Abstract
Background
Considering the limited accuracy of clinical examination for early diagnosis of rheumatic heart disease (RHD), echocardiography has emerged as an important epidemiological tool. The ideal setting for screening is yet to be defined. We aimed to evaluate the prevalence and pattern of latent RHD in schoolchildren (aged 5–18 years) and to compare effectiveness of screening between public schools, private schools, and primary care centers in Minas Gerais, Brazil.
Methods and Results
The PROVAR (Rheumatic Valve Disease Screening Program) study uses nonexperts and portable and handheld devices for RHD echocardiographic screening, with remote interpretation by telemedicine, according to the 2012 World Heart Federation criteria. Compliance with study consent and prevalence were compared between different screening settings, and variables associated with RHD were analyzed. In 26 months, 12 048 students were screened in 52 public schools (n=10 901), 2 private schools (n=589), and 3 primary care centers (n=558). Median age was 12.9 years, and 55.4% were girls. Overall RHD prevalence was 4.0% borderline (n=486) and 0.5% definite (n=63), with statistically similar rates between public schools (4.6%), private schools (3.5%), and primary care centers (4.8%) (P=0.24). The percentage of informed consents signed was higher in primary care centers (84.4%) and private schools (66.9%) compared with public schools (38.7%) (P<0.001). Prevalence was higher in children ≥12 years (5.3% versus 3.1%; P<0.001) and girls (4.9% versus 4.0%; P=0.02). Only age (odds ratio, 1.12; 95% confidence interval, 1.09–1.17; P<0.001) was independently associated with RHD.
Conclusions
RHD screening in primary care centers seems to achieve higher coverage rates. Prevalence among schoolchildren is significantly high, with rates higher than expected in private schools of high‐income areas. These data are important for the formulation of public policies to confront RHD.
Keywords: echocardiography, prevalence, rheumatic heart disease, screening, telemedicine
Subject Categories: Echocardiography, Health Services, Quality and Outcomes, Rheumatic Heart Disease, Valvular Heart Disease
Clinical Perspective
What Is New?
Data from the first large‐scale Rheumatic Heart Disease program in Brazil showed that integration of echocardiographic screening with the existing primary care system is feasible and seems to achieve higher participation rates.
Disease prevalence (both borderline and definite) among schoolchildren in the southeastern state of Minas Gerais is significantly high in low‐income areas, but rates higher than expected were also observed in private schools located in high‐income neighborhoods.
Among demographic and socioeconomic factors, age seems to be the only variable independently associated with echocardiographic prevalence, with a progressive pattern.
What Are the Clinical Implications?
These data are important for the formulation of public policies and development of surveillance approaches to confront rheumatic heart disease in high‐prevalence regions.
The epidemiological features of streptococcal infections may be more complex than anticipated in these areas, and other factors besides socioeconomic status should be further evaluated.
Strategies tested in the PROVAR (Rheumatic Valve Disease Screening Program) study, such as task shifting, computer‐based training, use of portable and handheld affordable devices, telemedicine for education purposes, and remote diagnosis and diagonal integration with existing health systems, may help overcome challenges for rheumatic disease control programs in endemic areas.
Introduction
Rheumatic heart disease (RHD) is an important complication that affects patients after an acute rheumatic fever episode.1 It accounts for the highest morbidity and mortality among heart valve diseases worldwide, particularly in middle‐ and low‐income countries, and poses substantial burden on health systems that struggle to meet the demands.2 In 2015, the age‐standardized prevalence of RHD in endemic countries was 444/100 000 inhabitants,3 and the inclusion of latent disease found in screening programs4, 5, 6 may increase this rate. Echocardiography is the most sensitive screening tool for early detection of latent RHD, identifying 10 times more subclinical disease when compared with auscultation.7, 8 In 2012, the World Heart Federation published a consensus document with standardized echocardiographic criteria, and screening studies have followed them since.9
RHD has been consistently associated with poor socioeconomic conditions, lower‐income countries, and rural areas. However, serological and bacteriological data about group A streptococcal epidemiological features10, 11, 12 propose the existence of geographical determinants, suggesting that socioeconomic status may not be the unique factor. In Brazil, an upper‐middle income country still classified among the most socially unequal in the world,13 the PROVAR (Rheumatic Valve Disease Screening Program) study (the first large‐scale screening program of the country) was implemented in 201414 and revealed an echocardiographic prevalence of 42/1000 in preliminary analyses. However, public schools of underserved neighborhoods highlighted a marginal participation (<40%).15 Similar to other publications,16, 17, 18 this was presumably related to the following: (1) lack of involvement by authorities and schools' representatives; (2) low parental engagement, with poorest participation in areas with lowest socioeconomic conditions; and (3) difficulties in monitoring whether the consents were actually being delivered, because parents are not present at the time of interaction.14, 15 In addition, difficulties related to simple procedures that affect school routine signify that school screening may not be the ideal approach.
The identification of the most effective screening strategies is a crucial step for the incorporation of RHD detection as a public policy in Brazil. To meet this goal, subsequent steps of the program included integration of echocardiographic screening within the primary care delivery system and evaluation of children from high‐income areas. In this study, we aimed to evaluate the impact of these measures by comparing the participation rate and prevalence and pattern of latent RHD between public schools, private schools, and primary care centers in school‐aged children.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure, from the corresponding author on reasonable request. The PROVAR study took place between 2014 and 2017 in the state of Minas Gerais, southeast Brazil. The study was conducted in the metropolitan areas of Belo Horizonte (the capitol; 4.9 million inhabitants; mean Human Development Index [HDI], 0.774; 92.9% coverage by primary care) and Montes Claros (north of the state; 0.58 million inhabitants; mean HDI, 0.770; 99.1% coverage by primary care), under the auspices of the Universidade Federal de Minas Gerais and the Telehealth Network of Minas Gerais,19 in collaboration with the Children's National Health System (Washington, DC). Ethics approval was obtained from the institutional review boards of the participant institutions and from the state and city Boards of Health and Education.
The method and the implementation of the PROVAR study have been described elsewhere14, 15: the study uses nonexperts for image acquisition, on portable (Vivid Q; GE Healthcare, Milwaukee, WI) and/or handheld (Vscan; GE Healthcare) devices for RHD echocardiographic screening, and telemedicine interpretation by experts in Brazil and the United States, according to the 2012 World Heart Federation9 criteria. The screening team consisted of 2 nurse research coordinators (KO and CG), 1 biomedical technician, and 1 imaging technician (CO); during the preparatory phase, these individuals underwent a combination of completing online RHD educational modules and at least 6 weeks of hands‐on training.20
Public schools and primary care centers were enrolled according to socioeconomic variables, following priorities of health and education authorities, mostly based on low socioeconomic indexes, considering the local HDI. Private schools of high‐income neighborhoods were randomly invited to participate. An RHD educational curriculum was delivered before screening in the following: (1) In public and private schools, a school‐wide educational process was provided to all students, teachers, and school staff, involving slide lectures on RHD basic concepts (replaced by a tablet‐based interactive module for 1400 random children), informative brochures for family awareness, and explanatory letters.14 Informed consents were sent home with the students. (2) In primary care, house‐to‐house education, with brochures and letters, was provided to families by community health agents during scheduled visits. Additional group education sessions (so‐called operative groups) were scheduled at the primary care centers for children and families. Any person attending the facility was also invited to participate. Informed consents were actively collected during visits and group activities.
Any asymptomatic children whose parents provided written informed consent (mandatory according to Brazilian regulations) answered a brief sociodemographic questionnaire and underwent a simplified echocardiographic protocol focusing on mitral and aortic valves. World Heart Federation criteria9 were modified for handheld devices, in the absence of spectral Doppler. Cloud computing environments, with online reading for DICOM files and Dropbox with proprietary offline software for Vscan files, were used for remote telemedicine interpretation in Brazil and the United States.21 Readers were blinded to the screening scenario.
All screen‐positive cases were blindly reviewed by 2 experts (M.N. and C.S.), and discrepancies were consensually solved by a third blind reader (A.B.). Patients with confirmed echocardiographic abnormalities (borderline or definite RHD and other abnormalities) were referred to the university hospital for clinical and echocardiographic follow‐up, and continuing care was left to the discretion of the caring pediatric cardiologist. In general, every 21 days, penicillin injections were prescribed to all definite cases, and children with borderline RHD were enrolled in scheduled medical surveillance. Patients with other structural abnormalities were also referred for specialized care (Figure 1). In this study, we present data from 26 months (802 days) of the PROVAR study, from October 2014 to December 2016.
Figure 1.
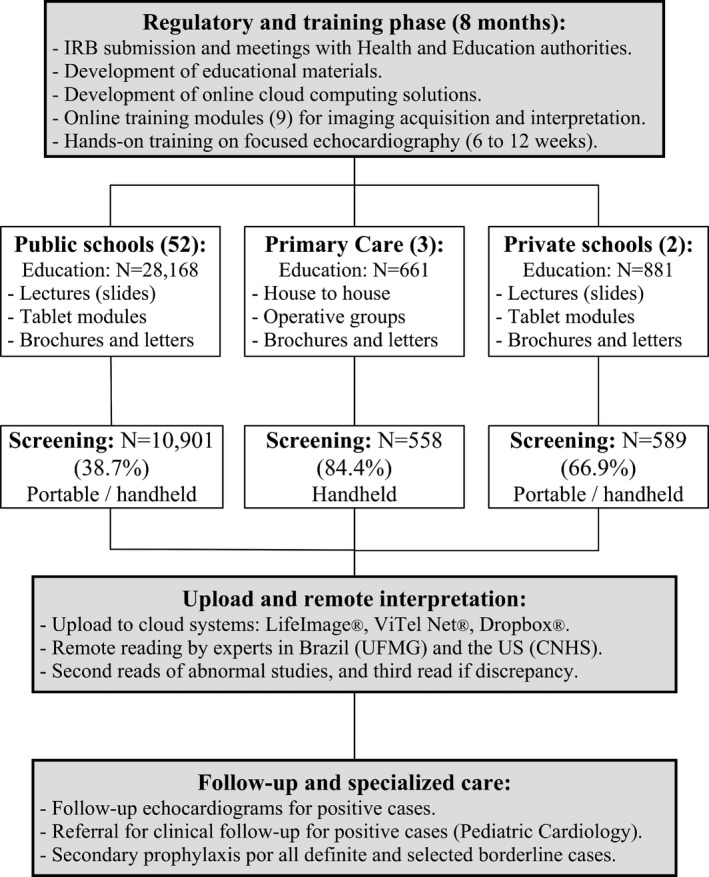
Flow chart of the PROVAR (Rheumatic Valve Disease Screening Program) study. CNHS indicates Children's National Health System; IRB, institutional review board; and UFMG, Universidade Federal de Minas Gerais.
Statistical Analysis
All data were systematically entered to the RedCap online database.22 Statistical analysis was performed using SPSS software, version 23.0, for Mac OSX (SPSS Inc, Chicago, IL). Categorical variables, expressed as numbers and percentages, were compared between groups (public schools, primary care centers, and private schools) using Fisher's exact test (with Bonferroni's correction for multiple comparisons). Continuous data, expressed as mean±SD or median and quartile1/quartile3 (25%/75%), were compared using 1‐way ANOVA (and Student's unpaired t test for pairwise comparisons) or the Kruskal‐Wallis test (and Mann‐Whitney U test for pairwise comparisons), as appropriate. Bivariate logistic regression was used to look for individual predictors of RHD (any RHD and definite cases, separately), and factors that were significant at P<0.10 were put into a multivariable model. The variables evaluated were age (years), sex, number of inhabitants per house, number of inhabitants aged <15 years, screening setting, and the neighborhood's HDI (as a surrogate for socioeconomic status). When necessary, transformations were done for analysis of variance. A 2‐tailed significance level of 0.05 was considered statistically significant.
Results
The RHD educational curriculum was successfully delivered to 29 695 children, and 12 048 (40.6%) consented students were enrolled in 52 public schools (Belo Horizonte, n=6353; and Montes Claros, n=4548), 3 primary care centers (Montes Claros, n=558), and 2 private schools (Belo Horizonte, n=589). Of those from public schools, 5996 were enrolled during the first 14 months of the project, before the initiation of the program in primary care centers and private schools. The median age was 13.0 (quartile 1–3, 11.0–15.0) years, 55.4% were girls, and 55.4% were enrolled in the Belo Horizonte metropolitan area. There were significant differences between the groups on age, sex, number of inhabitants per house, and HDI (Table 1).
Table 1.
Demographic and Clinical Characteristics of Children Undergoing Echocardiographic Screening in Public Schools, Primary Care Centers, and Private Schools
| Variable | Public Schools (n=10 901) | Primary Care Centers (n=558) | Private Schools (n=589) | P Value | Pairwise Comparisons |
|---|---|---|---|---|---|
| Age, median (quartile 1–3) | 12.8 (10.8–15.0) | 13.7 (11.8–16.0) | 15.7 (14.3–16.5) | <0.001 |
P<0.001a
P<0.001b P<0.001c |
| Age >12 y, N (%) | 6510 (60.2) | 397 (71.1) | 544 (93.6) | <0.001 |
P<0.001a
P<0.001b P<0.001c |
| Male sex, N (%) | 4845 (44.8) | 267 (47.9) | 224 (38.1) | 0.002 |
P=0.16a
P=0.001b P=0.001c |
| No. of inhabitants, median (quartile 1–3) | 4.0 (4.0–5.0) | 4.0 (4.0–5.0) | 4.0 (3.0–4.0) | <0.001 |
P=0.65a
P<0.001b P<0.001c |
| No. of inhabitants <15 y, median (quartile 1–3) | 2.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (0–2.0) | <0.001 |
P<0.001a
P<0.001b P<0.001c |
| HDI, median (quartile 1–3) | 0.770 (0.762–0.801) | 0.770 (0.770–0.770) | 0.955 (0.955–0.955) | <0.001 |
P=0.38a
P<0.001b P<0.001c |
HDI indicates Human Development Index; and quartile 1–3, 25%–75%.
Public schools vs primary care.
Public schools vs private schools.
Primary care vs private schools.
In primary care, education (house‐to‐house and operative groups) was successfully delivered to ≈270 families, from which 661 children met the inclusion criteria and 84.4% (n=558) were consented. In public and private schools, 38.7% (10 901/28 168) and 66.9% (589/881) of eligible students, respectively, undergoing the educational process returned the signed informed consent (P<0.001). In public schools, overall compliance improved from the first 14 months (35.3%) to the following 12 months of screening (42.9%) (P<0.001), and was higher in the north (66.2%) compared with the capitol (29.5%) (P<0.001) (Figure 1).
Overall RHD prevalence was 4.0% borderline (n=478) and 0.5% definite (n=63), with statistically similar rates between public schools (4.0% and 0.6%, respectively), primary care centers (4.2% and 0.7%, respectively), and private schools (3.2% and 0.3%, respectively) (P=0.24). The prevalence of definite RHD was also similar between the different screening settings (P=0.41) (Figure 2, Table 2). Among RHD screen‐positive patients, median age was higher in private schools (15.8 [quartile 1–3, 14.7–16.4] years) compared with public schools (14.0 [quartile 1–3, 11.7–15.8] years) (P=0.005), but similar to primary care centers (14.6 [quartile 1–3, 13.2–16.2] years) (P=0.08). A total of 78 children (0.6%) had other echocardiographic abnormalities not related to RHD; the most prevalent were mitral valve prolapse (n=29), atrial septal defects (n=10), bicuspid aortic valve (n=7), and ventricular septal defects (n=3).
Figure 2.
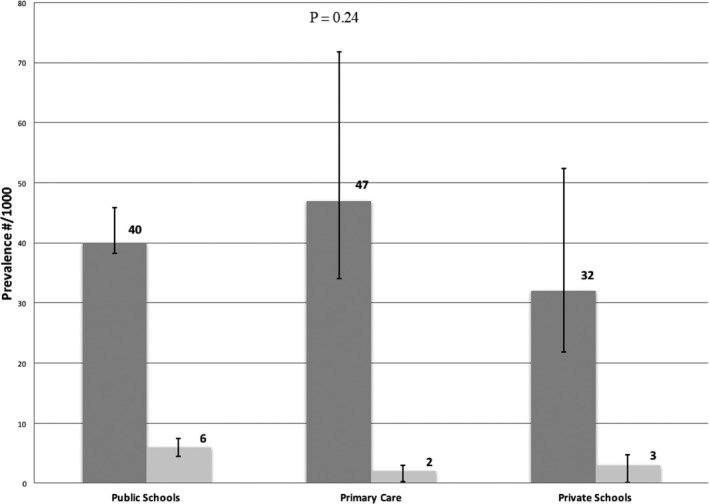
Echocardiographic prevalence of rheumatic heart disease according to screening location: public schools, primary care centers, and private schools.
Table 2.
Prevalence of RHD Echocardiographic Findings and WHF Criteria for Borderline and Definite Disease in Children Undergoing Screening in Public Schools, Primary Care Centers, and Private Schools
| Variable | Public Schools (n=10 901) | Primary Care Centers (n=558) | Private Schools (n=589) | P Valuea |
|---|---|---|---|---|
| Borderline RHD, N (% [95% CI]) | 433 (4.0 [3.8–4.6]) | 26 (4.7 [3.4–7.2]) | 19 (3.2 [2.2–5.2]) | 0.46 |
| Definite RHD, N (% [95% CI]) | 60 (0.6 [0.5–0.8]) | 1 (0.2 [0.0–1.0]) | 2 (0.3 [0.1–1.2]) | 0.41 |
| Other, N (% [95% CI]) | 65 (0.6 [0.5–0.8]) | 6 (1.1 [0.5–2.3]) | 7 (1.2 [0.6–2.4]) | 0.10 |
| WHF criteria for borderline RHD, N (%) | ||||
| A. At least 2 morphological features of RHD of the MV without pathological MR or MS | 15 (3.5) | 1 (3.8) | 0 | 0.35 |
| B. Pathological MR | 360 (83.1) | 18 (69.2) | 16 (84.2) | |
| C. Pathological AR | 58 (13.4) | 7 (26.9) | 3 (15.8) | |
| WHF criteria for definite RHD, N (%) | ||||
| A. Pathological MR and at least 2 morphological features of RHD of the MV | 40 (66.7) | 1 (100) | 2 (100) | 0.83 |
| B. MS mean gradient >4 mm Hg | 0 | 0 | 0 | |
| C. Pathological AR and at least 2 morphological features of RHD of the AV | 5 (8.3) | 0 | 0 | |
| D. Borderline disease of both the AV and MV | 15 (25.0) | 0 | 0 | |
AR indicates aortic regurgitation; AV, aortic valve; CI, confidence interval; MR, mitral regurgitation; MS, mitral stenosis; MV, mitral valve; RHD, rheumatic heart disease; and WHF, World Heart Federation.
Fisher's exact test was used for multiple comparisons of proportions between groups.
The pattern of valve disease is detailed in Table 2. In patients with definite disease, 68.3% had mitral regurgitation plus at least 2 morphological criteria of the mitral valve (100% among children with definite RHD screened in primary care centers and private schools), and 23.8% had mixed valve disease. The World Heart Federation criteria for both borderline and definite RHD were statistically similar between groups (Table 2). RHD prevalence was higher in children ≥12 years (5.3% versus 3.1%; P<0.001) and girls (4.9% versus 4.0%; P=0.02), and similar between the northern and central regions (4.4% versus 4.5%; P=0.66). A progressive increase of RHD (borderline and definite) with age was observed, reaching 6.0% in children aged ≥14 years.
In the bivariate regression analyses, age (odds ratio [OR], 1.13), female sex (OR, 1.24), and number of inhabitants aged <15 years in the household (OR, 0.89) were associated with the presence of any RHD, and age (OR, 1.22) was the only variable associated with definite disease. In multivariable analysis, age (OR, 1.12) was the only predictor of any RHD, with a borderline significance observed for sex. There was no association between the different screening settings and the prevalence observed (Table 3).
Table 3.
Factors Associated With the Presence of Echocardiographic Findings of RHD (Any or Definite): Bivariate and Multivariate Analyses
| Diagnosis | Any RHD (n=541) | Definite RHD (n=63) | ||
|---|---|---|---|---|
| Variable | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Age (each 1 y) | 1.13 (1.10–1.16) | <0.001a | 1.22 (1.11–1.34) | <0.001a |
| Sex (female) | 1.24 (1.04–1.48) | 0.016a | 1.37 (0.82–2.30) | 0.23 |
| No. of inhabitants | 0.99 (0.94–1.05) | 0.79 | 1.05 (0.91–1.21) | 0.54 |
| No. of inhabitants <15 y | 0.89 (0.82–0.97) | 0.007a | 0.85 (0.66–1.09) | 0.20 |
| Screening in public schoolsb | 1.09 (0.80–1.47) | 0.60 | 2.11 (0.66–6.74) | 0.21 |
| Screening in primary care centersb | 1.09 (0.73–1.61) | 0.68 | 0.33 (0.05–2.39) | 0.27 |
| Screening in private schoolsb | 0.78 (0.50–1.21) | 0.29 | 0.64 (0.16–2.61) | 0.53 |
| HDI | 1.12 (0.34–3.69) | 0.85 | 0.79 (0.03–24.5) | 0.89 |
| Multivariable analysis | ||||
| Age (each 1 y) | 1.12 (1.09–1.17) | <0.001a | ··· | ··· |
| Sex (female) | 1.18 (0.98–1.42) | 0.09 | ··· | ··· |
| No. of inhabitants <15 y | 1.01 (0.93–1.10) | 0.84 | ··· | ··· |
CI indicates confidence interval; HDI, Human Development Index; OR, odds ratio; and RHD, rheumatic heart disease.
P<0.05.
Three models were run for the bivariate analysis.
Discussion
The data from >12 000 children screened in 26 months of the PROVAR study (the first large‐scale screening program in Brazil) highlight the much better screening coverage observed in primary care centers and private schools (84% and 67%, respectively, in contrast with 39% in public schools). Findings also show the high prevalence of RHD echocardiographic findings in this region of the country, with surprising similar rates and pattern of disease between the 3 study settings, contrasting with previously published data from other regions.16, 18, 23 These data reinforce the need for diagonal integration of RHD control with the existing health system.
The high overall prevalence of RHD reinforces the preliminary findings of the PROVAR study,15 and again helps explain the great burden of severe late valvular sequelae, leading to interventions and posing great costs to the Brazilian public health system (SUS).24 Despite these observations, no integrating national programs or public policies for disease surveillance, as recommended by international documents,25, 26 have been implemented in the past decades. Notwithstanding the existing administrative SUS database, the paucity of consistent data on the efficacy and cost‐effectiveness of different preventive strategies may be among the reasons for that. The limitations of the databases (noticeably, the absence of specific codes for RHD in surgical procedures) along with the nonincorporation of subclinical disease and the lack of different models for specific population subsets (eg, teenagers versus adults) may explain the underestimation of RHD prevalence in Brazil and Latin America in the Global Burden of Disease study.3 To develop adequate public health policies and lower the impact of RHD, it is key to target higher‐risk populations with the most comprehensive approach possible, to diagnose early‐stage disease and initiate secondary prophylaxis to prevent progression.27
RHD prevalence in private schools situated in high‐income areas of Belo Horizonte was greater than anticipated. This finding contrasts with findings of previously published studies,16, 18 and we speculate about the contribution of several factors. First, the prevalence of group A streptococcus among children presenting with acute pharyngotonsillitis in Brazil seems to be higher than in other developing countries,28, 29 possibly as a result of a complex interaction between climatic factors, behavioral patterns, and genetic predisposition. Previous studies have also demonstrated that factors related with RHD onset, such as persistently elevated anti–streptolysin O titers, are not necessarily paralleled with socioeconomic status.10 Moreover, it is suggested that some group A streptococcal emm clusters may have a tropism for defined geographical areas,11, 12 besides their social determinants. These findings highlight the complex nature of group A streptococcal epidemiological features. Second, despite the wider access of families with private insurance to specialized health care in Brazil, primary care and health promotion initiatives are still scarce in this setting, which may affect primary prevention. Third, there is no geographical circumscription for private schools in Brazil, as there is for public education, and children from different areas share the same institutions. Thus, despite the high costs of these schools, the demographic and socioeconomic strata may not reflect those of the regions where the schools are located. Fourth, the higher median age and the greater proportion of girls in private schools may have overestimated prevalence. However, further studies are needed to know if this trend is real and to determine the driving factors behind the higher than expected prevalence in these middle‐ to high‐income children, because systematic data from other developing countries are scarce.
Our data point toward low compliance with study consent in public schools, in contrast with the high rates in primary care centers and private schools, although sample size was limited in the last 2 locations. Besides the previously recognized factors (mainly related to suboptimal involvement of school representatives and staff and to involvement of parents14), the low coverage in public schools is also attributed to some particularities of Brazilian health regulations. First, nonphysicians are not allowed to acquire echocardiographic images outside research protocols or to make official diagnoses. Thus, informed consents are mandatory for such procedures. However, in resource‐limited health systems, like the Brazilian SUS, task shifting has proved to be a crucial tool to improve availability and reduce costs of screening, freeing high‐level personnel for more complex tasks.27 The efficacy of nurses, technicians, and other professionals in performing simplified protocols has been demonstrated, as has their accuracy for identifying positive cases, even in simplified protocols and with limited training.20, 30, 31, 32 Second, individual consents (and not collective consents) are required for any research procedure. This poses a significant burden for school‐based programs while RHD screening is not incorporated as part of regular health care, and favors the primary care strategy.
A significant innovation of Brazilian public health was the implementation of the SUS in the 1990s, with a rapid scaling up of a community approach to deliver primary care through community health agents (members of the population hired by the health system) responsible for a defined population or area. The close contact favors health promotion and allows a deep integration between community needs and the health system, facilitating preventive interventions.33 Integration of echocardiography screening strategies with the existing SUS may be a future direction and has been successfully evaluated in different settings. Even in high‐resourced regions, like the United Kingdom, population‐based echocardiographic screening of asymptomatic elderly individuals was feasible, and the presence of valve disease was associated with socioeconomic status.34 In low‐income areas, such as India, a mass screening strategy in rural areas with focused handheld echocardiography performed by nonexperts and remote interpretation, similar to the PROVAR study flow chart, was also successful and revealed an alarming 16% of major echocardiographic abnormalities (32.9% valve disease), with an important contribution of RHD.35
Telemedicine has been successfully applied since the beginning of the PROVAR study. The project has collaborated with the development of cloud storage and reading systems dedicated to cardiovascular imaging and used a collaborative reading routine between Brazil and the United States, an innovation for large‐scale RHD programs.21 The incorporation of the RHD project was also an improvement for the Telehealth Network of Minas Gerais. The network was established in 2005 to connect hospitals from 5 public universities in the state. Since then, it has been successfully delivering health care to remote and underserved areas, providing cost‐effective36 services (teleelectrocardiography, teleconsultations,19 diabetes mellitus care,37 and support to systems of care) that give support to primary care through a network of experts. RHD programs can benefit from the existing infrastructure, political support, community involvement, and adaptation to local work routines for integration with primary care.19 Telemedicine and several adaptable strategies of the surveillance model applied by the PROVAR study can intersect with RHD programs of underserved regions and contribute to overcome challenges, as depicted in Table 4.
Table 4.
Adaptable Strategies for Successful Active RHD Surveillance
| PROVAR Design Feature | Challenge Addressed | Translational Strategy for LMIC |
|---|---|---|
| Diagonal integration | ||
| Screening for RHD within primary healthcare clinics | Vertical (RHD‐specific) vs diagonal (integration into existing systems) program |
Identification of platforms for accessing at‐risk populations (women and children) Integration with other disease‐specific programs or primary care (when available) |
| Follow‐up of screen‐positive cases at the primary healthcare center | Low rates of follow‐up for screen+examinations, patient barriers to travel for care | Consider outreach strategies to bring follow‐up locally, task shifting to lower providers after initial confirmatory diagnosis |
| Use of handheld echocardiography machines | Equipment costs, multifunctional uses | Consider point‐of‐care (not fully functional) echocardiographic equipment to reduce costs, and equipment that can be multipurpose (obstetrical and vascular imaging) |
| Use of equipment for all cardiac concerns and all ages |
Single focus (RHD) vs assessment for other common cardiac conditions Improved overall care delivery |
Explore expansion of indications for screening echocardiography to address other cardiac conditions |
| Educational programs for community awareness (house‐to‐house education by community health workers already visiting homes) | Suboptimal participation in screening. Low community knowledge of RHD | Identification of existing educational resources (village health teams, schools, and community organizations) to add RHD education |
| Task shifting | ||
| Use of nonspecialty physicians and nonphysicians for image acquisition | Constrained human resources | Identify a workforce available for continuous training of nonexperts, task shift as appropriate |
| Web‐based initial training | Trainer and trainee time, need for ongoing training of new staff | Consider asynchronous learning platforms |
| Telemedicine/web‐based applications | ||
| Telemedicine for diagnosis | Constrained human resources, large geographic areas |
Consider best staffing for final diagnosis in your setting Can experts be virtually accessed thorough telemedicine |
| Continuous competency assessment | Maintenance of high standards and competency | Consider remote monitoring and retraining of staff through telemedicine |
| Web‐based RHD registry | Case tracking and provision of secondary prophylaxis | Consider centralized registers, making RHD a reportable condition |
LMIC indicates low‐ and middle‐income countries; PROVAR, Rheumatic Valve Disease Screening Program; and RHD, rheumatic heart disease.
In summary, although the study was conducted in a single state, the PROVAR study data bring some aspects of RHD control in Brazil to discussion: (1) evaluation of populations of higher social strata should be considered, because socioeconomic variables may not be the only determinants of the disease; (2) a combined strategy, with a school‐based approach and integration with the existing SUS, may improve coverage and efficacy of screening; (3) the high burden of disease (both subclinical and advanced) urges the incorporation of some recommendations of international documents on RHD control.26 These recommendations may include the development of prospective RHD registers, maintenance of adequate benzathine penicillin supplies, decentralization of technical expertise, establishment of excellence centers for integrated national control programs, and structuring of partnerships with different sectors of society, with the common goal of fighting RHD.
Limitations
Our study has several limitations, especially related to the lack of association between socioeconomic variables and RHD prevalence. First, the HDI estimation has some inaccuracies: some northern municipalities have estimates for large areas, and not for specific locations and neighborhoods (eg, Montes Claros metropolitan area). It is also common that public schools enroll children from geographically related areas with discrepant socioeconomic conditions. Also, even private schools in the capitol, with their considerable costs, are usually a reference for children from different regions of the state, sometimes with different sociodemographic backgrounds. Second, despite the multiple engagement strategies applied by the PROVAR study (markedly, the multiple educational strategies14, 15), student participation in public schools remained marginal, which may bias prevalence estimates. This contrasts with the higher coverage in the primary care setting, reinforcing the need for diagonal integration. Third, because the PROVAR study was primarily designed for public schools, the smaller sample size in primary care centers and private schools may have underpowered the analysis to access the differences in prevalence, especially for the latter. Fourth, all consented children were consecutively included, without stratified sampling procedures, increasing the risk of bias associated with differences between groups (eg, higher median age in private schools). Fifth, because follow‐up confirmatory echocardiograms were not considered, prevalence estimates may be biased upward, especially for handheld devices.20 Last, the study was conducted in 2 metropolitan areas of the state of Minas Gerais, and the findings cannot be directly extrapolated to the entire country. Despite these limitations, the importance of the PROVAR study for the development of public policies is undoubtable, as the biggest RHD screening data in Latin America to date and the most reliable estimates for the Brazilian territory.
Conclusions
Our data show that the echocardiographic prevalence of RHD among schoolchildren from the Brazilian southeastern state of Minas Gerais is significantly high, especially in public schools, but with rates higher than expected in private schools of high‐income areas. Screening in primary care seems to achieve significantly higher participation rates. These data are important for the formulation of public policies and development of diagnostic approaches to confront RHD in high prevalence regions.
Disclosures
None.
PROVAR (Rheumatic Valve Disease Screening Program) Investigators
Adriana C. Diamantino, MD, MSc; Allison Tompsett, MD; Amanda O. Lauar, MD; Ana Luísa M. Costa, MD; Andrea Z. Beaton, MD; Antonio Luiz P. Ribeiro, MD; PhD; Bruno R. Nascimento, MD, MSc, PhD; Camila G. Ferreira, BSN; Cassio M. Oliveira, PhD; Catherine L. Webb, MD; Craig A. Sable, MD; Eduardo L. V. Lopes, MD; Gabriela Z. L. Ruiz, MD; Gabriel A. L. Carmo, MD, PhD; Graziela Chequer, MD, MSc, PhD; Hedda Richards, RDCS; Iara M. Castro, MD; Isabella M. Teixeira, MD; Júlia P. A. Santos, MD; Lindsay Perlman, MPh; Luciana C. X. Lafeta, BSN; Luise Cristina T. R. Barros, MD; Kaciane K. B. Oliveira, BSN, MSc; Letícia Maria M. Rabelo, MD; Maria do Carmo P. Nunes, MD, PhD; Michelle C. Galbas, MD; Sandra Regina T. Castilho, MD; Tainá V. Lourenço, MD; Vitória M. L. R. de Rezende, MD; Zilda Maria A. Meira, MD, PhD.
Sources of Funding
Verizon supported and funded the rheumatic heart disease (RHD) screening program in Brazil, General Electric Healthcare provided echocardiography equipment, Vitel Net collaborated and contributed to the development of the RHD cloud platform, and Edwards Lifesciences funds the ongoing phases of the primary care screening program. The Telehealth Network of Minas Gerais was funded by the State Government of Minas Gerais, by its Health Department (Secretaria de Estado da Saúde de Minas Gerais) and FAPEMIG (Foundation for Research Support of the State of Minas Gerais) (in Portugese, Fundação de Amparo à Pesquisa de Minas Gerais), and by the Brazilian Government, including the Health Ministry and the Science and Technology Ministry and its research and innovation agencies, CNPq (National Council for Scientific and Technological Development) (in Portugese, Conselho Nacional de Desenvolvimento Científico e Tecnológico) and FINEP (Financier of Studies and Projects) (in Portugese, Financiadora de Estudos e Projetos). Ribeiro was supported in part by CNPq (Bolsa de produtividade em pesquisa, 310679/2016‐8) and by FAPEMIG (Programa Pesquisador Mineiro, PPM‐00428‐17). Medical students received scholarships from the National Institute of Science and Technology for Health Technology Assessment (IATS, project: 465518/2014‐1). The funder did not have any relationship with the conduct of the study, the collection, analysis, and interpretation of the data, and the preparation, review, or approval of this article.
(J Am Heart Assoc. 2018;7:e008039 DOI: 10.1161/JAHA.117.008039.)29444774
The abstract of this work was presented at the American Heart Association Scientific Sessions, November 11 to 15, 2017, in Anaheim, CA.
Contributor Information
Bruno R. Nascimento, Email: ramosnas@gmail.com.
the PROVAR (Rheumatic Valve Disease Screening Program) Investigators:
Amanda O. Lauar, Ana Luísa M. Costa, Camila G. Ferreira, Catherine L. Webb, Eduardo L. V. Lopes, Gabriela Z. L. Ruiz, Graziela Chequer, Hedda Richards, Iara M. Castro, Isabella M. Teixeira, Lindsay Perlman, Luciana C. X. Lafeta, Luise Cristina T. R. Barros, Michelle C. Galbas, Tainá V. Lourenço, and Vitória M. L. R. de Rezende
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 2. Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102:75–85. [DOI] [PubMed] [Google Scholar]
- 3. Watkins DA, Johnson CO, Colquhoun SM, Karthikeyan G, Beaton A, Bukhman G, Forouzanfar MH, Longenecker CT, Mayosi BM, Mensah GA, Nascimento BR, Ribeiro ALP, Sable CA, Steer AC, Naghavi M, Mokdad AH, Murray CJL, Vos T, Carapetis JR, Roth GA. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med. 2017;377:713–722. [DOI] [PubMed] [Google Scholar]
- 4. Nulu S, Bukhman G, Kwan GF. Rheumatic heart disease: the unfinished global agenda. Cardiol Clin. 2017;35:165–180. [DOI] [PubMed] [Google Scholar]
- 5. Weinberg J, Beaton A, Aliku T, Lwabi P, Sable C. Prevalence of rheumatic heart disease in African school‐aged population: extrapolation from echocardiography screening using the 2012 World Heart Federation Guidelines. Int J Cardiol. 2016;202:238–239. [DOI] [PubMed] [Google Scholar]
- 6. Paar JA, Berrios NM, Rose JD, Caceres M, Pena R, Perez W, Chen‐Mok M, Jolles E, Dale JB. Prevalence of rheumatic heart disease in children and young adults in Nicaragua. Am J Cardiol. 2010;105:1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, Paquet C, Jacob S, Sidi D, Jouven X. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–476. [DOI] [PubMed] [Google Scholar]
- 8. Roberts KV, Brown AD, Maguire GP, Atkinson DN, Carapetis JR. Utility of auscultatory screening for detecting rheumatic heart disease in high‐risk children in Australia's Northern Territory. Med J Aust. 2013;199:196–199. [DOI] [PubMed] [Google Scholar]
- 9. Remenyi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J, Maguire G, Marijon E, Mirabel M, Mocumbi AO, Mota C, Paar J, Saxena A, Scheel J, Stirling J, Viali S, Balekundri VI, Wheaton G, Zuhlke L, Carapetis J. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease: an evidence‐based guideline. Nat Rev Cardiol. 2012;9:297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahin MS, Yalcin MU, Kocyigit D. Prevalence of rheumatic heart disease in patients with recurrent tonsillitis and elevated anti‐streptolysin O titers. Int J Pediatr Otorhinolaryngol. 2016;89:133–135. [DOI] [PubMed] [Google Scholar]
- 11. Shulman ST, Tanz RR, Dale JB, Steer AC, Smeesters PR. Added value of the emm‐cluster typing system to analyze group A Streptococcus epidemiology in high‐income settings. Clin Infect Dis. 2014;59:1651–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tartof SY, Reis JN, Andrade AN, Ramos RT, Reis MG, Riley LW. Factors associated with Group A Streptococcus emm type diversification in a large urban setting in Brazil: a cross‐sectional study. BMC Infect Dis. 2010;10:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morrow JS. Toward a more normative assessment of maldistribution: the Gini Index. Inquiry. 1977;14:278–292. [PubMed] [Google Scholar]
- 14. Santos J, Carmo G, Beaton AZ, Lourenco TV, Diamantino AC, Nunes M, Sable C, Nascimento BR. Challenges for the implementation of the first large‐scale rheumatic heart disease screening program in Brazil: the PROVAR study experience. Arq Bras Cardiol. 2017;108:370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nascimento BR, Beaton AZ, Nunes MC, Diamantino AC, Carmo GA, Oliveira KK, Oliveira CM, Meira ZM, Castilho SR, Lopes EL, Castro IM, Rezende VM, Chequer G, Landay T, Tompsett A, Ribeiro AL, Sable C; PROVAR (Programa de RastreamentO da VAlvopatia Reumática) Investigators . Echocardiographic prevalence of rheumatic heart disease in Brazilian schoolchildren: data from the PROVAR study. Int J Cardiol. 2016;219:439–445. [DOI] [PubMed] [Google Scholar]
- 16. Periwal KL, Gupta BK, Panwar RB, Khatri PC, Raja S, Gupta R. Prevalence of rheumatic heart disease in school children in Bikaner: an echocardiographic study. J Assoc Physicians India. 2006;54:279–282. [PubMed] [Google Scholar]
- 17. Roberts K, Maguire G, Brown A, Atkinson D, Remenyi B, Wheaton G, Kelly A, Kumar RK, Su JY, Carapetis JR. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation. 2014;129:1953–1961. [DOI] [PubMed] [Google Scholar]
- 18. Sadoh WE, Omuemu VO, Israel‐aina YT. Prevalence of rheumatic heart disease among primary school pupils in mid‐western Nigeria. East Afr Med J. 2013;90:28–32. [PubMed] [Google Scholar]
- 19. Alkmim MB, Figueira RM, Marcolino MS, Cardoso CS, Pena de Abreu M, Cunha LR, da Cunha DF, Antunes AP, Resende AG, Resende ES, Ribeiro AL. Improving patient access to specialized health care: the Telehealth Network of Minas Gerais, Brazil. Bull World Health Organ. 2012;90:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beaton A, Nascimento BR, Diamantino AC, Pereira GT, Lopes EL, Miri CO, Bruno KK, Chequer G, Ferreira CG, Lafeta LC, Richards H, Perlman L, Webb CL, Ribeiro AL, Sable C, Nunes MD. Efficacy of a standardized computer‐based training curriculum to teach echocardiographic identification of rheumatic heart disease to nonexpert users. Am J Cardiol. 2016;117:1783–1789. [DOI] [PubMed] [Google Scholar]
- 21. Lopes EL, Beaton AZ, Nascimento BR, Tompsett A, Dos Santos JP, Perlman L, Diamantino AC, Oliveira KK, Oliveira CM, Nunes MD, Bonisson L, Ribeiro AL, Sable C; Programa de RastreamentO da VAlvopatia Reumática (PROVAR) Investigators . Telehealth solutions to enable global collaboration in rheumatic heart disease screening. J Telemed Telecare. Available at https://hsrc.himmelfarb.gwu.edu/smhs_peds_facpubs/1737/. Accessed February 3, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimbally‐Kaky G, Gombet T, Voumbo Y, Ikama‐Meo S, Elenga‐Mbola B, Mbika‐Cardorelle A, Dilou L, Ekoba J, Nkoua JL, Moyen G, Bouramoue C. Rheumatic heart disease in schoolchildren in Brazzaville. Med Trop (Mars). 2008;68:603–605. [PubMed] [Google Scholar]
- 24. Ribeiro GS, Tartof SY, Oliveira DW, Guedes AC, Reis MG, Riley LW, Ko AI. Surgery for valvular heart disease: a population‐based study in a Brazilian urban center. PLoS One. 2012;7:e37855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayosi BM, Gamra H, Dangou JM, Kasonde J; 2nd All‐Africa Workshop on Rheumatic Fever and Rheumatic Heart Disease participants . Rheumatic heart disease in Africa: the Mosi‐o‐Tunya call to action. Lancet Glob Health. 2014;2:e438–e439. [DOI] [PubMed] [Google Scholar]
- 26. Watkins D, Zuhlke L, Engel M, Daniels R, Francis V, Shaboodien G, Kango M, Abul‐Fadl A, Adeoye A, Ali S, Al‐Kebsi M, Bode‐Thomas F, Bukhman G, Damasceno A, Goshu DY, Elghamrawy A, Gitura B, Haileamlak A, Hailu A, Hugo‐Hamman C, Justus S, Karthikeyan G, Kennedy N, Lwabi P, Mamo Y, Mntla P, Sutton C, Mocumbi AO, Mondo C, Mtaja A, Musuku J, Mucumbitsi J, Murango L, Nel G, Ogendo S, Ogola E, Ojji D, Olunuga TO, Redi MM, Rusingiza KE, Sani M, Sheta S, Shongwe S, van Dam J, Gamra H, Carapetis J, Lennon D, Mayosi BM. Seven key actions to eradicate rheumatic heart disease in Africa: the Addis Ababa communique. Cardiovasc J Afr. 2016;27:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nascimento BR, Nunes MC, Lopes EL, Rezende VM, Landay T, Ribeiro AL, Sable C, Beaton AZ. Rheumatic heart disease echocardiographic screening: approaching practical and affordable solutions. Heart. 2016;102:658–664. [DOI] [PubMed] [Google Scholar]
- 28. Barbosa Junior AR, Oliveira CD, Fontes MJ, Lasmar LM, Camargos PA. Diagnosis of streptococcal pharyngotonsillitis in children and adolescents: clinical picture limitations [in Portuguese]. Rev Paul Pediatr. 2014;32:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group a streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. [DOI] [PubMed] [Google Scholar]
- 30. Colquhoun SM, Carapetis JR, Kado JH, Reeves BM, Remenyi B, May W, Wilson NJ, Steer AC. Pilot study of nurse‐led rheumatic heart disease echocardiography screening in Fiji: a novel approach in a resource‐poor setting. Cardiol Young. 2013;23:546–552. [DOI] [PubMed] [Google Scholar]
- 31. Ploutz M, Lu JC, Scheel J, Webb C, Ensing GJ, Aliku T, Lwabi P, Sable C, Beaton A. Handheld echocardiographic screening for rheumatic heart disease by non‐experts. Heart. 2016;102:35–39. [DOI] [PubMed] [Google Scholar]
- 32. Diamantino A, Beaton A, Aliku T, Oliveira K, Oliveira C, Xavier L, Perlman L, Okello E, Nascimento B, Ribeiro ARP, Nunes MCP, Sable C. A focussed single‐view hand‐held echocardiography protocol for the detection of rheumatic heart disease. Cardiol Young. 2018;28:108–117. [DOI] [PubMed] [Google Scholar]
- 33. Macinko J, Harris MJ. Brazil's family health strategy: delivering community‐based primary care in a universal health system. N Engl J Med. 2015;372:2177–2181. [DOI] [PubMed] [Google Scholar]
- 34. d'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson‐Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, Myerson SG, Prendergast BD. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE Population Cohort Study. Eur Heart J. 2016;37:3515–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh S, Bansal M, Maheshwari P, Adams D, Sengupta SP, Price R, Dantin L, Smith M, Kasliwal RR, Pellikka PA, Thomas JD, Narula J, Sengupta PP; ASE‐REWARD Study Investigators . American Society of Echocardiography: remote echocardiography with web‐based assessments for referrals at a distance (ASE‐REWARD) study. J Am Soc Echocardiogr. 2013;26:221–233. [DOI] [PubMed] [Google Scholar]
- 36. Andrade MV, Maia AC, Cardoso CS, Alkmim MB, Ribeiro AL. Cost‐benefit of the telecardiology service in the state of Minas Gerais: Minas Telecardio Project. Arq Bras Cardiol. 2011;97:307–316. [DOI] [PubMed] [Google Scholar]
- 37. Maia JX, de Sousa LA, Marcolino MS, Cardoso CS, da Silva JL, Alkmim MB, Ribeiro AL. The impact of a clinical decision support system in diabetes primary care patients in a developing country. Diabetes Technol Ther. 2016;18:258–263. [DOI] [PubMed] [Google Scholar]


