Abstract
Background
Higher body mass index (BMI) is associated with lower circulating levels of N‐terminal‐pro‐b‐type natriuretic peptide (NT‐proBNP). The Interaction between BMI and NT‐proBNP with respect to clinical outcomes is not well characterized in patients with acute heart failure.
Methods and Results
A total of 686 patients from the biomarker substudy of the ASCEND‐HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated HF ) clinical trial with documented NT‐proBNP levels at baseline were included in the present analysis. Patients were classified by the World Health Organization obesity classification (nonobese: BMI <30 kg/m2, Class I obesity: BMI 30–34.9 kg/m2, Class II obesity BMI 35–39.9 kg/m2, and Class III obesity BMI ≥40 kg/m2). We assessed baseline characteristics and 30‐ and 180‐day outcomes by BMI class and explored the interaction between BMI and NT‐proBNP for these outcomes. Study participants had a median age of 67 years (55, 78) and 71% were female. NT‐proBNP levels were inversely correlated with BMI (P<0.001). Higher NT‐proBNP levels were associated with higher 180‐day mortality (adjusted hazard ratio for each doubling of NT‐proBNP, 1.40; 95% confidence interval, 1.16, 1.71; P<0.001), but not 30‐day outcomes. The effect of NT‐proBNP on 180‐day death was not modified by BMI class (interaction P=0.24).
Conclusions
The prognostic value of NT‐proBNP was not modified by BMI in this acute heart failure population. NT‐proBNP remains a useful prognostic indicator of long‐term mortality in acute heart failure even in the obese patient.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00475852.
Keywords: acute heart failure, body mass index, N‐terminal‐pro‐b‐type natriuretic peptide, Obesity
Subject Categories: Biomarkers, Heart Failure
Clinical Perspective
What Is New?
Higher baseline N‐terminal‐pro‐b‐type natriuretic peptide levels are associated with poor long‐term clinical outcomes.
While body mass index changes the distribution of N‐terminal‐pro‐b‐type natriuretic peptide (ie, an inverse relationship between natriuretic peptides and body mass index), it does not modify the prognostic significance of N‐terminal‐pro‐b‐type natriuretic peptide for post–acute heart failure outcomes.
What Are the Clinical Implications?
The prognostic significance of N‐terminal‐pro‐b‐type natriuretic peptide testing during acute heart failure exacerbation remains useful in patients with the highest or lowest body mass indices.
Introduction
Heart failure (HF) causes significant morbidity, mortality, and financial burden.1 Use of b‐type natriuretic peptide (BNP) and the physiologically inert cleaved fragment, N‐terminal‐pro‐b‐type natriuretic peptide (NT‐proBNP), are guideline recommended by for the evaluation of dyspnea.2, 3 BNP and/or NT‐proBNP levels correlate with HF diagnosis, severity, and mortality.4, 5 However, obesity may be associated with lower circulating levels of BNP and/or NT‐proBNP.6, 7, 8, 9 Thus, the prognostic utility of NT‐proBNP may differ between obese and nonobese patients, though this has not been well characterized in patients with acute HF. Given that the diagnostic and prognostic utility of NT‐proBNP may be strongest in the setting of acute decompensated HF,3 we examined the effect modification of body mass index (BMI) on the prognostic value of NT‐proBNP levels during acute HF exacerbation in patients enrolled in the ASCEND‐HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) randomized, clinical trial.
Methods
Study Overview
The authors are willing to make available the data, analytical methods, and study materials to other researchers for purposes of reproducing the results or replicating the procedure. The design and results of the ASCEND‐HF trial have been previously reported.10, 11 Briefly, ASCEND‐HF was a global, randomized, double‐blind, placebo‐controlled trial evaluating the efficacy of nesiritide versus placebo, both in addition to standard care, in 7141 patients with acute HF. The trial enrolled patients from 398 centers across 30 countries. Detailed inclusion and exclusion criteria have been described previously.11 Enrolled patients were required to have dyspnea at rest or with minimal activity, ≥1 accompanying sign, and ≥1 objective measure of HF. Of note, patients with BNP or NT‐proBNP within normal limits (ie, BNP <100 pg/mL or NT‐proBNP <125 pg/mL for subjects aged <75 years; NT‐proBNP <425 pg/mL for subjects aged ≥75 years) were excluded. The primary end point of the trial was a composite of all‐cause mortality or HF rehospitalization at 30 days postrandomization.
Study Population
The present analysis included patients enrolled in the biomarker substudy of ASCEND‐HF, which enrolled 687 patients in whom NT‐proBNP levels were documented at baseline prior to randomization and at 48 to 72 hours after admission. If discharge occurred before 48 hours, blood samples were collected at discharge. If 1 of these values were missing, these patients were excluded from the biomarker substudy. Postbaseline values of NT‐proBNP collected <36 or >96 hours were also excluded. Blood samples were obtained from venous sampling and placed in EDTA plasma tubes and were immediately centrifuged and stored at −80°C for analysis. NT‐proBNP levels were determined by the VITROS NT‐proBNP Assay (Ortho‐Clinical Diagnostics, Raritan, NJ). The full analysis of the analytical and clinical performance of the VITROS NT‐proBNP assay has been previously reported.12 The assay has been shown to have acceptable levels of imprecision, a limit of detection of 4.29 ng/L, and a limit of quantification estimated to be 5.0 ng/L. All subjects gave informed consent.
Study Definitions and End Points
Data on patient characteristics were collected during the index hospitalization. Patients were classified by BMI in accordance with the World Health Organization (WHO) obesity classification.13 BMI <30 kg/m2 was categorized as nonobese, BMI 30 to 34.9 kg/m2 as Class I obesity, BMI 35 to 39.9 kg/m2 as Class II obesity, and BMI ≥40 kg/m2 as Class III obesity. Health status was measured at baseline using the general EuroQol‐5 Dimensions survey.14
The primary end point for the present analysis was the composite of 30‐day death or rehospitalization for HF. Secondary end points were 30‐day death or all‐cause rehospitalization, in‐hospital worsening renal function, and 180‐day all‐cause death. The primary end point was adjudicated by an independent, blinded, clinical events committee. Hospitalization for HF was defined as admission for typical clinical manifestations of worsening HF resulting in the new administration of intravenous therapies, mechanical or surgical intervention, or provision of ultrafiltration, hemofiltration, or dialysis for the management of persistent or worsening HF. Worsening renal function was defined as an increase of serum creatinine ≥0.3 mg/dL (26.5 μmol/L) or ≥25% relative increase in serum creatinine from baseline to discharge.
Statistical Analysis
Patients were divided into groups based on WHO obesity classification. Baseline clinical characteristics including demographics, medical history, signs/symptoms, laboratory values, medication use, and quality of life were compared between groups. Presenting signs/symptoms, baseline medications, and medical history were reported as counts/percentages for discrete factors and as 25th, 50th, and 75th percentiles for continuous variables. Comparisons for continuous variables were based on the Wilcoxon (Kruskal–Wallis) test, whereas categorical variables were assessed using χ2 test or Fisher's exact test, as appropriate. Where Fisher's exact test could not be computed, a Monte Carlo estimate was provided.
Interaction between baseline WHO BMI class and both baseline NT‐proBNP levels and change in NT‐proBNP from baseline to 48 to 72 hours was evaluated using logistic regression for 30‐day outcomes and Cox proportional hazards regression for 180‐day outcomes. Similar interaction analysis was performed using continuous BMI as the interaction term. In addition, Cox proportional hazards and logistic regression were used to evaluate the association between baseline NT‐proBNP and change in NT‐proBNP at 48 to 72 hours and the primary and secondary end points. The nonlinear relationship between change in NT‐proBNP and the primary and secondary end points was modeled using linear piece‐wise splines with a knot at zero. Odds ratios (ORs) were calculated for short‐term outcomes and hazard ratios (HRs) for time to death censoring at 180 days. For the relationship between baseline NT‐proBNP and outcomes, HRs or ORs estimate the change in risk associated with doubling of the baseline NT‐proBNP. For the relationship between 48 and 72 hours change in NT‐proBNP and outcomes, HRs or ORs estimate the change in risk associated with an increase/decrease of 1000 pg/mL from the baseline value.
Models were adjusted for covariates previously identified as being associated with clinical outcomes.15, 16, 17 Outcomes of death or death/rehospitalization were adjusted for the following: region (North America or other), age, blood urea nitrogen, serum creatinine, serum sodium, chronic obstructive pulmonary disease, cerebrovascular disease, depression, dyspnea, systolic blood pressure, elevated jugular venous pulsation at baseline, randomized treatment assignment, and HF hospitalization within 1 year.15 The outcome of worsening renal function was adjusted for randomized treatment assignment, blood urea nitrogen, systolic and diastolic blood pressures, and weight gain.17 A 2‐sided P<0.05 was selected as the threshold for statistical significance. There was no adjustment for multiple comparisons given the exploratory nature of this analysis.
All statistical computations were generated using SAS software (version 9.4; SAS Institute Inc, Cary, NC). The institutional review board at each participating site approved the study. The Duke Clinical Research Institute performed database management and statistical analysis. Scios Inc (Mountain View, CA) provided financial and material support for the ASCEND‐HF trial.
Results
Biomarker Study Population
A total of 686 patients were enrolled in the biomarker substudy population with documented NT‐proBNP levels at baseline and 48 to 72 hours. Study participants had a median (25th, 75th) age of 67 years (55, 78), 71% were female, and median ejection fraction (EF) was 26% (20, 40). Participants enrolled in the biomarker substudy were more often white (P<0.001), female (P=0.003), and recruited from North America (n=608; P<0.001), as compared with those not included in the biomarker substudy (Tables S1 and S2). Substudy patients had a higher BMI of 30 kg/m2 (26, 36) as compared with 28 kg/m2 (24, 33) in those who were not enrolled in the substudy (P<0.001).
Baseline Clinical Characteristics by WHO Obesity Class
Patients in a higher WHO obesity class were significantly younger (P<0.001) and had a higher prevalence of medical comorbidities, including hypertension (P=0.033), diabetes mellitus (P<0.001), and chronic respiratory disease (P=0.024; Table 1). Patients in a higher obesity class had significantly lower prevalence of coronary artery disease and were less likely to have an ischemic etiology of HF compared with patients within lower WHO obesity classes. EF, smoking status, and alcohol use did not differ significantly between groups.
Table 1.
Patient Characteristics by BMI Category
| Characteristic | Overall (N=686) | Obesity Class | P Value | |||
|---|---|---|---|---|---|---|
| Nonobese (N=346) | Class I (N=147) | Class II (N=86) | Class III (N=107) | |||
| Patient characteristics | ||||||
| Age, y (median, 25th–75th) | 67 (55–78) | 74 (61–82) | 64 (56–73) | 64 (56–74) | 54 (45–63) | <0.001 |
| Female sex, % | 486 (71) | 240 (69) | 112 (76) | 60 (70) | 74 (69) | 0.46 |
| Race, % | 0.047 | |||||
| White | 472 (69) | 257 (74) | 101 (69) | 57 (66) | 57 (53) | |
| Black | 193 (28) | 72 (21) | 44 (30) | 28 (33) | 49 (46) | |
| Asian | 9 (1) | 8 (2) | 0 (0) | 1 (1) | 0 (0) | |
| Other | 12 (2) | 9 (3) | 2 (1) | 0 (0) | 1 (1) | |
| BMI, kg/m2, median (25th–75th) | 30 (26–36) | 26 (23–28) | 32 (31–33) | 37 (36–38) | 45 (42–50) | <0.001 |
| SBP, mm Hg, median (25th–75th) | 125 (111–140) | 124 (110–137) | 126 (112–144) | 128 (113–140) | 129 (111–147) | 0.065 |
| HR, bpm, median (25th–75th) | 78 (70–89) | 78 (70–89) | 79 (70–88) | 78 (68–86) | 80 (70–93) | 0.74 |
| HF Hosp within 1 y, % | 282 (41) | 135 (39) | 60 (41) | 35 (41) | 52 (49) | 0.39 |
| Ischemic etiology | 42 (62) | 232 (67) | 93 (63) | 50 (58) | 48 (45) | <0.001 |
| Ejection fraction, % median (25th–75th) | 26 (20–40) | 25 (20–40) | 29 (20–40) | 28 (20–41) | 30 (20–45) | 0.53 |
| EF ≥50% (%) | 87 (16) | 43 (16) | 19 (15) | 8 (11) | 17 (21) | 0.45 |
| NYHA Class, % | 0.025 | |||||
| I | 30 (6) | 15 (6) | 5 (5) | 6 (10) | 4 (5) | |
| II | 108 (22) | 61 (26) | 24 (22) | 13 (21) | 10 (13) | |
| III | 243 (50) | 98 (41) | 60 (54) | 33 (52) | 52 (66) | |
| IV | 110 (22) | 64 (27) | 22 (20) | 11 (18) | 13 (17) | |
| Comorbidities, % | ||||||
| Previous MI | 250 (36) | 137 (40) | 51 (35) | 31 (36) | 31 (29) | 0.23 |
| Hypertension | 536 (78) | 259 (75) | 120 (82) | 64 (74) | 93 (87) | 0.033 |
| Diabetes mellitus | 332 (48) | 129 (37) | 80 (54) | 51 (59) | 72 (67) | <0.001 |
| Atrial fibrillation/flutter | 277 (40) | 152 (44) | 57 (39) | 37 (43) | 31 (29) | 0.046 |
| Coronary artery disease | 408 (60) | 221 (64) | 91 (62) | 48 (56) | 48 (45) | 0.004 |
| Laboratory values, median (25th–75th) | ||||||
| Sodium, mmol/L | 139 (136–141) | 139 (136–141) | 139 (136–141) | 139 (137–142) | 139 (136–141) | 0.77 |
| Creatinine, mg/dL | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.3 (1.0–1.7) | 1.3 (1.1–1.6) | 1.3 (1.0–1.8) | 1.00 |
| Hemoglobin, g/dL | 12.5 (11.1–13.7) | 12.5 (11.1–13.6) | 12.8 (11.3–14.0) | 12.3 (11.1–13.4) | 12.4 (10.9–13.6) | 0.60 |
| Patient quality of life | ||||||
| EQ5D VAS, median (25th–75th) | 0.71 (0.51–0.82) [665] | 0.71 (0.53–0.83) [335] | 0.71 (0.52–0.83) [143] | 0.71 (0.46–0.78) [85] | 0.60 (0.38–0.78) [102] | <0.001 |
| Measures of congestion | ||||||
| Dyspnea, % | 0.49 | |||||
| At rest | 384 (56) | 187 (54) | 80 (54) | 53 (62) | 64 (60) | |
| With minimal activity | 302 (44) | 159 (46) | 67 (46) | 33 (38) | 43 (40) | |
| Orthopnea, % | 560 (82) | 274 (79) | 116 (79) | 79 (92) | 91 (85) | 0.030 |
| Nocturnal dyspnea, % | 420 (61) | 204 (59) | 88 (60) | 59 (69) | 69 (65) | 0.35 |
| Weight gain, % | 504 (74) | 221 (64) | 117 (80) | 68 (79) | 98 (92) | <0.001 |
| Pulmonary congestion/edema with rales/crackles, % | 545 (79) | 289 (84) | 120 (82) | 66 (77) | 70 (65) | <0.001 |
| Peripheral edema, % | 554 (81) | 262 (76) | 118 (80) | 76 (88) | 98 (92) | <0.001 |
| Elevated JVP, % | 413 (60) | 208 (60) | 88 (60) | 54 (63) | 63 (59) | 0.96 |
| Medications/devices at enrollment | ||||||
| ACE‐I or ARB, % | 434 (63) | 210 (61) | 99 (67) | 57 (66) | 68 (64) | 0.50 |
| Beta‐blocker, % | 524 (76) | 269 (78) | 111 (76) | 62 (72) | 82 (77) | 0.73 |
| Aldosterone antagonists, % | 165 (24) | 79 (23) | 39 (27) | 18 (21) | 29 (27) | 0.62 |
| ICD, % | 217 (32) | 106 (31) | 46 (31) | 27 (31) | 38 (36) | 0.82 |
| CRT, % | 117 (17) | 57 (17) | 25 (17) | 19 (22) | 16 (15) | 0.58 |
| Medications/devices at enrollment—EF <40% | N=406 | N=206 | N=91 | N=49 | N=60 | |
| ACE‐I or ARB, % | 276 (68) | 135 (66) | 71 (78) | 34 (69) | 36 (60) | 0.086 |
| Beta‐blocker, % | 325 (80) | 169 (82) | 70 (77) | 38 (78) | 48 (80) | 0.74 |
| Aldosterone antagonists, % | 122 (30) | 55 (27) | 30 (33) | 15 (31) | 22 (37) | 0.44 |
| ICD, % | 174 (43) | 83 (40) | 38 (42) | 22 (45) | 31 (52) | 0.46 |
| CRT, % | 90 (22) | 42 (20) | 21 (23) | 14 (29) | 13 (22) | 0.66 |
ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CRT, cardiac resynchronization therapy; EF, ejection fraction; EQ5D, EuroQol‐5 Dimensions survey; HF, heart failure; Hosp, hospitalization; HR, heart rate; ICD, implantable cardioverter‐defibrillator; JVP, jugular venous pulsation; MI, myocardial infarction; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; VAS, Visual Analog Scale.
Patients with HF with reduced EF (EF <40%) had similar rates of utilization of guideline‐recommended medical and device therapies across all obesity classes (Table 1). Presence of baseline loop diuretic use did not differ across groups. Patients with Class III obesity had significantly lower perceived quality of life as compared with patients within a lower obesity class. During the hospitalization, greater numbers of patients in higher obesity classes experienced weight gain, orthopnea, and peripheral edema, but had fewer signs of pulmonary congestion.
Baseline NT‐proBNP differed markedly across BMI groups (Table 2). Patients within a high obesity class had significantly lower baseline NT‐proBNP levels (P<0.001; Figure 1). Median (25th, 75th) baseline NT‐proBNP level was 8760 pg/mL (4395–15125) for nonobese patients, 5289 pg/mL (2641–10754) in Class I obesity, 3573 pg/mL (2349–7748) in Class II obesity, and 3107 pg/mL (1454–5930) in Class III obesity. Patients within higher obesity classes also had smaller absolute reductions in NT‐proBNP from baseline to 48 to 72 hours, though percent reductions did not demonstrate clinically meaningful differences by obesity classes (Figure 2).
Table 2.
Biomarkers by BMI Category
| Biomarker | Overall (N=686) | Obesity Class | P Value | |||
|---|---|---|---|---|---|---|
| Nonobese (N=346) | Class I (N=147) | Class II (N=86) | Class III (N=107) | |||
| Baseline NT‐proBNP, pg/mL | 5782 (3011, 11 971) | 8760 (4395, 15 125) | 5289 (2641, 10 754) | 3573 (2349, 7748) | 3107 (1454, 5930) | <0.001 |
| NT‐proBNP at 48 to 72 h, pg/mL | 3022 (1183, 6623) | 4209 (1975, 8938) | 2699 (1116, 6270) | 1797 (1038, 3882) | 1115 (573, 3468) | <0.001 |
| Change in NT‐proBNP at 48 to 72 h, pg/mL | −2187 (−5510, −727) | −2959 (−7401, −1044) | −2194 (−4836, −610) | −1568 (−3070, −705) | −1299 (−2993, −303) | <0.001 |
| Percent change in NT‐proBNP at 48 to 72 h, % | −47 (−68, −21) | −46 (−66, −22) | −46 (−69, −20) | −51 (−72, −25) | −54 (−69, −25) | <0.001 |
BMI indicates body mass index; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide.
Figure 1.
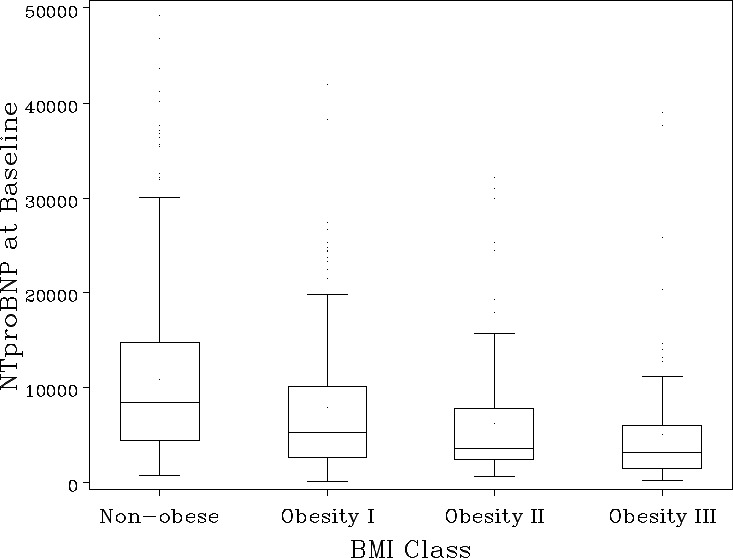
Baseline NT‐proBNP by BMI class. Values of NT‐proBNP >50 000 (n=12) are excluded from plot area. Line represents median, box represents interquartile range (IQR), upper bar represents 75% percentile+1.5 (IQR), lower bar represents 25% percentile—1.5 (IQR), and dots represent outliers included in this analysis. BMI indicates body mass index; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide.
Figure 2.
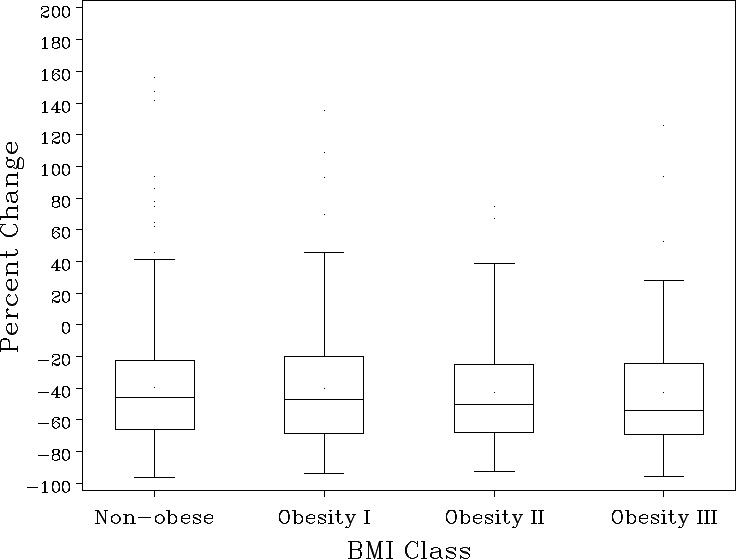
Percent change in NT‐proBNP at 48/72 hours by BMI class. Line represents median, box represents interquartile range (IQR), upper bar represents 75% percentile+1.5 (IQR), lower bar represents 25% percentile—1.5 (IQR), and dots represent outliers included in this analysis. BMI indicates body mass index; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide.
NT‐proBNP and Clinical Outcomes
The primary end point of 30‐day death/hospitalization for HF occurred in 86 patients, 42 of which were designated as nonobese. With respect to the outcome of 180‐day death, there were 78 events in the overall study population, 53 of which occurred in patients designated as nonobese.
Higher baseline NT‐proBNP levels were associated with higher rates of the primary end point, 30‐day death/hospitalization for HF, in unadjusted analysis (Table 3). This association was no longer significant following adjustment (adjusted P=0.25). Similarly, rates of the secondary outcome of 30‐day death or all‐cause hospitalization were higher in patients with higher baseline NT‐proBNP levels, though these differences were also not significant in adjusted analyses (adjusted P=0.35).
Table 3.
Outcomes by Baseline NT‐proBNP
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Clinical Outcome | OR for BL NT‐proBNP (95% CI) | P Value | OR for BL NT‐proBNP (95% CI) | P Value |
| Short‐term outcomes | ||||
| 30‐d death or HF‐rehosp.a | 1.19 (1.02, 1.39) | 0.027 | 1.12 (0.93, 1.34) | 0.25 |
| 30‐d death or all‐cause‐rehosp.a | 1.17 (1.03, 1.33) | 0.015 | 1.07 (0.93, 1.25) | 0.35 |
| Worsening renal functionb | 1.02 (0.86, 1.22) | 0.79 | 1.13 (0.93, 1.38) | 0.22 |
| Long‐term outcomes | HR for BL NT‐proBNP (95% CI) | P Value | HR for BL NT‐proBNP (95% CI) | P Value |
|---|---|---|---|---|
| 180‐d deatha | 1.58 (1.36, 1.83) | <0.001 | 1.40 (1.16, 1.71) | <0.001 |
Analyses adjusted for prespecified variables found to be correlated with outcomes. Hazard ratio (HR) and odds ratio (OR) estimate the change in risk associated with doubling of the baseline value based on a base‐2 logarithm model. BL indicates baseline; CI, confidence interval; HF, heart failure; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide; rehosp., rehospitalization.
Adjusted for region (North America or other), age, blood urea nitrogen (BUN), serum creatinine, serum sodium, chronic obstructive pulmonary disease, cerebrovascular disease, depression, dyspnea, systolic blood pressure, elevated jugular venous pulsation at baseline, randomized treatment assignment, and heart failure hospitalization within 1 year.
Adjusted for randomized treatment assignment, BUN, systolic and diastolic blood pressures, and weight gain.
Higher baseline NT‐proBNP levels were strongly associated with increased risk of all‐cause death rates censored at 180 days before and after adjustment (adjusted HR per doubling of baseline NT‐proBNP 1.40; 95% confidence interval, 1.16, 1.71; P<0.001). Median follow‐up time for this end point was 180 days. For patients who did have a reduction in NT‐proBNP levels at 48 to 72 hours (n=604; 87.9%), unadjusted analysis showed that the risk of all‐cause death rates censored at 180 days decreased as NT‐proBNP decreased (unadjusted HR, 0.93 per 1000 pg/mL decrease in NT‐proBNP from baseline; 95% confidence interval, 0.87, 0.98; P=0.014), though this relationship was not statistically significant after adjustment (P=0.41). Change in NT‐proBNP levels at 48 to 72 hours was not associated with significant changes the rates of primary end point or other secondary end points (Table 4).
Table 4.
Outcomes by 48/72 Hours Δ in NT‐proBNP
| Clinical Outcome | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR for Increase/Decrease of 1000 pg/mL in NT‐proBNP (95% CI) | P Value | OR for Increase/Decrease of 1000 pg/mL in NT‐proBNP (95% CI) | P Value | |
| Short‐term outcomes | ||||
| 30‐d death or HF‐rehosp.a | ||||
| Δ NT‐proBNP ≤0 | 0.90 (0.84, 0.97) | 0.007 | 0.95 (0.88, 1.02) | 0.14 |
| Δ NT‐proBNP >0 | 0.97 (0.86, 1.10) | 0.65 | 0.99 (0.87, 1.12) | 0.86 |
| 30‐d death or all‐cause‐rehosp.a | ||||
| Δ NT‐proBNP ≤0 | 0.92 (0.86, 0.97) | 0.005 | 0.95 (0.90, 1.01) | 0.13 |
| Δ NT‐proBNP >0 | 0.97 (0.88, 1.08) | 0.64 | 0.98 (0.88, 1.10) | 0.76 |
| Worsening renal functionb | ||||
| Δ NT‐proBNP ≤0 | 1.04 (0.96, 1.13) | 0.38 | 1.04 (0.62, 1.14) | 0.35 |
| Δ NT‐proBNP >0 | 1.05 (0.87, 1.27) | 0.63 | 1.06 (0.88, 1.28) | 0.54 |
| Long‐term outcomes | HR for increase/decrease of 1000 pg/mL in NT‐proBNP (95% CI) | P Value | HR for increase/decrease of 1000 pg/mL in NT‐proBNP (95% CI) | P Value |
|---|---|---|---|---|
| 180‐d deatha | ||||
| Δ NT‐proBNP ≤0 | 0.93 (0.87, 0.98) | 0.01 | 0.94 (0.88, 1.00) | 0.04 |
| Δ NT‐proBNP >0 | 0.99 (0.92, 1.06) | 0.76 | 0.95 (0.87, 1.03) | 0.20 |
Analyses adjusted for prespecified variables found to be correlated with outcomes. All models include baseline NT‐proBNP as a covariate. Hazard ratio (HR) and odds ratio (OR) estimate the change in risk associated with an increase/decrease of 1000 pg/mL from the baseline value. The relationship between ΔNT‐proBNP at 48 to 72 hours is nonlinear. Regression used piece‐wise linear splines with knot at ΔNT‐proBNP=0. Thus, the linear relationship between delta NT‐proBNP and clinical outcomes is modeled for patients with a decreased or no change in NT‐proBNP (Δ NT‐proBNP ≤0) and those with increased NT‐proBNP (Δ NT‐proBNP >0) separately in the model. CI indicates confidence interval; HF, heart failure; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide; rehosp., rehospitalization.
Adjusted for region (North America or other), age, blood urea nitrogen (BUN), serum creatinine, serum sodium, chronic obstructive pulmonary disease, cerebrovascular disease, depression, dyspnea, systolic blood pressure, elevated jugular venous pulsation at baseline, randomized treatment assignment, and HF hospitalization within 1 year.
Adjusted for randomized treatment assignment, BUN, systolic and diastolic blood pressures, and weight gain.
Modifying Effect of BMI on NT‐ProBNP Relationship to Outcomes
Categorical BMI class did not modify the relationship between baseline NT‐proBNP and the end point of all‐cause death rates censored at 180 days (adjusted interaction, P=0.24; Table 5). Specifically, the relationship observed between baseline NT‐proBNP and all‐cause death rates censored at 180 days (adjusted HR per doubling of baseline NT‐proBNP, 1.40; 95% confidence interval, 1.16, 1.71; P<0.001) did not differ with respect to a patient's WHO obesity class. There was also no significant interaction when continuous BMI was used as the interaction term.
Table 5.
Interaction Between BMI Class and NT‐proBNP
| Clinical Outcome | Unadjusted Interaction P Value | Adjusted Interaction P Value |
|---|---|---|
| Baseline NT‐proBNP | ||
| 30‐d death or HF‐rehosp.a | 0.63 | 0.64 |
| 30‐d death or all‐cause‐rehosp.a | 0.48 | 0.48 |
| Worsening renal functionb | 0.34 | 0.24 |
| 180‐d deatha | 0.42 | 0.24 |
| 48/72 h Δ in NT‐proBNP | ||
| 30‐d death or HF‐rehosp.a | ||
| Δ NT‐proBNP ≤0 | 0.72 | 0.88 |
| Δ NT‐proBNP >0 | 0.16 | 0.54 |
| 30‐d death or all‐cause‐rehosp.a | ||
| Δ NT‐proBNP ≤0 | 0.64 | 0.90 |
| Δ NT‐proBNP >0 | 0.11 | 0.25 |
| Worsening renal functionb | ||
| Δ NT‐proBNP ≤0 | 0.44 | 0.27 |
| Δ NT‐proBNP >0 | 0.59 | 0.75 |
| 180‐d deatha | ||
| Δ NT‐proBNP ≤0 | 0.93 | 0.77 |
| Δ NT‐proBNP >0 | 0.26 | 0.72 |
Analyses adjusted for prespecified variables found to be correlated with outcomes. Nonobese: BMI <30 kg/m2, Class I obesity: BMI 30 to 34.9 kg/m2, Class II obesity BMI 35 to 39.9 kg/m2, and Class III obesity BMI ≥40 kg/m2. BMI indicates body mass index; HF, heart failure; NT‐proBNP, N‐terminal‐pro‐b‐type natriuretic peptide; rehosp., rehospitalization.
Adjusted for region (North America or other), age, blood urea nitrogen (BUN), serum creatinine, serum sodium, chronic obstructive pulmonary disease, cerebrovascular disease, depression, dyspnea, systolic blood pressure, elevated jugular venous pulsation at baseline, randomized treatment assignment, and HF hospitalization within 1 year.
Adjusted for randomized treatment assignment, BUN, systolic and diastolic blood pressures, and weight gain.
Discussion
In this analysis, we found that patients within higher WHO obesity classes had significantly lower baseline NT‐proBNP levels. Baseline NT‐proBNP levels were significantly associated with 180‐day mortality. BMI class did not modify the prognostic value of NT‐proBNP on this outcome. Obesity was highly prevalent in this study population, with 16% of patients categorized as severely obese. Similar rates of severe obesity were observed in an analysis using pooled data of 795 patients from 3 acute HF clinical trials: (DOSE [Diuretic Strategies Optimization Evaluation], CARRESS‐HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure], and ROSE [Renal Optimization Strategies Evaluation in Acute Heart Failure]).18 In our study, the high rate of severe obesity is particularly striking given that the cohort consisted predominantly of patients with reduced EF, as our analysis and others have shown that HF patients with preserved EF tend to be more obese.18 The rise in severe obesity underscores the relevance of understanding how obesity may affect traditional signs, symptoms, laboratory values, and biomarkers commonly used in the diagnosis and prognosis of HF.
Our study supports the inverse relationship between baseline BMI and NT‐proBNP. Median levels of NT‐proBNP were >5500 pg/mL lower in patients with Class III obesity as compared with nonobese patients. Multiple analyses have reported similar findings.7, 8, 18 Mechanisms may include increased clearance of natriuretic peptides attributed to enzymes found in adipocytes and elevated glomerular filtration rates in the obese patient.19 It has been hypothesized that the widely observed obesity paradox may be mediated by reduced myocardial stretch, as evidenced by lower circulating natriuretic peptides, and may be indicative of less‐severe forms of HF, though this remains controversial and not empirically validated.20 Notably, whereas absolute 48 to 72 hours reductions in NT‐proBNP were greater in nonobese patients, percent reductions were similar across all obesity classes. Although requiring further validation, our analysis suggests that serial NT‐proBNP levels to evaluate for adequate decongestion may still be useful in the most obese patients.
Clinical Implications
Our study evaluated the complex relationship between NT‐proBNP, BMI, and outcomes. BNP and/or NT‐proBNP is affected by many variables, including age, sex, comorbidities, and BMI.21 Our study finds that higher NT‐proBNP levels are associated with poor long‐term posthospitalization mortality levels, a finding consistently reflected across many studies. Consequently, a number of studies have indicated that more‐obese patients have improved survival.22, 23, 24, 25 Proposed mechanisms for the protective role of obesity on HF outcomes have included increased cardiac reserve and/or less‐severe forms of HF.26 Given (1) the inverse relationship between NT‐proBNP and BMI and (2) proposed mechanisms that improved HF outcomes in more‐obese patients are observed in conjunction with lower NT‐proBNP levels, assessing whether BMI modifies the relationship between NT‐proBNP and outcomes by obesity class is important and relevant.
Our study shows that whereas BMI changes the distribution of NT‐proBNP (ie, an inverse relationship between natriuretic peptide and BMI), it does not modify the prognostic significance of NT‐proBNP on post–acute HF outcomes. Patients with higher NT‐proBNP values had consistently worse long‐term outcomes, regardless of their obesity class. Our study does not refute the presence of an obesity paradox, but rather suggests that a higher NT‐proBNP was a universally poor prognostic sign in HF patients with no differential effect based on obesity class. Past studies have sought to understand how the prognostic value of NT‐proBNP is modified by BMI in a number of scenarios, mostly in chronic HF. Nadruz et al similarly found that higher NT‐proBNP levels correlated with greater rates of cardiovascular death or HF hospitalization regardless of BMI.27 Horwich et al found that BNP was a predictor of 1‐year mortality independent of BMI class.23 Another study of 618 chronic HF patients found similar prognostic power of NT‐proBNP irrespective of BMI class.28 These studies focused exclusively on a chronic HF cohort. Other analyses have assessed the interaction of BNP or NT‐proBNP and BMI on outcomes in other clinical settings. An analysis of 12230 patients from the ARIC (Atherosclerosis Risk in Communities) Study found that higher NT‐proBNP levels were associated with an increased risk of the development of HF across all BMI classes.29 Krauser et al used the acute HF model to assess interaction of BMI and NT‐proBNP, but focused on the diagnosis of HF, not overall clinical prognosis.30 A recent analysis of the PROTECT (Placebo‐Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) trial similarly found no interaction between obesity class and BNP on 180‐day death.31 To our knowledge, this is the first investigation to assess the effect modification of BMI on the prognostic utility of NT‐proBNP levels on clinical outcomes in an acute HF population. Baseline and change in NT‐proBNP levels have been shown to be powerful prognostic factors in acute HF.32, 33, 34 With the prevalence of obesity rising in North America, as evidenced by 50% of patients in our study population categorized as obese and 16% severely obese, our analysis provides evidence that NT‐proBNP testing during acute HF exacerbation remains useful in patients with the highest or lowest BMIs.
Limitations
There are several limitations of the data that should be acknowledged. First, this analysis was post hoc and is subject to the biases of exploratory analyses. Furthermore, this analysis utilized a biomarker substudy population from the original ASCEND‐HF study. The biomarker substudy was a nonrandomized sample, and includes a fraction of the total patients enrolled in ASCEND‐HF, and therefore may be underpowered with respect to the interaction analysis and results should be viewed as hypothesis generating. Our analysis excluded patients with low NT‐proBNP levels (<125 pg/mL for subjects aged <75 years; <425 pg/mL for subjects aged ≥75 years). Patients with HF with preserved EF have been shown to have significantly lower natriuretic peptide levels, even during acute decompensation, which may have skewed our analysis to a more‐reduced EF cohort.38 In addition, because of high missingness (>20% of patients) of EF measurements within the data, EF was not included as a covariate in the adjustment models, placing the results at risk for confounding.
Findings in our analyses are subject to risk of other residual confounding. Because we used covariates previously identified as associated with outcomes in other ASCEND‐HF analyses, we may not have included potential covariates which could affect the results. The evidence for the effect of race and sex on NT‐proBNP as a useful prognostic tool in HF is unclear and conflicting35; a substudy of the Dallas Heart Study36 found that whites had higher NT‐proBNP levels as compared with blacks and Hispanics whereas a Get With the Guidelines analysis found contradictory results.37 For example, findings of increased rates of peripheral edema and orthopnea in more‐obese patients may be attributed to confounding factors, such as venous stasis, obstructive sleep apnea, and right heart dysfunction observed in higher prevalence among obese patients. It can be conceived that the 48 to 72 NT‐proBNP data assessment may be too early in the decongestion process and is therefore not reflective of full decongestion. Although this is plausible, greater than 40% reductions in NT‐proBNP were noted across all BMI classes. A recent meta‐analysis found that >30% reductions in NT‐proBNP levels are suggestive of adequate decongestion and reduced all‐cause and cardiovascular mortality.39
Conclusion
In conclusion, whereas patients in higher obesity classes were generally younger and had more comorbid conditions, they were generally well balanced with respect to adherence to guideline‐based therapies. Patients within higher obesity classes had lower baseline NT‐proBNP levels, though percent reductions with decongestion at 48 to 72 hours did not significantly differ. In an acute HF cohort, baseline NT‐proBNP levels were strongly associated with the long‐term outcome of 180‐day death. BMI did not modify the relationship of NT‐proBNP on the primary and secondary outcomes. Given the rapidly rising prevalence of obesity, additional studies are needed to assess the impact of obesity on the prognostic utility of biomarkers in HF.
Sources of Funding
ASCEND‐HF was supported by Johnson & Johnson. Dr. Bhatt was supported by internal funding from Duke Clinical Research Institute.
Disclosures
Dr Butler reports research grants from NIH, EU, and PCORI; consulting fees from Amgen, Bayer, Boehringer Ingelheim, Cardiocell, Jansen, Novartis, Relypsa, Trevena, Z pharma, StealthPeptide, Pharmaln, and Merck. Dr Felker reports research funding from the NIH, Amgen, BG Medicine, Cytokinetics, Johnson & Johnson, Roche Diagnostic Corp., and Otsuka and consulting fees from Amgen, Cytokinetics, Roche, Otsuka, and Novartis. Dr Ezekowitz reports consulting fees from Pfizer, Abbott Labs, and Servier,and research support from Amgen and Johnson & Johnson. Dr Armstrong reports research support from Johnson & Johnson. Dr Hernandez reports consulting fees from Sanofi, Johnson & Johnson, AstraZeneca, and Corthera and research support from Amylin and Scios/Johnson & Johnson. Dr O'Connor reports consulting fees from Novella and Amgen, ownership/partnership/principal in Biscardia, LLC, and research support from Otsuka, Roche Diagnostics, BG Medicine, Critical Diagnostics, Astellas, Gilead, GE Healthcare, and ResMed. Dr Mentz reports research support from NIH, Amgen, AstraZeneca, Bristol‐Myers Squibb, GlaxoSmithKline, Gilead, Medtronic, Novartis, Otsuka, and ResMed; honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, and Thoratec/St Jude; and has served on an advisory board for Luitpold Pharmaceuticals, Inc., and Boehringer Ingelheim. The remaining authors have no disclosures to report.
Supporting information
Table S1. Comparison of Baseline Characteristics Between Biomarker Cohort and Other ASCEND‐HF Patients in the Intention to Treat Population
Table S2. Event Rates by Obesity Class
(J Am Heart Assoc. 2018;7:e006740 DOI:10.1161/JAHA.117.006740.)29431103
References
- 1. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Køber L, Lip GYH, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Ž, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Ørn S, Parissis JT, Ponikowski P. ESC, guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. [DOI] [PubMed] [Google Scholar]
- 3. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 4. Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B‐type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. [DOI] [PubMed] [Google Scholar]
- 6. Horwich TB, Hamilton MA, Fonarow GC. B‐type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. [DOI] [PubMed] [Google Scholar]
- 7. Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, Canham RM, Chung AK, Leonard D, Wians FH, de Lemos JA. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. [DOI] [PubMed] [Google Scholar]
- 8. Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED. Obesity and suppressed B‐type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–1595. [DOI] [PubMed] [Google Scholar]
- 9. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 10. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJV, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KFJ, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 11. Hernandez AF, O'Connor CM, Starling RC, Reist CJ, Armstrong PW, Dickstein K, Lorenz TJ, Gibler WB, Hasselblad V, Komajda M, Massie B, McMurray JJ, Nieminen M, Rouleau JL, Swedberg K, Califf RM. Rationale and design of the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND‐HF). Am Heart J. 2009;157:271–277. [DOI] [PubMed] [Google Scholar]
- 12. Januzzi JL Jr, Lewandrowski KB, Bashirians G, Jackson S, Freyler D, Smith K, Murakami MM, Apple FS. Analytical and clinical performance of the Ortho‐Clinical Diagnostics VITROS amino‐terminal pro‐B type natriuretic peptide assay. Clin Chim Acta. 2008;387:48–54. [DOI] [PubMed] [Google Scholar]
- 13. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii, 1–253. [PubMed] [Google Scholar]
- 14. EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 15. Khazanie P, Heizer GM, Hasselblad V, Armstrong PW, Califf RM, Ezekowitz J, Dickstein K, Levy WC, McMurray JJV, Metra M, Wilson Tang WH, Teerlink JR, Voors AA, O'Connor CM, Hernandez AF, Starling R. Predictors of clinical outcomes in acute decompensated heart failure: Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure outcome models. Am Heart J. 2015;170:290–297.e1. [DOI] [PubMed] [Google Scholar]
- 16. Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, Atar D, Teerlink JR, Califf RM, Massie BM, Hasselblad V, Starling RC, O'Connor CM, Ponikowski P. Predictors of early dyspnoea relief in acute heart failure and the association with 30‐day outcomes: findings from ASCEND‐HF. Eur J Heart Fail. 2013;15:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Deursen VM, Hernandez AF, Stebbins A, Hasselblad V, Ezekowitz JA, Califf RM, Gottlieb SS, O'Connor CM, Starling RC, Tang WH, McMurray JJ, Dickstein K, Voors AA. Nesiritide, renal function, and associated outcomes during hospitalization for acute decompensated heart failure: results from the Acute Study of Clinical Effectiveness of Nesiritide and Decompensated Heart Failure (ASCEND‐HF). Circulation. 2014;130:958–965. [DOI] [PubMed] [Google Scholar]
- 18. Joyce E, Lala A, Stevens SR, Cooper LB, AbouEzzeddine OF, Groarke JD, Grodin JL, Braunwald E, Anstrom KJ, Redfield MM, Stevenson LW. Prevalence, profile, and prognosis of severe obesity in contemporary hospitalized heart failure trial populations. JACC Heart Fail. 2016;4:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT‐proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. [DOI] [PubMed] [Google Scholar]
- 21. Raymond I, Groenning BA, Hildebrandt PR, Nilsson JC, Baumann M, Trawinski J, Pedersen F. The influence of age, sex and other variables on the plasma level of N‐terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenchaiah S, Pocock SJ, Wang D, Finn PV, Zornoff LA, Skali H, Pfeffer MA, Yusuf S, Swedberg K, Michelson EL, Granger CB, McMurray JJ, Solomon SD. Body mass index and prognosis in patients with chronic heart failure: insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;116:627–636. [DOI] [PubMed] [Google Scholar]
- 23. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. [DOI] [PubMed] [Google Scholar]
- 24. Oreopoulos A, Padwal R, Kalantar‐Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta‐analysis. Am Heart J. 2008;156:13–22. [DOI] [PubMed] [Google Scholar]
- 25. Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153:74–81. [DOI] [PubMed] [Google Scholar]
- 26. Kalantar‐Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. [DOI] [PubMed] [Google Scholar]
- 27. Nadruz W Jr, Claggett BL, McMurray JJ, Packer M, Zile MR, Rouleau JL, Desai AS, Swedberg K, Lefkowitz M, Shi VC, Prescott MF, Solomon SD. Impact of body mass index on the accuracy of N‐terminal pro‐brain natriuretic peptide and brain natriuretic peptide for predicting outcomes in patients with chronic heart failure and reduced ejection fraction: insights from the PARADIGM‐HF Study (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial). Circulation. 2016;134:1785–1787. [DOI] [PubMed] [Google Scholar]
- 28. Frankenstein L, Remppis A, Nelles M, Schaelling B, Schellberg D, Katus H, Zugck C. Relation of N‐terminal pro‐brain natriuretic peptide levels and their prognostic power in chronic stable heart failure to obesity status. Eur Heart J. 2008;29:2634–2640. [DOI] [PubMed] [Google Scholar]
- 29. Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N‐terminal pro‐brain natriuretic peptide and heart failure risk among individuals with and without obesity: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krauser DG, Lloyd‐Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, Chen A, Tung R, Januzzi JL Jr. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149:744–750. [DOI] [PubMed] [Google Scholar]
- 31. Streng KW, Ter Maaten JM, Cleland JG, O'Connor CM, Davison BA, Metra M, Givertz MM, Teerlink JR, Ponikowski P, Bloomfield DM, Dittrich HC, Hillege HL, van Veldhuisen DJ, Voors AA, van der Meer P. Associations of body mass index with laboratory and biomarkers in patients with acute heart failure. Circ Heart Fail. 2017;10:e003350. [DOI] [PubMed] [Google Scholar]
- 32. Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N‐terminal–pro‐brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–2174. [DOI] [PubMed] [Google Scholar]
- 33. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, Pinto YM, Richards M. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT‐proBNP Study. Eur Heart J. 2006;27:330–337. [DOI] [PubMed] [Google Scholar]
- 34. Januzzi JL Jr, Sakhuja R, O'Donoghue M, Baggish AL, Anwaruddin S, Chae CU, Cameron R, Krauser DG, Tung R, Camargo CA Jr, Lloyd‐Jones DM. Utility of amino‐terminal pro‐brain natriuretic peptide testing for prediction of 1‐year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. 2006;166:315–320. [DOI] [PubMed] [Google Scholar]
- 35. Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL Jr. Neither race nor gender influences the usefulness of amino‐terminal pro‐brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Substudy. J Card Fail. 2006;12:452–457. [DOI] [PubMed] [Google Scholar]
- 36. Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial differences in natriuretic peptide levels the Dallas Heart Study. JACC Heart Fail. 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krim SR, Vivo RP, Krim NR, Qian F, Cox M, Ventura H, Hernandez AF, Bhatt DL, Fonarow GC. Racial/ethnic differences in B‐type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure findings from Get With the Guidelines‐Heart Failure. JACC Heart Fail. 2013;1:345–352. [DOI] [PubMed] [Google Scholar]
- 38. Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT, Clopton P, Steg G, Aumont MC, Westheim A, Knudsen CW, Perez A, Kamin R, Kazanegra R, Herrmann HC, McCullough PA. Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol. 2003;41:2010–2017. [DOI] [PubMed] [Google Scholar]
- 39. McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain‐type natriuretic peptide and amino‐terminal pro‐brain‐type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med. 2017;166:180–190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Baseline Characteristics Between Biomarker Cohort and Other ASCEND‐HF Patients in the Intention to Treat Population
Table S2. Event Rates by Obesity Class


