Abstract
STUDY QUESTION
Are parity and the timing of menarche associated with premature and early natural menopause?
SUMMARY ANSWER
Early menarche (≤11 years) is a risk factor for both premature menopause (final menstrual period, FMP <40 years) and early menopause (FMP 40–44 years), a risk that is amplified for nulliparous women.
WHAT IS KNOWN ALREADY
Women with either premature or early menopause face an increased risk of chronic conditions in later life and of early death. Findings from some studies suggest that early menarche and nulliparity are associated with early menopause, however overall the evidence is mixed. Much of the evidence for a direct relationship is hampered by a lack of comparability across studies, failure to adjust for confounding factors and inadequate statistical power.
STUDY DESIGN, SIZE, DURATION
This pooled study comprises 51 450 postmenopausal women from nine observational studies in the UK, Scandinavia, Australia and Japan that contribute to the International collaboration for a Life course Approach to reproductive health and Chronic disease Events (InterLACE).
PARTICIPANTS/MATERIALS, SETTING, METHODS
Age at menarche (categorized as ≤11, 12, 13, 14 and 15 or more years) and parity (categorized as no children, one child and two or more children) were exposures of interest. Age at FMP was confirmed by at least 12 months of cessation of menses where this was not the result of an intervention (such as surgical menopause due to bilateral oophorectomy or hysterectomy) and categorized as premature menopause (FMP before age 40), early menopause (FMP 40–44 years), 45–49 years, 50–51 years, 52–53 years and 54 or more years. We used multivariate multinomial logistic regression models to estimate relative risk ratio (RRR) and 95% CI for associations between menarche, parity and age at FMP adjusting for within-study correlation.
MAIN RESULTS AND THE ROLE OF CHANCE
The median age at FMP was 50 years (interquartile range 48–53 years), with 2% of the women experiencing premature menopause and 7.6% early menopause. Women with early menarche (≤11 years, compared with 12–13 years) were at higher risk of premature menopause (RRR 1.80, 95% CI 1.53–2.12) and early menopause (1.31, 1.19–1.44). Nulliparity was associated with increased risk of premature menopause (2.26, 1.84–2.77) and early menopause (1.32, 1.09–1.59). Women having early menarche and nulliparity were at over 5-fold increased risk of premature menopause (5.64, 4.04–7.87) and 2-fold increased risk of early menopause (2.16, 1.48–3.15) compared with women who had menarche at ≥12 years and two or more children.
LIMITATIONS, REASONS FOR CAUTION
Most of the studies (except the birth cohorts) relied on retrospectively reported age at menarche, which may have led to some degree of recall bias.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings support early monitoring of women with early menarche, especially those who have no children, for preventive health interventions aimed at mitigating the risk of adverse health outcomes associated with early menopause.
STUDY FUNDING/COMPETING INTEREST(S)
InterLACE project is funded by the Australian National Health and Medical Research Council project grant (APP1027196). G.D.M. is supported by Australian Research Council Future Fellowship (FT120100812). There are no competing interests.
Keywords: menarche, parity, premature menopause, early menopause, reproductive health, final menstrual period, InterLACE
Introduction
Women are usually defined as having ‘early menopause’ if they experience their final menstrual period (FMP) between the ages 40–44 years, which is well before the median age of natural menopause of 51 years for Western countries (North American Menopause Society, 2007). Women diagnosed with ‘premature ovarian failure’ failure following extended amenorrhoea, hypergonadotropinemia or oestrogen deficiency (Nippita and Baber, 2007) may experience menopause before the age of 40 and are usually classified as having ‘premature menopause’ (Shuster et al., 2010). These categories for the timing of natural menopause are distinct from when cessation of menses has occurred as a result of medical interventions, such as chemotherapy or bilateral oophorectomy (sometimes referred to as surgical menopause). Women with either premature or early menopause face increased risk of early death and are more likely to suffer from chronic conditions in later life, including cardiovascular disease, Type 2 diabetes and osteoporosis (Gold, 2011; Muka et al., 2016).
Numerous factors influence the timing of the menopause, from genetic and developmental factors to the cumulative effects of hormonal, environmental and lifestyle exposures. For instance, a mother's age at menopause is correlated with her daughter's age at menopause (van Asselt et al., 2004; Forman et al., 2013; He and Murabito, 2014); higher parity is associated with older age at menopause (Gold, 2011); and smoking and teetotalism are established risk factors for younger age at menopause (Gold, 2011; Schoenaker et al., 2014; Taneri et al., 2016). Early menarche is suggested to be associated with the early menopause. However, studies that have examined the links between the timing of menarche and the age at FMP show mixed findings (Hardy and Kuh, 1999; Gold, 2011; Farahmand et al., 2013). Much of the evidence for a direct relationship is hampered by a lack of comparability across studies, including definitional differences of FMP, and lack of adjustment for confounding factors (Gold, 2011; Forman et al., 2013). Lack of statistical power also remains a key issue (Nippita and Baber, 2007).
This study used data from over 50 000 postmenopausal women from populations in the UK, Scandinavia, Australia and Japan to examine associations between the age at menarche and parity with premature menopause and early menopause, while taking into account a range of potential confounding factors. To achieve this objective, we pooled participant-level data from selected studies that contribute to the International collaboration for a Life course Approach to reproductive health and Chronic disease Events (InterLACE) (Mishra et al., 2013, 2016).
Materials and Methods
Ethics
Each study in the InterLACE has been undertaken with ethical approval from the institutional review boards at each participating institution, and all participants provided written informed consent.
Study participants
InterLACE has brought together 20 observational, mostly longitudinal cohort studies with data on women's health. A more detailed description of the InterLACE collaboration has been published previously (Mishra et al., 2013, 2016). Participating studies collected prospective as well as retrospective self-reported survey data on key reproductive, sociodemographic and lifestyle variables. For these analyses, studies were included only if their criteria for sample selection were not based on the menopausal status of the women and data collection included information on the key variables of interest for this analysis, such as age at menarche and age at menopause. The resultant nine studies used for pooling data at the individual level were Australian Longitudinal Study on Women's Health (ALSWH) (Lee et al., 2005), Melbourne Collaborative Cohort Study (MCCS) (Giles and English, 2002), MRC National Survey of Health and Development (NSHD) (Wadsworth et al., 2006), National Child Development Study (NCDS) (Power and Elliott, 2006), English Longitudinal Study of Ageing (ELSA) (Steptoe et al., 2013), UK Women's Cohort Study (UKWCS) (Cade et al., 2015), Women's Lifestyle and Health Study (WLHS) (Roswall et al., 2015), Danish Nurse Cohort Study (DNCS) (Hundrup et al., 2012) and the Japan Nurses’ Health Study (JNHS) (Hayashi et al., 2007). The pooled study sample consisted of 51 450 women who had reported their FMP and had complete information on the covariates used.
Main outcome and exposure variables
Age at FMP was confirmed by at least 12 months of cessation of menses where this was not the result of an intervention (such as surgical menopause due to bilateral oophorectomy or hysterectomy). If the age at FMP was reported multiple times, data reported at the last available survey were used. Using established age at FMP categories for premature and early menopause, the timing of the FMP were classified as premature menopause (before age 40), early menopause (40–44 years), 45–49 years, 50–51 years, 52–53 years and 54 or more years. Age at menarche (categorized as ≤11, 12, 13, 14 and 15 or more years) and parity (categorized as no children, one child and two or more children) were exposure of interest after adjusting for other covariates.
Covariates
The following sociodemographic and lifestyle factors reported at baseline surveys (or at mid age surveys for the birth cohorts) were included in the analysis as covariates: education level (≤10 years, 11–12 years and >12 years), marital status (married or partnered, separated/divorced/widowed and never married/single), smoking status (never smokers, past smokers and current smokers), BMI (<25, 25–30 and ≥30 kg/m2) and year of birth (born before 1940, between 1940 and 1949 and between 1950 and 1969).
Statistical analysis
Multinomial (polytomous) logistic regression model with five categories of outcome for FMP <40, 40–44, 45–49, 50–51, 52–53 and 54+ years was used to examine the associations between age at menarche and parity with age at FMP adjusted for the covariates mentioned above. Age at FMP of 50–51 was used as a reference category for the outcome, and the regression model was adjusted for birth year, education, smoking status, BMI and marital status as categorical covariates. Categorized variables for age at menarche and parity were first analysed as independent exposure variables and relative risk ratios (RRRs) and 95% CI associated with menarche and parity were estimated separately for each FMP category with age 50–51 as the reference, corresponding to a generalized logit model. Furthermore, to understand whether the joint effect of early menarche and nulliparity on early menopause were simply additive or had a synergistic effect, we included an interaction term between the two exposures in the model and analysed their combined effects. For the combined variable, age at menarche was dichotomized as early (≤11) versus all other (12 or more) and combined with three levels of parity, resulting in six categories in total and the combination of later menarche (menarche age ≥12) and having two or more children was used as a reference category. The higher order categories for age at menarche were collapsed since there were no significant differences in their estimated effects. The SURVEYLOGISTIC procedure (SAS Institute Inc, 2008.) in SAS Version 9.4 was used for the multinomial logistic regression, with the generalized logit link that estimates sampling errors based on the clustered sample survey from multiple studies and incorporates that in the estimates. All tests of statistical hypothesis were done at the two-sided 5% of significance. We also performed study-specific regression and random-effect meta-analysis to estimate the between-study heterogeneity in the effect size estimates.
Results
Study characteristics
There were 51 450 women who have reported their age at FMP and also had complete data on the covariates. Most women were born before 1960, with two-thirds born between 1930 and 1949 (Table I). The mean age at menarche (Table II) was 13.2 years (median 13 years, range 8–20 years), with 14.1% of the women having early menarche (age 11 or less). Three-quarters of the women had two or more children, while 11% had one child and 12% remained nulliparous. Across studies, the prevalence of nulliparity varied from 8.2% (ALSWH) to 20.1% (DNCS). Mean age at FMP (Table III) was 49.9 years (median 50, interquartile range 48–53 years). Overall, 2% of the women experienced premature menopause (ranging across studies from 1% for the DNCS and NSHD to 3.6% for UKWCS), with a further 7.6% having early menopause (ranging from 4.9% for NSHD and JNHS to 9.4% for MCCS). Although women with premature menopause had a mean age at FMP of 36.5 years (SD: 2.5) and median 37.0 (IQR: 35.0, 39.0), two-thirds (68%) had more than one child (results not shown).
Table I.
Characteristics of individual longitudinal studies of a subset of women past their FMP (with no intervention) in the InterLACE consortium.
| Study | Country | N | Age at baseline | Age at last follow-up | Women's year of birth (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (Q1, Q3) | Mean (Q1, Q3) | <1930 | 1930–1939 | 1940–1949 | 1950–1959 | 1960+ | |||
| Australian Longitudinal Study on Women's Health (ALSWH) | Australia | 6327 | 47.6 (46.4, 48.9) | 63.4 (62.6, 65.5) | 75.1 | 24.9 | |||
| Melbourne Collaborative Cohort Study (MCCS) | Australia | 12 185 | 58.7 (53.6, 64.6) | 67.8 (62.4, 73.6) | 35.4 | 42.4 | 20.1 | 2.04 | |
| Danish Nurse Cohort Study (DNCS) | Denmark | 8885 | 59.6 (54.0, 64.0) | 69.8 (64.0, 76.0) | 29.4 | 50.8 | 19.4 | 0.34 | |
| Women's Lifestyle and Health Study (WLHS) | Sweden/Norway | 5922 | 44.4 (42.0, 47.0) | 55.4 (53.0, 58.0) | 72.3 | 27.7 | 0.05 | ||
| MRC National Survey of Health and Development (NSHD)a | UK | 572 | 47.0 | 53.9 | 100 | ||||
| National Child Development Study (NCDS)b | UK | 1907 | 50.0 | 54.8 | 100 | ||||
| English Longitudinal Study of Ageing (ELSA) | UK | 3516 | 60.0 (52.0, 67.0) | 68.6 (61.0, 76.0) | 16.0 | 25.5 | 35.8 | 22.4 | 0.23 |
| UK Women's Cohort Study (UKWCS) | UK | 7290 | 58.1 (52.9, 63.5) | 61.0 (55.6, 66.3) | 13.1 | 42.8 | 39.1 | 4.84 | 0.04 |
| Japan Nurses’ Health Study (JNHS)c | Japan | 4846 | 54.7 (52.0, 57.0) | 54.7 (52.0, 57.0) | 0.02 | 1.55 | 63.6 | 34.2 | 0.68 |
| Total | 51450 | 55.0 (48.5, 61.0) | 63.4 (56.0, 69.4) | 16.4 | 26.8 | 40.8 | 15.9 | 0.1 | |
FMP, final menstrual period; InterLACE, International collaboration for a Life course Approach to reproductive health and Chronic disease Events.
a1946 British birth cohort.
b1958 British birth cohort. For birth cohorts studies, data from mid age survey (prior to final menstrual period) have been used as baseline and corresponding ages as baseline age. Q1—25th percentile, Q3—75th percentile.
cOnly cross-sectional data were available for analysis for JNHS.
Table II.
Study-specific and overall reproductive characteristics of subset of women past their FMP (with no intervention) in the InterLACE Consortium.
| Study | Age at menarche | Parity distribution | |||
|---|---|---|---|---|---|
| No children (n = 6199) | One child (n = 5546) | ≥2 children (n = 39 705) | |||
| Mean (SD) | Median (Q1, Q3) | (%) | (%) | (%) | |
| ALSWH | 12.9 (1.5) | 13 (12, 14) | 8.2 | 8.8 | 83.0 |
| MCCS | 13.2 (1.6) | 13 (12, 14) | 12.7 | 7.8 | 79.4 |
| DNCS | 13.8 (1.5) | 14 (13, 15) | 20.1 | 12.7 | 67.1 |
| WLHSa | 13.1 (1.4) | 13 (12, 14) | 10.0 | 13.4 | 76.6 |
| MRC NSHD | 12.7 (1.2) | 13 (12, 13) | 15.4 | 13.1 | 71.5 |
| NCDS | 12.7 (1.2) | 13 (12, 14) | 13.0 | 15.9 | 71.2 |
| ELSA | 13.1 (1.7) | 13 (12, 14) | 13.4 | 16.5 | 70.2 |
| UKWCS | 12.9 (1.6) | 13 (12, 14) | 12.1 | 11.8 | 76.0 |
| JNHS | 13.1 (1.4) | 13 (12, 14) | 13.4 | 10.2 | 76.4 |
| Overall | 13.2 (1.6) | 13 (12, 14) | 12.0 | 10.8 | 77.2 |
This study included all women who had complete data on education, BMI, smoking and marital status at the baseline.
aIn WLHS, marital status was only recorded from mothers’ birth registry, thus the data were missing for all women who did not give birth. The distribution of parity for WLHS provided in the table is for all women in the study instead of those with complete data on marital status (i.e. the sample used for analysis). Q1—25th percentile, Q3—75th percentile.
Table III.
Average age at FMP and its categorical distribution by studies in the InterLACE Consortium.
| Study | Age at FMP | Categorical distribution of FMP | ||||||
|---|---|---|---|---|---|---|---|---|
| <40a (n = 1048) | 40–44b (n = 3927) | 45–49 (n = 14 547) | 50–51 (n = 12 788) | 52–53 (n = 10 152) | 54+ (n = 8988) | |||
| Mean (SD) | Median (Q1, Q3) | (%) | (%) | (%) | (%) | (%) | (%) | |
| ALSWH | 51.0 (4.3) | 51 (49, 54) | 1.2 | 5.8 | 20.4 | 23.4 | 20.1 | 29.0 |
| MCCS | 49.7 (4.5) | 50 (47, 53) | 2.7 | 9.4 | 26.5 | 24.5 | 18.6 | 18.3 |
| DNCS | 49.4 (3.7) | 50 (47, 52) | 1.0 | 8.2 | 34.8 | 25.9 | 18.4 | 11.7 |
| WLHS | 50.3 (3.7) | 51 (48, 53) | 1.2 | 5.1 | 28.2 | 24.7 | 22.0 | 18.8 |
| MRC NSHD | 50.7 (3.3) | 51 (49, 53) | 1.0 | 4.9 | 28.5 | 28.5 | 23.8 | 13.3 |
| NCDS | 49.3 (3.8) | 50 (48, 52) | 2.3 | 7.6 | 36.4 | 18.8 | 26.1 | 8.9 |
| ELSA | 50.1 (4.9) | 50 (48, 53) | 3.1 | 8.6 | 23.6 | 25.0 | 17.0 | 22.6 |
| UKWCS | 49.4 (4.6) | 50 (47, 52) | 3.6 | 9.3 | 27.8 | 23.5 | 19.3 | 16.4 |
| JNHS | 49.9 (3.6) | 50 (48, 52) | 1.2 | 4.9 | 31.7 | 29.9 | 21.3 | 11.0 |
| Total | 49.9 (4.2) | 50 (48, 53) | 2.0 | 7.6 | 28.3 | 24.9 | 19.7 | 17.5 |
aPremature menopause.
bEarly menopause, Q1—25th percentile, Q3—75th percentile
Menarche, parity and FMP
Both the timing of menarche and parity were independently associated with age at the FMP and adjusting for confounders or mutual adjustment made no significant difference to their effect estimates. The estimated RRRs for menarche and parity for various age at FMP groups, after mutual adjustment and adjustment for study and confounders (birth year, education level, marital status, smoking status, and BMI) are presented in Table IV. Compared with those who had menarche at age 13 years, women with early menarche had almost twice the relative risk of experiencing premature menopause (RRR 1.80, 95% CI 1.53–2.12) and 31% higher risk of early menopause (RRR 1.31, 1.19–1.44). Similarly, compared with women with two or more children, nulliparous women had over twice the risk of experiencing premature menopause (RRR 2.26, 1.84–2.77), 32% higher risk for experiencing early menopause (RRR 1.32, 1.09–1.59) and 13% higher risk of having menopause at age 45–49 (RRR 1.13, 1.03–1.23).
Table IV.
Multivariable adjusted RRR and their two-sided 95% CI of reproductive characteristics and their association with age at FMP using multinomial logistic regression.
| Age at FMP | ||||||
|---|---|---|---|---|---|---|
| <40 | 40–44 | 45–49 | 52–53 | 54+ | ||
| Variable | Categories | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) |
| Age at menarche | ≤11 | 1.80 (1.53, 2.12) | 1.31 (1.19, 1.44) | 1.10 (1.00, 1.21) | 1.07 (0.99, 1.15) | 1.05 (0.91, 1.21) |
| 12 | 1.04 (0.87, 1.25) | 1.05 (0.88, 1.26) | 0.96 (0.91, 1.02) | 0.98 (0.92, 1.05) | 0.95 (0.88, 1.02) | |
| 13 | Reference | Reference | Reference | Reference | Reference | |
| 14 | 1.04 (0.79, 1.37) | 0.99 (0.90, 1.09) | 0.94 (0.86, 1.04) | 0.96 (0.90, 1.02) | 1.00 (0.95, 1.05) | |
| ≥15 | 1.10 (0.90, 1.33) | 0.98 (0.88, 1.10) | 0.94 (0.90, 0.99) | 0.91 (0.85, 0.98) | 1.09 (1.04, 1.15) | |
| Parity | 0 | 2.26 (1.84, 2.77) | 1.32 (1.09, 1.59) | 1.13 (1.03, 1.23) | 0.92 (0.81, 1.04) | 0.89 (0.76, 1.03) |
| 1 | 1.53 (1.14, 2.06) | 1.23 (1.04, 1.45) | 1.12 (1.05, 1.19) | 0.94 (0.89, 0.98) | 0.90 (0.80, 1.00) | |
| ≥2 | Reference | Reference | Reference | Reference | Reference | |
RRR, relative risk ratio. Reference category for polytomous outcome was the FMP at age 50–51 which was the most common FMP age group. The multivariable model included study, birth year, education, marital status, smoking status, BMI, menarche and parity.
Combined exposure of early menarche and nulliparity
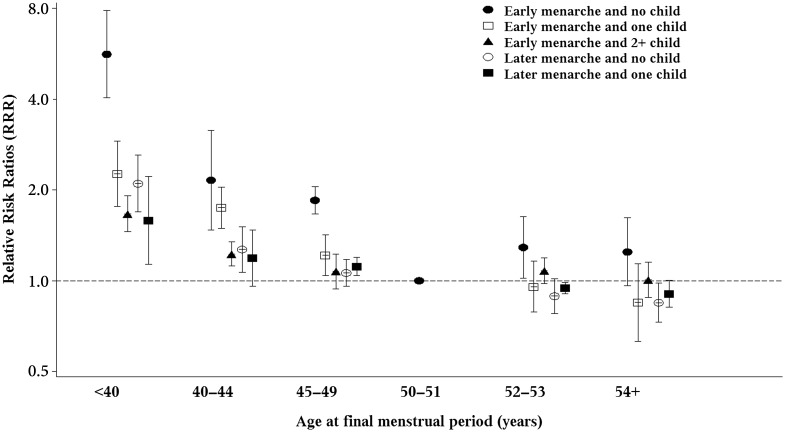
There was a significant interaction between age at menarche and parity associated with age at the FMP (P < 0.0001). The combination of having both early menarche and no children was associated with five times the relative risk of premature menopause (RRR 5.64, 4.04–7.87) and twice the risk of early menopause (RRR 2.16, 1.48–3.15), compared with the reference group (women with menarche at age 12 or later who had two or more children). The increased risks for premature and early menopause were also statistically significant for the combination of having early menarche and only one child (Fig. 1) but to a lesser extent. Meanwhile, nulliparous women with early menarche were also at slightly increased risk of having FMP later than age 51 years.
Figure 1.
Relative risk ratios (RRRs) and two-sided 95% CI for menopausal age <40, 40–44, 45–49, 52–53 or ≥54 with reference to age 50–51 among women with early (≤11 years) and later age of menarche (12 years or more) in combination with no, one, or two or more children (combination of late age at menarche and two or more children were used as reference group; y-axis on log scale). The estimates were fully adjusted for study cluster, birth year, education, marital status, smoking status and BMI.
Meta-analysis
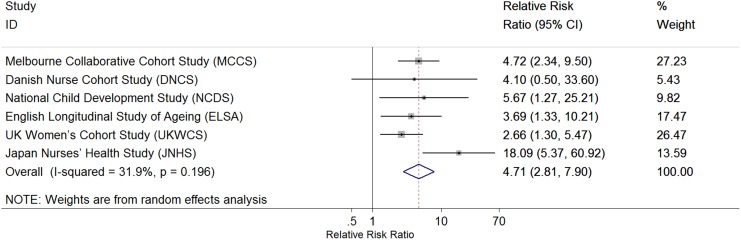
Of the nine studies, six had sufficient data to contribute to the study-specific analysis. Random-effect meta-analysis of the estimates from the six studies observed a pooled RRR estimate of 4.71 (95% CI 2.81–7.90) for the combined association of early menarche and nulliparity with premature menopause, with no significant heterogeneity between studies (test for heterogeneity P = 0.20, I2 = 31.9%) in the effect estimate (Fig. 2). The estimated effect size was much larger for JNHS compared with all other studies. When the JNHS was excluded, the combined effect was attenuated slightly to 3.75 (95% CI 2.46–5.72).
Figure 2.
Forest plot of study-specific effect estimates of the association between premature menopause (<40 years) and the combined exposure of early menarche and nulliparity (RRR on log scale). The estimates were fully adjusted for birth year, education, marital status, smoking status and BMI.
Discussion
To our knowledge, this is the first large-scale multinational study that has found robust evidence for a number of key associations with respect to the timing of the FMP, and particularly for premature and early menopause. In our study, almost 1 in 10 women had premature or early menopause. Having early menarche increased the risk of premature and early menopause by 80%, while the risk doubled for women without children. Furthermore, the combination of early menarche and nulliparity resulted in a 5-fold increased risk of premature menopause and twice the risk of early menopause compared with women having later menarche and two or more children.
Our findings are consistent with previous studies that have established the association between parity and age at natural menopause (Dorjgochoo et al., 2008; Gold, 2011). Some individual studies included in the InterLACE, such as JNHS (Yasui et al., 2012) and NSHD (Hardy and Kuh, 1999), have also previously shown that nulliparity was associated with early menopause. In contrast with previous reviews that concluded a lack of evidence on the relationship between menarche and menopause (Gold et al., 2001; Gold, 2011; Forman et al., 2013), this study showed associations between early menarche and both early and premature menopause (but no associations were evident for late menarche and the timing of the FMP). Again these findings are consistent with previous results from two individual studies (JNHS (Yasui et al., 2011) and NSHD (Hardy and Kuh, 1999)) that were included in the InterLACE study.
Previous studies have shown that early menarche is associated with poor reproductive functioning, including irregular periods (Hunter, 1992; Mishra et al., 2009), PCOS (Ibanez et al., 2000) and a slightly increased risk of endometriosis (Nnoaham et al., 2012). Although some women may have used fertility controls to remain childless, parity rates for the generation of women in this study should still reflect fertility, because the childbearing years occurred when general fertility rates were relatively high and prior to the wide availability of advanced treatments for infertility. Further, since the majority of women with premature menopause had their FMP between 35 and 40 years, they had sufficient time to have children, as most did. In this study, 50% of the women had their first child by age 25, 86% by age 30 and 97% by age 35. It is possible, however, that reproductive decline preceded FMP by 5–10 years and could have impacted fertility. Thus, the greatly increased risk of premature and early menopause among nulliparous women with early menarche is consistent with sub-fertility and accelerated ovarian ageing (Kok et al., 2003). A ‘dose response’ was also evident: women with early menarche who had one child also faced increased risk of premature and early menopause, but to a lesser extent than nulliparous women. As the timing of menarche is influenced by factors early in life, including by maternal weight gain, childhood obesity and psychosocial stress in childhood (e.g. infant–parent attachment security), the findings may reflect an underlying common cause for poor reproductive health through the life course (Belsky et al., 2010; Forman et al., 2013). Genetic studies also have shown that a number of menarche-related single-nucleotide polymorphisms (SNPs) collectively predicted age at natural menopause (Day et al., 2015), supporting a causal relationship between the timing of these two reproductive factors. More studies are needed to understand the cumulative and interactive effects of genetic and environmental factors on the association between menarche and menopause.
The main strength of this study was access to individual-level data across several populations across different geographic regions and cultures. The scale of this study was sufficiently large to provide the heterogeneity and statistical power needed to examine premature menopause. The participant-level data in InterLACE enabled harmonization of variables using common definitions, coding and cut points not normally possible with meta-analyses of published results. However, a number of limitations need to be acknowledged. Although InterLACE comprises mainly of longitudinal studies of women in mid-life, most of the studies (except the birth cohorts) relied on retrospectively reported age at menarche which may have led to some degree of recall bias. The NSHD has information on age at menarche collected prospectively and retrospectively and found that validity was improved when age categories for menarche were used (as this was the case in this study) (Cooper et al., 2006). Even though 70% of women retrospectively reported their age at menopause at baseline, misclassification was less likely to occur among women with premature or early menopause since women would notice if their menstrual periods stop much earlier than expected. The accuracy of recall could be influenced by educational level and having experienced a stillbirth or miscarriage (Cooper et al., 2006). Women with a history of these pregnancy complications might provide more accurate information as part of understanding their gynaecological history.
Findings in this study of a rather small but statistically significant higher relative risks of later FMP compared with the reference group among nulliparous women with early menarche were unexpected. Study-specific analysis (data not shown) suggested only DNCS contributed to the statistical significance of the finding, with the pooled effect estimate no longer significant when DNCS was excluded. This could be due to some unmeasured confounding associated with that study.
Cohort differences between the women in our study and women who are younger now are unavoidable since our cohorts’ members (and any similar study) have to be postmenopausal. In many high-income countries, there has been a long-term decline in the age at menarche, a decline in the mean fertility rate of women, and increased use of fertility treatments (Forman et al., 2013). The robust relationships evident in this study suggest that they are likely to be highly relevant, even if they should be applied with some caution to the current generation of young and mid-aged women.
In summary, this study provides strong evidence for early menarche as a risk factor for both premature and early menopause, a risk that was amplified for nulliparous women. Current guidelines for the clinical management of the menopausal transition, such as those given by National Institute for Health and Care Excellence in the UK, address the diagnosis and treatment options for premature ovarian failure/insufficiency (National Institute for Health and Care Excellence, 2015). It suggests that if the findings of this study were incorporated into clinical guidelines for advising nulliparous women from around the age of 35 years who had an early menarche (≤11 years), clinicians would gain valuable time to prepare these women for the possibility of premature ovarian failure/insufficiency or early menopause. The evidence also strengthens the case for early preventive strategies and clinical surveillance for these women to address the increased risks of chronic diseases associated with earlier menopause.
Acknowledgements
The data on which this research is based were drawn from several observational studies including: Australian Longitudinal Study on Women's Health (ALSWH) (funded by the Australian Government Department of Health), Melbourne Collaborative Cohort Study (MCCS) (funded by VicHealth and the Cancer Council, Victoria, Australia), Danish Nurse Cohort Study (DNCS) (funded by the National Institute of Public Health, Denmark), Swedish Women's Lifestyle and Health Study (WLH) (funded by the Swedish Research Council Grant 521-2011-2955), MRC National Survey of Health and Development (1946) (NSHD) (funded by the UK Medical Research Council), National Child Development Study (1958) (NCDS) (funded by the UK Medical Research Council), English Longitudinal Study of Ageing (ELSA) (funded by the National Institute on Aging grant: 2RO1AG7644 and 2RO1AG017644-01A1, and a consortium of UK government departments), UK Women's Cohort Study (UKWCS) (funded by the World Cancer Research Fund) and Japan Nurses’ Health Study (JNHS) (funded by the Japan Society for the Promotion of Science and the Japan Menopause Society). All studies would like to thank the participants for volunteering their time to be involved in the respective studies. The findings and views in this paper are not necessarily those of the original studies or their respective funding agencies.
The InterLACE study team also includes Nancy E. Avis, Sybil L. Crawford, Ellen B. Gold, Daniel Brown, Lynette L. Sievert, Eric Brunner, Rachel Cooper, Rebecca Hardy, Carla Makhlouf Obermeyer, Kathryn A. Lee, Toyoko Yoshizawa, Nancy F. Woods and Ellen S. Mitchell.
Authors’ roles
G.D.M. conceptualized the study and drafted the manuscript; N.P. performed the statistical analysis and contributed to interpretation of results; H.F.C. performed the literature review and assisted in data management and draft preparation; A.J.D., D.A., D.K., S.S., G.G.G., F.B., K.H., J.S.L., H.M., J.E.C., V.B., D.C.G., A.G., M.K.S., H.O.A., P.D. and E.W. contributed data and provided critical revision of the manuscript for important intellectual content. All authors have read and approved the final version.
Funding
InterLACE project is funded by the Australian National Health and Medical Research Council project grant (APP1027196). G.D.M. is supported by Australian Research Council Future Fellowship (FT120100812). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
None declared.
References
- Belsky J, Houts RM, Fearon RM. Infant attachment security and the timing of puberty: testing an evolutionary hypothesis. Psychol Sci 2010;21:1195–1201. [DOI] [PubMed] [Google Scholar]
- Cade JE, Burley VJ, Alwan NA, Hutchinson J, Hancock N, Morris MA, Threapleton DE, Greenwood DC. Cohort profile: the UK Women's Cohort Study (UKWCS). Int J Epidemiol 2015. doi:10.1093/ije/dyv173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Blell M, Hardy R, Black S, Pollard TM, Wadsworth ME, Pearce MS, Kuh D. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 2006;60:993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorjgochoo T, Kallianpur A, Gao YT, Cai H, Yang G, Li H, Zheng W, Shu XO. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause 2008;15:924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmand M, Tehrani FR, Pourrajabi L, Najafi M, Azizi F. Factors associated with menopausal age in Iranian women: Tehran Lipid and Glucose Study. J Obstet Gynaecol Res 2013;39:836–841. [DOI] [PubMed] [Google Scholar]
- Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther 2013;4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles GG, English DR. The Melbourne Collaborative Cohort Study. Lyon: International Agency for Research on Cancer, 2002. [PubMed] [Google Scholar]
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- Hardy R, Kuh D. Reproductive characteristics and the age at inception of the perimenopause in a British National Cohort. Am J Epidemiol 1999;149:612–620. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Mizunuma H, Fujita T, Suzuki S, Imazeki S, Katanoda K, Matsumura Y, Kubota T, Aso T. Design of the Japan Nurses’ Health Study: a prospective occupational cohort study of women's health in Japan. Ind Health 2007;45:679–686. [DOI] [PubMed] [Google Scholar]
- He C, Murabito JM. Genome-wide association studies of age at menarche and age at natural menopause. Mol Cell Endocrinol 2014;382:767–779. [DOI] [PubMed] [Google Scholar]
- Hundrup YA, Simonsen MK, Jørgensen T, Obel EB. Cohort profile: the Danish nurse cohort. Int J Epidemiol 2012;41:1241–1247. [DOI] [PubMed] [Google Scholar]
- Hunter M. The south-east England longitudinal study of the climacteric and postmenopause. Maturitas 1992;14:117–126. [DOI] [PubMed] [Google Scholar]
- Ibanez L, Potau N, de Zegher F. Recognition of a new association: reduced fetal growth, precocious pubarche, hyperinsulinism and ovarian dysfunction. Ann Endocrinol 2000;61:141–142. [PubMed] [Google Scholar]
- Kok HS, van Asselt KM, van der Schouw YT, Grobbee DE, te Velde ER, Pearson PL, Peeters PH. Subfertility reflects accelerated ovarian ageing. Hum Reprod 2003;18:644–648. [DOI] [PubMed] [Google Scholar]
- Lee C, Dobson AJ, Brown WJ, Bryson L, Byles J, Warner-Smith P, Young AF. Cohort profile: the Australian longitudinal study on women's health. Int J Epidemiol 2005;34:987–991. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Chung HF, Pandeya N, Dobson AJ, Jones L, Avis NE, Crawford SL, Gold EB, Brown D, Sievert LL et al. The InterLACE study: design, data harmonization and characteristics across 20 studies on women's health. Maturitas 2016;92:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra GD, Anderson D, Schoenaker DA, Adami HO, Avis NE, Brown D, Bruinsma F, Brunner E, Cade JE, Crawford SL et al. InterLACE: a new International Collaboration for a Life Course Approach to Women's Reproductive Health and Chronic Disease Events. Maturitas 2013;74:235–240. [DOI] [PubMed] [Google Scholar]
- Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health 2009;5:175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence Menopause: Diagnosis and Management (NG23) NICE Guideline 2015. nice.org.uk/guidance/ng23. [PubMed]
- Nippita TA, Baber RJ. Premature ovarian failure: a review. Climacteric 2007;10:11–22. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril 2012;98:702–712. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North American Menopause Society Menopause Practice: A Clinician's Guide, 3rd edn Cleveland, OH: North American Menopause Society, 2007. [Google Scholar]
- Power C, Elliott J. Cohort profile: 1958 British birth cohort (national child development study). Int J Epidemiol 2006;35:34–41. [DOI] [PubMed] [Google Scholar]
- Roswall N, Sandin S, Adami HO, Weiderpass E. Cohort profile: the Swedish Women's Lifestyle and Health cohort. Int J Epidemiol 2015. doi:10.1093/ije/dyv089. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc SAS/STAT 9.2 User's Guide, The SURVEYLOGISTIC Procedure. Cary, NC: SAS Institute Inc, 2008. [Google Scholar]
- Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014;43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol 2013;42:1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneri PE, Kiefte-de Jong JC, Bramer WM, Daan NM, Franco OH, Muka T. Association of alcohol consumption with the onset of natural menopause: a systematic review and meta-analysis. Hum Reprod Update 2016;22:516–528. [DOI] [PubMed] [Google Scholar]
- van Asselt KM, Kok HS, Pearson PL, Dubas JS, Peeters PH, Te Velde ER, van Noord PA. Heritability of menopausal age in mothers and daughters. Fertil Steril 2004;82:1348–1351. [DOI] [PubMed] [Google Scholar]
- Wadsworth M, Kuh D, Richards M, Hardy R. Cohort profile: the 1946 National Birth Cohort (MRC National Survey of Health and Development). Int J Epidemiol 2006;35:49–54. [DOI] [PubMed] [Google Scholar]
- Yasui T, Hayashi K, Mizunuma H, Kubota T, Aso T, Matsumura Y, Lee JS, Suzuki S. Association of endometriosis-related infertility with age at menopause. Maturitas 2011;69:279–283. [DOI] [PubMed] [Google Scholar]
- Yasui T, Hayashi K, Mizunuma H, Kubota T, Aso T, Matsumura Y, Lee JS, Suzuki S. Factors associated with premature ovarian failure, early menopause and earlier onset of menopause in Japanese women. Maturitas 2012;72:249–255. [DOI] [PubMed] [Google Scholar]