Abstract
BACKGROUND
Endometrial hyperplasia (EH) is a uterine pathology representing a spectrum of morphological endometrial alterations. It is predominantly characterized by an increase in the endometrial gland-to-stroma ratio when compared to normal proliferative endometrium. The clinical significance of EH lies in the associated risk of progression to endometrioid endometrial cancer (EC) and ‘atypical’ forms of EH are regarded as premalignant lesions. Traditional histopathological classification systems for EH exhibit wide and varying degrees of diagnostic reproducibility and, as a consequence, standardized patient management can be challenging.
OBJECTIVE AND RATIONALE
EC is the most common gynaecological malignancy in developed countries. The incidence of EC is rising, with alarming increases described in the 40–44-year-old age group. This review appraises the current EH classification systems used to stratify women at risk of malignant progression to EC. In addition, we summarize the evidence base regarding the use of immunohistochemical biomarkers for EH and discuss an emerging role for genomic analysis.
SEARCH METHODS
PubMed, Medline and the Cochrane Database were searched for original peer-reviewed primary and review articles, from January 2000 to January 2016. The following search terms were used: ‘endometrial hyperplasia’, ‘endometrial intraepithelial neoplasia’, ‘atypical hyperplasia’, ‘complex atypical hyperplasia’, ‘biomarker’, ‘immunohistochemistry’, ‘progression’, ‘genomic’, ‘classification’ and ‘stratification’.
OUTCOMES
Recent changes to EH classification reflect our current understanding of the genesis of endometrioid ECs. The concept of endometrial intraepithelial neoplasia (EIN) as a mutationally activated, monoclonal pre-malignancy represents a fundamental shift from the previously held notion that unopposed oestrogenic stimulation causes ever-increasing hyperplastic proliferation, with accumulating cytological atypia that imperceptibly leads to the development of endometrioid EC. Our review highlights several key biomarker candidates that have been described as both diagnostic tools for EH and markers of progression to EC. We propose that, moving forwards, a ‘panel’ approach of combinations of the immunohistochemical biomarkers described in this review may be more informative since no single candidate can currently fill the entire role.
WIDER IMPLICATIONS
EC has historically been considered a predominantly postmenopausal disease. Owing in part to the current unprecedented rates of obesity, we are starting to see signs of a shift towards a rising incidence of EC amongst pre- and peri-menopausal woman. This creates unique challenges both diagnostically and therapeutically. Furthering our understanding of the premalignant stages of EC development will allow us to pursue earlier diagnosis and facilitate appropriate stratification of women at risk of developing EC, permitting timely and appropriate therapeutic interventions.
Keywords: biomarkers, endometrioid endometrial cancer, endometrial hyperplasia, endometrial intraepithelial neoplasia, progression, genomic classification, immunohistochemistry, patient stratification, personalized medicine
Introduction
Endometrial cancer (EC) is the most common gynaecological malignancy affecting women in developed countries and the second most common gynaecological malignancy world-wide, due to the higher rates of cervical cancer in the developing world (Ferlay et al., 2015). The incidence of EC is steadily increasing, largely owing to an ageing population and escalating rates of obesity (Renehan et al., 2010; Ferlay et al., 2015; Wise et al., 2016). In spite of the frequency of this disease, awareness amongst the general population is low and EC research is somewhat underfunded relative to its societal burden (Carter and Nguyen, 2012). If diagnosed and treated at Stage I or II (International Federation of Gynaecology and Obstetrics [FIGO] stages), EC 5-year survival figures stand at ~92% and 75%, respectively (Creasman et al., 2006; Murali et al., 2014). Women diagnosed with advanced EC, FIGO stages III and IV, have 5-year survival figures reported at 57–66% and 20–26%, respectively (Murali et al., 2014).
ECs have classically been described via a dualistic model which divides them into ‘Type 1’ and ‘Type 2’ carcinomas, based upon histological, clinical and metabolic features (Bokhman, 1983). The Type 1 carcinomas, of which the endometrioid histological subtype accounts for ~75%, typically represent low-grade tumours which are often amenable to surgical treatment (Silverberg et al., 2003; Murali et al., 2014). Type 1 ECs are considered oestrogen-dependent and are frequently associated with hyperplastic proliferation of the endometrial glands; they are characteristically seen in postmenopausal obese women. In our own studies, we have demonstrated differential expression of oestrogen receptors alpha and beta in Type 1 ECs according to tumour grade (Collins et al., 2009).
Conversely, Type 2 ECs tend to be oestrogen-independent and include the clinically aggressive ‘serous’ and ‘clear cell’ histological subtypes. Type 2 ECs are more often associated with endometrial atrophy in the postmenopausal woman rather than with endometrial hyperplasia (EH) as in Type 1 EC. They are linked with a much poorer clinical prognosis (Creasman et al., 2006; Abu-Rustum et al., 2010; Matias-Guiu and Prat, 2013). Despite the seemingly intuitive division of ECs into these two types, this classification is far from perfect. There is a significant overlap between Type 1 and Type 2 ECs. For example, 10–19% of endometrioid ECs are deemed high-grade and have clinical, histopathological and molecular features that are more akin to Type 2 ECs (Voss et al., 2012; Brinton et al., 2013). Mixed histological patterns incorporating endometrioid and serous morphology also exist (Mackenzie et al., 2015).
Large-scale, next-generation sequencing projects and advanced molecular pathology methodologies are spearheading an expansion of personalized medicine within cancer care. Notably ‘pre-cancer’ detection and patient risk stratification are increasingly important for early diagnosis and prevention of cancer (Berman et al., 2006). There are several lines of evidence that a diagnosis of EH may precede the development of endometrioid EC and that the two share common predisposing risk factors (Table I). The incidence of EH is roughly three times higher than EC and certain atypical forms of EH are considered to represent direct precursor lesions to endometrioid EC (Reed et al., 2009; Ellenson et al., 2011). In the current literature, two main classification systems have been used to subdivide EH. The more recent of the two places greater emphasis on robust diagnostic reproducibility and develops the entity endometrial intraepithelial neoplasia (EIN) as a premalignant lesion with significant oncogenic potential (Mutter, 2000a).
Table I.
Risk factors for the development of endometrial hyperplasia (EH).
| Risk factor category | Risk factor |
|---|---|
| Non-modifiable | Age >35 years Caucasian ethnicity Family history |
| Menstrual | Postmenopausal status Early menarche/late menopause Prolonged perimenopause Null parity |
| Co-morbid conditions | Obesity Diabetes mellitus Polycystic ovarian syndrome (PCOS) Functional tumours, e.g. granulosa cell Lynch syndrome/hereditary non-polyposiscolorectal cancer (HNPCC) |
| Iatrogenic | Long-term Tamoxifen therapy Oestrogen only hormone replacement therapy(HRT) Exogenous oestrogen exposure |
| Others | Smoking Genetic mutations |
As we will explore in this review, classifying EHs can be troublesome, largely due to the heterogeneity of EH lesions. This makes the task of stratifying women at risk of progression to endometrioid EC all the more challenging. We aim to appraise the current EH classification systems used for patient risk stratification. In addition, we summarize the evidence base regarding the use of immunohistochemical biomarkers for EH and discuss the future potential of genomic analysis.
Methods
PubMed, Ovid® Medline and the Cochrane Collaborative database were searched for high-quality, peer-reviewed primary papers and review articles, from January 2000 to January 2016. Using Boolean operators, several search variants using the following keyword terms were combined: ‘endometrial hyperplasia’, ‘endometrial intraepithelial neoplasia’, ‘atypical hyperplasia’, ‘complex atypical hyperplasia’, ‘biomarker’, ‘immunohistochemistry’, ‘progression’, ‘genomic’, ‘classification’ and ‘stratification’. We reviewed all identified manuscripts and included them, where appropriate, in the scope of this review. The reference lists of included manuscripts were also searched for any older, relevant sources and included where appropriate. In addition, a hand-search identified several pertinent websites, guidelines and policy documents that were included. Non-English language texts were excluded.
Endometrial hyperplasia
EH represents a spectrum of irregular morphological alterations, whereby abnormal proliferation of the endometrial glands results in an increase in gland-to-stroma ratio when compared to endometrium from the proliferative phase of the cycle (Ellenson et al., 2011; Kurman et al., 2014). The proliferating glands in EH can vary greatly in size and shape, and cytological atypia may be present (Fig. 1). Historically, several different terms have been employed to describe this abnormal proliferation of the endometrium, including: ‘adenomatous hyperplasia’, ‘atypical hyperplasia’ and ‘carcinoma-in-situ’ (Giuntoli et al., 2014). In developed countries, there are an estimated 200 000 new cases of EH per annum (Ozdegirmenci et al., 2011). However, this is likely an underestimation since epidemiological registry data on EH patients can differ significantly between institutions.
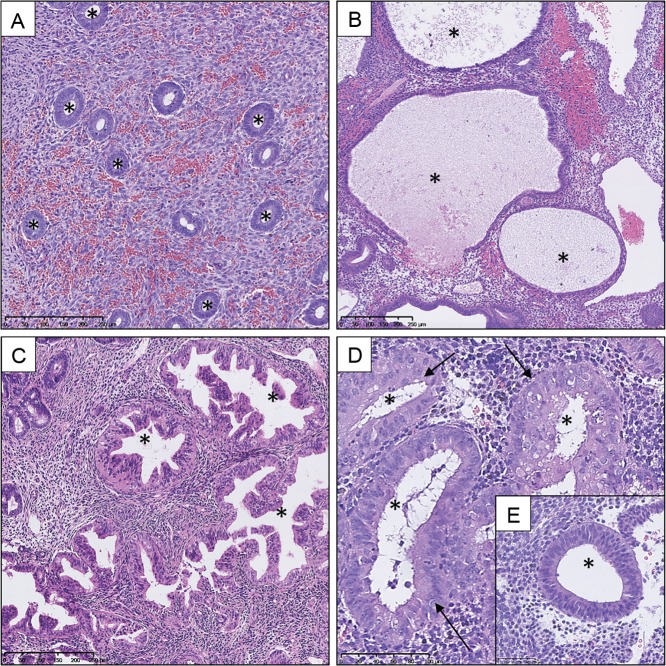
Figure 1.
Haematoxylin and eosin (H&E) stained sections demonstrating variation in size and shape of endometrial glands within a spectrum of endometrial hyperplasia (EH) lesions compared to proliferative endometrium (PE). Selection of glands marked by * in lumen. (A) PE, (B) hyperplasia without atypia: large cystically dilated glands, (C) endometrial intraepithelial neoplasia (EIN): varied and irregular gland morphology, (D) high-power EIN lesion: cytological atypia within glands (arrow) and (E) excerpt of a phenotypically ‘normal’ gland cytology within the same section as D for comparison. Varying magnifications: see scale bars.
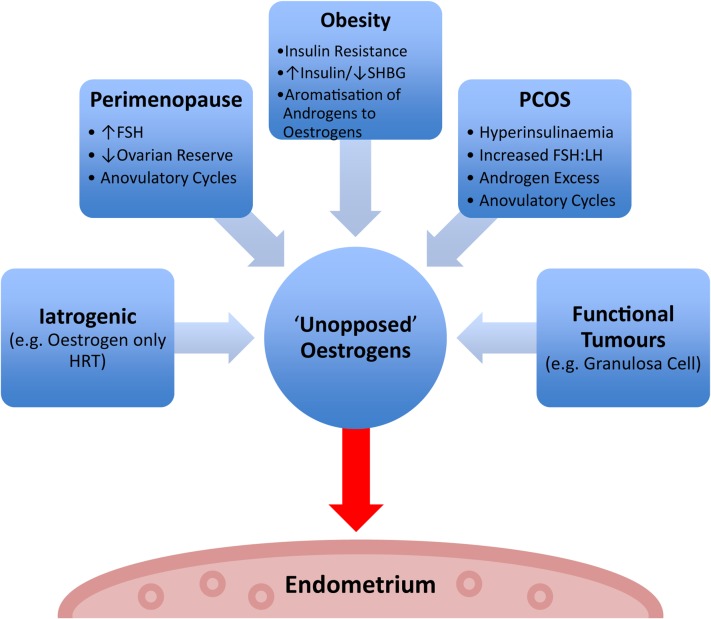
It is presumed that most EHs develop in a background of chronic stimulation of the endometrium by oestrogens unopposed by a progestin, occurring secondary to a number of possible conditions (Trimble et al., 2012) (Fig. 2). The majority of women with EH will present clinically with abnormal uterine bleeding (AUB) and EHs have previously been estimated to account for 15% of all cases of postmenopausal bleeding (Lidor et al., 1986). The main risk factors for the development of EH (Table I) are similar to those associated with EC. Two particularly high-risk patient populations are (i) obese peri/postmenopausal women, owing in part to peripheral aromatization of androgens to oestrogens in adipose tissue, coupled with erratic anovulatory cycles and (ii) premenopausal patients with polycystic ovarian syndrome (PCOS), due to hyperandrogenic anovulation. Although stimulation of the endometrium by oestrogens is considered the main risk factor for developing EH, other causes such as immunosuppression have been suggested (Bobrowska et al., 2006). A retrospective study of 45 immunosuppressed renal transplant recipients with AUB found a ∼2-fold increase in the incidence of EH (69% versus 34%) compared to non-transplanted immunocompetent controls with AUB (Bobrowska et al., 2006).
Figure 2.
Factors contributing to ‘unopposed’ oestrogen stimulation of the endometrium. SHBG = sex hormone binding globulin, FSH = follicle stimulating hormone, FSH:LH = follicle stimulating hormone to luteinizing hormone ratio, HRT = hormone replacement therapy, PCOS = polycystic ovarian syndrome.
The clinical importance of a diagnosis of EH relates to the long-term risk of progression to endometrioid EC and it is generally accepted that cytological atypia is the principal histological characteristic when assessing EHs for malignant potential (Ellenson et al., 2011). However, not all EHs will progress to malignancy; some EHs occur secondary to oestrogenic proliferation without an underlying malignant mechanism. These patients may be asymptomatic and in some cases the EH may regress without ever being detected.
Analysis and classification of EH is not without challenge. Firstly, the endometrium is a dynamic, multicellular tissue structure that undergoes hormonally driven cyclical proliferation, shedding and rapid healing. In premenopausal women, this renders a consistently ‘normal’ or ‘control’ state difficult to establish (Ellenson et al., 2011). This is especially challenging in peri-menopausal women who will often have erratic menstrual cycles. Secondly, EHs can be very heterogeneous and may present as focal or diffuse lesions, often with multifaceted architectural and cytological features. An EH lesion may be shed with menses, may be entirely removed or under-sampled with a diagnostic biopsy, or may regress with progestin treatment or even spontaneously without intervention (Allison et al., 2008; Trimble et al., 2012). As such, classifying EHs into clinically meaningful groups that permit correlation with potential for malignant transformation can be fraught with difficulty.
Several histological classification methods have been proposed aiming to correlate EH architecture and cytological features with the risk of progression to endometrioid EC (Chandra et al., 2016). The two prominent classification systems are (i) The World Health Organisation (WHO) system, established in 1994 with revision in 2003, which is widely known within current clinical gynaecological practice and (ii) The endometrial intraepithelial neoplasia (EIN) system, introduced in 2000 (Mutter, 2000a) and was endorsed in 2014 by the WHO as part of their most recent classification of tumours of the female reproductive organs (Kurman et al., 2014).
The World Health Organization (WHO) 1994 classification system
Multiple pathological classification systems, stretching back to 1963, have been used to describe EH (reviewed in Chandra et al., 2016). Each system struggles in some part to describe the spectrum of heterogeneity observed between individual EH lesions and correlate this with clinical management.
In 1994, the WHO recommended a classification system based upon the histological features of EH lesions, in an attempt to stratify EHs based on their potential for malignant transformation (Scully et al., 1994). The system focused on the glandular/stromal architectural pattern of the endometrium and the presence or absence of cytological atypia. Four groups emerged and are detailed in Table II, although the simple atypical hyperplasia group is very rarely seen and some would question its reproducibility and clinical relevance as a category (Kendall et al., 1998; Bergeron et al., 1999).
Table II.
The World Health Organisation 1994 classification of EH (Palmer et al. 2008; Ellenson et al. 2011; Chandra et al., 2016).
| WHO94 categories | Histological and cytological features |
|---|---|
| Simple hyperplasiawithout atypia (SH) |
|
| Simple atypical hyperplasia(SAH) |
|
| Complex hyperplasiawithout atypia (CH) |
|
| Complex atypicalhyperplasia (CAH) |
|
Nuclear atypia = enlarged and rounded nuclei, irregular, clumped chromatin, thickened nuclear membrane and prominent nucleoli.
These four groups appeared to correlate with long-term follow-up studies of patients diagnosed with EH that had been conducted previously (Kurman et al., 1985; Ferenczy and Gelfand, 1989). Arguably, the most influential EH follow-up study was reported in 1985 by Kurman et al. (1985). In this study, the authors performed a retrospective analysis of 170 ‘untreated’ EH patients who had been diagnosed with EH on uterine curettage. The mean follow-up period for the women was 13.4 years, during which time a hysterectomy was not performed <1 year following the index diagnosis. Of the 170 women in the study, 13 progressed to EC during the follow-up period (Kurman et al., 1985). The authors published EC progression rates of 1% (simple hyperplasia, SH, without atypia), 3% (complex hyperplasia, CH, without atypia), 8% (simple atypical hyperplasia, SAH) and 29% (complex atypical hyperplasia, CAH), respectively, for the four categories (Kurman et al., 1985). However, the differences in progression between the four categories were not statistically significant and given the small number of cancer patients and a lack of controls, there is difficulty extrapolating a true rate of progression (Lacey et al., 2008a; Ellenson et al., 2011).
Lacey et al. conducted a nested case–control study in 2007. The authors analysed 138 cases of EH (and 241 matched controls) who progressed to EC at least 1 year following an index EH diagnosis. They demonstrated a 40% probability of developing EC following a diagnosis of atypical hyperplasia (incorporating both simple and complex variants), compared to a 10% probability when atypia was not present (Lacey et al., 2008a). Lacey et al. (2008a) commented on the need to increase sensitivity and specificity when diagnosing atypical hyperplasia and to find methods of identifying the rare non-atypical EH lesions that are also likely to progress to EC.
When first introduced, the WHO94 system was considered an improved approach to EH classification since it correlated the histological features of EH lesions with clinical outcome data (Baak and Mutter, 2005). However, the subjective nature of this system has meant that significant diagnostic variation occurs between pathologists and overall reproducibility is poor (Skov et al., 1997; Kendall et al., 1998). Cytological atypia is not always uniformly seen in individual EH samples and the use of subjective atypia grading scales, i.e. mild, moderate and severe by individuals has been problematic, especially when translating the scheme to clinical management (Kendall et al., 1998). These issues were highlighted by Trimble et al. in a study in which 289 endometrial specimens with a community diagnosis of atypical EH were re-reviewed by specialized gynaecological pathologists using WHO94 criteria; 25% of cases were downgraded to a less severe histology than atypical EH and 29% were upgraded to EC (Trimble et al., 2006).
Another difficulty identified within the WHO94 system is the relationship between the diagnostic groups and clinical treatments. The four-tier WHO94 system does not straightforwardly correspond to the separate therapeutic options available (i.e. surgical, medical or observational) (Baak and Mutter, 2005), which may contribute to a tendency for surgical overtreatment due to the fear of malignant progression for lesions with no underlying sinister mechanism (Baak et al., 2001).
Clinical treatment based upon WHO94 classification of EH varies between institutions, with patient-specific factors, i.e. age, co-morbid status and future fertility wishes, influencing decision-making. EHs without nuclear atypia have been documented to regress back to normal endometrium in around 90% of cases, with no progression to malignancy but a recurrence rate of ~10% (Hannemann et al., 2010). When atypia is seen, definitive surgical treatment in the form of a total hysterectomy is normally offered, since the risk of progression to endometrioid EC is so much higher. Furthermore, there is the risk of a concurrent EC already being present in the uterus that may have gone undetected on biopsy.
The endometrial intraepithelial neoplasia (EIN) 2000 classification system
As reviewed above, it is well established that nuclear atypia within hyperplastic lesions confers the highest risk of progression to endometrioid EC (Sherman and Brown, 1979; Kurman et al., 1985). Research in the 1980s, spearheaded by Jan Baak, developed a prognostic tool designed to predict EC risk based upon morphometric analysis of the nuclear features within EH lesions (Ausems et al., 1985). It was subsequently found that, through a combined analysis of nuclear and architectural features, the prognostic value of morphometric analysis could be increased (Baak et al., 1988). This work culminated in the development of a weighted likelihood ratio called the ‘D-score’. The D-score centres on three key EH features: (i) volume percentage of stroma, (ii) outer surface density of the glands and (iii) the standard deviation, SD of the shortest nuclear axis within glandular cells (Baak et al., 1988; Baak and Mutter, 2005). The following equation is used:
D-score = 0.6229 + 0.0439 × (volume percentage stroma) − 3.9934 × natural logarithm, Ln (SD shortest nuclear axis) − 0.1592 × (glands outer surface density) (Baak et al., 1988).
By applying the D-score, hyperplastic biopsies with a score of ≤1 have a high rate of progression to EC, whereas biopsies with a score of >1 almost never progress to EC (Baak et al., 1992, 2001). In addition, this system has been shown to be highly reproducible (Baak and Mutter, 2005). Advances in molecular genetics, occurring at around a similar time as the progress being made with morphometric analysis, recognized a shared monoclonal pattern of development between atypical hyperplastic lesions and ECs (Jovanovic et al., 1996). These findings would be consistent with mutated cells stemming from a common progenitor, proliferating more rapidly than their neighbours and resulting in clonal expansions of aberrant cells detected as lesions (Jovanovic et al., 1996).
A multicentre European study was conducted in 1999 by Bergeron et al. to investigate and assess both intra- and inter-observer variability in the diagnosis of 56 endometrial samples using the WHO94 classification system (Bergeron et al., 1999). The investigators noted significant disagreement in the diagnoses of CH and atypical hyperplasia between pathologists (Bergeron et al., 1999). They concluded that histological classification should be simplified into two groups; a combined category for SH and CH, referred to as ‘hyperplasia’, and a combined category for atypical hyperplasia and well-differentiated adenocarcinoma, called ‘endometrial neoplasia’. The rationale for this being that by utilizing two groups, one benign and one neoplastic, reproducibility would be increased and a two-tier system would align easily with therapeutic interventions, i.e. medical or surgical (Bergeron et al., 1999).
Acknowledging the deficiencies within the WHO94 system, in 2000 the Endometrial Collaborative Group introduced the notion of EIN, as part of a newer classification system (Mutter, 2000a). The EIN concept incorporated advances in morphometric understanding and recognized the novel molecular research occurring in the field of endometrial precancers at that time (Jovanovic et al., 1996; Mutter et al., 1996; Mutter, 2000a). The EIN classification system divides hyperplastic endometrial lesions into two groups: (i) benign EH and (ii) EIN. This is based on objective diagnostic criteria (Table III) that can be determined from a haematoxylin and eosin (H&E) stained endometrial section. In essence, these criteria emulate what the D-score achieves; however, they can be ascertained quickly by a pathologist using routine light microscopy (Owings and Quick, 2014).
Table III.
Haematoxylin and eosin section diagnostic criteria for endometrial intraepithelial neoplasia (EIN).
| EIN criterion | Comments |
|---|---|
| Architecture | Area of glands exceeds that of stroma (VPS < 55%) |
| Cytology | Cytology differs between architecturally crowdedfocus and background |
| Diameter >1 mm | Maximum linear dimension of the lesion exceeds1 mm |
| Exclude mimics | Benign conditions with overlapping criteria: basalis,secretory, polyps, repair, etc. |
| Exclude cancer | Carcinoma if maze-like meandering glands, solidareas or appreciable cribriforming |
NB: All criteria must be met in order for a diagnosis of EIN to me made.VPS = volume percentage stroma.Reproduced with permission from Baak and Mutter (2005).
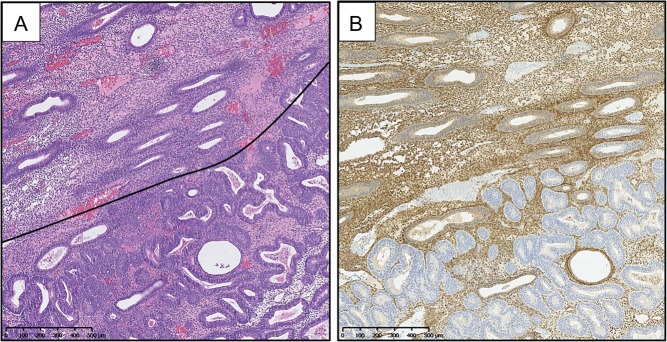
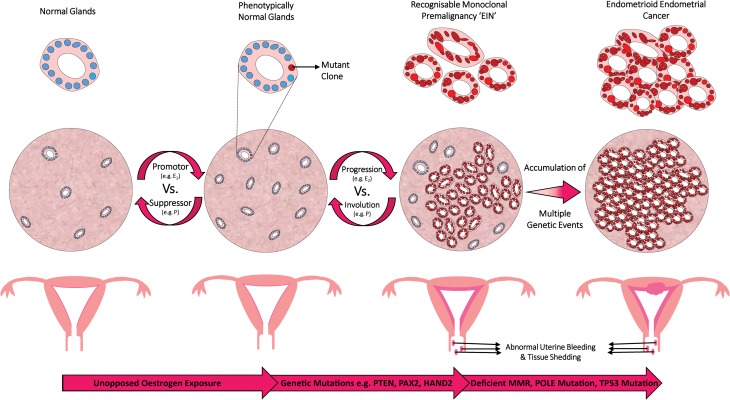
EIN lesions are defined as monoclonal proliferations of architecturally and cytologically altered premalignant endometrial glands, which are prone to transformation to endometrioid EC (Mutter, 2000a) (Fig. 3). Prior to the inception of EIN, a general belief was held that unopposed oestrogenic stimulation caused ever-increasing endometrial proliferation, with accumulating cytological atypia that imperceptibly led to the development of endometrioid EC. The EIN mechanism proposes that genetic alterations within the endometrium initially occur at a level undetectable by light microscopy. These ‘latent’ genetically transformed cells can be present for numerous years within cycling endometrium (Mutter et al., 2007). Through the ongoing accrual of genetic damage, higher risk mutated clones assert themselves phenotypically, exhibiting architectural and cytological characteristics that are indicative of EIN (Table III). The mutant clones are subject to endocrine modifiers, with oestrogens acting as promoters and progestins (natural or synthetic) acting as suppressors. Variations in the balance of endocrine modifiers can alter the balance of progression to EC versus hyperplastic lesion involution (Mutter et al., 2007). In contrast, benign EH lesions are deemed diffuse and polyclonal, occurring globally due to an unopposed oestrogenic stimulus (Mutter, 2000a; Baak and Mutter, 2005). Crucially, these lesions do not exhibit cytological differences between architecturally crowded and uncrowded glandular regions; their appearance at any one time point is entirely dependent on the predisposing hormonal milieu (Mutter et al., 2007).
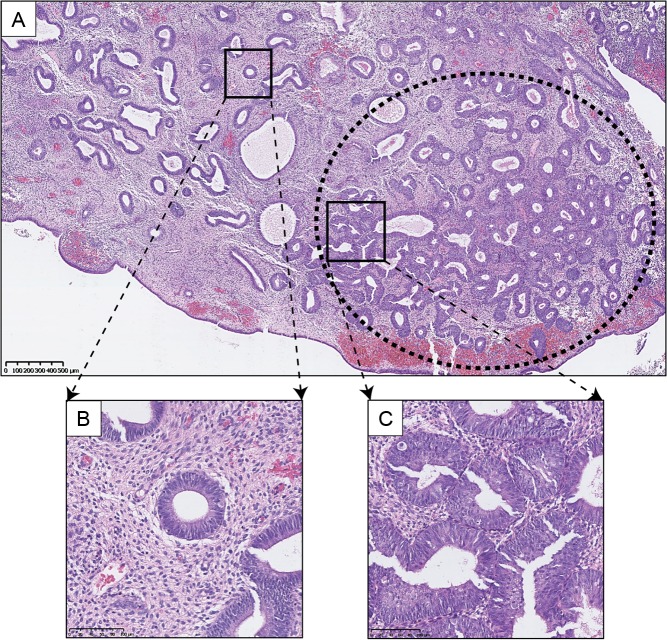
Figure 3.
Clonal expansion of EIN. H&E staining of an endometrial biopsy. (A) Low power view of a clonal expansion of EIN, with prominent gland crowding (marked in oval with bold dashes), in a background endometrium demonstrating benign EH, (B) high-power view of background endometrium, (C) high-power view of EIN lesion glands. Varying magnifications: see scale bars.
The EIN concept recognizes the importance of unopposed oestrogenic stimulation; however, it distinguishes it from the separate event of a mutationally activated clone developing an oestrogenic background (Mutter, 2000b; Mutter et al., 2007; Owings and Quick, 2014). Histologically, this can prove difficult to segregate, as early lesions can have a combination of appearances that may include the clone (EIN) within an oestrogen stimulated tissue background. The idea of separating the two events, mutational activation and oestrogenic promotion, not only permits examination of the two components separately, but gives a more comprehensible model of the multistep carcinogenic process that is similar to that described in many other tissue types (Vineis et al., 2010).
The EIN classification system has been shown to be reproducible between observers and straightforward to establish in standard pathological practice (Kane and Hecht, 2012; Usubutun et al., 2012). Hecht et al. analysed the use of the D-score compared to EIN criteria in their 2005 retrospective study of 97 EH biopsies. They demonstrated that subjective EIN assessment correlates well with objective morphometric analysis. All EHs that progressed to EC occurred in patients whose endometrial biopsies were deemed high risk by both methods, although interestingly 15 samples were given a D-score of ≤1 (i.e. high risk) and yet were subjectively classified as non-EIN (Hecht et al., 2005).
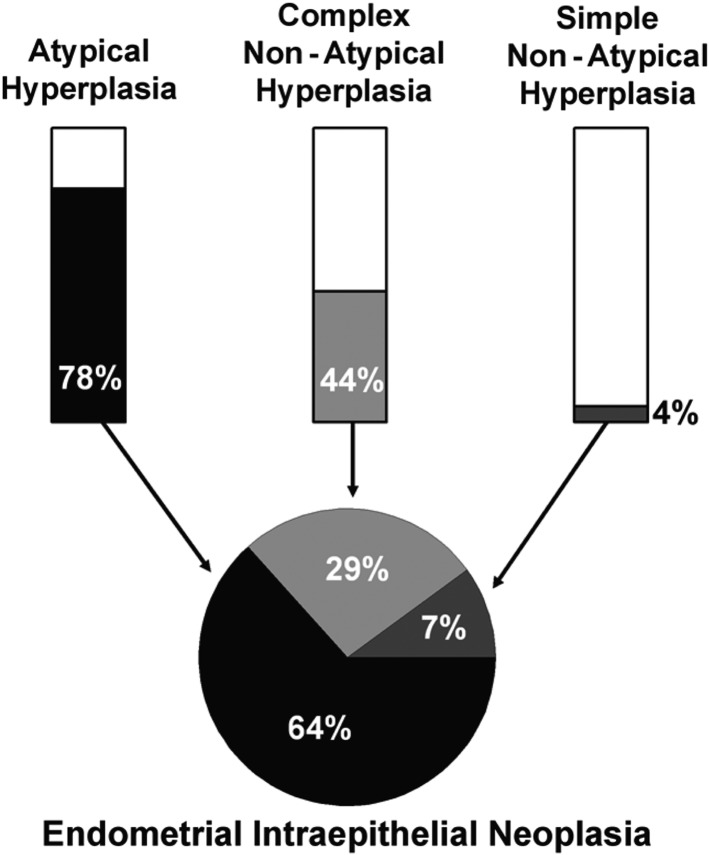
EIN classification categories do not correspond directly to specific categories in the WHO94 system (Mutter, 2000b), although, there is an element of recognizable overlap. Most SH and some CHs will align into the benign EH category and many CHs and most CAHs will align into the EIN category. A useful visual summary was provided by Hecht and colleagues and this is reproduced, with permission, in Fig. 4 (Hecht et al., 2005). Both the EIN and WHO94 systems are governed by different diagnostic elements and so the two systems are not directly comparable (Mutter et al., 2007).
Figure 4.
Correlation of WHO and EIN diagnoses (Hecht et al., 2005). (1) The bar graphs show the approximate percentage of each WHO94 category that would be considered as EIN. Residual WHO94 EHs that are not diagnostic of EIN (i.e. the white areas of the bars) are attributed to unopposed oestrogen (anovulatory cycles), polyps and other causes. (2) The pie chart demonstrates the relative contributions of each hyperplasia subtype to the EIN diagnostic category in a series of 97 cases with 28 EIN examples by Hecht et al. from their 2005 study. Republished with permission from Hecht et al. (2005).
Endorsement of the EIN system: the WHO 2014 classification system
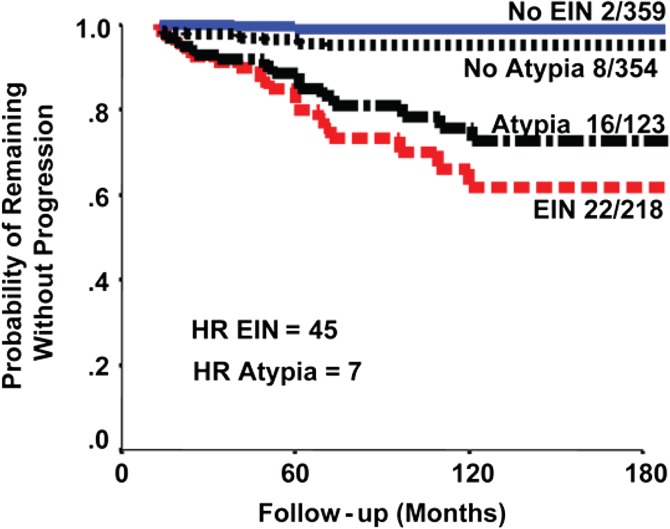
The EIN system has several proposed clinic-pathological advantages over the WHO94 system, most notably diagnostic reproducibility and correlation with clinical management. Clinical outcome data suggest that ~40% of women diagnosed with EIN will have an EC diagnosed within 12 months of index biopsy (Baak et al., 2005a; Mutter et al., 2008). The mostly likely explanation for this is the presence of a concurrent EC that was not sampled on initial biopsy. Those women who do not develop EC within 12 months are 45 times more likely to develop a future EC (Baak et al., 2005a). Baak et al. (2005a) also argued that the EIN classification system more accurately predicts progression to EC than the WHO94 system (Fig. 5, reproduced with permission). A later study reported that both EIN and atypical hyperplasia have similar risks of progression to EC when followed-up for 12 months after the index diagnosis (Lacey et al., 2008b). It is only in the last few years that the EIN classification system has started to gain widespread recognition. This may reflect regional variation in gynaecological and gynae-pathological practices and, until recently, the lack of a standardized approach to the clinical management and surveillance of EH.
Figure 5.
Data to suggest that EIN classification system more accurately predicts progression to EC than the WHO94 system (Baak et al., 2005a). Patients with at least 1-year follow-up. The graph compares the WHO94 ‘Atypia’ and EIN systems in terms of prognostic accuracy. The study reported that the EIN classification is superior to the WHO94 classification for discerning cases at risk of progression to future EC. The fractions are the number that progressed to cancer over the total in that subgroup. HR = hazard ratio. Republished with permission from Baak et al. (2005a).
In 2014, the WHO published its 4th edition of Classification of Tumours of the Female Reproductive Organs (Kurman et al., 2014). The new edition clarifies the WHO position on the classification of EH and it endorses the EIN diagnostic system (Table III). WHO 2014 differentiates EHs into two categories: (i) hyperplasia without atypia and (ii) atypical hyperplasia/EIN (both terms synonymous) (Zaino et al., 2014; Emons et al., 2015). The American College of Obstetricians and Gynecologists (ACOG) released a committee opinion paper detailing EIN in 2015 (Committee on Gynecologic Practice, 2015). It is intended for dissemination to all interested stakeholders regarding the EIN classification method and provides advice on the clinical management of EH and EIN. The ACOG favours the use of EIN terminology over ‘atypical hyperplasia’ providing recognition that these lesions are distinctly neoplastic and harbour significant malignant potential. In the UK, the Royal College of Obstetricians and Gynaecologists (RCOG) released their first guideline for the management of EH in 2016 (Gallos et al., 2016). The RCOG refer to ‘atypical hyperplasia’ as the premalignant lesion, although they acknowledge that the term is interchangeable with EIN (Gallos et al., 2016). The RCOG guidance goes one step further and includes a management algorithm for EH, detailing preferred treatments and advising on timing of endometrial biopsy for patients undergoing conservative or medical management.
The EIN system offers a robust and reproducible classification method that correlates well with the risk of progression of EH to EC. Subjective histopathological assessment based upon the objective EIN criteria can be undertaken on H&E stained sections of tissue and so a potential role for immunohistochemical biomarkers has been established as a way of further stratifying ‘at risk’ EH patients for malignant progression.
Immunohistochemical biomarkers for diagnosis of endometrial hyperplasia and predicting progression of endometrial hyperplasia to endometrial cancer
Biomarkers are defined as ‘characteristics that can be objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention’ (Atkinson et al., 2001). Several immunohistochemical biomarkers have already been investigated for use as diagnostic adjuncts to H&E staining to: (i) aid diagnosis and classification of EHs; and (ii) predict the likelihood of transition from EH to EC. The optimal molecular biomarker would be one that could both reliably and reproducibly distinguish between normal/benign, premalignant and malignant endometrium, in addition to indicating/predicting transition between these three groups. To date, no single candidate has been found to fulfil this role entirely and so the search continues. In the following sections, we review several promising candidates identified from the literature and appraise their role as biomarkers for EH diagnosis and progression to EC.
Regulators of steroid action or inflammation
Oestrogen receptors alpha and beta
Oestrogens bind to one of two nuclear receptors (Oestrogen receptors alpha and beta, ERα and ERβ), which are both encoded by independent genes (reviewed in Gibson and Saunders, 2012). The full-length receptors classically operate as ligand-dependent transcription factors with subsequent modulation of gene expression. Several immunohistochemical studies have reported nuclear expression of both oestrogen-receptor subtypes (ERs) in the glandular and stromal regions of the normal premenopausal and postmenopausal endometrium, with differences in their distribution described between the phases of the normal cycling endometrium (Lessey et al., 1988; Snijders et al., 1992; Fujishita et al., 1997; Critchley et al., 2001). These and other studies have consistently demonstrated the importance of oestrogens in regulating endometrial cell proliferation, angiogenesis and inflammation (Gibson and Saunders, 2012). The causative relationship between excess oestrogen exposure, EH and endometrioid ECs has been unequivocally established and concerns have been expressed about the potential for environmental compounds, classified as endocrine disruptors, to increase the risk of malignant transformation (reviewed in Gibson and Saunders, 2014). Numerous studies have examined ER status of patients diagnosed with malignant endometrial disease, endeavouring to analyse the association between ER status, lesion histology, progression and survival (Geisinger et al., 1986; Sutton et al., 1989; Chamber et al., 1990; Lukes et al., 1994; Sivridis et al., 2001; Ashton et al., 2009; Zannoni et al., 2013). These remain active areas of investigation to date.
Our review identified several studies that have compared ER expression between normal endometrium, EH and EC tissues with conflicting findings reported (Supplementary Table SI). For example, Uchikawa et al. and Bircan et al. both described increased ERα expression within EHs when compared to normal secretory endometrium (Uchikawa et al., 2003; Bircan et al., 2005). In a normal fertile cycle, ERα expression in the epithelial cells is down-regulated in the secretory phase in response to progesterone driven changes in gene expression (Critchley et al., 2001; Gibson and Saunders, 2012), hence sustained expression of ERα may reflect reduced action of progesterone (see below).
Hu et al. assessed 114 patient samples (15 normal, 37 ECs, 30 SHs, 13 CHs and 20 atypical hyperplasias) for both ERα and ERβ expression using fixed tissue sections in 2008 (Hu et al., 2008). They reported a significant increase in the positivity index (% of stained cells) of ERα in samples of SH and CH compared with proliferative endometrium (PE). In contrast, Chakravarty et al. (2008) did not report any difference in the expression levels of either ERα or ERβ between PE and SH in their 2008 study.
Owing to the complex interactions between the cycling endometrium and steroid hormones, it is perhaps not so surprising that there are conflicting literature findings regarding ER expression levels between normal, hyperplastic and malignant endometrial lesions. In Stage I EC, changes in ERα are also reported to be independent of ERβ and loss of expression of ERα is a feature of a more malignant phenotype (Collins et al., 2009). Several authors identified in our review have described that lower ERα expression can be found in atypical EH and ECs, implying that loss of receptor expression may occur as the lesions progress (Nunobiki et al., 2003; Uchikawa et al., 2003; Bircan et al., 2005; Hu et al., 2008). However, the significance levels differ between the studies and variations in methodology and immunohistochemical scoring have to be taken into account, which all limit the potential utility for EH/EIN.
Progesterone receptors
Progesterone is a steroid hormone that is essential to female reproductive function. Primarily produced by the corpus luteum following ovulation, it counteracts the proliferative effects of oestrogen by inducing secretory differentiation of the glandular and stromal compartments of the endometrium and down-regulates ERα (Graham and Clarke, 1997; Deligdisch, 2000). Progesterone asserts its actions via progesterone receptors (PR), which are members of the same superfamily of ligand-activated transcription factors as the oestrogen receptors (Graham and Clarke, 1997). There are two main isoforms of PR, namely PR-A and PR-B, which are both encoded by a single PGR gene (Gadkar-Sable et al., 2005). Extensive studies using in vitro cell systems as well as genomic analyses have identified PR as an oestrogen regulated gene (reviewed in Diep et al., 2016). PR isoforms are spatially and temporally controlled within the endometrial compartments across the menstrual cycle in response to fluctuating concentrations of ovarian steroids (Wang et al., 1998; Mote et al., 1999; Leyendecker et al., 2002).
Chronic exposure to oestrogens unopposed by progesterone is considered a key component in the development of EH and EC (Amant et al., 2005). The role of PR has, therefore, been extensively investigated in EC development and progression, with loss of PR being shown to be associated with poor survival and metastatic disease (Kleine et al., 1990; Kadar et al., 1993; Fukuda et al., 1998; Tangen et al., 2014). Progestin treatment is used as a medical therapy for women diagnosed with EH with reported regression rates of 89–96% (Gallos et al., 2010). For women diagnosed with EIN who wish to preserve their fertility or who are not suitable for surgery, progestins are also recommended as a first line medical therapy (Committee on Gynecologic Practice, 2015; Gallos et al., 2016). In addition, intrauterine progestin (e.g. Levonorgestrel, LNG/Mirena® IUS, Bayer, UK) has also been explored as a hormonal treatment for early stage endometrioid EC with varying success reported (Montz et al., 2002; Ramirez et al., 2004; Dhar et al., 2005). The delivery of progestins is challenging due to the short half-life and doses that are required.
This review captured five studies where PR expression was investigated within EH tissues (Supplementary Table SI). Three authors reported reduced trends of expression of PR within EHs compared to control endometrium (Nunobiki et al., 2003; Uchikawa et al., 2003; Pieczyńska et al., 2011). On the contrary, Ghabreau et al. (2004) demonstrated a progressive increase in PR expression from non-atypical EH to atypical EH. Orejuela et al. reported no significant difference in PR expression between normal endometrium and EHs; they also note a slight reduction in PR expression between ECs compared to EH and normal endometrium groups; however, this did not reach statistical significance (Orejuela et al., 2005).
Given the clinical application of progestins as a medical treatment for EH, it is perhaps a little surprising that studies investigating PR expression in EH tissues do not reach an agreement regarding its utility as either a diagnostic EH tool or marker of progression to EH. However, as is seen with ER expression, the interplay between the endometrium and steroid hormones and their co-receptors make any potential changes in PR expression pattern difficult to interpret. PR expression may be of novel use as a method of predicting response to progestin therapy in the treatment of EH. In 2012, Upson et al. (2012) published data to suggest that PR-B showed promise as a biomarker of progestin response. They performed a nested case–control study of women with CH and atypical hyperplasia who received treatment with oral progestins. Several biomarker candidates were investigated for protein expression and in women with atypical hyperplasia, the authors found higher PR-B expression in those with a 90% decreased risk of lesion persistence/progression (Upson et al., 2012).
Cyclooxygenase-2
Cyclooxygenase-2 (COX-2), also known as Prostaglandin-Endoperoxide Synthase 2 (PTGS2), is an isoform of the cyclooxygenase (COX) enzyme. It is involved in the conversion of arachidonic acid to prostaglandin H2, leading subsequently to the production of prostaglandin E2 (PGE2) (Erkanli et al., 2007; Faloppa et al., 2014). PGE2 has an established role in cell growth and development (Faloppa et al., 2014). In normal endometrial physiology, the expression of COX-2 and the metabolizing enzyme 15-Hydroxyprostaglandin Dehydrogenase (PGDH) are both regulated by progesterone (Baird et al., 1996; Hapangama et al., 2002). Increased COX-2 and PGE2 expression have been demonstrated to play key roles in the development of several malignancies (Williams et al., 1999) including EC (Tong et al., 2000; Jabbour and Boddy, 2003).
From our literature review, six studies investigated COX-2 expression within EH tissues (Supplementary Table SI). Three studies described trends of increasing expression of COX-2 from EH to EC (Orejuela et al., 2005; Erkanli et al., 2007; Nasir et al., 2007). Erkanli et al. (2007) demonstrated statistically significant COX-2 overexpression in EH and EC cases compared to PE. Orejuela et al., in their investigation of 43 retrospective endometrial biopsies, reported that COX-2 expression followed a trend of increased expression in EC and EH compared to normal endometrium. Their results, however, did not reach statistical significance and they recommended further studies utilizing much larger sample sizes. Nasir et al. performed qualitative and semi-quantitative COX-2 immunohistochemical staining scores based on the proportion of immunoreactive cells and the strength of cytoplasmic COX-2 expression (Nasir et al., 2007). These authors found increasing expression of COX-2 from EH to invasive ECs and concluded that COX-2 inhibition may have a potential utility to halt the progression of precursor lesions to invasive ECs (Nasir et al., 2007).
Faloppa et al. (2014) investigated COX-2 and nuclear factor-κB (NF-κB) expression in hyperplastic and malignant samples utilizing EIN diagnostic criteria, finding no significant difference in COX-2 expression between benign hyperplasia and EIN following post hoc analysis (Faloppa et al., 2014). Cao et al. (2002) demonstrated negative COX-2 expression in normal and hyperplastic endometrium.
Steinbakk et al. (2011b) published a research paper with associated review of potential EH biomarkers of progression to EC, in which they concluded that combining the morphometric D-score with negativity for COX-2 strongly predicted progression of EH to EC (Supplementary Table SII). To support this assertion, the authors described 8 out of 13 cases with a D-score < 1 (i.e. high progression risk for EC) and COX-2 negativity that progressed to EC as compared to 3 of 139 of all other cases (P < 0.0001) (Steinbakk, et al., 2011b).
Given the complex interplay between prostaglandins and steroid hormone responsiveness (reviewed in Wallace et al., 2010), it is maybe unsurprising that COX-2 is reported as a potential biomarker of progression of EH to EC. However, it is important to note that its role as a diagnostic biomarker for EH requires further investigation in well-characterized patient samples.
Tumour suppressors
Phosphatase and tensin homologue
Phosphatase and tensin homologue (PTEN) is a tumour suppressor gene, located on chromosome 10q23, that encodes a dual-specificity phosphatase with both protein and lipid actions (Latta and Chapman, 2002). PTEN regulates cellular proliferation and apoptosis, acting as an antagonist to growth factor-induced intracellular signalling pathways (Kimura et al., 2004; Allison et al., 2008). Loss-of-function mutations of the PTEN gene can cause up-regulation of endometrial glandular proliferation via the PI3K/Akt/mTOR pathway and evidence for an association with EH and endometrioid EC has been demonstrated using heterozygous Pten knockout mice (Stambolic et al., 2000; Wang et al., 2002; Daikoku et al., 2008).
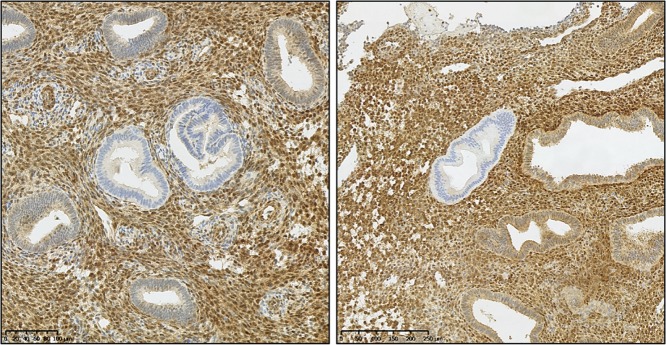
PTEN protein has been evaluated across the normal menstrual cycle with changes in concentrations occurring in response to changes in the hormonal environment across the different phases (Mutter et al., 2000a). PTEN expression is increased in both the glandular epithelium and stromal compartments during the proliferative phase (plausibly influencing proliferation), whilst decreased in the glandular epithelial compartment during the secretory phase (Mutter et al., 2000a). Numerous studies have investigated PTEN expression in EH and EC using immunohistochemical techniques, with mixed reports regarding the pattern of expression seen (an example of loss of PTEN expression in EIN from our own work can be seen in Fig. 6).
Figure 6.
PTEN immunohistochemical staining of an EIN lesion. (A) H&E stained endometrial biopsy section demonstrating a region of EIN (below black line). (B) PTEN immunohistochemical staining of the same tissue section as in A. PTEN-null glands demonstrated by a loss of brown (DAB) cytoplasmic and nuclear staining in the same region corresponding to the EIN lesion as seen in image A. (Mouse monoclonal anti-human PTEN clone 6H2.1, Dako, Ely, UK; Antigen retrieval: decloaking chamber in citrate pH6; Overnight incubation 1:300 at 4°C.) Magnification: see scale bars.
Several authors have demonstrated that immunohistochemical loss of glandular PTEN expression is more marked in endometrioid EC and EIN compared to PE and benign EH (Mutter et al., 2000b; Baak et al., 2005b; Monte et al., 2010; Steinbakk et al., 2011b) (Supplementary Tables SI and SII). Mutter et al. (2000b) determined that PTEN mutations were evident in up to 55% of EIN lesions, suggesting that PTEN inactivation is an early event in EC carcinogenesis. Xiong et al. (2010) suggested that loss of PTEN expression is not a robust diagnostic marker of EIN, since they demonstrated complete PTEN loss occurring in only 38% of EIN lesions. Furthermore, Cirpan et al. (2006) demonstrated no significant difference in ‘complete loss’ of PTEN expression between PE, EIN and endometrioid EC, with some differences shown with ‘incomplete-loss’ of PTEN expression. These two studies raise the question as to what constitutes ‘complete loss’ of PTEN expression within a lesion, a discussion point considered by Allinson et al. in their 2008 review, highlighting that some authors regard PTEN-null as a single negative gland within a lesion whilst others consider only a more extensive loss.
Authors using WHO94 criteria have reported along similar lines to their counterparts using EIN criteria (Erkanli et al., 2006; Kapucuoglu et al., 2007; Sarmadi et al., 2009; Lee et al., 2012). Lee et al. found PTEN expression loss in endometrioid EC and CAH was higher than in SH. Kapucuoglu et al. echoed this finding; however, they noted no significant PTEN expression differences between CAH and EC, nor between individual EH groups (Kapucuoglu et al., 2007). One study, examining a small group of samples (n = 11), showed no significant difference between normal endometrium and EH (Kimura et al., 2004). However, this study analysed PTEN nuclear staining via a nuclear stain scoring system rather than reporting PTEN loss of expression (Kimura et al., 2004).
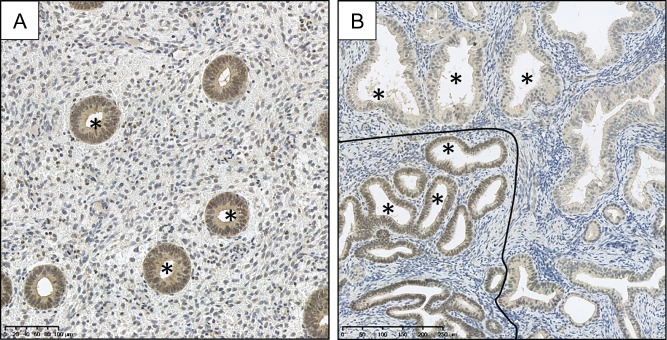
Isolated PTEN-null glands have also been demonstrated within macroscopically normal premenopausal endometrial samples in a reported 43% of cases (Mutter et al., 2001) (an example of isolated PTEN-null glands can be seen in Fig. 7). These glands do not express PTEN protein owing to a genetic mutation and/or deletion and notably they persist between menstrual cycles (Mutter et al., 2001). That being said, the available evidence suggests only a small proportion of these macroscopically normal, PTEN-null glands will progress to endometrioid EC (Ayhan et al., 2015). Important evidence was gathered during a study that compared PTEN immunohistochemistry using samples from a cohort of women with EIN or EC, as well as histologically benign biopsies taken from the same women (matched non-neoplastic controls were included) (Mutter et al., 2014). Where PTEN-null glands were identified in both the index neoplastic biopsy and historic ‘normal’ biopsy, DNA sequencing was performed on both samples for comparative PTEN somatic mutation analysis. The results demonstrated that in only 6.7% of cases the PTEN-null, macroscopically normal glands were the direct progenitors of the high-risk neoplasia subsequently detected (Mutter et al., 2014).
Figure 7.
PTEN immunohistochemical staining of hyperplasia without atypia. PTEN immunohistochemical staining demonstrating isolated PTEN-null glands (loss of brown (DAB) staining) seen within two separate tissue sections diagnosed as hyperplasia without atypia. (Mouse monoclonal anti-human PTEN clone 6H2.1, Dako, Ely, UK; antigen retrieval: decloaking chamber in citrate pH6; overnight incubation 1:300 at 4°C.) Magnification: see scale bars.
Three studies identified by this review retrospectively analysed PTEN expression in women who subsequently progressed from EH to EC and two studies looked at women who had a concurrent/coexisting EC after a biopsy result of EH (Supplementary Table SII). Steinbakk et al. noted lower PTEN expression in EC samples than in EH samples and using a univariant analysis suggested that PTEN negativity in EH was prognostic of progression to EC (P = 0.026) (Steinbakk et al., 2011b). Lacey et al. (2008a) concluded that loss of PTEN expression in EH was neither sensitive nor specific in predicting progression to EC. Baak et al. (2005b) determined in their study that all EH cases that progressed to EC were PTEN-null; however, only 16% of all PTEN-null cases progressed to EC. They concluded that the prognostic power of PTEN could be increased when combined with tissue analysis using the morphometric D-score (Baak et al., 2005b). The two studies of women who had a concurrent/coexisting EC after a biopsy result of EH were more divisive. Pavlakis et al. (2010) noted that loss of PTEN expression on its own was not predictive of concurrent EC, however that changed when analysed in conjunction with a finding of marked nuclear atypia within an EIN lesion. Orbo et al. (2003) found loss of function of PTEN was more likely in EH lesions when a concurrent EC was present or when EC subsequently developed.
When considered in isolation, PTEN immunostaining continues to elicit conflicting views when it comes to its utility as a biomarker of EH and its value as a predictor of progression from EH to EC. Undoubtedly, its role in the process of endometrial carcinogenesis is still of great importance and so investigation into including its evaluation in any biomarker ‘profile’ should be encouraged.
Tumour protein p53
Tumour protein p53, or simply p53, is a protein encoded by the TP53 gene that in humans is located on the short arm of chromosome 17 (17p13.1) (Isobe et al., 1986). When cellular DNA is damaged, the p53 protein regulates cell-cycle inhibition and apoptosis, thereby determining whether or not the damaged DNA should be repaired or destroyed (Ozkara and Corakci, 2004). Owing to its role in preventing damaged DNA from dividing, p53 is considered a tumour suppressor and is often referred to as ‘the guardian of the genome’ (Lane, 1992).
Loss of expression of wild-type p53 due to mutation or gene inactivation leads to malignant transformation of tissues (Cinel et al., 2002). In ECs, most TP53 mutations are missense mutations, generally detected in serous/‘type 2’ ECs and associated with formation of a functionally defective p53 protein that is more stable and has a longer half-life than the wild-type p53 protein (Soong et al., 1996). The mutated protein product accumulates and is detected as overexpression in nuclei using immunohistochemistry. Typically wild-type p53 in cells cannot be detected by immunohistochemistry; however, if p53 is stabilized, due to overexpression in normal cells in response to DNA damage, a positive immunohistochemistry reaction (usually focal, weak and heterogeneous) can be detected in the absence of any mutation (Soong et al., 1996). To further complicate matters, nonsense or frame-shift mutations of TP53 can lead to a protein undetectable by immunohistochemistry and so a completely negative p53 immunohistochemistry reaction may also indicate a gene mutation (Garg et al., 2010).
During the course of this review, we identified several studies that had assessed p53 immunohistochemical expression in EH and EC (Supplementary Tables SI and SII). Horrée et al. noted p53 expression gradually increasing from nearly all negative cells in inactive endometrium, through to EH where only a few cells were positive, with the highest expression seen in ECs (Horrée et al., 2007). Both Cinel et al. and Elhafey et al. demonstrated higher expression scores in atypical hyperplasias, with the highest expression scores in non-endometrioid EC (Elhafey et al., 2001; Cinel et al., 2002).
In terms of using p53 as a marker of progression from EH to EC, the only study our review captured was by Steinbakk et al. Their retrospective analysis demonstrated that two out of eight patients who developed EC from EH had ≤1% positivity for p53 which, using a univariant analysis, they found was prognostic of progression (P = 0.038). Although, given the small number of patients progressing to EC captured by this study, the CI is notably wide (0.9–23.2) (Steinbakk et al., 2011b).
Overall, given the known complexities associated with p53 immunohistochemical analysis, especially the misinterpretation of wild-type p53 staining, its use as a biomarker for EH diagnosis and progression to EC seems doubtful. This is in stark contrast with difficult to diagnose serous ECs or mixed ECs where a clear p53 overexpression pattern provides valuable diagnostic information.
AT-rich interactive domain-containing protein 1 A
AT-rich interactive domain-containing protein 1 A (ARID1A), also known BAF250A, is an important component of the SWItch/Sucrose Non-Fermentable (SWI/SNF) nucleosome remodelling complex. It is encoded by ARID1A which is located on chromosome 1p36.11 (Takeda et al., 2015). The SWI/SNF complex is involved in the regulation of cellular differentiation, tissue development and DNA repair (Guan et al., 2011; Werner et al., 2012). ARID1A is required for SWI/SNF complexes to suppress DNA synthesis and as such ARID1A is considered a tumour suppressor since it regulates cell proliferation and functions to prevent genomic instability (Mao and Shih, 2013). Mutations of ARID1A have been described in ~29–40% of cases of EC (Guan et al., 2011; Wiegand et al., 2011; Kandoth et al., 2013) ARID1A mutations are normally insertions or deletions that lead to the formation of truncated proteins (Guan et al., 2011).
Three studies identified by our review have investigated the role of ARID1A expression within EH tissues (Supplementary Table SI). Mao et al. (2013) performed an immunohistochemical investigation of 246 endometrial tissue samples spanning a range from normal endometrium to CAH and high-grade endometrioid EC. They specifically analysed tissues for ‘clonal’ loss of ARID1A, rather than loss of expression across the entire tissue section (Mao et al., 2013). The authors reported that all samples of normal endometrium retained ARID1A expression, with 16% of CAH demonstrating clonal but not complete loss of expression. In contrast, the samples lacking expression of ARID1A increased with EC tumour grade, from 25% in low-grade to 44% in high-grade tumours (Mao et al., 2013). The same group went on to compare ARID1A expression, along with that of PTEN and the proliferation marker Ki67, utilizing a cohort of 114 endometrial samples with a diagnosis of atypical hyperplasia/EIN (Ayhan et al., 2015). They noted that all specimens (N = 17) with focal ARID1A loss also exhibited concurrent loss of PTEN expression and that this was correlated with a significant increase in proliferation when compared to adjacent areas in the same tissue without concurrent loss of both markers (Ayhan et al., 2015). The authors used these findings to suggest that ARID1A may act to prevent PTEN inactivation from furthering cellular proliferation in the transition from pre-malignancy to EC (Ayhan et al., 2015).
Werner et al. adopted a semi-quantitative intensity staining score when analysing ARID1A expression in their retrospective study of 679 endometrial tissue samples (n = 641 ECs, n = 38 EH). Their findings echoed those of Mao et al. demonstrating a stepwise reduction in staining intensity of ARID1A with progression from hyperplasia without atypia (no loss of protein expression) to hyperplasia with atypia (16% loss of expression) and endometrioid tumours (19% loss of expression) (Werner et al., 2012).
ARID1A has emerged from molecular and genomic studies as an important candidate and tumour suppressor in gynaecological malignancies (Kandoth et al., 2013; Takeda et al., 2015). The current literature suggests we should recognize the potential of ARID1A as a valuable biomarker of progression from EH to EC and it, therefore, warrants further investigation in larger (more diverse) tissue sample sets.
Transcription factors
Paired box gene 2
Paired box gene 2 (PAX2) is a member of a large gene family of pair box genes that are implicated in transcriptional regulation during the process of embryogenesis (Mansouri et al., 1994; Ryan et al., 1995). PAX2 gene expression has been connected to the normal growth of the central nervous system, eyes, ears and urogenital system (Allison et al., 2012). Expression of PAX2 has been described as a marker of the Müllerian duct derivatives (fallopian tubes, uterus, cervix and upper vagina) (Tong et al., 2007). According to Tong et al. epithelial cells within the uterine glands normally demonstrate nuclear expression of PAX2 (Tong et al., 2007). The interest in PAX genes as a predictor of EH/EC have been stimulated by reports that they can act as proto-oncogenes through regulation of cell proliferation, survival and apoptosis (Robson et al., 2006; Lang et al., 2007).
Loss of PAX2 expression has been implicated in the development of EIN (Fig. 8) by several authors and has found potential utility as a tool when diagnosing difficult EIN cases (e.g. where there is no ‘normal’ tissue in a sample to act as in internal control when assessing nuclear morphology) (Quick et al., 2012). Five studies were captured by our review of the literature (Supplementary Table SI). For example, in a recent study, Joiner et al. (2015) built on earlier recommendations that PAX2 aids EIN diagnosis (Quick et al., 2012) and compared the WHO94 and EIN classification systems for EH using PAX2 immunohistochemistry. In their study, the authors considered complete loss of nuclear staining, or reduced nuclear staining as compared to background endometrium, to be indicative of reduced PAX2 expression (Joiner et al., 2015). Reduced PAX2 expression was noted in 92% of EIN cases and 88% of atypical EHs. Although the authors concluded that loss of PAX2 immunoexpression is a useful finding when deciding whether lesions are premalignant, they also advocated careful comparison to H&E sections when considering the findings (Joiner et al., 2015). Loss of PAX2 expression was also found in 71% of EIN cases by Monte et al. and in 74% of atypical EHs by Allison et al. (Monte et al., 2010; Allison et al., 2012). Allison et al. proposed that PAX2 loss occurs early in the process of endometrial carcinogenesis as they did not detect loss of expression in their proliferative or secretory endometrial samples. They added the caveat that the expression pattern does not discriminate between diagnostic categories of EH since its expression is ubiquitously lost amongst all EH groups (Allison et al., 2012). Monte et al. (2010) also corroborated this, adding that the greatest stepwise change in PAX2 expression occurs between normal and premalignant endometrium. Only one study reported alternative findings; Kahraman et al. (2012) demonstrated an increase in PAX2 expression with progression from premalignant states to EC.
Figure 8.
PAX2 immunohistochemical staining. Selection of glands marked by * in lumen. (A) PAX2 stained section of proliferative endometrium demonstrating strong brown nuclear (DAB) PAX2 staining within the glands, (B) loss of brown (DAB) PAX2 nuclear staining within the glands of an EIN lesion above/right of the black line. Crowded glandular background endometrium can also be seen. (Rabbit anti-PAX2 polyclonal Z-RX2, Invitrogen, Camarillo, CA; Antigen retrieval: decloaking chamber in citrate pH6; Overnight incubation 1:500 at 4°C.) Magnification: see scale bars.
The available literature regarding PAX2 as a diagnostic biomarker of EH appears to suggest promise due to its ability to discern normal cycling endometrium from EH and EC; however, as emphasized by Quick et al., up to a third of EIN lesions may not demonstrate loss of PAX2 and thus caution should be exercised with its interpretation (Quick et al., 2012). However based on these reports, the utility of PAX2 in a panel of diagnostic biomarkers warrants further investigation.
Heart and neural crest derivatives expressed transcript 2
Heart and neural crest derivatives expressed transcript 2 (HAND2) belongs to the basic helix-loop-helix (bHLH) family of transcription factors; it plays crucial roles during embryological cardiac morphogenesis (VanDusen et al., 2014) and knockout mice are infertile due to failure of implantation (Li et al., 2011). In mice, Hand2 has been shown to be a PR-regulated gene and its expression in endometrial stromal cells inhibits epithelial cell proliferation via suppression of several fibroblast growth factors (Li et al., 2011).
When Jones et al. (2013) conducted a comprehensive epigenome-transcriptome-interactome analysis, they found HAND2 was at the centre of the most highly ranked differential hotspot in EC, leading them to propose that epigenetic deregulation of HAND2 was a crucial step in endometrial carcinogenesis. They reported that methylation of HAND2 was increased in premalignant endometrial lesions when compared to normal endometrium and that this was associated with a reduction in HAND2 expression (Jones et al., 2013).
Buell-Gutbrod et al. (2015) also hypothesized that HAND2 plays a role in the development of EH and Type 1 endometrioid EC (Supplementary Table SI). In their immunohistochemical study, 56 archival hysterectomy specimens with a known pathological diagnosis of either disordered proliferative endometrium, SH or CH with or without atypia and EC, were investigated for expression of HAND2 (Buell-Gutbrod et al., 2015). The results demonstrated a statistically significant (P ≤ 0.001) reduction in the stromal expression of HAND2 between benign endometrium and both simple and CH with atypia and EC (Buell-Gutbrod et al., 2015). But there was no statistically significant difference between benign endometrium and SH without atypia or disordered proliferative endometrium (Buell-Gutbrod et al., 2015). The authors noted that the HAND2 antibody cannot distinguish between SH with atypia, CH with atypia and EC (Buell-Gutbrod et al., 2015).
These studies suggest that this progesterone-regulated transcription factor shows promise as a potential diagnostic biomarker of EH with an ability to differentiate neoplastic from endocrine driven EH. However, further work needs to be undertaken on larger and more diverse sample cohorts.
Mismatch repair
The DNA mismatch repair (MMR) pathways repair damaged DNA resulting from insertions/deletions in microsatellites, replication errors from damaged DNA polymerases and events such as single base mismatches (Poulogiannis et al., 2010; Diaz-Padilla et al., 2013). Microsatellites refer to repetitive nucleotide sequences within DNA, typically with 1–5 nucleotide repeats that can be repeats of mononucleotide, dinucleotide or larger units (Karamurzin and Rutgers, 2009).
Microsatellite instability (MSI) ensues where there is expansion or reduction in the length of microsatellite tracts in malignant tissue (Diaz-Padilla et al., 2013). Inactivation of any of the MMR genes (including MLH1, MSH2, MSH6 and PMS2) can cause MSI (Eshleman and Markowitz, 1996; Poulogiannis et al., 2010). The National Cancer Institute (NCI) consensus or ‘Bethesda’ panel was established as a panel of microsatellite markers to be used to diagnose MSI. Although initially designed for colorectal cancer, the system has also been adopted for use in the endometrium (Boland et al., 1998). The panel comprises five microsatellite loci: two mononucleotide markers and three dinucleotide markers. MSI-high tumours are defined by their instability at two or more of the five loci (or >30% of loci if a larger panel of markers is used), whereas MSI-low tumours show instability at only one locus out of the five (or in 10–30% of loci in larger panels). Microsatellite stable (MSS) tumours are those without instability at any loci (or <10% of loci in larger panels) (Vilar and Gruber, 2010). MSI-high status has been demonstrated to be an indicator of poor prognosis in International Federation of Gynecology and Obstetrics (FIGO) stage 1, but not FIGO 2–4 endometrioid ECs (Steinbakk et al., 2011a).
Deficiencies in the MMR system are observed in 25–30% of somatic ECs and are commonly associated with endometrioid histology (Hecht and Mutter, 2006). ECs are observed both somatically and in association with germline mutations in MLH1, MSH2, MSH6 and PMS2 genes, as part of Lynch syndrome or hereditary non-polyposis colorectal cancer (HNPCC) syndrome (Hendriks et al., 2006). In sporadic EC, MMR deficiency is mainly caused by hypermethylation of the MLH1 promoter, silencing its expression, thus leading to MSI. This is responsible for the lack of immunohistochemically detectable MLH1 protein expression in the majority of sporadic ECs with MSI (Simpkins et al., 1999). Woo et al. (2014) demonstrated the utility of MMR immunohistochemistry in their 2014 study of MMR proteins in women with EC.
Studies involving immunohistochemical expression of MLH1, MSH2 and MSH6 were captured by our literature search (Supplementary Tables SI and SII). Our search did not identify any studies examining PMS2 expression in EH lesions. Berends et al. suggest that loss of MLH1 or MSH2 protein may be an early event in endometrial carcinogenesis. Their study of 62 cases was interesting in that they looked at patients with EC who had germline HNPCC/Lynch syndrome mutations, patients with HNPCC and no EC and patients with EC without HNPCC. In patients with EH, both with and without a germline MLH1 mutation, loss of the corresponding protein was detected using immunohistochemistry (Berends et al., 2001). In six cases of EH and concurrent EC, loss of MLH1 or MSH2 proteins was seen in both areas within the tissue (Berends et al., 2001). Hamid et al. analysed endometrial samples from 123 women (including 51 EH cases) for MSH2 expression. They noted that all SHs showed a normal positive expression of MSH2, with some complex and atypical EHs demonstrating weak or no MSH2 expression at all (Hamid et al., 2002). However, this was not significant enough to be able to infer utility as a diagnostic marker for distinguishing between EH categories (Hamid et al., 2002). The authors also commented that loss of MSH2 expression is rarely observed in sporadic EC cases (Hamid et al., 2002). Orbo et al. looked at EC progression from EH and analysed expression of MLH1, MSH2 and MSH6. They found loss of expression in these markers was higher in EH cases where there was either a concurrent EC or subsequent progression to EC (Orbo et al., 2003).
Molecular evidence connecting an absence of expression of MLH1 with tumour-specific promoter hypermethylation in EH has previously been described, suggesting ECs with MSI may acquire this feature as precancers (Jovanovic et al., 1996). Esteller et al. share this view; they found that aberrant MLH1 methylation is almost exclusively restricted to atypical EHs (Esteller et al., 1999). In addition, the group noted that the atypical EHs methylated at MLH1 which demonstrate a MSI phenotype are usually those also associated with a concurrent EC that also have MSI and MLH1 methylation (Esteller et al., 1999). These reports and the trends observed from the above literature imply a role for the detection and categorization of deficiencies in the MMR system within EHs, with suggestions that deficiencies in the MMR system may be useful in predicting malignant progression.
Cell adhesion and signalling
Beta-catenin
Beta-catenin (β-catenin) is a protein encoded by the CTNNB1 gene (Norimatsu et al., 2007) that plays a critical role in cell–cell adhesion and is a constituent of the Wnt pathway (Morin, 1999). Canonical Wnt signalling through β-catenin is thought to have a significant role in regulating cell and tissue proliferation, differentiation and carcinogenesis (Villacorte et al., 2012). In normal cells, β-catenin is rapidly degraded by the proteasome and factors that impair this turnover lead to an excess of cytoplasmic protein (Scholten et al., 2003). This results in simultaneous translocation of β-catenin into the cell nucleus, where it may form transcriptionally active complexes with T-cell factor/lymphoid enhancer factors (Tcf/Lef), resulting in activation of downstream targets (Morin, 1999; Scholten et al., 2003).
Seven studies were captured by this review as having analysed the immunohistochemical expression of β-catenin with respect to EH and EC (Supplementary Tables SI and SII). Saegusa et al. (2001) and Liao et al. (2009) both noted more intense nuclear expression of β-catenin in atypical EHs and EC compared to non-atypical/benign hyperplasia and normal endometrium. Furthermore, Moreno-Bueno et al. (2003) suggested that nuclear accumulation of β-catenin is characteristic of endometrioid EC and may be an early event in the carcinogenesis process. The EIN classification system was utilized by Norimatsu et al. (2007), who demonstrated 26% of EIN cases had strong nuclear staining of β-catenin, in contrast to normal endometrium where no expression was detected. Xiong et al. (2010) noted abnormal β-catenin expression in 10% of benign hyperplasia/non-EIN, rising to 50% and 67% in EIN and EC, respectively; they infer that detection of β-catenin may be of use in distinguishing benign hyperplasia from EIN. In terms of progression of EH to EC, Ashihara et al. (2002) noted that 55% of EH with a concurrent EC had positive or intensively positive β-catenin nuclear staining. Steinbakk et al., in their retrospective study, found that 40% of their EHs that progressed to EC demonstrated nuclear staining (two of five) (Steinbakk, et al., 2011b).
Changes in nuclear β-catenin expression between normal, neoplastic and frankly malignant endometrial tissues provide insight into the activation of the beta-catenin/Wnt signal transduction pathway, highlighting potential for further mechanistic studies into the role played by Wnt-dependent target genes in the process of EH progression and carcinogenesis.
E-cadherin
E-cadherin is a trans-membrane epithelial cell adhesion protein containing a cytoplasmic domain that connects the actin cytoskeleton via a complex with its related cytoplasmic proteins: α-, β- and γ-catenins (Carico et al., 2010). Several in vitro studies have associated low expression of E-cadherin with progression of malignancy and abnormal expression of E-cadherin and β-catenin have been implicated in the invasive and metastatic ability of various epithelial tumours (Hashimoto et al., 1989; Frixen et al., 1991).
Three studies were identified by our literature search relating to E-cadherin and neoplastic endometrial lesions (Supplementary Table SI). Two studies reported decreased expression of E-cadherin in EC compared with atypical hyperplasias (Moreno-Bueno et al., 2003; Carico et al., 2010). Carico et al. (2010) analysed the entire spectrum of endometrial lesions from benign to malignant, noting a progressive reduction in glandular expression at each stage, from normal endometrium through to EC. Moreno-Bueno et al. (2003) noted that in EC, the largest reduction in E-cadherin expression is seen in high-grade malignancies. In contrast Ahmed et al. reported E-cadherin expression was higher in EC than in atypical hyperplasia (Ahmed and Muhammad, 2014).
These studies suggest a role for E-cadherin in regulating cell adhesion during endometrial carcinoma progression; however, only limited information can be drawn at present from the current literature on the utility of E-cadherin as a diagnostic marker of EH or for its capacity to predict progression to EC.
Regulators of cell survival or migration
B-cell lymphoma 2/Bcl-2 associated x protein
Programmed cell death (apoptosis) plays an essential role in homeostatic mechanisms during cyclical endometrial breakdown, piecemeal shedding and tissue restoration at menses (Harada et al., 2004; Marshall et al., 2011). The B-cell lymphoma 2 (Bcl-2) gene forms part of a group of proto-oncogenes that extend cellular longevity by counteracting the apoptotic process (Reed, 1994). In contrast, the Bcl-2 associated x protein (BAX) gene is an apoptosis-promoting member of the Bcl-2 gene family (Oltvai et al., 1993). It is thought that the Bcl-2 and BAX proteins forms heterodimers in vivo (Kokawa et al., 2001), and the cellular Bcl-2: BAX ratio is an important factor in the regulation of apoptosis, with a high ratio resulting in cells becoming resistant to apoptotic stimuli and a low ratio inducing cell death (Sedlak et al., 1995; Vaskivuo et al., 2002). The BAX protein is expressed throughout the menstrual cycle, but Bcl-2 appears to be regulated by oestrogen, and demonstrates a rise in the proliferative phase, before falling to a plateau in the secretory and menstrual phases (Saegusa et al., 1996; Critchley et al., 1999; Allison et al., 2008).
Many authors have studied expression of Bcl-2 and BAX in the hyperplastic endometrium and EC (Supplementary Tables SI and SII). Four studies from our literature search demonstrated reduced Bcl-2 expression with a trend from normal endometrium (highest expression), through the hyperplasias, to EC (lowest expression) (Peiró et al., 2001; Risberg et al., 2002; Sakuragi et al., 2002; Vaskivuo et al., 2002). In addition, three studies noted Bcl-2 expression to be higher in non-atypical hyperplasias compared with atypical hyperplasias (Morsi et al., 2000; Kokawa et al., 2001; Nunobiki et al., 2009). Mitselou et al. (2003) referred to ‘adenomatous hyperplasia’ in their cohort, but also found lower levels of expression of Bcl-2 in the EC group. Cinel et al. (2002) published findings to the contrary noting a pattern of increasing expression between the EH groups. BAX immunohistochemistry expression was more divisive with two studies demonstrating an increasing trend from normal endometrium, via EH, to EC (Kokawa et al., 2001; Peiró et al., 2001) and two studies suggesting the inverse (Sakuragi et al., 2002; Vaskivuo et al., 2002). Kapucuoglu et al. (2007) found no significant change in BAX expression between EH groups, nor between EH and EC.
From the studies described above, it seems that increased Bcl-2 expression is a feature of benign EHs with a reduction in atypical EHs and ECs. Since Bcl-2 promotes cell survival, this pattern would fit with the observation of increased apoptosis in atypical EHs and ECs (Arends, 1999). In terms of utility as a EH biomarker, although unlikely to be of great diagnostic benefit on its own, the reports suggest that Bcl-2 expression may have some utility as a marker of EH progression.
Others
Additional candidate markers deemed of potential relevance, which were identified by our review of the literature, are Survivin, p16, p21 and p27. The literature findings for these markers are summarized in Supplementary Tables SI and SII.
Conclusions and what is still missing: towards a genomic future?
Ongoing challenges
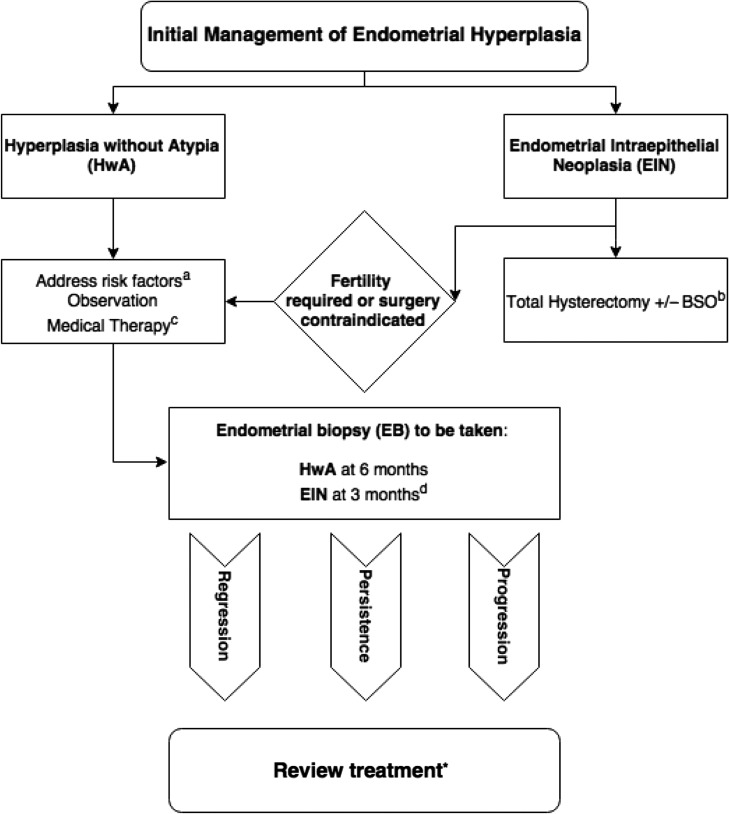
A biopsy diagnosis of EIN carries a 45 times greater risk of progression to endometrioid EC after 1 year (Lacey et al., 2008b), meaning that surgery in the form of a total hysterectomy is usually the treatment of choice (Trimble et al., 2012). Alternatively, if a patient's co-morbid status or fertility wishes preclude hysterectomy, treatment with progestins and a programme of repeat observation can be considered, albeit with caution (Fig. 9).
Figure 9.
Flow diagram detailing proposed initial management of EHs based upon guidance released by the Royal College of Obstetricians and Gynaecologists (RCOG), UK in 2016 (Gallos et al., 2016). *A comprehensive treatment review and plan for ongoing management should be made at this point dependant on the outcome of the preceding endometrial biopsy and in keeping with local and national guidelines/practise. aRisk factors for EH are specified in Table I. bOvarian conservation should be considered according to patients age, menopausal status and preferences. Total hysterectomy may also be indicated where there are (i) adverse effects with medical treatments, (ii) concerns over medication compliance and (iii) patient preference, e.g. elevated anxiety. cMedical progestin therapy has varying forms; for further recommendations, refer to national guidelines (Committee on Gynecologic Practice, 2015; Gallos et al., 2016). dFollow-up intervals for patients undergoing medical treatment of EIN should be tailored to individual patients and must reflect any ongoing risk factors, symptomatology and treatment responses. BSO = bilateral salpingo-oophorectomy.
It is highly likely that difficult EH/EIN clinical scenarios will become more commonplace in the future, due to escalating levels of obesity, the ageing population and increasing trends in delaying childbearing (Mackintosh and Crosbie, 2013; Daniluk and Koert, 2016). The clinical need for robust diagnostic biomarkers, capable of (i) differentiating neoplastic EIN lesions from benign hyperplasia and (ii) predicting their progression to EC, has, therefore, never been more apparent. For example, a premenopausal nulliparous patient with PCOS, found to have EIN on endometrial biopsy and who wishes to have future children, would benefit from a biomarker test able to predict the likelihood of her EIN progressing to EC versus EIN lesion involution. In addition, a diagnostic biomarker test may aid pathological analysis of sub-diagnostic EIN lesions, as described by Owings et al. (2014), whereby crowding of cytologically suspicious glands is a concern; however, the overall lesion size may be insufficient for EIN diagnosis. To date, a single ‘holy grail’ biomarker has not been found, although the search continues. Our review highlights several prominent immunohistochemical candidates from the current medical literature. We would hypothesize that moving forwards, a collective ‘panel’ approach of multiple candidates may provide greater diagnostic and prognostic value than what can currently be achieved by any single marker in isolation. This reflects the heterogeneous nature of these lesions, since the underlying pathogenesis is undoubtedly complex, multifactorial and may involve several different molecular pathways.
Towards a genomic future?
Molecular classification is expanding our understanding of ECs and appears to correlate well with clinical outcome data, representing an important step forward from the traditional morphological diagnostic methods and the dichotomous ‘Type 1’ and ‘Type 2’ categories (Salvesen et al., 2009; Le Gallo et al., 2012; McConechy et al., 2012; Kandoth et al., 2013; Talhouk et al., 2015).
In 2013, an integrated molecular classification drawing on proteomic, genomic and transcriptomic analyses of over 370 ECs performed by The Cancer Genome Atlas (TCGA) resulted in new insights into EC subtypes (Kandoth et al., 2013). Briefly, employing array-based and sequencing methodologies, four major EC groups were characterized: (i) ultramutated cancers with DNA polymerase epsilon (POLE) mutations (7%), (ii) hypermutated cancers with MSI due to MLH1 promoter methylation (28%), (iii) ECs with low mutation rate and low frequency of DNA copy-number alterations (CNA, 39%) and (iv) ECs with low mutation rate but high-frequency DNA CNA (26%) (Kandoth et al., 2013). The TGCA suggested that EC patients harbouring POLE mutations (more commonly seen in Grade 3 endometrioid ECs in their cohort) had a less aggressive clinical course and improved progression-free survival when compared with the three other groups identified (Kandoth et al., 2013). In 2014, Meng et al. reported that POLE mutations could function as a prognostic marker for management of Grade 3 endometrioid EC. A suggested mechanism for these observations is that ECs with POLE mutations have an enhanced antitumor T-cell response combined with an enrichment of antigenic neopeptides (van Gool et al., 2015). Owing to the expense and technical expertise required when undertaking molecular classification of ECs, surrogate and clinically applicable methods for use with formalin-fixed paraffin-embedded tissues have been proposed (Talhouk et al., 2015). Through analysis of 152 historic ECs using a combination of p53 immunohistochemistry (as a surrogate marker for copy-number status), MMR (MLH1, MSH2, MSH6 and PMS2) immunohistochemistry and POLE mutation analysis, Talhouk et al. (2015) were able to replicate the survival curves as demonstrated by the TGCA.
Molecular classification has novel implications for diagnostics and personalizing treatment options for individual patients based upon prognostic outcomes. Given that the ECs seen in TGCA groups 1–3 were virtually all endometrioid (Kandoth et al., 2013), genomic profiling of EH/EIN as the precursor lesion to endometrioid EC may warrant further investigation. Owing in part to the escalating problem of obesity, rates of EHs and ECs and particularly the incidence of premenopausal disease are rising (Mackintosh and Crosbie, 2013; Wise et al., 2016). As already eluded to, these women potentially face difficult decisions regarding fertility limiting treatments and would, therefore, benefit from individualized risk stratification to help guide the decision-making process.
Concluding remarks
EHs embody a uniquely heterogeneous group of lesions occurring in a dynamic, multicellular tissue that is exquisitely sensitive to changes in the hormonal environment. Historically, due to the heterogeneous nature of EH lesions, there has been considerable difficulty classifying them into clinically relevant and pathologically reproducible groups that correlate risk of malignancy with treatment options and clinical outcome. The introduction of EIN represents a fundamental change in our understanding of the development of EH (Fig. 10). EIN is considered a direct precursor lesion for the development of endometrioid EC, which is backed by molecular evidence that proposes a monoclonal lineage with significant malignant potential. The EIN system has been endorsed by the World Health Organisation in 2014 (Zaino et al., 2014).
Figure 10.
A schematic diagram to illustrate a proposed mechanism for monoclonal development of EIN. The endometrium is exposed to unopposed oestrogens via several possible routes (as described in Fig. 2). Oestrogen (E2), acting as a promotor, drives proliferation of the endometrial glands. This process can be reversible, e.g. with progestin (P) therapy acting as a suppressor. In ‘at risk’ individuals, a mutant clone may develop in this environment. The mutant clone occurs within phenotypically normal appearing endometrial glands. The mutant clone is selected for and progresses, aided by the influence of unopposed oestrogens. Over time, with the accrual of further genetic damage, not yet fully elucidated (bottom arrow shows suggestions), the mutant clone proliferates and an EIN lesion can be diagnosed during routine light microscopic examination of an H&E stained section. Endocrine modifiers can alter the balance of EIN progression versus involution. The patient may present with symptoms of abnormal uterine bleeding (AUB) and a thickened endometrium on ultrasound imaging. With the continued accumulation of further genetic damage, not yet fully elucidated (bottom arrow shows suggestions), the EIN lesion undergoes malignant transformation to EC.
We acknowledge that there may be a small number of candidate biomarkers that have fallen outside the search criteria employed by this review, despite every effort taken to include all relevant sources of information. This unfortunately is inevitable. Our focus on post-2000 literature aimed to ensure that we had the best coverage of current and up-to-date studies with an element of consistency between methodologies. We have chosen to focus on immunohistochemical biomarkers for this review. This technique is widely available and a there is a plethora of literature information detailing application of the technique in both EH and EC tissues. We felt that an attempt to summarize this in a comprehensive manner was needed. We recognize that there are limits to the conclusions that can be drawn when comparing a non-standardized semi-quantitative technique like immunohistochemistry, with researchers using different antibodies for the same antigen and employing different staining and scoring systems. In addition, the complexity of attempting to compare studies with a lack of consistency between diagnostic terminologies (e.g. EIN versus WHO94) has meant that a simple descriptive comparison is the ceiling of what can be achieved for the vast majority of cases.
The recent genomic classification of ECs heralds an exciting opportunity for EH risk stratification and, moving forwards, a role for qualitative genomic biomarkers is apparent. We are starting to see the age-specific incidence of EC increasing sharply at ages 40–44 years (Cancer Research UK, 2016) and what has previously been considered a predominantly postmenopausal disease is becoming an increasing concern for women of childbearing age, obese individuals and those with PCOS (Hardiman et al., 2003; Li et al., 2014; Shafiee et al., 2014). Since EH/EIN often precedes EC by several years (with approximate intervals dependent on the EH classification system adopted) the chance to intervene early with informative risk stratification and thus appropriate timely treatment is an opportunity not to be missed.
Supplementary Material
Acknowledgements
The authors wish to thank Dr Douglas Gibson and Mrs Frances Collins for their proofreading of the manuscript and Ms Arantza Esnal-Zufiaurre for her invaluable technical support.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors’ roles
P.A.S. and P.T.K.S. conceived and planned the manuscript. P.A.S., P.T.K.S., H.O.D.C., A.R.W.W. and M.J.A. agreed the proposal submission. P.A.S. undertook literature searching, data acquisition and drafted the manuscript with support from P.T.K.S. All authors critically appraised the drafts and revisions of the manuscript and approved its intellectual content.
Funding
P.A.S. is supported by a Cancer Research UK Clinical Research Fellowship awarded from the Edinburgh Cancer Research Centre, UK. All work undertaken in the P.T.K.S. laboratory is supported by an MRC Programme Grant (G1100356/1) awarded to P.T.K.S. No additional funding was required to complete this work.
Conflicts of interest
P.A.S., M.J.A. and P.T.K.S. report no conflicts of interest relating to this work. A.R.W.W. reports consultancy for Preglem SA, Gedeon Richter, HRA Pharma and Bayer Pharma AG outside the submitted work. H.O.D.C. reports research grant support from Bayer Pharma AG and consultancy for Bayer Pharma AG, PregLem SA, Gedeon Richter, Vifor Pharma UK Ltd and AbbVie Inc. outside the submitted work.
References
- Abu-Rustum NR, Zhou Q, Gomez JD, Alektiar KM, Hensley ML, Soslow RA, Levine DA, Chi DS, Barakat RR, Iasonos A. A nomogram for predicting overall survival of women with endometrial cancer following primary therapy: toward improving individualized cancer care. Gynecol Oncol 2010;116:399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed ARH, Muhammad EMS. E-cadherin and CD10 expression in atypical hyperplastic and malignant endometrial lesions. J Egypt Natl Canc Inst 2014;26:211–217. [DOI] [PubMed] [Google Scholar]
- Allison KH, Tenpenny E, Reed SD, Swisher EM, Garica RL. Immunohistochemical markers in endometrial hyperplasia: is there a panel with promise. Appl Immunohistochem Mol Morphol 2008;16:329–343. [DOI] [PubMed] [Google Scholar]
- Allison KH, Upson K, Reed SD, Jordan CD, Newton KM, Doherty J, Swisher EM, Garcia RL. PAX2 loss by immunohistochemistry occurs early and often in endometrial hyperplasia. Int J Gynecol Pathol 2012;31:159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet 2005;366:491–505. [DOI] [PubMed] [Google Scholar]
- Arends MJ. Apoptosis in the endometrium. Histopathology 1999;35:174–178. [DOI] [PubMed] [Google Scholar]
- Ashihara K, Saito T, Mizumoto H, Nishimura M, Tanaka R, Kudo R. Mutation of beta-catenin gene in endometrial cancer but not in associated hyperplasia. Med Electron Microsc 2002;35:9–15. [DOI] [PubMed] [Google Scholar]
- Ashton KA, Proietto A, Otton G, Symonds I, McEvoy M, Attia J, Gilbert M, Hamann U, Scott RJ. Estrogen receptor polymorphisms and the risk of endometrial cancer. BJOG 2009;116:1053–1061. [DOI] [PubMed] [Google Scholar]
- Atkinson AJJ, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, Peck CC, Schooley RT, Spilker BA et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- Ausems EWMA, van der Kamp J-K, Baak JPA. Nuclear morphometry in the determination of the prognosis of marked atypical endometrial hyperplasia. Int J Gynecol Pathol 1985;4:180–185. [DOI] [PubMed] [Google Scholar]
- Ayhan A, Mao T-L, Suryo Rahmanto Y, Zeppernick F, Ogawa H, Wu R-C, Wang T-L, Shih I-M. Increased proliferation in atypical hyperplasia/endometrioid intraepithelial neoplasia of the endometrium with concurrent inactivation of ARID1A and PTEN tumour suppressors. J Pathol Clin Res 2015;1:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak JPA, Mutter GL, Robboy S, van Diest PJ, Uyterlinde AM, Ørbo A, Palazzo J, Fiane B, Løvslett K, Burger C et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer 2005. a;103:2304–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak JPA, Mutter GL. EIN and WHO94. J Clin Pathol 2005;58:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baak JPA, Nauta JJP, Wisse-Brekelmans ECM, Bezemer PD. Architectural and nuclear morphometrical features together are more important prognosticators in endometrial hyperplasias than nuclear morphometrical features alone. J Pathol 1988;154:335–341. [DOI] [PubMed] [Google Scholar]
- Baak JPA, Wisse-Brekelmans ECM, Fleege JC, van der Putten HWHM, Bezemer PD. Assessment of the risk on endometrialcancer in hyperplasia, by means of morphological and morphometrical features. Pathol – Res Pract 1992;188:856–859. [DOI] [PubMed] [Google Scholar]
- Baak JPA, Ørbo A, van Diest PJ, Jiwa M, de Bruin P, Broeckaert M, Snijders W, Boodt PJ, Fons G, Burger C et al. Prospective multicenter evaluation of the morphometric D-score for prediction of the outcome of endometrial hyperplasias. Am J Surg Pathol 2001;25:930–935. [DOI] [PubMed] [Google Scholar]
- Baak JPA, van Diermen B, Steinbakk A, Janssen E, Skaland I, Mutter GL, Fiane B, Løvslett K. Lack of PTEN expression in endometrial intraepithelial neoplasia is correlated with cancer progression. Hum Pathol 2005. b;36:555–561. [DOI] [PubMed] [Google Scholar]
- Baird DT, Cameron ST, Critchley HO, Drudy TA, Howe A, Jones RL, Lea RG, Kelly RW. Prostaglandins and menstruation. Eur J Obstet Gynecol Reprod Biol 1996;70:15–17. [DOI] [PubMed] [Google Scholar]
- Berends MJW, Hollema H, Wu Y, Van Sluis T Der, Mensink RGJ, Ten Hoor KA, Sijmons RH, De Vries EGE, Pras E, Mourits MJE et al. MLH1 and MSH2 protein expression as a pre-screening marker in hereditary and non-hereditary endometrial hyperplasia and cancer. Int J Cancer 2001;92:398–403.. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Nogales FF, Masseroli M, Abeler V, Duvillard P, Müller-Holzner E, Pickartz H, Wells M. A multicentric european study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol 1999;23:1102–1108. [DOI] [PubMed] [Google Scholar]
- Berman JJ, Albores-Saavedra J, Bostwick D, DeLellis R, Eble J, Hamilton SR, Hruban RH, Mutter GL, Page D, Rohan T et al. Precancer: a conceptual working definition. Cancer Detect Prev 2006;30:387–394. [DOI] [PubMed] [Google Scholar]
- Bircan S, Ensari A, Ozturk S, Erdogan N, Dundar I, Ortac F. Immunohistochemical analysis of c-myc, c-jun and estrogen receptor in normal, hyperplastic and neoplastic endometrium. Pathol Oncol Res 2005;11:32–39. [DOI] [PubMed] [Google Scholar]
- Bobrowska K, Kamiński P, Cyganek A, Pietrzak B, Jabiry-Zieniewicz Z, Durlik M, Paczek L. High rate of endometrial hyperplasia in renal transplanted women. Transplant Proc 2006;38:177–179. [DOI] [PubMed] [Google Scholar]
- Bokhman J V. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983;15:10–17. [DOI] [PubMed] [Google Scholar]
- Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN et al. A national cancer institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248–5257. [PubMed] [Google Scholar]
- Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, Cohn DE, Walker JL, Moore RG, Downs LS et al. Etiologic heterogeneity in endometrial cancer: evidence from a gynecologic oncology group trial. Gynecol Oncol 2013;129:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell-Gutbrod R, Cavallo A, Lee N, Montag A, Gwin K. Heart and neural crest derivatives expressed transcript 2 (HAND2): a novel biomarker for the identification of atypical hyperplasia and type I endometrial carcinoma. Int J Gynecol Pathol 2015;34:65–73. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK. Uterine Cancer Statistics. 2016. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence#ref-1. (28 March 2016, date last accessed).
- Cao QJ, Einstein MH, Anderson PS, Runowicz CD, Balan R, Jones JG. Expression of COX-2, Ki-67, cyclin D1, and P21 in endometrial endometrioid carcinomas. Int J Gynecol Pathol 2002;21:147–154. [DOI] [PubMed] [Google Scholar]
- Carico E, Atlante M, Giarnieri E, Raffa S, Bucci B, Giovagnoli MR, Vecchione A. E-cadherin and alpha-catenin expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res 2010;30:4993–4998. [PubMed] [Google Scholar]
- Carter AJR, Nguyen CN. A comparison of cancer burden and research spending reveals discrepancies in the distribution of research funding. BMC Public Health 2012;12:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D, Srinivasan R, Ghosh S, Rajwanshi A, Gopalan S. Estrogen receptor beta (ERbeta) in endometrial simple hyperplasia and endometrioid carcinoma. Appl Immunohistochem Mol Morphol 2008;16:535–542. [DOI] [PubMed] [Google Scholar]
- Chamber JT, Carcangiu ML, Voynick IM, Pirro M, Schwartz P. Immunohistochemical evaluation of estrogen and progesterone receptor content in 183 patients with endometrial carcinoma. Am J Clin Pathol 1990;94:247–254. [DOI] [PubMed] [Google Scholar]
- Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R. Therapeutic options for management of endometrial hyperplasia. J Gynecol Oncol 2016;27:712–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinel L, Polat A, Aydin Ö, Düşmez D, Eğilmez R. Bcl-2, iNOS, p53 and PCNA expression in normal, disordered proliferative, hyperplastic and malignant endometrium. Pathol Int 2002;52:384–389. [DOI] [PubMed] [Google Scholar]
- Cirpan T, Terek MC, Mgoyi L, Zekioglu O, Iscan O, Ozsaran A. Immunohistochemical evaluation of PTEN protein in patients with endometrial intraepithelial neoplasia compared to endometrial adenocarcinoma and proliferative phase endometrium. Eur J Gynaecol Oncol 2006;27:389–392. [PubMed] [Google Scholar]
- Collins F, MacPherson S, Brown P, Bombail V, Williams ARW, Anderson RA, Jabbour HN, Saunders PTK. Expression of oestrogen receptors, ERalpha, ERbeta, and ERbeta variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERalpha. BMC Cancer 2009;9:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee on Gynecologic Practice – Society of Gynecologic Oncology The American College of Obstetricians and Gynecologists Committee Opinion No. 631. Endometrial intraepithelial neoplasia. Obstet Gynecol 2015;125:1272–1278. [DOI] [PubMed] [Google Scholar]
- Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, Heintz APM, Ngan HYS, Pecorelli S. Carcinoma of the corpus uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95:S105–S143. [DOI] [PubMed] [Google Scholar]
- Critchley HOD, Tong S, Cameron ST, Drudy TA, Kelly RW, Baird DT. Regulation of bcl-2 gene family members in human endometrium by antiprogestin administration in vivo. J Reprod Fertil 1999;115:389–395. [DOI] [PubMed] [Google Scholar]
- Critchley HOD, Brenner RM, Henderson TA, Williams K, Nayak NR, Slayden OD, Millar MR, Saunders PTK. Estrogen receptor beta, but not estrogen receptor alpha, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab 2001;86:1370–1378. [DOI] [PubMed] [Google Scholar]
- Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SHK. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 2008;68:5619–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniluk JC, Koert E. Childless women's beliefs and knowledge about oocyte freezing for social and medical reasons. Hum Reprod 2016;1–8. doi:10.1093/humrep/dew189 Advanced Access published 2 September 2016. [DOI] [PubMed] [Google Scholar]
- Deligdisch L. Hormonal pathology of the endometrium. Mod Pathol 2000;13:285–294. [DOI] [PubMed] [Google Scholar]
- Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol 2005;97:924–927. [DOI] [PubMed] [Google Scholar]
- Diaz-Padilla I, Romero N, Amir E, Matias-Guiu X, Vilar E, Muggia F, Garcia-Donas J. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2013;88:154–167. [DOI] [PubMed] [Google Scholar]
- Diep CH, Ahrendt H, Lange CA. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids 2016;114:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhafey AS, Papadimitriou JC, El-Hakim MS, El-Said AI, Ghannam BB, Silverberg SG. Computerized image analysis of p53 and proliferating cell nuclear antigen expression in benign, hyperplastic, and malignant endometrium. Arch Pathol Lab Med 2001;125:872–879. [DOI] [PubMed] [Google Scholar]
- Ellenson LH, Ronnett BM, Kurman RJ. Precursor lesions of endometrial carcinoma In: Kurman RJ, Ellenson LH, Ronnett BM (eds).. Blaustein's Pathology of the Female Genital Tract. Boston, MA: Springer, 2011;359–392. [Google Scholar]
- Emons G, Beckmann M, Schmidt D, Mallmann P. New WHO classification of endometrial hyperplasias. Geburtshilfe Frauenheilkd 2015;75:135–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkanli S, Kayaselcuk F, Kuscu E, Bagis T, Bolat F, Haberal A, Demirhan B. Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int J Gynecol Cancer 2006;16:1412–1418. [DOI] [PubMed] [Google Scholar]
- Erkanli S, Bolat F, Kayaselcuk F, Demirhan B, Kuscu E. COX-2 and survivin are overexpressed and positively correlated in endometrial carcinoma. Gynecol Oncol 2007;104:320–325. [DOI] [PubMed] [Google Scholar]
- Eshleman JR, Markowitz SD. Mismatch repair defects in human carcinogenesis. Hum Mol Genet 1996;5:1489–1494. [DOI] [PubMed] [Google Scholar]
- Esteller M, Catasus L, Matias-Guiu X, Mutter GL, Prat J, Baylin SB, Herman JG. hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol 1999;155:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faloppa CC, Baiocchi G, Cunha IW, Fregnani JHTG, Osorio CABT, Fukazawa EM, Kumagai LY, Badiglian-Filho L, Pinto GLS, Soares FA. NF-κB and COX-2 expression in nonmalignant endometrial lesions and cancer. Am J Clin Pathol 2014;141:196–203. [DOI] [PubMed] [Google Scholar]
- Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol 1989;160:126–131. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J cancer 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991;113:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishita A, Chavez RO, Nakane PK, Yamabe T, Koji T, Ishimaru T, Masuzaki H. Expression of estrogen and progesterone receptors in endometrium and peritoneal endometriosis: an immunohistochemical and in situ hybridization study. Fertil Steril 1997;67:856–864. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol 1998;69:220–225. [DOI] [PubMed] [Google Scholar]
- Gadkar-Sable S, Shah C, Rosario G, Sachdeva G, Puri C. Progesterone receptors: various forms and functions in reproductive tissues. Front Biosci 2005;10:2118–2130. [DOI] [PubMed] [Google Scholar]
- Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol 2010;203:547.e1–10. [DOI] [PubMed] [Google Scholar]
- Gallos ID, Alazzam M, Clark T, Faraj R, Rosenthal A, Smith P, Gupta J RCOG Green-top Guideline: Management of Endometrial Hyperplasia. 2016. https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf. (2 May 2016, date last accessed).
- Garg K, Leitao MM, Wynveen CA, Sica GL, Shia J, Shi W, Soslow RA. P53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol 2010;23:80–92. [DOI] [PubMed] [Google Scholar]
- Geisinger KR, Homesley HD, Morgan MT, Kute TE, Marshall R. Endometrial adenocarcinoma: a multiparameter clinicopathologic analysis including the DNA profile and the sex steroid hormone receptors. Cancer 1986;58:1518–1525. [DOI] [PubMed] [Google Scholar]
- Ghabreau, Roux JP, Niveleau A, Fontanière B, Mahe CC, Mokni M, Frappart L. Correlation between the DNA global methylation status and progesterone receptor expression in normal endometrium, endometrioid adenocarcinoma and precursors. Virchows Arch 2004;445:129–134. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Saunders PTK. Estrogen dependent signaling in reproductive tissues - a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol 2012;348:361–372. [DOI] [PubMed] [Google Scholar]
- Gibson DA, Saunders PTK. Endocrine disruption of oestrogen action and female reproductive tract cancers. Endocr Relat Cancer 2014;21:13–31. [DOI] [PubMed] [Google Scholar]
- Giuntoli R, Zacur H, Goff B, Garcia R, Falk S Classification and Diagnosis of Endometrial Hyperplasia. 2014. http://www.uptodate.com/contents/classification-and-diagnosis-of-endometrial-hyperplasia?source=see_link. (2 May 2016, date last accessed).
- van Gool IC, Eggink FA, Freeman-Mills L, Stelloo E, Marchi E, de Bruyn M, Palles C, Nout RA, de Kroon CD, Osse EM et al. POLE proofreading mutations elicit an anti-tumor immune response in endometrial cancer. Clin Cancer Res 2015;21:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev 1997;18:502–519. [DOI] [PubMed] [Google Scholar]
- Guan B, Mao T, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng Y, Wang T, Shih I. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol 2011;35:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Mandai M, Konishi I, Nanbu K, Tsuruta Y, Kusakari T, Kariya M, Kita M, Fujii S. Cyclical change of hMSH2 protein expression in normal endometrium during the menstrual cycle and its overexpression in endometrial hyperplasia and sporadic endometrial carcinoma. Cancer 2002;94:997–1005. [PubMed] [Google Scholar]
- Hannemann MM, Alexander HM, Cope NJ, Acheson N, Phillips A. Endometrial hyperplasia: a clinician's review. Obstet Gynaecol Reprod Med 2010;20:116–120. [Google Scholar]
- Hapangama DK, Critchley HOD, Henderson TA, Baird DT. Mifepristone-induced vaginal bleeding is associated with increased immunostaining for cyclooxygenase-2 and decrease in prostaglandin dehydrogenase in luteal phase endometrium. J Clin Endocrinol Metab 2002;87:5229–5234. [DOI] [PubMed] [Google Scholar]
- Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E, Terakawa N. Apoptosis in human endometrium and endometriosis. Hum Reprod Update 2004;10:29–38. [DOI] [PubMed] [Google Scholar]
- Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet 2003;361:1810–1812. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Niwa O, Nitta Y, Takeichi M, Yokoro K. Unstable expression of E-cadherin adhesion molecules in metastatic ovarian tumor cells. Jpn J Cancer Res 1989;80:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol 2006;24:4783–4791. [DOI] [PubMed] [Google Scholar]
- Hecht JL, Ince TA, Baak JPA, Baker HE, Ogden MW, Mutter GL. Prediction of endometrial carcinoma by subjective endometrial intraepithelial neoplasia diagnosis. Mod Pathol 2005;18:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks YMC, de Jong AE, Morreau H, Tops CMJ, Vasen HF, Wijnen JT, Breuning MH, Bröcker-Vriends AHJT. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 2006;56:213–225. [DOI] [PubMed] [Google Scholar]
- Horrée N, van Diest PJ, Sie-Go DMDS, Heintz APM. The invasive front in endometrial carcinoma: higher proliferation and associated derailment of cell cycle regulators. Hum Pathol 2007;38:1232–1238. [DOI] [PubMed] [Google Scholar]
- Hu K, Zhong G, He F. Expression of estrogen receptors ERalpha and ERbeta in endometrial hyperplasia and adenocarcinoma. Int J Gynecol Cancer 2008;15:537–541. [DOI] [PubMed] [Google Scholar]
- Isobe M, Emanuel BS, Givol D, Oren M, Croce CM. Localization of gene for human p53 tumour antigen to band 17p13. Nature 1986;320:84–85. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Boddy SC. Prostaglandin E2 induces proliferation of glandular epithelial cells of the human endometrium via extracellular regulated kinase 1/2-mediated pathway. J Clin Endocrinol Metab 2003;88:4481–4487. [DOI] [PubMed] [Google Scholar]
- Joiner AK, Quick CM, Jeffus SK. Pax2 expression in simultaneously diagnosed WHO and EIN classification systems. Int J Gynecol Pathol 2015;34:40–46. [DOI] [PubMed] [Google Scholar]
- Jones A, Teschendorff AE, Li Q, Hayward JD, Kannan A, Mould T, West J, Zikan M, Cibula D, Fiegl H et al. Role of DNA methylation and epigenetic silencing of HAND2 in endometrial cancer development. PLoS Med 2013;10:e1001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic AS, Boynton KA, Mutter GL. Uteri of women with endometrial carcinoma contain a histopathological spectrum of monoclonal putative precancers, some with microsatellite instability. Cancer Res 1996;56:1917–1921. [PubMed] [Google Scholar]
- Kadar N, Malfetano JH, Homesley HD. Steroid receptor concentrations in endometrial carcinoma: effect on survival in surgically staged patients. Gynecol Oncol 1993;50:281–286. [DOI] [PubMed] [Google Scholar]
- Kahraman K, Kiremitci S, Taskin S, Kankaya D, Sertcelik A, Ortac F. Expression pattern of PAX2 in hyperplastic and malignant endometrium. Arch Gynecol Obstet 2012;286:173–178. [DOI] [PubMed] [Google Scholar]
- Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane SE, Hecht JL. Endometrial intraepithelial neoplasia terminology in practice: 4-year experience at a single institution. Int J Gynecol Pathol 2012;31:160–165. [DOI] [PubMed] [Google Scholar]
- Kapucuoglu N, Aktepe F, Kaya H, Bircan S, Karahan N, Ciriş M, Çiriş M. Immunohistochemical expression of PTEN in normal, hyperplastic and malignant endometrium and its correlation with hormone receptors, bcl-2, bax, and apoptotic index. Pathol Res Pract 2007;203:153–162. [DOI] [PubMed] [Google Scholar]
- Karamurzin Y, Rutgers JKL. DNA mismatch repair deficiency in endometrial carcinoma. Int J Gynecol Pathol 2009;28:239–255. [DOI] [PubMed] [Google Scholar]
- Kendall BS, Ronnett BM, Isacson C, Cho KR, Hedrick L, Diener-West M, Kurman RJ. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol 1998;22:1012–1019. [DOI] [PubMed] [Google Scholar]
- Kimura F, Watanabe J, Hata H, Fujisawa T, Kamata Y, Nishimura Y, Jobo T, Kuramoto H. PTEN immunohistochemical expression is suppressed in G1 endometrioid adenocarcinoma of the uterine corpus. J Cancer Res Clin Oncol 2004;130:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol 1990;38:59–65. [DOI] [PubMed] [Google Scholar]
- Kokawa K, Shikone T, Otani T, Nishiyama R, Ishii Y, Yagi S, Yamoto M. Apoptosis and the expression of Bax and Bcl-2 in hyperplasia and adenocarcinoma of the uterine endometrium. Hum Reprod 2001;16:2211–2118. [DOI] [PubMed] [Google Scholar]
- Kurman R, Carcangiu M, Herrington C, Young R. World Health Organisation Classification of Tumors of Female Reproductive Organs, 4th edn Lyon France: International Agency for Research on Cancer (IARC) Press, 2014. [Google Scholar]
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of ‘untreated’ hyperplasia in 170 patients. Cancer 1985;56:403–412. [DOI] [PubMed] [Google Scholar]
- Lacey JV, Ioffe OB, Ronnett BM, Rush BB, Richesson DA, Chatterjee N, Langholz B, Glass AG, Sherman ME. Endometrial carcinoma risk among women diagnosed with endometrial hyperplasia: the 34-year experience in a large health plan. Br J Cancer 2008. a;98:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey JV, Mutter GL, Nucci MR, Ronnett BM, Ioffe OB, Rush BB, Glass AG, Richesson DA, Chatterjee N, Langholz B et al. Risk of subsequent endometrial carcinoma associated with endometrial intraepithelial neoplasia classification of endometrial biopsies. Cancer 2008. b;113:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP. Cancer. p53, guardian of the genome. Nature 1992;358:15–16. [DOI] [PubMed] [Google Scholar]
- Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol 2007;73:1–14. [DOI] [PubMed] [Google Scholar]
- Latta E, Chapman WB. PTEN mutations and evolving concepts in endometrial neoplasia. Curr Opin Obstet Gynecol 2002;14:59–65. [DOI] [PubMed] [Google Scholar]
- Le Gallo M, O'Hara AJ, Rudd ML, Urick ME, Hansen NF, O'Neil NJ, Price JC, Zhang S, England BM, Godwin AK et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet 2012;44:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Choi HJ, Kang CS, Lee HJ, Lee WS, Park CS. Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma. Mod Pathol 2012;25:1508–1515. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 1988;67:334–340. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod 2002;17:2725–2736. [DOI] [PubMed] [Google Scholar]
- Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011;331:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Feng Y, Lin J-F, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci 2014;21:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Siu MKY, Au CWH, Chan QKY, Chan HY, Wong ESY, Ip PPC, Ngan HYS, Cheung ANY. Aberrant activation of hedgehog signaling pathway contributes to endometrial carcinogenesis through beta-catenin. Mod Pathol 2009;22:839–847. [DOI] [PubMed] [Google Scholar]
- Lidor A, Ismajovich B, Confino E, David MP. Histopathological findings in 226 women with post-menopausal uterine bleeding. Acta Obstet Gynecol Scand 1986;65:41–43. [DOI] [PubMed] [Google Scholar]
- Lukes AS, Kohler MF, Pieper CF, Kerns BJ, Bentley R, Rodriguez GC, Soper JT, Clarkepearson DL, Bast RC, Berchuck A. Multivariable analysis of DNA ploidy, p53, and Her-2/Neu as prognostic factors in endometrial cancer. Cancer 1994;73:2380–2385. [DOI] [PubMed] [Google Scholar]
- Mackenzie R, Talhouk A, Eshragh S, Lau S, Cheung D, Chow C, Le N, Cook LS, Wilkinson N, McDermott J et al. Morphologic and molecular characteristics of mixed epithelial ovarian cancers. Am J Surg Pathol 2015;39:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh ML, Crosbie EJ. Obesity-driven endometrial cancer: is weight loss the answer. BJOG 2013;120:791–794. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Stoykova A, Gruss P. Pax genes in development. J Cell Sci Suppl 1994;18:35–42. [DOI] [PubMed] [Google Scholar]
- Mao T-L, Shih I-M. The roles of ARID1A in gynecologic cancer. J Gynecol Oncol 2013;24:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T-L, Ardighieri L, Ayhan A, Kuo K-T, Wu C-H, Wang T-L, Shih I-M. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol 2013;37:1342–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall E, Lowrey J, MacPherson S, Maybin JA, Collins F, Critchley HOD, Saunders PTK. In silico analysis identifies a novel role for androgens in the regulation of human endometrial apoptosis. J Clin Endocrinol Metab 2011;96:E1746–E1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology 2013;62:111–123. [DOI] [PubMed] [Google Scholar]
- McConechy MK, Ding J, Cheang MCU, Wiegand KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 2012;228:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng B, Hoang LN, McIntyre JB, Duggan MA, Nelson GS, Lee CH, Köbel M. POLE exonuclease domain mutation predicts long progression-free survival in grade 3 endometrioid carcinoma of the endometrium. Gynecol Oncol 2014;134:15–19. [DOI] [PubMed] [Google Scholar]
- Mitselou A, Ioachim E, Kitsou E, Vougiouklakis T, Zagorianakou N, Makrydimas G, Stefanaki S, Agnantis NJ. Immunohistochemical study of apoptosis-related Bcl-2 protein and its correlation with proliferation indices (Ki67, PCNA), tumor suppressor genes (p53, pRb), the oncogene c-erbB-2, sex steroid hormone receptors and other clinicopathological features, in no. In Vivo 2003;17:469–477. [PubMed] [Google Scholar]
- Monte NM, Webster KA, Neuberg D, Dressler GR, Mutter GL. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res 2010;70:6225–6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol 2002;186:651–657. [DOI] [PubMed] [Google Scholar]
- Moreno-Bueno G, Hardisson D, Sarrió D, Sánchez C, Cassia R, Prat J, Herman JG, Esteller M, Matías-Guiu X, Palacios J. Abnormalities of E- and P-cadherin and catenin (beta-, gamma-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol 2003;199:471–478. [DOI] [PubMed] [Google Scholar]
- Morin PJ. Beta-catenin signaling and cancer. Bioessays 1999;21:1021–1030. [DOI] [PubMed] [Google Scholar]
- Morsi HM, Leers MP, Jager W, Bjorklund V, Radespiel-Troger M, el Kabarity H, Nap M, Lang N. The patterns of expression of an apoptosis-related CK18 neoepitope, the bcl-2 proto-oncogene, and the Ki67 proliferation marker in normal, hyperplastic, and malignant endometrium. Int J Gynecol Pathol 2000;19:118–126. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 1999;84:2963–2971. [DOI] [PubMed] [Google Scholar]
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014;15:e268–e278. [DOI] [PubMed] [Google Scholar]
- Mutter G, Lin M, Fitzgerald J, Kum J, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle 1. J Clin Endocrinol Metab 2000. a;85:2334–2338. [DOI] [PubMed] [Google Scholar]
- Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol 2000. a;76:287–290. [DOI] [PubMed] [Google Scholar]
- Mutter GL. Histopathology of genetically defined endometrial precancers. Int J Gynecol Pathol 2000. b;19:301–309. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Boynton KA, Faquin WC, Ruiz RE, Jovanovic AS. Allelotype mapping of unstable microsatellites establishes direct lineage continuity between endometrial precancers and cancer. Cancer Res 1996;56:4483–4486. [PubMed] [Google Scholar]
- Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000. b;92:924–930. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Ince TA, Baak JPA, Kust GA, Zhou XP, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res 2001;61:4311–4314. [PubMed] [Google Scholar]
- Mutter GL, Zaino RJ, Baak JPA, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol 2007;26:103–114. [DOI] [PubMed] [Google Scholar]
- Mutter GL, Kauderer J, Baak JPA, Alberts D. Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: a Gynecologic Oncology Group study. Hum Pathol 2008;39:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter GL, Monte NM, Neuberg D, Ferenczy A, Eng C. Emergence, involution, and progression to carcinoma of mutant clones in normal endometrial tissues. Cancer Res 2014;74:2796–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir A, Boulware D, Kaiser HE, Lancaster JM, Coppola D, Smith PV, Hakam A, Siegel SE, Bodey B. Cyclooxygenase-2 (COX-2) expression in human endometrial carcinoma and precursor lesions and its possible use in cancer chemoprevention and therapy. In Vivo 2007;21:35–43. [PubMed] [Google Scholar]
- Norimatsu Y, Moriya T, Kobayashi TK, Sakurai T, Shimizu K, Tsukayama C, Ohno E. Immunohistochemical expression of PTEN and beta-catenin for endometrial intraepithelial neoplasia in Japanese women. Ann Diagn Pathol 2007;11:103–108. [DOI] [PubMed] [Google Scholar]
- Nunobiki O, Taniguchi E, Ishii A, Tang W, Utsunomiya H, Nakamura Y, Mori I, Kakudo K. Significance of hormone receptor status and tumor vessels in normal, hyperplastic and neoplastic endometrium. Pathol Int 2003;53:846–852. [DOI] [PubMed] [Google Scholar]
- Nunobiki O, Nakamura M, Taniguchi E, Utsunomiya H, Mori I, Tsubota Y, Mabuchi Y, Kakudo K. Adrenomedullin, Bcl-2 and microvessel density in normal, hyperplastic and neoplastic endometrium. Pathol Int 2009;59:530–536. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993;74:609–619. [DOI] [PubMed] [Google Scholar]
- Orbo A, Nilsen MN, Arnes MS, Pettersen I, Larsen K. Loss of expression of MLH1, MSH2, MSH6, and PTEN related to endometrial cancer in 68 patients with endometrial hyperplasia. Int J Gynecol Pathol 2003;22:141–148. [DOI] [PubMed] [Google Scholar]
- Orejuela FJ, Ramondetta LM, Smith J, Brown J, Lemos LB, Li Y, Hollier LM. Estrogen and progesterone receptors and cyclooxygenase-2 expression in endometrial cancer, endometrial hyperplasia, and normal endometrium. Gynecol Oncol 2005;97:483–488. [DOI] [PubMed] [Google Scholar]
- Owings RA, Quick CM. Endometrial intraepithelial neoplasia. Arch Pathol Lab Med 2014;138:484–491. [DOI] [PubMed] [Google Scholar]
- Ozdegirmenci O, Kayikcioglu F, Bozkurt U, Akgul MA, Haberal A. Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecol Obstet Invest 2011;72:10–14. [DOI] [PubMed] [Google Scholar]
- Ozkara SK, Corakci A. Significantly decreased P27 expression in endometrial carcinoma compared to complex hyperplasia with atypia (correlation with p53 expression). Pathol Oncol Res 2004;10:89–97. [DOI] [PubMed] [Google Scholar]
- Palmer JE, Perunovic B, Tidy JA. Endometrial hyperplasia. The Obstetrician & Gynaecologist 2008;10:211–216. [Google Scholar]
- Pavlakis K, Messini I, Vrekoussis T, Panoskaltsis T, Chrissanthakis D, Yiannou P, Stathopoulos EN. PTEN-loss and nuclear atypia of EIN in endometrial biopsies can predict the existence of a concurrent endometrial carcinoma. Gynecol Oncol 2010;119:516–519. [DOI] [PubMed] [Google Scholar]
- Peiró G, Diebold J, Baretton GB, Kimmig R, Löhrs U. Cellular apoptosis susceptibility gene expression in endometrial carcinoma: correlation with Bcl-2, Bax, and caspase-3 expression and outcome. Int J Gynecol Pathol 2001;20:359–367. [DOI] [PubMed] [Google Scholar]
- Pieczyńska B, Wojtylak S, Zawrocki A, Biernat W. Analysis of PTEN, estrogen receptor α and progesterone receptor expression in endometrial hyperplasia using tissue microarray. Pol J Pathol 2011;62:133–138. [PubMed] [Google Scholar]
- Poulogiannis G, Frayling IM, Arends MJ. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology 2010;56:167–179. [DOI] [PubMed] [Google Scholar]
- Quick CM, Laury AR, Monte NM, Mutter GL. Utility of PAX2 as a marker for diagnosis of endometrial intraepithelial neoplasia. Am J Clin Pathol 2012;138:678–684. [DOI] [PubMed] [Google Scholar]
- Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol 2004;95:133–138. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mini-review: cellular mechanisms of disease series; Bcl-2 and the regulation of programmed cell death. J Cell Biol 1994;124:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, Newton KM, Clinton WL, Epplein M, Garcia R, Allison K, Voigt LF, Weiss NS. Incidence of endometrial hyperplasia. Am J Obstet Gynecol 2009;200:678.e1–678.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Soerjomataram I, Tyson M, Egger M, Zwahlen M, Coebergh JW, Buchan I. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer 2010;126:692–702. [DOI] [PubMed] [Google Scholar]
- Risberg B, Karlsson K, Abeler V, Lagrelius A, Davidson B, Karlsson MG. Dissociated expression of Bcl-2 and Ki-67 in endometrial lesions: diagnostic and histogenetic implications. Int J Gynecol Pathol 2002;21:155–160. [DOI] [PubMed] [Google Scholar]
- Robson EJD, He S-J, Eccles MR. A PANorama of PAX genes in cancer and development. Nat Rev Cancer 2006;6:52–62. [DOI] [PubMed] [Google Scholar]
- Ryan G, Steele-Perkins V, Morris JF, Rauscher FJ, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development 1995;121:867–875. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Kamata Y, Isono M, Okayasu I. Bcl-2 expression is correlated with a low apoptotic index and associated with progesterone receptor immunoreactivity in endometrial carcinomas. J Pathol 1996;180:275–282. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Hashimura M, Yoshida T, Okayasu I. Beta-catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 2001;84:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuragi N, Salah-eldin A, Watari H, Itoh T, Inoue S, Moriuchi T, Fujimoto S. Bax, Bcl-2, and p53 expression in endometrial cancer. Gynecol Oncol 2002;86:288–296. [DOI] [PubMed] [Google Scholar]
- Salvesen HB, Carter SL, Mannelqvist, Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB, Trovik J et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A 2009;106:4834–4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar S. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol 2009;4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten AN, Creutzberg CL, van den Broek LJCM, Noordijk EM, Smit VTHBM. Nuclear beta-catenin is a molecular feature of type I endometrial carcinoma. J Pathol 2003;201:460–465. [DOI] [PubMed] [Google Scholar]
- Scully RE, Bonfiglio TA, Kurman RJ, Silverberg SG, Wilkinson EJ. Histological Typing of Female Genital Tract Tumours. Heidelberg, Berlin, Germany: Springer, 1994;1–189. [Google Scholar]
- Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A 1995;92:7834–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiee MN, Khan G, Ariffin R, Abu J, Chapman C, Deen S, Nunns D, Barrett DA, Seedhouse C, Atiomo W. Preventing endometrial cancer risk in polycystic ovarian syndrome (PCOS) women: could metformin help. Gynecol Oncol 2014;132:248–253. [DOI] [PubMed] [Google Scholar]
- Sherman AI, Brown S. The precursors of endometrial carcinoma. Am J Obstet Gynecol 1979;135:947–956. [DOI] [PubMed] [Google Scholar]
- Silverberg S, Kurman R, Nogales F, Mutter G, Kubik-Huch R, Tavassoli F. Epithelial tumours and related lesions In: World Health Organization Classification of Tumours: Pathology and Genetics - Tumours of the Breast and Female Genital Organs. Lyon, France: IARC Press, 2003;271–232. [Google Scholar]
- Simpkins SB, Bocker T, Swisher EM, Mutch DG, Gersell DJ, Kovatich AJ, Palazzo JP, Fishel R, Goodfellow PJ. MLH1 promoter methylation and gene silencing is the primary cause of microsatellite instability in sporadic endometrial cancers. Hum Mol Genet 1999;8:661–666. [DOI] [PubMed] [Google Scholar]
- Sivridis E, Giatromanolaki A, Koukourakis M, Anastasiadis P. Endometrial carcinoma: association of steroid hormone receptor expression with low angiogenesis and bcl-2 expression. Virchows Arch 2001;438:470–477. [DOI] [PubMed] [Google Scholar]
- Skov BG, Broholm H, Engel U, Franzmann MB, Nielsen AL, Lauritzen AF, Skov T. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol 1997;16:33–37. [DOI] [PubMed] [Google Scholar]
- Snijders MP, de Goeij AF, Debets-Te Baerts MJ, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil 1992;94:363–371. [DOI] [PubMed] [Google Scholar]
- Soong R, Robbins PD, Dix BR, Grieu F, Lim B, Knowles S, Williams KE, Turbett GR, House AK, Iacopetta BJ. Concordance between p53 protein overexpression and gene mutation in a large series of common human carcinomas. Hum Pathol 1996;27:1050–1055. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten(+/-) mice. Cancer Res 2000;60:3605–3611. [PubMed] [Google Scholar]
- Steinbakk A, Malpica A, Slewa A, Gudlaugsson E, Janssen EAM, Arends M, Kruse AJ, Yinhua Y, Feng W, Baak JP. High frequency microsatellite instability has a prognostic value in endometrial endometrioid adenocarcinoma, but only in FIGO stage 1 cases. Cell Oncol (Dordr) 2011. a;34:457–465. [DOI] [PubMed] [Google Scholar]
- Steinbakk A, Gudlaugsson E, Aasprong OG et al. Molecular biomarkers in endometrial hyperplasias predict cancer progression. Am J Obstet Gynecol 2011. b;204:357–e1-e12. [DOI] [PubMed] [Google Scholar]
- Sutton GP, Geisler HE, Stehman FB, Young PCM, Kimes TM, Ehrlich CE. Features associated with survival and disease-free survival in early endometrial cancer. Am J Obstet Gynecol 1989;160:1385–1393. [DOI] [PubMed] [Google Scholar]
- Takeda T, Banno K, Okawa R, Yanokura M, Iijima M, Irie-Kunitomi H, Nakamura K, Iida M, Adachi M, Umene K et al. ARID1A gene mutation in ovarian and endometrial cancers (Review). Oncol Rep 2015;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 2015;113:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangen IL, Werner HMJ, Berg A, Halle MK, Kusonmano K, Trovik J, Hoivik EA, Mills GB, Krakstad C, Salvesen HB. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur J Cancer 2014;50:3003–3010. [DOI] [PubMed] [Google Scholar]
- Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBoist RN, Dey SK. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-δ in human endometrial adenocarcinoma1. Neoplasia 2000;2:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G-X, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Müllerian system origin. Mod Pathol 2007;20:856–863. [DOI] [PubMed] [Google Scholar]
- Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ, Alberts D, Curtin J, Mackey D. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer 2006;106:812–819. [DOI] [PubMed] [Google Scholar]
- Trimble CL, Method M, Leitao M, Lu K, Ioffe O, Hampton M, Higgins R, Zaino R, Mutter GL. Management of endometrial precancers. Obstet Gynecol 2012;120:1160–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchikawa J, Shiozawa T, Shih HC, Miyamoto T, Feng YZ, Kashima H, Oka K, Konishi I. Expression of steroid receptor coactivators and corepressors in human endometrial hyperplasia and carcinoma with relevance to steroid receptors and Ki-67 expression. Cancer 2003;98:2207–2213. [DOI] [PubMed] [Google Scholar]
- Upson K, Allison KH, Reed SD, Jordan CD, Newton KM, Swisher EM, Doherty JA, Garcia RL. Biomarkers of progestin therapy resistance and endometrial hyperplasia progression. Am J Obstet Gynecol 2012;207:36.e1–36.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usubutun A, Mutter GL, Saglam A, Dolgun A, Ozkan EA, Ince T, Akyol A, Bulbul HD, Calay Z, Eren F et al. Reproducibility of endometrial intraepithelial neoplasia diagnosis is good, but influenced by the diagnostic style of pathologists. Mod Pathol 2012;25:877–884. [DOI] [PubMed] [Google Scholar]
- VanDusen NJ, Casanovas J, Vincentz JW, Firulli BA, Osterwalder M, Lopez-Rios J, Zeller R, Zhou B, Grego-Bessa J, De La Pompa JL et al. Hand2 is an essential regulator for two Notch-dependent functions within the embryonic endocardium. Cell Rep 2014;9:2071–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaskivuo TE, Stenbäck F, Tapanainen JS. Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer 2002;95:1463–1471. [DOI] [PubMed] [Google Scholar]
- Vilar E, Gruber SB. Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol 2010;7:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villacorte, Suzuki K, Hirasawa A, Ohkawa Y, Suyama M, Maruyama T, Aoki D, Ogino Y, Miyagawa S, Terabayashi T et al. β-Catenin signaling regulates Foxa2 expression during endometrial hyperplasia formation. Oncogene 2012;32:3477–3482. [DOI] [PubMed] [Google Scholar]
- Vineis P, Schatzkin A, Potter JD. Models of carcinogenesis: an overview. Carcinogenesis 2010;31:1703–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer—A clinical and pathological evaluation. Gynecol Oncol 2012;124:15–20. [DOI] [PubMed] [Google Scholar]
- Wallace AE, Gibson DA, Saunders PTK, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. J Endocrinol 2010;206:141–157. [DOI] [PubMed] [Google Scholar]
- Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod 1998;4:407–412. [DOI] [PubMed] [Google Scholar]
- Wang H, Douglas W, Lia M, Edelmann W, Kucherlapati R, Podsypanina K, Parsons R, Ellenson LH. DNA mismatch repair deficiency accelerates endometrial tumorigenesis in Pten heterozygous mice. Am J Pathol 2002;160:1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner HMJ, Berg, Wik E, Birkeland E, Krakstad C, Kusonmano K, Petersen K, Kalland KH, Oyan AM, Akslen LA et al. ARID1A loss is prevalent in endometrial hyperplasia with atypia and low-grade endometrioid carcinomas. Mod Pathol 2012;26:428–434. [DOI] [PubMed] [Google Scholar]
- Wiegand KC, Lee AF, Al-Agha OM, Chow, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 2011;224:328–333. [DOI] [PubMed] [Google Scholar]
- Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999;18:7908–7916. [DOI] [PubMed] [Google Scholar]
- Wise MR, Jordan V, Lagas A, Showell M, Wong N, Lensen S, Farquhar CM. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol 2016;214:689.e1–689.e17. [DOI] [PubMed] [Google Scholar]
- Woo YL, Cheah PL, Shahruddin SI, Omar SZ, Arends M. The immunohistochemistry signature of mismatch repair (MMR) proteins in a multiethnic Asian cohort with endometrial carcinoma. Int J Gynecol Pathol 2014;33:554–559. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Xiong YY, Zhou YF. Expression and significance of beta-catenin, Glut-1 and PTEN in proliferative endometrium, endometrial intraepithelial neoplasia and endometrioid adenocarcinoma. Eur J Gynaecol Oncol 2010;31:160–164. [PubMed] [Google Scholar]
- Zaino R, Carinelli S, Ellenson L. Uterine corpus: epithelial tumors and precursors In: Kurman R, Carcangiu M, Herrington C, Young R (eds).. World Health Organization Classification of Tumors of Female Reproductive Organs, 4th edn Lyon, France: International Agency for Research on Cancer (IARC) Press, 2014;125–135. [Google Scholar]
- Zannoni GF, Monterossi G, De Stefano I, Gargini A, Salerno MG, Farulla I, Travaglia D, Vellone VG, Scambia G, Gallo D. The expression ratios of estrogen receptor α (ERα) to estrogen receptor β1 (ERβ1) and ERα to ERβ2 identify poor clinical outcome in endometrioid endometrial cancer. Hum Pathol 2013;44:1047–1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.