Abstract
Background
The 2013 American College of Cardiology/American Heart Association cholesterol guidelines recommend high‐intensity statins for patients after myocardial infarction (MI) rather than treating to a low‐density lipoprotein cholesterol goal, as the previous ATP III (Adult Treatment Panel third report) guidelines had advised.
Methods and Results
To evaluate the frequency of postdischarge lipid testing and high‐intensity statin use among MI patients discharged on a statin during the ATP III guidelines era, we linked ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry data to Medicare claims for 11 046 MI patients aged ≥65 years who were discharged alive on a statin from 347 hospitals (2007–2009). Multivariable regression was used to evaluate the association between lipid testing and 1‐year high‐intensity statin use. Only 21% of MI patients were discharged on a high‐intensity statin. By 90 days after MI, 44% of patients discharged on a statin underwent lipid testing (43% on low‐ or moderate‐intensity statins and 49% on high‐intensity statins; P=0.001). Follow‐up lipid testing rates were 47% among patients with in‐hospital low‐density lipoprotein cholesterol ≥100 mg/dL and 47% among newly prescribed statin recipients. By 1 year, only 14% of patients were on high‐intensity statins. Only 4% of patients discharged on low‐ or moderate‐dose statin were uptitrated to high intensity; postdischarge lipid testing was associated with a slightly higher likelihood of high‐intensity statin use by 1 year (5.4% versus 2.9%, adjusted odds ratio: 1.92; 95% confidence interval, 1.52–2.41).
Conclusions
Previous guidelines recommended low‐density lipoprotein cholesterol goal‐directed statin therapy, but lipid testing and high‐intensity statin use were infrequent after MI. The American College of Cardiology/American Heart Association guidelines may promote more intensive cardiovascular risk reduction by eliminating treatment dependence on lipid testing.
Keywords: lipid testing, myocardial infarction, statin dosing
Clinical Perspective
What Is New?
Previous guidelines recommended low‐density lipoprotein cholesterol goal‐directed statin therapy, but lipid testing and high‐intensity statin use were infrequent after myocardial infarction.
High‐intensity statin use was infrequently prescribed at discharge and use rates were even lower by 1 year after discharge. Low‐density lipoprotein cholesterol levels may be suboptimally reduced in post–myocardial infarction patients as a result of these practices.
What Are the Clinical Implications?
The 2013 American College of Cardiology/American Heart Association cholesterol guidelines promote more intensive cardiovascular risk reduction by eliminating treatment dependence on lipid testing after myocardial infarction.
Introduction
Multiple primary1, 2, 3, 4, 5, 6 and secondary prevention trials7, 8, 9, 10, 11, 12, 13, 14 have consistently shown a strong, direct correlation between low‐density lipoprotein cholesterol (LDL‐C) level and atherosclerotic cardiovascular disease risk. As a result, the third report from the National Cholesterol Education Program Adult Treatment Panel (ATP III) promoted cardiovascular risk reduction via treatment to LDL‐C targets.15 The ATP III guidelines recommended an LDL‐C target of <100 mg/dL for patients with established coronary heart disease and a target of <70 mg/dL for acute coronary syndrome patients. Statins (3‐hydroxy‐3‐methylglutaryl‐coenzyme A reductase inhibitors) are considered a first‐line therapy to achieve such reductions in LDL‐C levels; however, despite guideline recommendations, the majority of acute coronary syndrome patients failed to achieve their LDL‐C goal of <70 mg/dL.16
In 2013, the American College of Cardiology (ACC) and the American Heart Association (AHA) updated the cholesterol guidelines to recommend high‐intensity statin therapy for patients with confirmed atherosclerotic cardiovascular disease, regardless of LDL‐C levels, and particularly among those aged <75 years.17 Nevertheless, some clinicians continue to advocate for an LDL‐C goal‐directed strategy to more aggressively reduce risk, and the 2016 European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) guidelines still call for treating patients to designated LDL‐C targets.18
We hypothesized that under the ATP III guidelines that pursued targeted LDL‐C goals, patients were suboptimally treated after myocardial infarction (MI) because of low rates of follow‐up lipid testing and treatment intensification. The de‐emphasis of lipid targets in current guidelines may optimize secondary‐prevention lipid lowering by removing the reliance of therapeutic titration on follow‐up lipid test results. Consequently, we evaluated the frequency and temporal trends of postdischarge lipid testing and 1‐year statin treatment intensity among MI patients who were discharged on a statin during the ATP III guidelines era.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Data Sources
The data source for this study was the National Cardiovascular Data Registry's ACTION (Acute Coronary Treatment and Intervention Outcomes Network) Registry, which was linked to the Centers for Medicare and Medicaid Services (CMS) claims database. The ACTION Registry is an ongoing quality‐improvement registry that captures detailed clinical information on consecutive patients with a primary diagnosis of either ST‐segment–elevation MI (STEMI) or non‐STEMI who were treated at >300 participating hospitals across the United States. Using previously described methodology,19 we used 5 indirect identifiers in combination (date of birth, sex, hospital identification, date of admission, date of discharge) to link patients from the ACTION Registry who were aged ≥65 years with their respective Medicare claims data. The linkage to CMS claims data provides information on postdischarge lipid testing rates (Medicare Part B), as well as statin use and dosage (Medicare Part D). This data linkage was supported by a Centers for Education and Research on Therapeutics grant (U19HS021092) from the Agency for Healthcare Research and Quality. The Duke University Medical Center institutional review board granted a waiver of informed consent and authorization for this study.
Study Population
Our study population started with all patients aged ≥65 years in the linked database who were treated for STEMI or non‐STEMI at hospitals participating in the ACTION Registry from July 1, 2007, to December 31, 2009, and enrolled in the Part D prescription coverage plan before discharge (n=23 029). We excluded patients who were not discharged to home (n=7809), who were not discharged on a statin (n=3140), or who did not survive to 90 days after discharge (n=552). We also excluded patients who were not enrolled in Medicare Part A (inpatient coverage), Part B (outpatient coverage), and Part D (prescription coverage) in the 90 days after discharge (n=160). Finally, patients were counted once, and subsequent MI hospitalizations in the ACTION Registry were excluded (n=322). After these exclusions, the final analysis population comprised 11 046 patients from 347 United States hospitals.
Data Definitions
Postdischarge lipid testing was ascertained from Medicare Part B carrier files and outpatient revenue center file line items and was defined as any of the following Current Procedural Terminology codes occurring within 90 days after the index MI discharge: 80061, 82465, 83700, 83701, 83704, 93718, 83 721, and 84 478. As expected with administrative claims data, the presence of these codes indicates that the test was performed, but postdischarge laboratory test results are unknown. In‐hospital LDL‐C levels were captured in the ACTION Registry data collection form. Statin use before the index MI hospitalization was also captured in ACTION Registry data. Postdischarge statin type and dose were ascertained from Medicare Part D prescription fill data. High‐intensity statin was defined as atorvastatin ≥40 mg daily or rosuvastatin ≥20 mg daily; moderate‐intensity statin was defined as a daily dose of atorvastatin 10 to 39 mg, rosuvastatin 5 to 19 mg, simvastatin ≥20 mg, pravastatin ≥40 mg, lovastatin ≥40 mg, fluvastatin ≥80 mg, or pitavastatin ≥2 mg. All other statin type–dose combinations were considered low intensity. Statin use at 1 year was defined based on the dose and supply of the most recent refill before day 365 (with a 7‐day grace period) among the 10 099 patients alive at 1 year with continued Part D coverage.
Statistical Analyses
We summarized rates of lipid testing within 90 days after discharge over time and the variation in 90‐day testing rates at the hospital level among hospitals with at least 20 patients discharged on a statin. Patients were divided into those with and without follow‐up testing within 90 days; patient characteristics, including medical history, risk factors, and in‐hospital treatment, were described in the 2 groups. Categorical variables were presented as frequencies (percentages), and continuous variables were presented as median (interquartile range). We also examined rates of lipid testing among patients grouped by age, in‐hospital LDL‐C levels, statin therapy use before the index MI, and statin intensity at discharge.17 Rates of high‐intensity statin use at discharge were compared between age groups using a Pearson chi‐square test.
A multivariable logistic regression model was used to determine which patient and hospital factors are independently associated with follow‐up lipid testing. Variables entered into the model included demographics (age, sex, race), medical history (current/recent tobacco use, hypertension, dyslipidemia, dialysis status, diabetes mellitus, prior MI, prior heart failure, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior stroke, peripheral arterial disease, Charlson comorbidity index), presentation (STEMI versus non‐STEMI, signs of heart failure on arrival, shock on arrival, heart rate on admission, systolic blood pressure on admission, body mass index), statin use before admission, time from arrival to catheterization, in‐hospital LDL‐C, in‐hospital creatinine clearance (Cockcroft–Gault), discharge medications (angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, P2Y12 inhibitors, beta blockers, statin intensity, ezetimibe), hospital characteristics (geographic region, teaching status, total hospital beds, urban or rural location), and community characteristics (median household income, percentage that completed high school, urban or rural location, unemployment rate). Rates of missing data were <1% for all variables except body mass index (1.4% missing), and missing values were imputed using the sex‐ and STEMI‐specific median for patient‐level covariates and the overall median for hospital‐ or community‐level covariates. A hierarchical modeling approach was used, with patients clustered within hospitals and hospitals treated as a random effect. This approach considers that a patient's probability of postdischarge lipid testing may be dependent on the hospital at which he or she received care. Hospital effect was assessed with a median odds ratio (OR), which is the median of the distribution of all possible comparisons (ORs) of patients from different hospitals. Because the patient with higher odds is always in the numerator, the median OR is always ≥1, and a larger median OR indicates more between‐hospital variability,20 suggesting that the treating hospital contributes to individual patient probability of having a lipid test performed. The final model was selected using forward stepwise selection. For variables not included in the final model, significance tests were generated by adding them one at a time to the final model. In addition, a multivariable regression model was used to examine the association of 90‐day lipid testing with 1‐year statin use and intensity among patients still alive and enrolled in Medicare Part D at 1 year. Variables included in the model were the same as above with the addition of an interaction term between statin intensity at discharge and 90‐day lipid testing.
Based on patients' in‐hospital LDL‐C levels and the intensity of statin therapy that they were prescribed at discharge, we estimated the proportion of patients who would reach the guideline‐recommended target of <70 mg/dL by 1 year after discharge, assuming that high‐intensity statins decrease LDL‐C levels by 50%, moderate‐intensity statins decrease LDL‐C by 30%, and low‐intensity statins decrease LDL‐C by 15%.17 In addition, we estimated the proportion of patients who would reach the target of <70 mg if all patients were prescribed high‐intensity statins at discharge. Finally, although linked CMS data were not available beyond 2014, we presented discharge high‐intensity statin use rates among MI patients aged ≥65 years discharged alive in 2014, which was the year after the ACC/AHA guidelines were released.
All analyses were conducted at the Duke Clinical Research Institute using SAS version 9.4 or higher (SAS Institute).
Results
Patterns of Postdischarge Lipid Testing
Among 11 046 MI patients discharged on a statin between July 2007 and December 2009, only 21% were discharged on a high‐intensity statin. This rate varied only slightly by patient age; high‐intensity statins were used in 24% of patients aged <75 years and in 18% of patients aged ≥75 years (P<0.0001).
Lipid testing was performed in 44% of patients within 90 days after discharge. The rate of follow‐up lipid testing remained relatively unchanged through our study period (Figure 1). The proportion of patients with lipid testing by 90 days varied across hospitals, ranging between 20% and 70% of discharged patients with a median testing rate of 45% (Figure 2).
Figure 1.
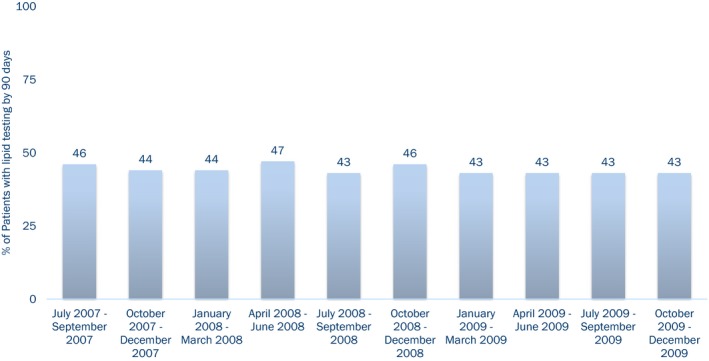
Trend in lipid testing within 90 days after myocardial infarction in the United States from 2007 to 2009.
Figure 2.
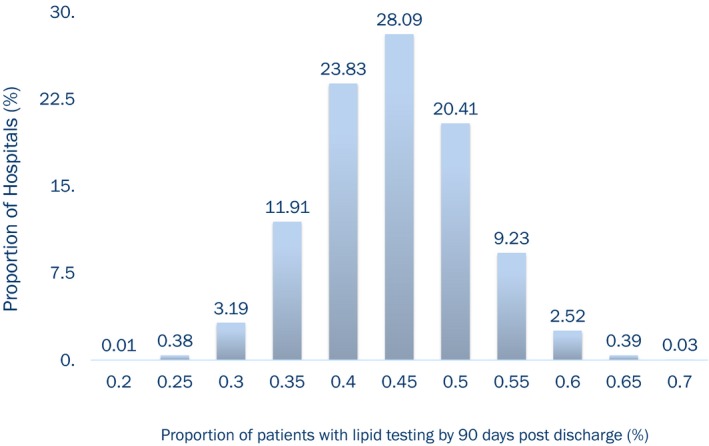
Rates of lipid testing in US hospitals from 2007 to 2009.
Characteristics Associated With Postdischarge Lipid Testing
When we compared patients with and without follow‐up lipid testing, no major differences were observed in the location or type of hospitals from which the patients were discharged (rural hospital: 15% versus 14%; teaching hospital: 28% versus 29%; median bed size: 418 versus 418; P>0.05 for all). Similarly, we did not observe major differences in the age, sex, race, or socioeconomic status of patients with and without follow‐up lipid testing (Table). Patients with follow‐up lipid testing were more likely to present with STEMI and less likely to have a history of MI, heart failure, percutaneous coronary intervention, coronary artery bypass grafting, atrial fibrillation/flutter, stroke, or peripheral arterial disease; these patients were as likely to get lipids measured during the initial hospitalization as patients who did not have follow‐up testing.
Table 1.
Patient Characteristics
| Lipid Testing Within 90 Days (n=4884) | No Lipid Testing Within 90 Days (n=6162) | |
|---|---|---|
| Patient demographics | ||
| Age, y, median (IQR) | 74 (69–80) | 75 (70–82) |
| Female, % | 49.3 | 51.9 |
| White race, % | 88.9 | 86.9 |
| Household income (×$1000), median (IQR)a | 45 (40–53) | 45 (40–52) |
| Unemployment rate, %a | 6.6 | 6.8 |
| College degree, %a | 23.4 | 23.4 |
| Medical history | ||
| Current/recent smoker, % | 17.3 | 18.3 |
| Hypertension, % | 78.3 | 81.5 |
| Dyslipidemia, % | 64.8 | 65.1 |
| Currently on dialysis, % | 2.9 | 2.2 |
| Chronic lung disease, % | 23.1 | 28.3 |
| Diabetes mellitus, % | 33.3 | 34.2 |
| Prior MI, % | 23.3 | 30.1 |
| Prior HF, % | 11.2 | 16.9 |
| Prior CABG, % | 16.0 | 22.1 |
| Prior PCI, % | 21.1 | 27.3 |
| Atrial fibrillation/flutter, % | 18.2 | 21.4 |
| Prior stroke, % | 8.5 | 11.1 |
| Peripheral arterial disease, % | 11.9 | 14.1 |
| Charlson comorbidity index, median (IQR) | 2.0 (1.0–4.0) | 3.0 (2.0–4.0) |
| Presentation | ||
| STEMI | 36.1 | 29.7 |
| Signs of HF | 15.1 | 20.2 |
| Cardiogenic shock | 2.1 | 2.0 |
| HR on admission, bpm, median (IQR) | 79 (66–93) | 80 (68–96) |
| SBP on admission, mm Hg, median (IQR) | 145 (125–165) | 144 (124–165) |
| BMI, kg/m2, median (IQR) | 27.6 (24.5–31.2) | 27.1 (23.8–31.1) |
| Measurements and labs | ||
| Creatinine, mg/dL, median (IQR) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
| Hemoglobin, g/dL, median (IQR) | 13.5 (12.2–14.67) | 13.2 (11.9–14.4) |
| Lipids measured in hospital, % | 79.4 | 78.9 |
| In‐hospital LDL‐C, mg/dL, median (IQR) | 92 (70–117) | 87 (66–113) |
| Discharge medications | ||
| ACEI or ARB | 74.9 | 74.6 |
| Beta blocker | 97.2 | 96.0 |
| High‐intensity statin | 23.4 | 19.1 |
All variables in this table missing in <1% of patients except for BMI, which was missing in 1.4% of patients. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; HF, heart failure; HR, heart rate; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST‐segment–elevation myocardial infarction.
Census data based on patient ZIP code of residence.
In the multivariable regression model, patient characteristics associated with a greater likelihood of follow‐up lipid testing included prior history of dyslipidemia and a high‐intensity statin prescribed at discharge, as well as higher body mass index and higher household income (Figure 3). Statin use before admission was associated with a lower likelihood of follow‐up lipid testing. In addition, older patients, those of nonwhite race, and current/recent smokers were less likely to receive follow‐up lipid testing. Patients' probability of undergoing postdischarge lipid testing was also significantly dependent on the hospital at which they were cared for during their index MI (median OR: 1.28; 95% confidence interval, 1.21–1.36).
Figure 3.
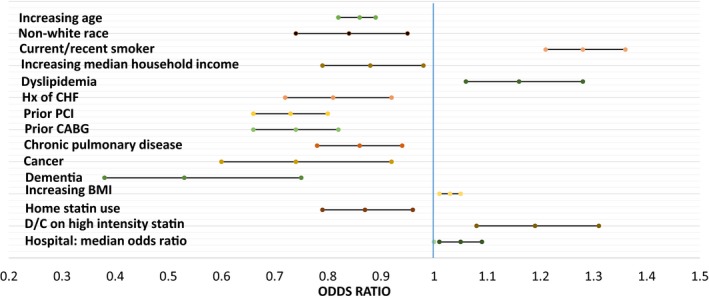
Characteristics associated with postdischarge lipid testing. *For each 5‐year increase above age 74 years. †For each $10 000 increase. ‡For each 1‐point increase. §The median OR is the median of the distribution of ORs for all possible pair of people with similar covariates but treated at different hospitals. The median OR allows examination of the magnitude of hospital effect relative to the other factors in the multivariable model. BMI indicates body mass index; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CI, confidence interval; D/C, discharged; Hx, history; OR, odds ratio; PCI, percutaneous coronary intervention.
Patients had a median in‐hospital LDL‐C level of 90 mg/dL (25th and 75th percentiles: 68 and 115, respectively), and 39% (n=3320) of patients had an in‐hospital LDL‐C ≥100 mg/dL. Rates of follow‐up lipid testing did not vary significantly based on in‐hospital LDL‐C levels (47% of patients with in‐hospital LDL‐C ≥100 mg/dL versus 43% of patients with LDL‐C <100 mg/dL, P=0.19 when added to the multivariable model). Among patients aged <75 years, the rates of follow‐up testing were 51% in those with in‐hospital LDL‐C ≥100 mg/dL and 45% in those with LDL‐C <100 mg/dL. Rates of follow‐up testing were modestly higher among patients newly prescribed a statin compared with those already on a statin before their MI (47% versus 41%, P=0.004 from multivariable model). At discharge, 21% (n=2315) of patients were prescribed a high‐intensity statin; follow‐up lipid testing was more common among high‐intensity statin users than those discharged on lower intensity statins (49% versus 43%, P=0.0005 from multivariable model). Lipid testing rates were 51% versus 47% among patients aged <75 years prescribed high‐ versus lower intensity statins at discharge.
Postdischarge Lipid Testing and Subsequent Treatment Changes
Rates of overall and high‐intensity statin use at 1 year are shown in Figure 4. Patients who had received lipid testing within 90 days after discharge were more likely to remain on a statin at 1 year (adjusted OR: 1.17; 95% confidence interval, 1.07–1.29), regardless of whether they were discharged on a high‐intensity statin or a lower intensity statin (P interaction=0.29). Among patients discharged on high‐intensity statins, 51.4% remained on a high‐intensity statin by 1 year. For patients prescribed a high‐intensity statin at discharge, lipid testing within 90 days was not associated with remaining on a high‐intensity statin at 1 year (adjusted OR: 1.07; 95% confidence interval, 0.90–1.28). Among patients who were discharged on a low‐ or moderate‐intensity statin, those who received lipid testing within 90 days were nearly twice as likely to be titrated up to a high‐intensity statin at 1 year compared with those who did not receive lipid testing (adjusted OR: 1.92; 95% confidence interval, 1.52–2.41), but the rate of high‐intensity statin use was still very low (5.4%) in the lipid‐tested group.
Figure 4.
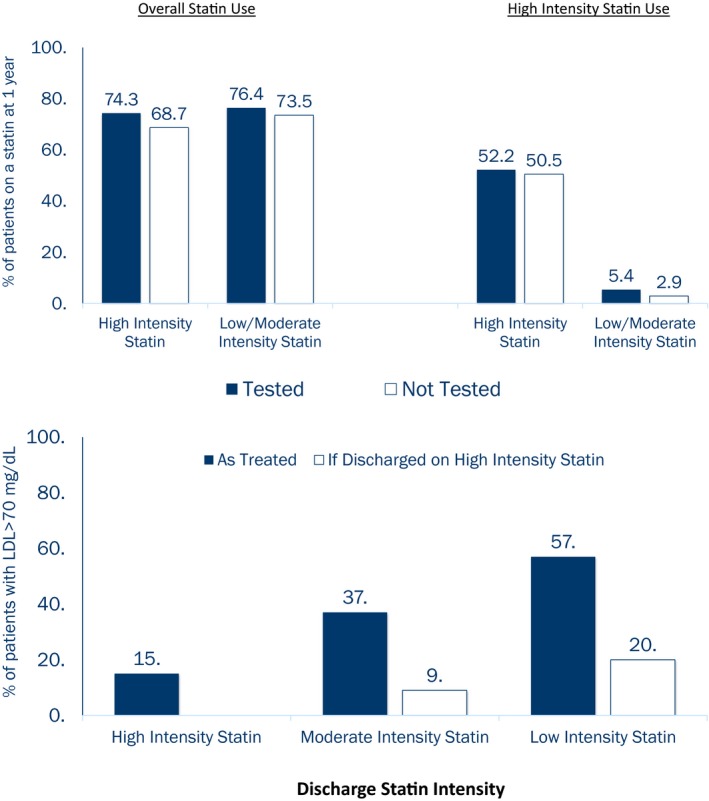
Overall and high‐intensity statin use at 1 year after discharge. Overall and high‐intensity statin use at 1 year after discharge among patients with and without lipid testing within 90 days.
Guidelines during this study period recommended an LDL goal <70 mg/dL for post‐MI patients. Although administrative claims data show performance of lipid testing, measured lipid levels are not available. Based on the patients' in‐hospital LDL‐C levels and discharge statin therapy intensity and on the assumption that high‐intensity statins decrease LDL‐C levels by 50%, moderate‐intensity statins decrease LDL‐C by 30%, and low‐intensity statins decrease LDL‐C by 15%,17 we estimate that only ≈66% of the post‐MI patients in our study population would reach LDL‐C levels <70 mg/dL. Among patients who were discharged on moderate‐ and low‐intensity statins, 37% and 57%, respectively, would remain undertreated with estimated LDL‐C ≥70 mg/dL (Figure 5). The 2013 ACC/AHA guidelines favor consideration of high‐intensity statin for secondary prevention. If all patients discharged on low‐ or moderate‐intensity statin in our study were instead given high‐intensity statins, we estimate that 90% of all MI patients would reach LDL‐C <70 mg/dL.
Figure 5.
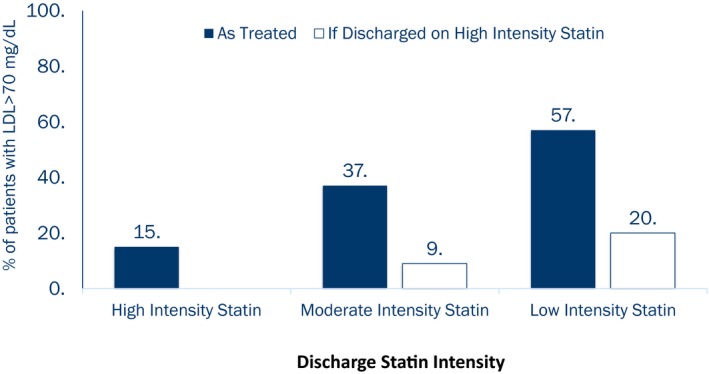
Projected proportion of patients failing to reach guideline‐recommended LDL‐C <70 mg/dL based on in‐hospital LDL‐C level and statin intensity prescribed at discharge. *Under the assumption that high‐intensity statins decrease LDL‐C levels by 50%, moderate‐intensity statins decrease LDL‐C by 30%, and low‐intensity statins decrease LDL‐C by 15%.18 LDL‐C indicates low‐density lipoprotein cholesterol.
Among patients aged <75 years, an estimated 64% would potentially reach LDL‐C levels <70 mg/dL based on in‐hospital LDL‐C levels and discharge statin intensity; 57% and 40% of patients discharged on low‐ and moderate‐intensity statin, respectively, would remain undertreated with estimated LDL‐C ≥70 mg/dL. If these patients were instead given high‐intensity statins, we estimate that 90% would reach LDL‐C <70 mg/dL. Among MI patients aged ≥65 years discharged in 2014 in the ACTION Registry, the rate of high‐intensity statins prescribed at discharge had increased to 49%; among patients aged between 65 and 75 years, the rate of high‐intensity statin use was 54%.
Discussion
Previous guidelines recommended that statins be dosed for individual patients based on measured LDL‐C levels, with the goal of lowering LDL‐C to <70 mg/dL after acute MI. In this national study, we showed that this strategy likely failed to optimally lower LDL‐C because follow‐up lipid testing was performed in only a minority (44%) of post‐MI patients, and only 1 in 7 patients was treated with a high‐intensity statin at 1 year. Among patients discharged on low‐ or moderate‐intensity statin, follow‐up lipid testing was associated with a greater likelihood of high‐intensity statin use at 1 year after discharge. If high‐intensity statin was initiated in all MI patients at discharge, per current guideline recommendations, we estimate that a greater proportion of patients would reach LDL‐C <70 mg/dL.
Any LDL‐C goal‐based strategy for lipid‐lowering therapy must necessarily rely on lipid testing. Under the ATP III guidelines, lipid testing rates remained consistently in the 40% range, and we did not see evidence that testing was used selectively for patients with greater need of statin uptitration. Even among patients with in‐hospital LDL‐C levels above conservative guideline‐recommended goals (LDL‐C ≥100 mg/dL) who would benefit from lipid testing to ensure treatment target reached, less than half (47%) received lipid testing within 90 days following hospital discharge. Similarly, testing rates were low for patients with newly prescribed statin therapy (43%) and among patients discharged on low‐ or moderate‐intensity statins (47%)—groups that may be expected to require active testing and statin intensification. Surprisingly, patients discharged on a high‐intensity statin were more likely to undergo lipid testing. Older patients and those with clinical comorbidities were less likely to undergo lipid testing; these may be patients for whom statin dose titration is not a high priority. In addition, we showed that the likelihood of postdischarge lipid testing varies significantly by the hospital at which patients received index MI treatment, suggesting that hospital discharge practices and the coordination of follow‐up care within or between healthcare systems may be a driver of postdischarge lipid testing, rather than patient factors. The transition from the hospital to home setting has been identified as an important period that may influence patients' downstream care and risk of adverse cardiovascular events.21
Our study shows that the prior LDL‐C goal‐directed strategy did not yield as high of a proportion of patients on high‐intensity statin therapy. By 1 year after MI, only 14% of patients were treated with a high‐intensity statin. Of the 79% of post‐MI patients discharged on low‐ or moderate‐intensity statin, those with follow‐up lipid testing were more likely to be uptitrated than those who did not, but overall, only 4% of these patients were uptitrated to a higher intensity statin by 1 year after MI. Based on in‐hospital LDL‐C levels and intensity of statins prescribed at discharge, we project that only ≈66% of all patients discharged on a statin in our study population would reach the guideline‐recommended LDL‐C goal of <70 mg/dL. The actual rate of achieving LDL‐C <70 mg/dL may be lower than this estimate, as this estimate is calculated based on in‐hospital LDL‐C levels, which are lower during an acute MI.22 Our results suggest that suboptimal lipid‐lowering in post‐MI patients can be attributed (1) to low use of high‐intensity statin at discharge, despite trial evidence suggesting benefit7, 8, 11, 23, 24; (2) to underutilization of follow‐up lipid testing to guide statin dose titration; and (3) to inertia in uptitrating statin dose to achieve guideline‐recommended LDL‐C, even when lipid testing was performed.
Targeted lipid management is a multistep process in which the post‐MI patient discharged on a statin is required to have their lipid levels followed after a few months of treatment. If LDL‐C levels have not reached guideline‐recommended targets, then statin intensification would be considered. The cycle of lipid remeasurement and dose titration is repeated until the LDL‐C goal is reached, which introduces barriers or delays to treatment intensification. In shifting toward a strategy of treating post‐MI patient populations uniformly with high‐intensity statins, the ACC/AHA guidelines effectively replaced the prior paradigm of tailoring statin dosing to target LDL‐C level goals, thereby reducing the reliance of dose titration on follow‐up lipid testing. These current guidelines may promote a more aggressive approach to lipid management and cardiovascular risk reduction; our study data project that implementation of these guidelines could substantially raise the likelihood of reaching LDL‐C <70 mg/dL. This simplified treatment strategy also streamlines the transition‐of‐care process after hospital discharge and reduces potential therapeutic inertia in responding to lipid test results. Examination of MI patients in the year after the 2013 ACC/AHA guidelines already showed an increase in high‐intensity statin use at discharge. Of course, patients discharged on a high‐intensity statin may not necessarily continue on a high‐intensity statin long term. However, prior studies have shown that patients are more likely to be adherent to therapies when these therapies are prescribed at discharge.25
Some clinicians view the current guidelines as a sledgehammer approach to a nuanced problem. The 2016 ESC/EAS cholesterol guidelines18 have inspired new momentum to return to pursuing LDL‐C goals, advocating >50% LDL‐C reduction for patients with high or very high cardiovascular disease risk. Arguably, the role of lipid‐level monitoring extends beyond simply assessing progress toward an LDL‐C goal. Regular lipid measurements may help ascertain patient medication adherence, monitor safety of statin treatment, and offer an opportunity to actively educate and engage post‐MI patients in risk factor modification behaviors. Early and routine follow‐up outpatient visits, as required with lipid testing, have been associated with improved adherence to lipid‐lowering therapy.26, 27 Recent trials have emerged that suggest improved patient outcomes with targeted lipid management. Results from IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) demonstrate significant reductions in cardiovascular events when ezetimibe was added to statin therapy to lower LDL‐C levels below previous targets.28 Novel lipid‐lowering therapies, such as PCSK9 inhibitors, have shown substantial LDL‐C–lowering capability, with imminent outcomes trial results.29, 30, 31 How these data will influence future guideline updates is unknown, but our results encourage reengineering of clinical processes if we return to a lipid‐level–driven treatment strategy, because only a minority of post‐MI patients actually completed follow‐up lipid testing in routine clinical practice during the time period when this follow‐up was strongly recommended by national guidelines, and the association between lipid testing and therapy intensification was significant but modest.
Study Limitations
This study should be interpreted in the context of a few limitations. First, although the ACTION Registry provides data on LDL‐C values from in‐hospital lipid testing, the linked administrative data can only assess whether postdischarge lipid tests were performed; they do not provide follow‐up lipid test results. We used in‐hospital LDL‐C values to project the proportion of patients with controlled LDL‐C levels based on the dose of statin prescribed at discharge; however, this approach may overestimate the proportion of patients with LDL‐C <70 mg/dL, because in‐hospital LDL‐C values may be depressed when measured in the setting of acute coronary syndrome. Second, because statin type and dosing are gathered from Medicare data, our study population was limited to patients aged ≥65 years, and results cannot be generalized to younger patients. Third, the ACTION Registry may represent hospitals with interest in quality improvement and may not be representative of all practices in the United States. Fourth, as in any observational study, there is the potential for unmeasured confounding factors. We cannot causally link patients who had received lipid testing to the use of statins or high‐intensity statins downstream. Finally, this analysis examined a study period before the consideration and dissemination of guidelines that changed our lipid management strategy. More recent Medicare data are not currently available to examine lipid testing practices and postdischarge statin use patterns after implementation of the 2013 ACC/AHA guidelines.
Conclusion
Although previous guidelines recommended lipid‐lowering targets, follow‐up lipid measurements were done in only a minority of post‐MI patients in routine clinical practice. High‐intensity statin use was infrequently prescribed at discharge, and use rates were even lower by 1 year after discharge. LDL‐C levels may be suboptimally reduced in post‐MI patients as a result of these practices. The 2013 ACC/AHA cholesterol guidelines promote more intensive cardiovascular risk reduction by eliminating treatment dependence on post‐MI lipid testing.
Sources of Funding
This work was supported by grant number U19HS021092 from the Agency for Healthcare Research and Quality.
Disclosures
Dr Peterson reports grant support from American College of Cardiology, American Heart Association, Janssen; and consulting from Bayer, Boehringer Ingelheim, Merck, Valeant, Sanofi, Astra Zeneca, Janssen, Regeneron, Genentech. Dr Wang reports research funding from AstraZeneca, Gilead, Lilly, The Medicines Company, and Canyon Pharmaceuticals (all significant); educational activities or lectures (generates money for Duke) for AstraZeneca (modest); consulting (including continuing medical education) for Medco (modest) and American College of Cardiology (significant). The remaining authors have no disclosures to report.
Acknowledgments
The authors would like to thank Erin Campbell for her editorial contributions to this article. Ms Campbell did not receive compensation for her assistance, apart from her employment at the institution where this study was conducted.
(J Am Heart Assoc. 2018;7:e006460 DOI: 10.1161/JAHA.117.006460.)29371200
References
- 1. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 2. Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 3. Sever PS, Dahlöf B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J; ASCOT investigators . Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial‐Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 4. Crouse JR III, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, Grobbee DE, Bots ML; METEOR Study Group . Effect of rosuvastatin on progression of carotid intima‐media thickness in low‐risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–1353. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ; JUPITER Study Group . Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 6. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López‐Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJ, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez‐Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E; HOPE‐3 Investigators . Cholesterol lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 7. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM; Pravastatin or Atorvastatin Evaluation and Infection Therapy‐Thrombolysis in Myocardial Infarction 22 Investigators . Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 8. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets (TNT) Investigators . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 9. Pedersen TR, Kjekshus J, Berg K, Haghfelt T, Faergeman O, Faergeman G, Pyorala K, Miettinen T, Wilhelmsen L, Olsson AG, Wedel H. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [DOI] [PubMed] [Google Scholar]
- 10. Long‐Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group . Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 11. Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group . High‐dose atorvastatin vs usual‐dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 12. Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group , Armitage J, Bowman L, Wallendszus K, Bulbulia R, Rahimi K, Haynes R, Parish S, Peto R, Collins R. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double‐blind randomised trial. Lancet. 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bezafibrate Infarction Prevention (BIP) study . Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation. 2000;102:21–27. [DOI] [PubMed] [Google Scholar]
- 14. Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 15. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 16. Chinwong D, Patumanond J, Chinwong S, Siriwattana K, Gunapam S, Hall JJ, Phrommintikul A. Statin therapy in patients with acute coronary syndrome: low‐density lipoprotein cholesterol goal attainment and effect of statin potency. Ther Clin Risk Manag. 2015;11:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S46–S48. [DOI] [PubMed] [Google Scholar]
- 18. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL; Authors/Task Force Members; Additional Contributor . 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 19. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ko DT, Alter DA, Newman AM, Donovan LR, Tu JV. Association between lipid testing and statin therapy in acute myocardial infarction patients. Am Heart J. 2005;150:419–425. [DOI] [PubMed] [Google Scholar]
- 22. Fyfe T, Baxter RH, Cochran KM, Booth EM. Plasma‐lipid changes after myocardial infarction. Lancet. 1971;2:997–1001. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association between intensity of statin therapy and mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:47–54. [DOI] [PubMed] [Google Scholar]
- 24. Stone PH, Lloyd‐Jones DM, Kinlay S, Frei B, Carlson W, Rubenstein J, Andrews TC, Johnstone M, Sopko G, Cole H, Orav J, Selwyn AP, Creager MA; Vascular Basis Study Group . Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111:1747–1755. [DOI] [PubMed] [Google Scholar]
- 25. Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. [DOI] [PubMed] [Google Scholar]
- 26. Benner JS, Tierce JC, Ballantyne CM, Prasad C, Bullano MF, Willey VJ, Erbey J, Sugano DS. Follow‐up lipid tests and physician visits are associated with improved adherence to statin therapy. Pharmacoeconomics. 2004;22(suppl 3):13–23. [DOI] [PubMed] [Google Scholar]
- 27. Faridi KF, Peterson ED, McCoy LA, Thomas L, Enriquez J, Wang TY. Timing of first postdischarge follow‐up and medication adherence after acute myocardial infarction. JAMA Cardiol. 2016;1:147–155. [DOI] [PubMed] [Google Scholar]
- 28. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 29. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 30. Colhoun HM, Robinson JG, Farnier M, Cariou B, Blom D, Kereiakes DJ, Lorenzato C, Pordy R, Chaudhari U. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord. 2014;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabatine MS, Giugliano RP, Keech A, Honarpour N, Wang H, Liu T, Wasserman SM, Scott R, Sever PS, Pedersen TR. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94–101. [DOI] [PubMed] [Google Scholar]


