vergallo R, Hu S, Tian J, Kim SJ, Lee H, McNulty I, Lee S, Uemura S, Jang Y, Park SJ, Mizuno K, Yu B, Jang IK. Nonculprit coronary plaque characteristics of chronic kidney disease. Circ Cardiovasc Imaging. 2013;6:448–456. [DOI] [PubMed] [Google Scholar]
- 31. Kashiyama K, Sonoda S, Muraoka Y, Suzuki Y, Kamezaki F, Tsuda Y, Araki M, Tamura M, Takeuchi M, Abe H, Okazaki M, Fujino Y, Otsuji Y. Coronary plaque progression of non‐culprit lesions after culprit percutaneous coronary intervention in patients with moderate to advanced chronic kidney disease: intravascular ultrasound and integrated backscatter intravascular ultrasound study. Int J Cardiovasc Imaging. 2015;31:935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, Witzenbichler B, Weisz G, Rinaldi MJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri EL Jr, Xu K, Parise H, Brodie BR, Stuckey TD, Stone GW. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the Assessment of Dual Antiplatelet Therapy with Drug‐Eluting Stents Registry. Circ Cardiovasc Interv. 2015;8:e001683. [DOI] [PubMed] [Google Scholar]
- 33. Miao Y, Yu‐Jie Z, Zhi‐Jian W, Dong‐Mei S, Yu‐Yang L, Ying‐Xin Z, Fei G, Shi‐Wei Y, De‐An J. Chronic kidney disease and the risk of stent thrombosis after percutaneous coronary intervention with drug‐eluting stents. Catheter Cardiovasc Interv. 2012;80:361–367. [DOI] [PubMed] [Google Scholar]
- 34. Nakazawa G, Tanabe K, Aoki J, Yamamoto H, Higashikuni Y, Onuma Y, Yachi S, Nakajima H, Hara K. Impact of renal insufficiency on clinical and angiographic outcomes following percutaneous coronary intervention with sirolimus‐eluting stents. Catheter Cardiovasc Interv. 2007;69:808–814. [DOI] [PubMed] [Google Scholar]
- 35. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA. 2008;300:1765–1773. [DOI] [PubMed] [Google Scholar]
- 37. Toth GG, Toth B, Johnson NP, De Vroey F, Di Serafino L, Pyxaras S, Rusinaru D, Di Gioia G, Pellicano M, Barbato E, Van Mieghem C, Heyndrickx GR, De Bruyne B, Wijns W. Revascularization decisions in patients with stable angina and intermediate lesions: results of the international survey on interventional strategy. Circ Cardiovasc Interv. 2014;7:751–759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics According to Complete Revascularization and Chronic Kidney Disease, After Adjustment With Using Stabilized Inverse Probability of Treatment Weighting
Table S2. Details of Nonfatal Target‐Vessel Myocardial Infarction at 3 Years According to Complete Revascularization and Chronic Kidney Disease
Tables S3. Clinical Outcomes at 3 Years, According to Completeness of Revascularization in Patients With eGFR <45 mL/min per 1.73 m2
Table S4. Clinical Outcomes at 3 Years, According to Completeness of Revascularization in Patients With Chronic Kidney Disease and Treated Using Only Second‐Generation Drug‐Eluting Stents
Figure S1. Clinical outcomes according to completeness of revascularization in patients with eGFR (mL/min per 1.73 m2) <45. Among the patients with more advanced CKD, defined as eGFR (mL/min per 1.73 m2) <45, the significant hazard of angiographic CR was similar to the total CKD population. Multivariate adjusted hazard ratio (HR) with 95% confidence intervals (95% CI) and P values are presented. CKD indicates chronic kidney disease; CR, complete revascularization; eGFR, estimated glomerular filtration rate; IR, incomplete revascularization.
Figure S2. According to completeness of revascularization in patients with CKD and treated using only second‐generation DES. The sensitivity analysis among patients who were revascularized using only second‐generation DES showed trends similar to the original one; however, statistical significance was not reached, probably because of limited sample size. Multivariable adjusted hazard ratio (HR) with 95% confidence intervals (95% CI) and P values are presented. CKD indicates chronic kidney disease; CR, complete revascularization; IR, incomplete revascularization DES, drug‐eluting stents.


