Abstract
Biologic therapies have become central to the long-term management of many chronic diseases, including inflammatory rheumatic diseases. Over recent years, the development and licensing pathways for biosimilars have become more standardized, and several biosimilars have been made available for patients with inflammatory rheumatic diseases, such as RA. Pre-licensing requirements for biosimilars mandate the demonstration of comparability with reference products in terms of clinical activity, safety and immunogenicity, whereas post-marketing surveillance and risk minimization requirements are set in place to ensure that long-term, real-world safety data are collected to assess biosimilars in clinical practice. These measures should provide a foundation for physician confidence in biosimilars, which can be established further through clinical experience. Biosimilars may help to fill an unmet need by improving patient access to effective biologic treatments for chronic diseases. Greater access may result in additional clinical benefits, with appropriate use of biologic therapies according to treatment guidelines being associated with improved outcomes and the potential for reduced costs of care. Key challenges for the integration of biosimilars into everyday practice include questions about interchangeability, switching and automatic substitution. Several switching studies have shown that biosimilars can be used in place of reference products while maintaining efficacy and safety. Additional ongoing studies and registries may help to optimize the process of switching, and different funding models are examining the optimal mechanisms to ensure effective uptake of these new treatments.
Keywords: biologics, immunogenicity, interchangeability, rheumatologic biosimilars, risk minimization, switching
Rheumatology key messages
Biosimilar licensing is based on robust non-clinical and clinical evaluation of comparability with reference products.
The NOR-SWITCH study has shown that switching to biosimilars does not affect clinical outcomes in chronic diseases.
Biosimilars may enable improved access to biologic treatments for chronic diseases, such as RA.
Introduction
The availability of biologic agents has affected the treatment algorithm for many chronic diseases, including inflammatory rheumatic diseases [1, 2]. For example, biologic DMARDs (bDMARDs) are well recognized as a crucial component of long-term therapy for RA [1], providing clinically meaningful benefits in pain and function for patients who do not respond to traditional DMARDs [2]. However, access to these biologic treatments for RA remains uneven [3], with availability primarily being limited by financial constraints [4].
Biosimilars can help to fulfil an unmet need by providing a new threshold for patient access to effective biologic treatments for chronic diseases [5, 6]. Greater access may result in additional clinical benefits, with potentially earlier and more appropriate use of biologic therapies, which are associated with better outcomes. As a result, both direct and indirect costs of care for chronic diseases may be improved [6, 7]. Within Europe, the European Medicines Agency (EMA) has licensed a number of biosimilar products, including epoetin-alfa, etanercept, filgrastim, follitropin-alfa, infliximab, insulins, interferons and somatropin [8]. Within the USA, the US Food and Drug Administration (FDA) has licensed biosimilars of filgrastim and infliximab [9, 10].
This review examines the integration of biosimilars into everyday clinical practice in Europe, focusing on the management of inflammatory rheumatic diseases. As well as considering the regulatory framework, pharmacovigilance and post-marketing safety monitoring, the challenges of switching and interchangeability are reviewed. The important question of how biosimilars may affect patient access to biologic treatments is also discussed.
Comparability of biosimilars: how similar is similar?
As described earlier in this supplement, the EMA defines a biosimilar as “a biological medicine that is developed to be similar to an existing biological medicine (reference product)” [11]. The EMA regulatory framework sets out requirements for demonstrating biosimilarity, with the goal of ensuring that any minor differences between biosimilars and reference products do not affect effectiveness or safety [11]. Owing to the size and complexity of biologic products, such as antibodies and soluble receptors, minor differences are inevitable for different batches of the same biologic product, and non-identicality is an accepted facet of biotechnology production processes [6, 12, 13]. For biosimilars, functional and structural aspects must be as similar as possible to reference products, with consistency being demonstrated in pharmacokinetics (PK), efficacy and safety, including risk of immunogenicity [6, 11, 14].
Non-clinical studies for biosimilars typically include in vitro receptor-binding assays or cell-based assays to establish comparability in reactivity [15]. If comparability is not demonstrated in these studies, the EMA advises additional animal studies, which should be focused on the outcomes that are most likely to answer questions that were not resolved by the non-clinical studies. These may include tests of pharmacodynamics (PD) activity and non-clinical dose toxicity (e.g. antibody titres, cross-reactivity and neutralizing capacity) [15]. In all cases, the EMA advises that drug developers give ongoing consideration to the use of emerging technologies so that the best current technologies are used for assessment. Certain non-clinical studies, such as safety, pharmacology, reproductive and developmental toxicity and carcinogenicity studies, are not required if a high level of similarity between the reference product and biosimilar has been demonstrated in structural and functional characterization studies [15]. With respect to clinical studies, a step-wise approach to clinical comparability is required. Specifically, the EMA suggests that biosimilar comparability testing should begin with PK and, if feasible, PD studies, followed by clinical efficacy and safety [15]. In certain cases, confirmatory PK/PD studies for demonstrating clinical biosimilar comparability may be required [15].
The European Union (EU) regulatory framework has enabled several biosimilar products to be licensed in the area of rheumatic diseases: infliximab (CT-P13: Remsima®/Inflectra®; SB2: Flixabi®) [16–18] and etanercept (Benepali®) [19], and a number of other biosimilars are in development (Table 1). In clinical studies, these biosimilar products have demonstrated PK equivalence to their reference products [37–39], as well as equivalent efficacy and comparable safety and immunogenicity [40–45]. An upcoming milestone in this therapeutic area is the projected expiry of patent protection for adalimumab in the EU in April 2018 [46].
Table 1.
Biosimilars of infliximab, etanercept and adalimumab, licensed or in development for rheumatic diseases
| Reference product | Biosimilar | Biosimilar manufacturer | Highest development status |
|---|---|---|---|
| Infliximab | Remsima® (CT-P13) [16] | Celltrion | Licensed in EU |
| Inflectra® [17] | Hospira | Licensed in Canada, USA | |
| Inflimab® (BOW015) [20] | Epirus | Licensed in India | |
| Flixabi®/Renflexis® (SB2) [21, 22] | Samsung Bioepis/Biogen | Licensed in EU and Korea | |
| PF-06438179 [23] | Pfizer/Sandoz | Phase III | |
| ABP710 [24] | Amgen | Phase I/II | |
| Etanercept | Benepali®/Brenzys® (SB4) [19, 25] | Samsung Bioepis/ Biogen | Licensed in EU, Korea |
| Davictrel® (HD203) [26] | Hanwha/Merck | Licensed in Korea | |
| GP-2015 [27] | Sandoz | Filed in USA | |
| CHS-0214 [28] | Coherus/Baxalta | Phase III | |
| Adalimumab | Exemptia® (ZRC-3197) [29] | Zydus | Licensed in India |
| ABP501 [30] | Amgen | Filed in USA | |
| BI695501 [31] | Boehringer Ingelheim | Phase III | |
| GP-2017 [27] | Sandoz | Phase III | |
| CHS-1420 [32] | Coherus | Phase III | |
| M923 [33] | Momenta/Baxalta | Phase III | |
| SB5 [34] | Samsung Bioepis/Biogen | Phase III | |
| PF-06410293 [35] | Pfizer | Phase III | |
| Rituximab [36] | ABP 798 | Amgen | Phase III |
| AcellBia | Biocad | Licensed in Russia | |
| CT-P10 | Celltrion/Hospira | Phase III | |
| Reditux | Dr Reddy’s Laboratories | Licensed in Bolivia, Chile, India, Peru | |
| Maball | Hetero group | Licensed in India | |
| MabTas | Intas Biopharmaceuticals | Licensed in India | |
| JHL1101 | JHL Biotech | Phase I | |
| MabionCD20 | Mabion | Phase III | |
| PF-05280586 | Pfizer | Phase I/II | |
| Kikuzubam | Probiomed | Licensed in Bolivia, Chile, Mexico, Peru | |
| GP2013 | Sandoz | EU application submitted | |
| HLX01 | Shanghai Henlius Biotech | Phase III | |
| TL011 | Teva | Phase I/II | |
| Rituximab | Zenotech Laboratories | Licensed in India |
Information current as of October 2016. EU: European Union; USA, United States of America.
The next-generation biologics (sometimes referred to as biobetter agents) aim to build on the available biologic agents by providing enhanced attributes [47–49]. Indeed, these next-generation biologics can be defined as having the same target as the originator but improved characteristics, such as PD or PK [50]. If these agents do indeed deliver improvements over available drugs, we can look forward to even greater choices in this therapeutic area.
Assessment and monitoring of biosimilars
Pre-licensing safety assessments, including immunogenicity
In addition to efficacy and PK testing, safety assessments are a key element of the pre-licensing of biosimilars and should involve sufficient patient data to enable the bio-similars and the reference product to be compared [51, 52]. In particular, there has been concern about the possibility of original drugs and their biosimilars differing in immunogenicity; any level of post-translational modification to the antibody structure could affect patterns of immunogenicity [51]. EMA guidance states that non-clinical immunogenicity findings do not predict potential immunogenic responses to biologics in humans [15]. As a result, clinical immunogenicity testing is a crucial component of the safety evaluation of biosimilars, including detection of anti-drug antibodies (ADAs) [15, 51, 52].
Immunogenicity with the infliximab biosimilar Remsima® appears to be similar to that observed with the reference product [41–43, 53]. In a phase III study comparing Remsima® with its reference product, the incidence of ADAs was very slightly higher for Remsima® (ADAs detected in 25.4 and 25.8% of patients for Remsima® and the reference product, respectively, at week 14) [41]. In this instance, the incidence of ADAs appeared to increase over time but did not appear to affect efficacy or safety [41]. In a phase III, randomized study comparing the etanercept biosimilar Benepali® with the etanercept reference product in patients with refractory RA, Benepali® was associated with a significantly lower incidence of ADAs compared with the etanercept reference product (0.7 and 13.1% of patients, respectively, tested positive for ADAs at least once up to week 24; P < 0.001) [40]. The ADAs appeared early and did not affect efficacy or safety, which was consistent with the reference product, and so were considered to have no bearing on establishing biosimilarity [40]. In response to questions regarding the interpretation of these results [54], the authors commented that there was no correlation between ADA incidence and safety profile, and suggested that differences in product aggregates, impurities and glycosylation for Benepali® compared with the etanercept reference product may have resulted in the lower incidence of ADAs with Benepali® in this study [55].
It is challenging to compare immunogenicity results across studies because of differences in assays, patient populations and the timing of testing [56]. In addition, immunogenicity assessments can be affected by previous exposure to a reference product or similar biologic therapeutic, co-administration of other drugs and the underlying disease [57]. The results with Remsima® and Benepali® suggest that immunogenicity for biosimilars must be considered on a case-by-case basis. This is in line with EMA recommendations, which recommend focusing on any differences in immunogenicity that translate into clinically meaningful changes in safety or efficacy [58]. In addition, monitoring of immunogenicity and any link with clinical activity is a crucial component of post-marketing pharmacovigilance [51].
Post-marketing pharmacovigilance and risk management
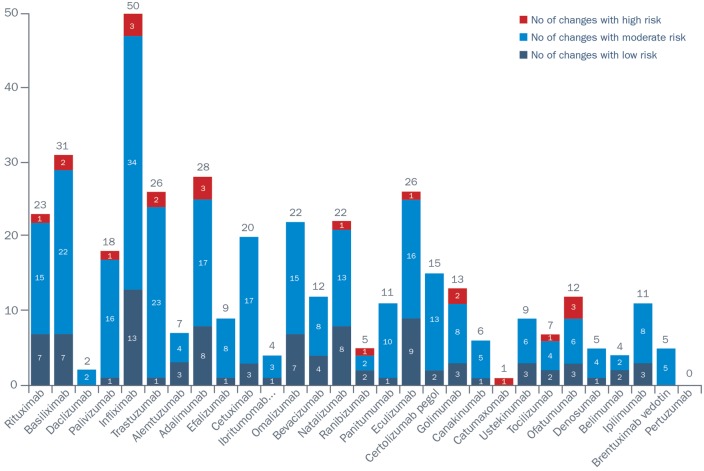
Biologic products vary over time as a result of modifications to the manufacturing process. This applies to both originator products and biosimiliars [59]. Indeed, results from a study that investigated the number and types of manufacturing changes for originator mAbs according to the European Public Assessment Report reported 404 manufacturing changes authorized by the EMA from 29 European Public Assessment Report reports [59]. Of these, 22 were categorized as high-risk, 286 as moderate-risk and 96 as low-risk manufacturing changes (Fig. 1). However, although the EMA has significant experience of process changes for originator mAbs, manufacturers of biosimilars are required to implement pharmacovigilance or risk management plans to assess potential product risks proactively after the biosimilar is made available, including the following [51, 60]: safety profile; how any risks will be prevented or minimized; plans for studies and other activities to gain more knowledge about the safety and efficacy of the medicine; risk factors for developing adverse reactions; and measuring the effectiveness of risk minimization measures.
Fig. 1.
Number of manufacturing changes for monoclonal antibodies in their European Public Assessment Reports according to risk category
Reproduced from: Vezér B, Buzás Z, Sebeszta M, Zrubka Z. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin 2016;32:829–34 [59]. Reprinted by permission of the publisher (Taylor & Francis).
In contrast to the requirements for non-biologic products, additional safety post-marketing monitoring requirements for biosimilars are mandated in view of their abbreviated approval pathway, which may not have enabled the collection of extensive, long-term safety data (Table 2) [51, 60, 61]. For example, a condition of the licensing of Remsima® in Europe was a mandatory post-marketing programme, including multiple registries that will continue up to the year 2026 [62, 63]. Examples of a post-marketing risk management plan and a post-authorization study programme are shown in Tables 3 and 4, respectively [51, 60, 61, 64]. The plan should lay out safety information acquired, any safety concerns and gaps in knowledge to be addressed in the post-authorization programme.
Table 2.
Example of risk minimization measures
| Risk identified | Risk minimization measure | Objective and rationale | Description |
|---|---|---|---|
| Serious infections (including opportunistic infections, tuberculosis, Legionella, Listeria, parasitic infection) | Patient alert card | To provide information to patients to make them aware that, during treatment with Benepali®, there is an increased risk of acquiring serious infections or that existing infections may get worse |
|
| Worsening heart failure (worsening of congestive heart failure in adult patients) | Patient alert card | To provide adequate information to patients to make them aware of the increased risk of worsening of heart failure during treatment with Benepali® |
|
| Potential for medication errors (pre-filled pen) | Educational material for healthcare professionals and patients | To alert patients and healthcare professionals to the risk of medication errors in patients using a pre-filled pen |
|
| Potential for pediatric off-label use | Educational material/patient alert card | To remind patients and healthcare professionals that Benepali® is not indicated for children under the age of 18 years |
|
Summary of information from [61]. Example shown is for a biosimilar etanercept (Benepali®).
Table 3.
Safety risk management requirements for biosimilars in Europe
| Module | Details | Required for biosimilars |
|---|---|---|
| Module S1 | Epidemiology of the indication(s) and target population(s) | No |
| Module SII | Non-clinical part of the safety specification | Yes |
| Module SIII | Clinical trial exposure | Yes |
| Module SIV | Populations not studied in clinical trials | Yes |
| Module SV | Post-authorization experience | Yes |
| Module SVI | Additional EU requirements for the safety specification | Yes |
| Module SVII | Identified and potential risks | Yes |
| Module SVIII | Summary of the safety concerns | Yes |
Table 4.
Example of post-authorization development
| Safety/efficacy concerns addressed | Study | Location(s) | Study overview | Estimated time lines |
|---|---|---|---|---|
| All safety concerns, including serious and/or opportunistic infections, cancers, heart failure and injection-site reactions | SB4-G31-RA (NCT01895309) | Europe |
|
100-week switching data reported at EULAR 2016 |
| All safety concerns, including serious and/or opportunistic infections, cancers, heart failure and injection-site reactions | British Society for Rheumatology Biologics Register–Rheumatoid Arthritis (BSRBR-RA) | UK |
|
|
| All safety concerns, including serious and/or opportunistic infections, cancers, heart failure and injection-site reactions | Rheumatoid Arthritis oBservation of BIologic Therapy (RABBIT) | Germany |
|
|
| All safety concerns, including serious and/or opportunistic infections, cancers, heart failure and injection-site reactions | Anti-Rheumatic Therapies In Sweden (ARTIS) | Sweden | Prospective, observational study to assess the risk of selected adverse events in patients with RA, juvenile idiopathic arthritis and other rheumatic diseases receiving Benepali® |
|
| Long-term safety of biologic treatments for psoriasis | British Association of Dermatologists Biologic Interventions Register (BADBIR) | UK | Nationwide registry to monitor the long-term safety of biologic treatments for psoriasis |
|
Summary of information from [61]. Example shown is for a biosimilar etanercept (Benepali®). DMARD: disease-modifying anti-rheumatic drug; EULAR: European League Against Rheumatism; PSUR: Periodic Safety Update Report; RA: rheumatoid arthritis; RMP: risk management plan; TNF: tumor necrosis factor.
Nomenclature and traceability
The World Health Organization has previously recommended that the standard international non-proprietary name (INN) system be used for biosimilars to enable physicians and regulatory authorities to recognize the active ingredient easily [65]. This approach is used within Europe, whereby a biosimilar that is designed to be identical to a reference product does not have a different INN [11, 65]. In contrast, FDA guidance for industry indicates that biosimilar products submitted under the Public Health Service Act should have a non-proprietary name that includes a four-letter suffix to distinguish the biosimilar from the reference product; for example, the biosimilar filgrastim has the non-proprietary name filgrastim-sndz in the USA [66].
Although emphasizing to clinicians and patients that biosimilars and reference products are to be seen as the same, the policy of using the same name could pose challenges for traceability [67]. Consistent pharmacovigilance systems are required that enable capture of both product (brand name) and batch manufacturing information in relationship to individual dispensed medications, as well as the transfer of exposure details to the relevant pharmacovigilance programmes [67]. Accordingly, European legislation requires that, for all reports of adverse drug reactions, all appropriate measures should be taken to identify the brand name and batch number, as well as the INN [51]. Although national traceability regulations and local procedures for monitoring the dispensing of biologics currently vary, post-marketing studies and monitoring programmes for licensed biosimilars should assist in facilitating traceability in the short and medium term [67].
Integrating biosimilars into clinical practice
Switching
Switching is defined as a decision by the treating physician to exchange one medicine for another medicine with the same therapeutic intent in patients who are undergoing treatment [68, 69]. Several studies (including open-label extensions to randomized controlled studies, observational studies and a randomized study) have examined the impact of using the biosimilar infliximab (Remsima®) in place of the reference product (switching) in patients receiving ongoing treatment for rheumatic diseases or IBD. The PLANETRA open-label extension study recruited 302 patients with RA who completed the 54-week randomized PLANETRA study, and either continued Remsima® (n = 158) or were switched from infliximab to Remsima® for 1 year of treatment in the extension, which ran from weeks 62 to 102 (n = 144) [70]. Comparable efficacy and tolerability were observed in the patients who switched to Remsima® in the extension and the patients who received Remsima® for 2 years (in the randomized study and extension) [70]. Likewise, in the PLANETAS open-label extension study in patients with ankylosing spondylitis, switching to Remsima® from infliximab from weeks 62 to 102, after 54 weeks of prior treatment, was associated with similar efficacy and tolerability compared with receipt of Remsima® throughout [71]. Additional studies in different countries have indicated that switching to Remsima® offers clinical efficacy that is comparable to the reference product [72–77]. However, in some observational studies, discontinuation rates for patients switching from infliximab to Remsima® have been attributed to a possible nocebo effect [72, 76, 77]. These findings, although suppositional, underscore the need for controlled switching studies in this therapeutic area. More recently, a government-funded, randomized, double-blind, multicenter, phase IV study in Norway (NOR-SWITCH) assessed the safety and efficacy of switching patients from reference infliximab (Remicade®) to Remsima® [78, 79]. Altogether, 498 patients were recruited into the study, including patients with RA (n = 77), spondyloarthritis (n = 91), PsA (n = 30), ulcerative colitis (n = 93), Crohn’s disease (n = 155) and chronic plaque psoriasis (n = 35) who had been treated with Remicade® for at least 6 months and who were experiencing stable disease. These patients were randomized (1:1) to switch to Remsima® (n = 241) or to continue treatment with Remicade® (n = 240) for 52 weeks. Disease worsening (primary end point; worsening in disease-specific composite measures and/or a consensus between investigator and patient leading to major change in treatment) occurred in 29.6% of patients receiving Remsima® and in 26.2% of patients receiving Remicade® (treatment difference -4.4%; 95% CI: -12.7% to 3.9%) which confirmed non-inferiority [79]. Evaluations of generic and specific disease measures were similar between the groups, as were the incidence of ADAs (8% Remsima®; 7% Remicade®), trough drug levels and the incidence of adverse events. An extension to the NOR-SWITCH study is also ongoing, in which eligible patients are followed up for a further 6 months while receiving Remsima®, enabling additional comparison of efficacy and safety between patients receiving Remsima® for 12 months and patients who have recently been switched [78].
In an open-label extension to the phase III, 52-week randomized study that compared SB4 with reference etanercept, 126 patients continued to receive SB4 (SB4/SB4) and 119 patients switched from reference etanercept to SB4 (etanercept/SB4) for a further 48 weeks [45]. At the end of this open-label treatment period, the efficacy, safety and immunogenicity profiles were again comparable for both SB4/SB4 and etanercept/SB4 groups. Switching from etanercept to SB4 had no detrimental effects. Of note, there was no decline in efficacy, increase in adverse events or increase in immunogenicity [45].
Against this background, there is a growing body of evidence to support switching from an originator to a biosmiliar, with the NOR-SWITCH study providing the first randomized data set in this respect [79]. National and regional registries will probably provide crucial, real-world data on switching to biosimilars. As more biosimilars become available, a key challenge will be the issue of multiple switching, which is not currently covered in regulatory guidelines and has not yet been addressed in clinical studies [6].
Interchangeability
Interchangeability can be defined as the medical practice of changing one medicine for another that is expected to achieve the same clinical effect in a given clinical setting and in any patients on the initiative, or with agreement of the prescriber [68, 69]. Interchangeability is also a regulatory term used for switching. A biosimilar is defined as being interchangeable with the reference product if biosimilarity has been demonstrated and if it can be expected to produce the same clinical result in any given patient [14]. The term interchangeability is often confused with automatic substitution (see next subsection). The regulatory framework in Europe and post-marketing pharmaco-vigilance commitments undertaken by manufacturers should provide reassurance to prescribers that an approved biosimilar can be administered safely to their patients, and is therefore interchangeable with the reference product [14].
Automatic substitution
Substitution is the practice of dispensing one medicine instead of another equivalent and interchangeable medicine at the pharmacy level without consulting the prescriber [68, 69]. Generic small molecules may be dispensed to patients at the pharmacy level through automatic substitution. In contrast, automatic substitution of biosimilars for reference products is not currently recommended in most countries [14]. Within Europe, the EMA notes that guidance on biosimilar substitution is the responsibility of individual member states and should take place only under the guidance of a healthcare professional [80]. Interpretation of this guidance varies across different European markets, with some countries prohibiting automatic substitution at the pharmacy level [14]. In the UK, for example, the National Health Service recommends against automatic substitution for any biologics at the pharmacy level, and leaves the decision on the use of a biosimilar with the prescriber, who must use brand-name prescribing [81]. In contrast, in many other settings, the use of biosimilars has been actively facilitated by national and local tender systems [82, 83], where the manufacturers are invited to tender a price for their product, thus creating open competition.
Extrapolation of evidence across indications
During the development and licensing of biosimilars, questions have been raised as to whether the demonstration of similar efficacy and safety in one disease justifies indication extrapolation to support licensing of the biosimilar for other diseases [84, 85]. Within the EMA regulatory framework, extrapolation across indications is possible, based on the total evidence of comparability if the reference product’s mechanisms of action are consistent across its different indications [84]. If different mechanisms of action across indications are suspected, then additional evidence is required.
In the case of Remsima®, for which pre-registration clinical trials focused on RA [41], the EMA license included extrapolation across all licensed indications [62]. Likewise, based on the quality of the evidence, the EMA permitted extrapolation of the PK, efficacy and safety data generated with the etanercept biosimilar Benepali® in healthy volunteers and patients with RA to the other adult-licensed indications of the reference product, Enbrel® [64]. Within the FDA framework, extrapolation of indications has also been permitted in the case of Inflectra® (biosimilar infliximab) [10], and this approach is accepted by other regulatory authorities on a case-by-case basis dependent on sufficient scientific rationale [86, 87]. An increasing number of articles are providing evidence that the extrapolation of the biosimilar infliximab from rheumatic to gastrointestinal indications is both efficient and safe [88].
Barriers to implementation—healthcare professional and patient opinions
In order for biosimilars to be adopted widely, both precribers and patients need to be fully aware of their attributes and benefits to be confident in their use. A recent physician Web-based survey of members of the European Crohn’s and Colitis Organization indicated high levels of awareness for biosimilar attributes and potential advantages. However, only 24% agreed with extrapolation of use to indications without direct clinical evidence, and 61% stated that they had little or no confidence in using biosimilars in their clinical practice [89]. This last point is interesting and may require further information and education. Indeed, in a more recent survey of US and European physicians, biosimilar awareness was again found to be high, and whereas 47% of respondents stated they felt these agents were sufficiently safe and effective for them to prescribe, 43% said that they required more information on biosimilars [90].
In a similar vein, patients have a need for education regarding biosimilars. Awareness of biosimilars is low among patients, with gaps in knowledge being notable for efficacy, safety and access to these agents [91]. Important issues relating to practical management, such as pharmacovigilance, interchangeability, switching and substitution, are causes of scepticism and anxiety among patients with rheumatic and musculoskeletal diseases [92]. It is not surprising, as previously noted, that switching to a biosimilar has been associated with a possible nocebo effect [72, 76, 77]. The possibility that secondary inefficacy or adverse effects are inappropriately attributed to the switch is a cause for concern. Overall, the need for patient and physician education and for effective communication between physician and patient are issues that need to be addressed in order for biosimilars to be integrated effectively into clinical practice.
Access to implementation—access to treatments
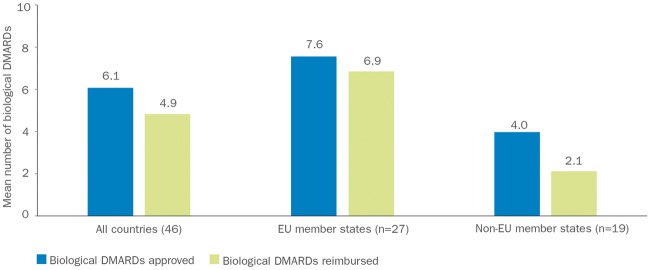
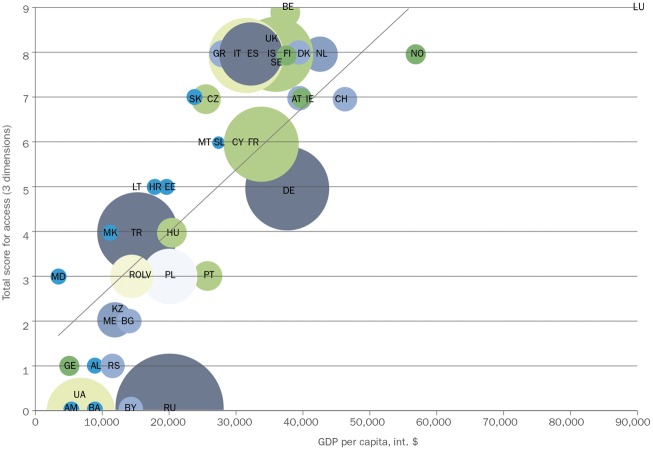
Access to bDMARDs varies considerably across Europe and is dependent on national and local guidelines, levels of funding and differing approaches to healthcare management [3, 4, 93]. In a study of 46 European countries published in 2015, access to bDMARDs differed by country, with 22% of the countries (10/46) having no reimbursement of any of the bDMARDs assessed [3]. Across all of the 46 countries, the mean (s.d.) number of bDMARDs reimbursed was 4.9 (3.3), and this varied according to EU member states and non-EU member states (Fig. 2) [3]. Further analyses of three dimensions of access (acceptability, affordability and availability) indicated a strong correlation between higher socio-economic status, assessed as gross domestic product per capita, and access to bDMARDs (Fig. 3) [3]. The study estimated that, in total, 320 million people with RA in the European region (∼40%) would have severe restrictions on their access to bDMARDs [3]. Barriers to access appeared to be primarily financial and administrative, but may also have been related to prescribing restrictions, which means that countries with lower socio-economic status have higher eligibility barriers for access to biologic treatments [4]. Among the countries in which bDMARDs were reimbursed (n = 36), clinical criteria were in place to regulate the initiation of treatment in all countries, whereas 39% (14/36) had regulations affecting stopping or maintaining treatment and 53% (19/36) provided guidance on switching between treatments [4]. In more than half of the countries (56%; 20/36), a DAS-28 of ⩾3.2 was required to initiate bDMARD treatment, and 61% of countries (22/36) required the failure of more than one traditional DMARD before bDMARD treatment could be started [4]. Of note, non-EU member states tended to have eligibility criteria for access to bDMARDs that were more stringent than recommendations from the EULAR [4]. A composite eligibility score indicated that one-third of all countries had highly restricted access to bDMARDs for RA [4].
Fig. 2.
Approval and reimbursement of biologic DMARDs in Europe
The mean number of bDMARDs approved and reimbursed varied across the 46 European countries in this study, with a different profile being observed for EU member states compared with non-EU member states. Data taken from [3]. bDMARDs: biologic DMARDs; EU: European Union.
Fig. 3.
Access to biologic DMARDs according to gross domestic product per capita
Analysis based on GDP in dollars in 44 countries. The size of the bubbles is proportional to the population size of the country. Reproduced from: Putrik P, Ramiro S, Kvien TK et al. Inequalities in access to biological and synthetic DMARDs across 46 European Countries. Ann Rheum Dis 2014;73:198–206, © 2014 [3]. With permission from BMJ Publishing Group Ltd. AL: Albania; AM: Armenia; AT: Austria; BA: Bosnia and Herzegovina; bDMARD: biologic DMARD; BE: Belgium; BG: Bulgaria; BY: Belarus; CH: Switzerland; CY: Cyprus; CZ: Czech Republic; DE: Germany; DK: Denmark; EE: Estonia; ES: Spain; FI: Finland; FR: France; GDP: gross domestic product; GE: Georgia; GR: Greece; HR: Croatia; HU: Hungary; IE: Ireland; IS: Iceland; IT: Italy; KZ: Kazakhstan; LT: Lithuania; LU: Luxembourg; LV: Latvia; MD: Moldova; ME: Montenegro; MK: Macedonia; MT: Malta; NL: The Netherlands; NO: Norway; PL: Poland; PT: Portugal; RO: Romania; RS: Serbia; RU: Russia; SE: Sweden; SK: Slovakia; SL: Slovenia; TR: Turkey; UA: Ukraine; UK: United Kingdom.
An important consideration for chronic diseases, such as RA, is that earlier intervention generally results in substantially improved outcomes. Treatment of RA, with effective treatments, may provide a unique opportunity to change the course of RA early: after the start of symptoms but before radiographic damage occurs [94]. In countries with spending limitations, early treatment may be less likely, and cost pressure may prevent patients from receiving optimal doses or continuing with appropriate treatments [95]. Budget impact analyses and feedback from countries where biosimilar products are being used suggest that substantial cost savings are possible, particularly when biosimilar substitution is favoured, which could enable many more patients to receive treatment [96, 97]. Changes to European prescribing practices and regulations are necessary to take advantage of the potential benefits of biosimilar products and to harmonize treatment within EU member states. However, alternative strategies before attempting bDMARD implementation need to be considered. These include a treat-to-target (or tight control) approach for example, combination of conventional synthetic DMARDs (csDMARDs) [98–101], following inadequate response to csDMARD monotherapy [102, 103]. Once bDMARDs are introduced, it is important also to consider strategies to reduce costs [104, 105].
Discussion and future directions
Biosimilars offer an important opportunity to improve patient access to effective biologic treatments, thereby not only enhancing the individual patient experience, but also contributing to a reduction in long-term care costs for chronic diseases [6, 7]. Inequity in the access to biologic treatment across European countries could be reduced by better access, with treatment of a greater number of patients at a more cost-effective level. Indeed, several cost-minimization analyses have shown these agents to be cost effective in this context, with biosimilars appearing to exert downward pressure on pricing [96, 97, 106–109]. For example, in Central and Eastern Europe, the introduction of CT-P13 has resulted in a 20–60% reduction in the cost of infliximab [110], and a 69% discount was offered for CT-P13 vs that offered for Remicade® for its national supply in Norway in 2015 [111].
Against this background, the development and licensing process for biosimilars has been shown to be effective and robust, with biologic similarity resulting in consistency with respect to efficacy and safety. Although small in number, switching studies have so far shown that consistent efficacy and safety can be achieved when switching between reference products and biosimilars. Crucially, the real-world experience that is being proactively gathered through post-marketing studies (including the NOR-SWITCH study) and patient registries will add to our knowledge concerning biosimilars, and may further enhance confidence in the use of these products in clinical practice.
There are many stakeholders with various attitudes to important questions, such as switching and interchangeability, and issues are becoming more complex as more biosimilars become available. Interchanging is not standard practice in Europe at present, and this may affect the uptake of biosimilars in some markets. Experience with different pricing and tender systems may be valuable to facilitate the use of biosimilars while leaving the decision for prescription with the physician. Specific mechanisms for tracing the use of specific biosimilars are essential, which must take into account differing approaches to their nomenclature in Europe and the USA.
In the future, biosimilars are expected to play an important role in providing patients with access to effective biologic treatment at the appropriate time during their disease course. Mechanisms are in place to monitor biosimilar effectiveness and safety in clinical practice, and evidence is growing regarding how patients may be switched safely to these treatments. An important issue for future clinical practice will be how to approach multiple switching, as the number of biosimilars increases.
Acknowledgments
The authors would like to acknowledge the editorial support provided by inVentiv Health Medical Communications. Philip Ford and Frances Gambling from inVentiv Health Medical Communications wrote the drafts of the article based on input from all authors, and styled the article per journal requirements. Biogen reviewed and provided feedback on the article to the authors. The authors had full editorial control of the article and provided their final approval of all content. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Funding: This work was supported by Biogen, who provided funding for medical writing and editorial support in the development of this article.
Disclosure statement: T.U. has received consultancy fees from AbbVie, Biogen, Bristol-Myers Squibb, Eli Lilly, MSD, Pfizer, Roche and UCB. G.L.G. has received honoraria/served a consultant for AbbVie, Novartis, Orion Pharma, Boehringer Ingelheim and Pfizer.
References
- 1. Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh JA, Hossain A, Ghogomu T. et al. Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2016;13:CD012183.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Putrik P, Ramiro S, Kvien TK. et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014;73:198–206. [DOI] [PubMed] [Google Scholar]
- 4. Putrik P, Ramiro S, Kvien TK. et al. Variations in criteria regulating treatment with reimbursed biologic DMARDs across European countries. Are differences related to country’s wealth? Ann Rheum Dis 2014;73:2010–21. [DOI] [PubMed] [Google Scholar]
- 5. Henry D, Taylor C.. Pharmacoeconomics of cancer therapies: considerations with the introduction of biosimilars. Semin Oncol 2014;41:S13–20. [DOI] [PubMed] [Google Scholar]
- 6. Dörner T, Strand V, Cornes P. et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 2016;75:974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aapro M. Biosimilars in oncology: current and future perspectives. Eur J Oncol Pharm 2014;8:33–6. [Google Scholar]
- 8. European Medicines Agency. European Public Assessment Reports (EPAR): biosimilars 2016. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000408.jsp (9 July 2017, date last accessed).
- 9. US Food and Drug Administration. FDA approves first biosimilar product Zarxio. 3 June 2015. http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm436648.htm (9 July 2017, date last accessed).
- 10. US Food and Drug Administration. FDA approves Inflectra, a biosimilar to Remicade. 5 April 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm (9 July 2017, date last accessed).
- 11. European Medicines Agency. Questions and Answers on Biosimilar Medicines (similar biological medicinal products). 27 September 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2009/12/WC500020062.pdf (9 July 2017, date last accessed).
- 12. Schneider CK. Biosimilars in rheumatology: the wind of change. Ann Rheum Dis 2013;72:315–8. [DOI] [PubMed] [Google Scholar]
- 13. Beck A, Reichert JM.. Approval of the first biosimilar antibodies in Europe: a major landmark for the biopharmaceutical industry. MAbs 2013;5:621–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ebbers HC, Crow SA, Vulto AGSH.. Interchangeability, immunogenicity and biosimilars. Nat Biotechnol 2012;30:1186–90. [DOI] [PubMed] [Google Scholar]
- 15. European Medicines Agency. Guideline on Similar Biological Medicinal Products Containing Biotechnology-derived Proteins as Active Substance: Quality Issues (revision 1). 22 May 2014. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500167838 (9 July 2017, date last accessed).
- 16. Electronic Medicines Compendium. Remsima 100 mg Powder for Concentrate for Solution for Infusion. 27 May 2016. https://www.medicines.org.uk/emc/medicine/29978 (9 July 2017, date last accessed).
- 17. Electronic Medicines Compendium. Inflectra 100 mg Powder for Concentrate for Solution for Infusion 2016. 3 February 2016. https://www.medicines.org.uk/emc/medicine/29980 (9 July 2017, date last accessed).
- 18. European Medicines Agency. Flixabi. 1 April 2016. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004020/human_med_001980.jsp&mid=WC0b01ac058001d124 (9 July 2017, date last accessed).
- 19. Electronic Medicines Compendium. Benepali 50 mg Solution for Injection in Prefilled Syringe and Benepali 50 mg solution for Injection in Prefilled pen 2015. 11 July 2016. https://www.medicines.org.uk/emc/medicine/31511 (9 July 2017, date last accessed).
- 20. Epirus Pharmaceuticals, Inc. Pipeline BOW015 (infliximab). 2015. http://www.epirusbiopharma.com/pipeline/bow015-infliximab.php (9 July 2017, date last accessed).
- 21. Samsung Bioepis. Samsung Bioepis’ RENFLEXIS® Infliximab Biosimilar Receives Regulatory Approval in Korea. 4 December 2015. http://www.businesswire.com/news/home/20151204005228/en/Samsung-Bioepis-RENFLEXIS®-Infliximab-Biosimilar-Receives-Regulatory (9 July 2017, date last accessed).
- 22. European Medicines Agency. CHMP Assessment Report. Flixabi. 1 April 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004020/WC500208358.pdf (9 July 2017, date last accessed).
- 23. Novartis. Sandoz Strengthens its Biosimilars Portfolio with Acquisition of Pfizer’s Biosimilar Infliximab in Europe. 12 February 2016. https://www.novartis.com/news/media-releases/sandoz-strengthens-its-biosimilars-portfolio-acquisition-pfizers-biosimilar (9 July 2017, date last accessed).
- 24. Amgen biosimilars. Our Pipeline. http://www.amgenbiosimilars.com/our-products/our-pipeline/ (9 July 2017, date last accessed).
- 25. Samsung Bioepis. Merck and Samsung Bioepis announce approval of BRENZYS™ (etanercept), a biosimilar of Enbrel in Korea. 8 September 2015. http://www.mercknewsroom.com/news-release/prescription-medicine-news/merck-and-samsung-bioepis-announce-approval-brenzys-etanerce (9 July 2017, date last accessed).
- 26. GaBI Online. Generics and Biosimilars Initiative. Hanwha to transfer biosimilar etanercept technology to Merck. 6 February 2015. http://www.gabionline.net/Biosimilars/News/Hanwha-to-transfer-biosimilar-etanercept-technology-to-Merck (9 July 2017, date last accessed).
- 27. Sandoz. Sandoz: Several Biosimilars in Late-stage Clinical Trials. http://www.sandoz-biosimilars.com/en/clinicaltrials/sandoz-clinical-trials.shtml (9 July 2017 date last accessed).
- 28. Coherus Biosciences. Coherus and Baxalta announce CHS-0214 (investigational etanercept biosimilar) met primary efficacy endpoints in Phase 3 psoriasis clinical study (Rapsody). 9 November 2015. https://globenewswire.com/news-release/2015/11/09/785367/0/en/Coherus-and-Baxalta-Announce-CHS-0214-Investigational-Etanercept-Biosimilar-Met-Primary-Efficacy-Endpoints-in-Phase-3-Psoriasis-Clinical-Study-RaPsODY.html (9 July 2017, date last accessed).
- 29. Zydus. Zydus launches world’s first biosimilar of adalimumab. 9 December 2014. http://www.zydususa.com/Press%20Releases/2014/December%209%20-%20Zydus%20Launches%20World's%20First%20Biosimilar%20of%20Adalimumab.pdf (9 July 2017, date last accessed).
- 30. Amgen. FDA accepts Amgen’s biosimilar biologics license application for ABP 501. 25 January 2016. http://www.prnewswire.com/news-releases/fda-accepts-amgens-biosimilar-biologics-license-application-for-abp-501-300209337.html (9 July 2017, date last accessed).
- 31. Boehringer Ingelheim. Boehringer Ingelheim Announces Completed Enrollment of Phase III Clinical Trial for Biosimilar Candidate to Adalimumab. 10 November 2015. http://www.businesswire.com/news/home/20151110005132/en/Boehringer-Ingelheim-Announces-Completed-Enrollment-Phase-III (9 July 2017, date last accessed).
- 32. Coherus Biosciences. CHS-1420 Adalimumab Biosimilar. 2016. http://www.coherus.com/our-products/CHS-1420 (9 July 2017, date last accessed).
- 33. Baxalta. Baxalta and Momenta announce M923, a proposed HUMIRA (adalimumab) biosimilar, met primary endpoint in pharmacokinetic study. 21 December 2015. https://globenewswire.com/news-release/2015/12/21/797225/0/en/Baxalta-and-Momenta-Announce-M923-a-Proposed-HUMIRA-adalimumab-Biosimilar-Met-Primary-Endpoint-in-Pharmacokinetic-Study.html (9 July 2017, date last accessed).
- 34. Weinblatt M, Baranauskaite A, Niebrzydowski J. et al. A Phase III, randomized, double-blind clinical study comparing SB5, an adalimumab biosimilar, with adalimumab reference product (Humira®) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy (24-week results). Presented at the 2015 ACR/AHRP Annual Meeting, 27 October 2015, San Francisco, USA (Abstract 8L).
- 35. Clinicaltrials.gov. A study of PF-06410293 (adalimumab Pfizer) and adalimumab (Humira) in combination with methotrexate in subjects with active rheumatoid arthritis (REFLECTIONS B53802) 2016;06410293:5–7. https://www.clinicaltrials.gov/ct2/show/NCT02480153?term=PF-06410293&rank=2 (9 July 2017, date last accessed).
- 36. GaBI On-line. Biosimilars of rituximab. 9 September 2016. http://www.gabionline.net/Biosimilars/General/Biosimilars-of-rituximab (9 July 2017, date last accessed)
- 37. Araújo F, Cordeiro I, Teixeira F, Gonçalves J, Fonseca J.. Pharmacology of biosimilar candidate drugs in rheumatology: a literature review. Acta Reumatol Port 2014;39:19–26. [PubMed] [Google Scholar]
- 38. Lee YJ, Shin D, Kim Y. et al. A randomized phase l pharmacokinetic study comparing SB4 and etanercept reference product (Enbrel®) in healthy subjects. Br J Clin Pharmacol 2016;82:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shin D, Kim Y, Kim YS, Körnicke T, Fuhr R.. A randomized Phase I pharmacokinetic study comparing SB2 and infliximab reference product (Remicade®) in healthy subjects. BioDrugs 2015;29:381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Emery P, Vencovský J, Sylwestrzak A. et al. A Phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoo DH, Hrycaj P, Miranda P. et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013;72:1613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Park W, Yoo DH, Jaworski J. et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther 2016;18:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choe J-Y, Prodanovic N, Niebrzydowski J. et al. A randomised, double-blind, Phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choe J-Y, Prodanovic N, Niebrzydowski J. et al. A randomized, double-blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product (Remicade®) in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy: 54-week results. Am Coll Rheumatol 2015;67:2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Emery P, Vencovský J, Sylwestrzak A. et al. Long term safety and efficacy of SB4 (etanercept biosimilar) in patients with rheumatoid arthritis: comparison between continuing SN4 and switching from etanercept reference product to SB4. Ann Rheum Dis 2016;75:236.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Business Finance News. Biosimilars threaten AbbVie Inc Humira as it approaches patent expiry. 9 February 2016. http://www.businessfinancenews.com/27672-biosimilars-threaten-abbvie-inc-humira-as-it-approaches-patent-expiration/ (9 July 2017, date last accessed).
- 47. Beck A. Biosimilar, biobetter and next generation therapeutic antibodies. MAbs 2011;3:107–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V.. The state-of-play and future of antibody therapeutics. Adv Drug Deliv Rev 2016. Dec 2 [Epub ahead of print]. doi: 10.1016/j.addr.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 49. Nickisch K, Bode-Greuel KM.. Biosimilars or biobetters: make your decisions wisely. RA J App Res 2016;2:530–5. [Google Scholar]
- 50. Correia IR. Stability of IgG isotypes in serum. MAbs 2010;2:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calvo B, Zuñiga L.. EU’s new pharmacovigilance legislation: considerations for biosimilars. Drug Saf 2014;37:9–18. [DOI] [PubMed] [Google Scholar]
- 52. Calvo B. Author’s reply to Borg et al. Comment on: “EU’s new pharmacovigilance legislation: Considerations for biosimilars”. Drug Saf 2014;37:125–6. [DOI] [PubMed] [Google Scholar]
- 53. Lambert J, Wyand M, Lassen C. et al. Bioavailability, safety and immunogenicity of biosimilar infliximab (BOW015) compared to reference infliximab. Int J Clin Pharmacol Ther 2016;54:315–22. [DOI] [PubMed] [Google Scholar]
- 54. Moots RJ, Balsa A, Wolbink G.. Reporting of potential immunogenicity with biologic drugs: clarity and accuracy required. Ann Rheum Dis 2016;75:e24.. [DOI] [PubMed] [Google Scholar]
- 55. Emery P, Vencovský J, Ghil J. et al. Response to: “Reporting of potential immunogenicity with biologic drugs: clarity and accuracy required” by Moots. Ann Rheum Dis 2016;75:e25. [DOI] [PubMed] [Google Scholar]
- 56. Chamberlain P. Assessing immunogenicity of biosimilar therapeutic monoclonal antibodies: regulatory and bioanalytical considerations. Bioanalysis 2013;5:561–74. [DOI] [PubMed] [Google Scholar]
- 57. Liu PM, Zou L, Sadhu C, Shen WD, Nock S.. Comparative immunogenicity assessment: a critical consideration for biosimilar development. Bioanalysis 2015;7:373–8. [DOI] [PubMed] [Google Scholar]
- 58. European Medicines Agency. Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. 24 May 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500128688.pdf (9 July 2017, date last accessed).
- 59. Vezér B, Buzás Z, Sebeszta M, Zrubka Z.. Authorized manufacturing changes for therapeutic monoclonal antibodies (mAbs) in European Public Assessment Report (EPAR) documents. Curr Med Res Opin 2016;32:829–34. [DOI] [PubMed] [Google Scholar]
- 60. European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module V – Risk management systems (Rev 2). 24 February 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2016/02/WC500202424.pdf (9 July 2017, date last accessed).
- 61. European Medicines Agency. Summary of the risk management plan (RMP) for Benepali (etanercept). December 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Risk-management-plan_summary/human/004007/WC500196679.pdf (9 July 2017, date last accessed).
- 62. European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Assessment report. REMSIMA. 27 June 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf (9 July 2017, date last accessed).
- 63. Isaacs JD, Cutolo M, Keystone EC, Park W, Braun J.. Biosimilars in immune-mediated inflammatory diseases: initial lessons from the first approved biosimilar anti-tumour necrosis factor monoclonal antibody. J Intern Med 2016;279:41–59. [DOI] [PubMed] [Google Scholar]
- 64. European Medicines Agency. Summary of opinion (initial authorization). Benepali. 19 November 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/004007/WC500196736.pdf (9 July 2017, date last accessed).
- 65. World Health Organization (WHO). WHO informal consultation on international nonproprietary names (INN) – policy for biosimilar products. 4–5th September 2006. http://www.who.int/medicines/services/inn/BiosimilarsINN_Report.pdf (9 July 2017, date last accessed).
- 66. FDA. Nonproprietary naming of biological products. Guidance for Industry. August 2015. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm459987.pdf (9 July 2017, date last accessed).
- 67. Vermeer NS, Spierings I, Mantel-Teeuwisse AK. et al. Traceability of biologicals: present challenges in pharmacovigilance. Expert Opin Drug Saf 2015;14:63–72. [DOI] [PubMed] [Google Scholar]
- 68. Ebers HC, Chamberlain P.. Interchangeability. An insurmountable fifth hurdle? GaBI J 2014;3:88–93. [Google Scholar]
- 69. Medicines for Europe Biosimilars Handbook. 2016. http://www.medicinesforeurope.com/wp-content/uploads/2016/03/biosimilars (9 July 2017, date last accessed).
- 70. Yoo DH, Prodanovic N, Jaworski J. et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 2017;76:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Park W, Yoo DH, Miranda P. et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis 2017;76:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nikiphorou E, Kautiainen H, Hannonen P. et al. Clinical effectiveness of CT-P13 (infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther 2015;15:1677–83. [DOI] [PubMed] [Google Scholar]
- 73. Bettey M, Downey L, Underhill C. et al. Outcomes of a managed switching programme changing IBD patients established on originator infliximab to biosimilar infliximab. J Crohns Colitis 2016;10:S43–4. [Google Scholar]
- 74. Kolar M, Duricova D, Brotlik M. et al. Switching of patients with inflammatory bowel disease from original infliximab (Remicade®) to biosimilar infliximab (Remsima™) is effective and safe. J Crohns Colitis 2016;10:S45–6. [Google Scholar]
- 75. Smits L, Derikx L, Drenth J. et al. Elective switching from Remicade® to biosimilar CT-P13 in inflammatory bowel disease patients: a prospective observational cohort study. J Crohns Colitis 2016;10:1287–93. [DOI] [PubMed] [Google Scholar]
- 76. Glintborg B, Sørensen IJ, Loft AG. et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017;76:1426–31. [DOI] [PubMed] [Google Scholar]
- 77. Tweehuysen L, van den Bemt BJF, van Ingen IL. et al. Clinical and Immunogenicity Outcomes after Switching Treatment from Innovator Infliximab to Biosimilar Infliximab in Rheumatic Diseases in Daily Clinical Practice. Arthritis Rheumatol 2016;68 (Suppl 10):822. [Google Scholar]
- 78. Clinicaltrials.gov. The NOR-SWITCH Study. https://www.clinicaltrials.gov/ct2/show/NCT02148640?term=nor-switch&rank=1 (9 July 2017, date last accessed).
- 79. Jørgensen KK, Olsen IC, Gol GL. et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017;389 (10086): 2304–2316. [DOI] [PubMed] [Google Scholar]
- 80. European Medicines Agency. Guideline on similar biological medicinal products. 23 October 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf (9 July 2017, date last accessed).
- 81. NHS England. What is a Biosimilar? https://www.england.nhs.uk/wp-content/uploads/2015/09/biosimilar-guide.pdf (9 July 2017, date last accessed).
- 82. Mack A. Norway, biosimilars in different funding systems. What works? GaBI J 2015;4:90–2. [Google Scholar]
- 83. Uhlig T, Moe RH, Kvien TK.. The burden of disease in rheumatoid arthritis. Pharmacoeconomics 2014;32:841–51. [DOI] [PubMed] [Google Scholar]
- 84. Feagan BG, Choquette D, Ghosh S. et al. The challenge of indication extrapolation for infliximab biosimilars. Biologicals 2014;42:177–83. [DOI] [PubMed] [Google Scholar]
- 85. Weise M, Kurki P, Wolff-Holz E, Bielsky MC, Schneider CK.. Biosimilars: the science of extrapolation. Blood 2014;124:3191–6. [DOI] [PubMed] [Google Scholar]
- 86. Health Canada. Guidance for sponsors: Information and Submission Requirements for Subsequent Entry Biologics (SEBs). 2010. https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/informationsubmission-requirements-biosimilar-biologic-drugs.html (9 July 2017, date last accessed).
- 87. Swissmedic. Questions and answers regarding the application of the administrative ordinance. Similar Biological Medicinal products (Biosimilars). https://www.swissmedic.ch/ueber/00134/00519/index.html?lang=en (9 July 2017, date last accessed).
- 88. Ben-Horin S, Casteele NV, Schreiber S, Lakatos P.. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol 2016;14:1685–96. [DOI] [PubMed] [Google Scholar]
- 89. Danese S, Fiorino G, Michetti P.. Viewpoint: knowledge and viewpoints on biosimilar monoclonal antibodies among members of the European Crohn’s and Colitis Organization. J Crohns Colitis 2014;8:1548–50. [DOI] [PubMed] [Google Scholar]
- 90. Dobrow L. What do physicians think about biosimilars. Medical Marketing and Media 2016; http://www.mmm-online.com/commercial/what-do-physicians-think-about-biosimilars/article/498542/ (9 July 2017, date last accessed). [Google Scholar]
- 91. Jacobs I, Singh E, Sewell KL. et al. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Pref Adher 2016;10:937–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Skingle D. Biosimilars: what do patients need to consider? RMD Open 2015;1:e000141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Putrik P, Ramiro S, Keszei A. et al. Lower education and living in countries with lower wealth are associated with higher disease activity in rheumatoid arthritis: results from the multinational COMORA study. Ann Rheum Dis 2015;75:540–6. [DOI] [PubMed] [Google Scholar]
- 94. Breedveld FC, Kalden JR.. Appropriate and effective management of rheumatoid arthritis. Ann Rheum Dis 2004;63:627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sokka T, Kautiainen H, Pincus T. et al. Disparities in rheumatoid arthritis disease activity according to gross domestic product in 25 countries in the QUEST-RA database. Ann Rheum Dis 2009;68:1666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brodszky V, Baji P, Balogh O, Péntek M.. Budget impact analysis of biosimilar infliximab (CT-P13) for the treatment of rheumatoid arthritis in six Central and Eastern European countries. Eur J Health Econ 2014;15:S65–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jha A, Upton A, Dunlop WCN, Akehurst R.. The budget impact of biosimilar infliximab (Remsima®) for the treatment of autoimmune diseases in five European countries. Adv Ther 2015;32:742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Möttönen T, Hannonen P, Leirisalo-Repo M.. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet 1999;353:1568–73. [DOI] [PubMed] [Google Scholar]
- 99. Grigor C, Capell H, Stirling A.. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 100. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 101. Haavardsholm EA, Aga AB, Olsen IC. et al. Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ 2016;354:i4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Scott DL, Ibrahim F, Farewell V. et al. Randomised controlled trial of tumour necrosis factor inhibitors against combination intensive therapy with conventional disease-modifying antirheumatic drugs in established rheumatoid arthritis: the TACIT trial and associated systematic reviews. Health Technol Assess 2014;18:i–xxiv. 1–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hazlewood GS, Barnabe C, Tomlinson G.. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016;353:i1777.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Porter D, van Melckebeke J, Dale J. et al. Tumour necrosis factor inhibition versus rituximab for patients with rheumatoid arthritis who require biological treatment (ORBIT): an open-label, randomised controlled, non-inferiority, trial. Lancet 2016;388:239–47. [DOI] [PubMed] [Google Scholar]
- 105. Smolen JS, Nash P, Durez P. et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2014;381:918–29. [DOI] [PubMed] [Google Scholar]
- 106. McCarthy G, Bitoun CE, Guy H.. Introduction of an infliximab biosimilar (CT-P13): a five-year budget impact analysis for the treatment of rheumatoid arthritis in Ireland. Value Health 2013;16:A558. [Google Scholar]
- 107. Gulacsi L, Brodszky V, Baji P, Péntek M.. Biosimilars for the management of rheumatoid arthritis: economic considerations. Expert Rev Clin Immunol 2015;11(Suppl. 1):S43e52.. [DOI] [PubMed] [Google Scholar]
- 108. Rencz F, Péntek M, Bortlik M. et al. Biological therapy in inflammatory bowel diseases: access in Central and Eastern Europe. World J Gastroenterol 2015;21:1728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Baji P, Gulácsi L, Lovász BD. et al. Treatment preferences of originator versus biosimilar drugs in Crohn’s disease; discrete choice experiment among gastroenterologists. Scand J Gastroenterol 2016;51:22–7. [DOI] [PubMed] [Google Scholar]
- 110. Braun J, Kudrin A.. Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals 2016;44:257–66. [DOI] [PubMed] [Google Scholar]
- 111. GaBI Online. Huge Discount on Biosimilar Infliximab in Norway. Mol: Pro Pharma Communications International. 2015. http://www.gabionline.net/Biosimilars/General/Huge-discount-on-biosi milar-infliximab-in-Norway (9 July 2017, date last accessed).