Abstract
Background
Children with single ventricle heart disease require frequent interventions and follow‐up. Low socioeconomic status (SES) may limit access to high‐quality care and place these children at risk for poor long‐term outcomes.
Methods and Results
Data from the SVR (Pediatric Heart Network Single Ventricle Reconstruction Trial Public Use) data set were used to examine the relationship of US neighborhood SES with 30‐day and 1‐year mortality or cardiac transplantation and length of stay among neonates undergoing the Norwood procedure (n=525). Crude rates of death or transplantation at 1 year after Norwood were highest for patients living in neighborhoods with low SES (lowest tertile 37.0% versus middle tertile 31.0% versus highest tertile 23.6%, P=0.024). After adjustment for patient demographics, birth characteristics, and anatomy, patients in the highest SES tertile had significantly lower risk of death or transplant than patients in the lowest SES tertile (hazard ratio 0.62, 95% confidence interval, 0.40, 0.96). When SES was examined continuously, the hazard of 1‐year death or transplant decreased steadily with increasing neighborhood SES. Hazard ratios for 30‐day transplant‐free survival and 1‐year transplant‐free survival were similar in magnitude. There were no significant differences in length of stay following the Norwood procedure by SES.
Conclusions
Low neighborhood SES is associated with worse 1‐year transplant‐free survival after the Norwood procedure, suggesting that socioeconomic and environmental factors may be important determinants of outcome in critical congenital heart disease. Future studies should investigate aspects of SES and environment amenable to intervention.
Clinical Trial Registration
URL:http://www.clinicaltrials.gov> http://www.clinicaltrials.gov. Unique identifier: NCT00115934.
Keywords: single ventricle, socioeconomic position, surgery, survival
Subject Categories: Mortality/Survival, Risk Factors, Congenital Heart Disease
Clinical Perspective
What Is New?
This study provides the first in‐depth exploration of the relationship of socioeconomic status with post‐Norwood outcomes in children with single ventricle physiology.
Patients in the highest neighborhood socioeconomic status tertile had a 38% lower risk of 1‐year mortality or cardiac transplantation after Norwood compared with patients in the lowest tertile.
This relationship was not explained by patient demographics, birth characteristics, or anatomy and was not limited to the first 30 days or postdischarge.
There was no difference in Norwood length of stay by socioeconomic status.
What Are the Clinical Implications?
Post‐Norwood care should focus not only on operative management strategies but also socioeconomic and environmental factors, which may be equally important determinants of patient outcomes.
Additional studies are needed to identify strategies for improving Norwood survival among low socioeconomic status patients and to reduce disparities in patient outcomes.
Introduction
Advances in medical and surgical care have significantly improved the outcomes for children born with single ventricle (SV) heart disease1; however, postoperative mortality rates remain high and long‐term outcomes poor.2 Therefore, risk factors for adverse outcome in the SV population have been the subject of many investigations. Among these, innate risk factors, such as low birth weight, anatomy, preterm birth, genetic abnormalities, and associated noncardiac congenital anomalies, are well‐established predictors for early mortality and transplantation.3, 4, 5, 6, 7, 8 Recently, societal factors such as poverty, medical insurance, and maternal education have emerged as predictors of adverse outcomes in children with complex congenital heart disease (CHD) and may explain some of the difference in survival between children with similar clinical characteristics.9 Specifically, low socioeconomic status (SES) in children with SV physiology has been associated with higher early mortality6, 8 and lower quality of life10 after surgery, and other studies have found similar associations in the larger CHD population.11, 12, 13, 14, 15, 16 Nevertheless, existing studies examining SES have been limited by cross‐sectional or case–control study designs,14, 15 single center populations,7, 9, 10, 14, 15 or limited outcome assessment.6, 8 To date, there has been no in‐depth exploration of the relationship of SES with post‐Norwood outcomes in children with SV physiology.
Insight into the relationship between SES and outcomes in these children may suggest additional strategies to improve health outcomes. Children with SV physiology require early medical and surgical interventions, frequent follow‐up, and intensive parental involvement in both inpatient and outpatient settings. Low SES could limit access to high‐quality care and postoperative follow‐up programs, and the economic burden posed by frequent hospitalizations and surgeries may place children with low SES at particularly high risk of poor longer‐term outcomes.17 Indeed, qualitative studies of parents with CHD have shown that lower SES is associated with higher levels of financial stress, emotional drain, and caregiver burden.18
Accordingly, the aim of this study was to examine the association of neighborhood SES with hospital length of stay and postoperative survival in children undergoing the Norwood procedure. In addition, we examine the relationship of SES with other clinical factors such as race and gestational age to understand how the effect of neighborhood SES varies by these factors.
Methods
Study Design
We used publicly available data from the SVR (Pediatric Heart Network Single Ventricle Reconstruction) trial. The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.19 Details of the trial design have been reported.20 Briefly, 555 neonates with single right ventricle anomalies and systemic outflow tract obstruction were randomized to receive either a modified Blalock‐Taussig shunt or a right ventricle‐to‐pulmonary artery shunt during the Norwood procedure. Enrollment occurred between May 2005 and July 2008 at 15 centers in the United States and Canada. The primary end point was death or cardiac transplantation at 1 year after randomization however, several additional outcomes were assessed including length of stay, complications, and additional interventions. The institutional review board or ethics committee of all institutions approved the trial, and all parents or legal guardians gave written informed consent before participation.
Patient Sample
Of the 555 neonates randomized in the trial, the overall analysis cohort included 549 children. Five subjects who did not undergo the Norwood procedure and 1 subject who withdrew from the trial within a week of randomization were excluded from the analyses. In addition, we excluded 14 children from Canada and 10 children with missing information on SES. The final sample included 525 patients.
Data Collection
Detailed information on patient demographics, birth characteristics, anatomy, medical history, perioperative characteristics, and postoperative hospital course were collected using standardized forms. Neighborhood SES was measured using a US census‐based score previously developed by Diez Roux et al.21 Briefly, the neighborhood SES score was derived from 6 measures related to wealth and income (log of the median household income, log of the median value of housing units, and the percentage of households receiving interest, dividend, or net rental income), education (among adults 25 years of age or older, the percentage who had completed high school and the percentage who had completed college), and occupation (the percentage of employed people 16 years of age or older in executive, managerial, or professional specialty occupations). Z‐scores for each census block group were calculated for each variable, and the neighborhood summary score was calculated as the sum of the z‐scores, with a higher score indicating higher neighborhood SES. We chose to examine neighborhood SES over family SES because the SES score is a more comprehensive measure than single measures of parental education or income and has been validated across several populations. Moreover, studies have shown that neighborhood SES is associated with health outcomes in patients with CHD,6, 8, 14, 22, 23, 24 and others have suggested that neighborhood SES may predict health above and beyond individual SES.25 We also compared our findings to another US Census‐based indicator of neighborhood SES defined as neighborhood with at least 20% of individuals living below the federal poverty level.
Outcome Measurement
We evaluated 3 outcomes including a composite outcome of mortality or transplantation within 30 days and 1 year of randomization and length of stay during Norwood hospitalization. Length of stay was only calculated in patients surviving the Norwood hospitalization (n=441).
Statistical Analyses
Descriptive characteristics were compared across SES score tertiles using χ2 tests for categorical variables and ANOVA or Kruskal–Wallis tests for continuous variables. To evaluate the relationship of neighborhood SES to 30‐day and 1‐year transplant‐free mortality, we performed 2 sets of analyses, modeling SES first as a continuous and then as a categorical variable. In the first set of analyses, we modeled the hazard of mortality or cardiac transplantation relative to the mean SES score of 0 using proportional hazards regression restricted cubic spline models with 5 knots.26, 27 This approach allows the log hazard ratio to vary nonlinearly with SES without requiring prespecification of the precise shape of the relationship.
In analyses of SES as a categorical variable, we used χ2 tests, Kaplan–Meier curves, and Cox proportional hazards regression to compare unadjusted and adjusted transplant‐free survival across SES tertiles at 30 days and 1 year. To evaluate whether the effect of neighborhood SES varied over the short (ie, first 30 days) and longer term (ie, >30 days to 1 year), we evaluated interactions between SES and time. We also estimated conditional hazard ratios for patients who survived to hospital discharge (n=441).
Multivariable models sequentially adjusted for patient demographic and clinical characteristics to determine whether certain covariates could explain the relationship between neighborhood SES and transplant‐free survival. For models evaluating 30‐day and 1‐year mortality or transplantation as well as the interaction between SES and time, we included only preoperative covariates (age at Norwood procedure, sex, race/ethnicity, gestational age, birth weight, and diagnosis of hypoplastic left heart syndrome) in the risk adjustment. For the model evaluating 1‐year survival among survivors to Norwood discharge, we added additional peri‐ and postoperative covariates (shunt type [right ventricle‐to‐pulmonary‐artery shunt versus modified Blalock‐Taussig shunt], receipt of extracorporeal membrane oxygenation, open sternum, and number of post‐Norwood inpatient complications, World Health Organization z‐score for weight‐for‐age at discharge, and tube feeding requirement at discharge) in order to determine whether hospital course and health status at discharge explained any differences in mortality or transplantation risk.
For length of stay, we modeled the relationship of neighborhood SES with log‐transformed length of stay during the Norwood hospitalization using linear regression. Multivariable models again adjusted for preoperative patient demographics and clinical characteristics. Finally, we considered interactions of neighborhood SES with race and gestational age to determine whether the effect of SES varied by these characteristics. Missing data were minimal, with <1% of patients missing any covariate data. For all analyses, a 2‐sided P value of <0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Our sample included 525 infants undergoing the Norwood procedure. The SES score ranged from −11 to +18, with mean (by design) of 0 and SD of 5. Table 1 presents sociodemographic and clinical characteristics of patients according to tertile of neighborhood SES (≤ −2.79, −2.79 to 1.96, >1.96). Compared with patients in the middle and highest SES tertiles, a greater percentage of patients in the lowest tertile were of Hispanic ethnicity and of nonwhite race. In addition, significantly more patients in the lowest tertile lived in areas with higher poverty. Patients in the highest SES tertile were more likely to have had a prenatal diagnosis. Procedural characteristics did not vary significantly across SES tertiles.
Table 1.
Sociodemographic and Clinical Characteristics by Neighborhood SES Tertile (N=525)
| Lowest Tertile (N=173) | Middle Tertile (N=174) | Highest Tertile (N=178) | P Valuea | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Female, n (%) | 72 (41.6) | 63 (36.2) | 67 (37.6) | 0.56 |
| Hispanic ethnicity, n (%) | 52 (30.6) | 32 (18.7) | 15 (8.6) | <0.001 |
| Nonwhite race, n (%) | 54 (32.0) | 31 (17.9) | 19 (10.7) | <0.001 |
| Missing=5 | ||||
| Age at Norwood (d), median (IQR) | 6 (4, 8) | 6 (4, 8) | 6 (4, 7) | 0.35 |
| Neighborhood with >20% of residents living below the federal poverty level, n (%) | 91 (52.6) | 11 (6.3) | 2 (1.1) | <0.001 |
| Birth characteristics | ||||
| Prenatal diagnosis, n (%) | 127 (73.4) | 122 (70.1) | 151 (84.8) | 0.003 |
| Gestational age, n (%) | 0.65 | |||
| <37 wks | 23 (13.3) | 21 (12.1) | 18 (10.1) | |
| ≥37 wks | 150 (86.7) | 153 (87.9) | 160 (89.9) | |
| Anatomy | ||||
| HLHS, n (%) | 145 (83.8) | 141 (86.8) | 156 (87.6) | 0.56 |
| Norwood procedure | ||||
| Treatment assignment, n (%) | 0.44 | |||
| RVPAS | 86 (49.7) | 82 (47.1) | 96 (53.9) | |
| MBTS | 87 (50.3) | 92 (52.9) | 82 (46.1) | |
| Sternum opened, n (%) | 135 (79.0) | 138 (79.8) | 134 (76.1) | 0.69 |
| Cross‐clamp time (min), mean±SD | 55.8±23.7 | 56.8±23.3 | 54.6±22.7 | 0.68 |
| Bypass time (min), mean±SD | 143.7±60.0 | 143.3±48.3 | 144.3±55.3 | 0.99 |
| ECMO, n (%) | 13 (7.5) | 10 (5.8) | 10 (5.6) | 0.72 |
| Duration of intubation (d), median (IQR) | 7 (4, 14) | 7 (5, 12) | 7 (5, 11) | 0.70 |
| Missing=8 | ||||
| Number of post‐Norwood inpatient complications, median (IQR) | 4 (2, 7) | 4 (2, 7) | 4 (2, 7) | 0.83 |
| Hospital characteristics | ||||
| Site volume (number of patients/y), n (%) | 0.35 | |||
| ≤15 | 34 (19.7) | 30 (17.2) | 29 (16.3) | |
| 16 to 20 | 23 (13.3) | 40 (23.0) | 29 (16.3) | |
| 21 to 30 | 60 (34.7) | 51 (29.3) | 62 (34.8) | |
| >30 | 56 (32.4) | 53 (30.5) | 58 (32.6) | |
| Outcomes | ||||
| 30‐d mortality or transplantation, n (%) | 26 (15.0) | 18 (10.3) | 15 (8.4) | 0.13 |
| 1‐y mortality or transplantation, n (%) | 64 (37.0) | 54 (31.0) | 42 (23.6) | 0.02 |
| 1‐y mortality or transplantation, among hospital transplant‐free survivors, n (%) | 27 (19.9) | 27 (18.4) | 22 (13.9) | 0.37 |
| Norwood length of stay, median (IQR) | 24.5 (16.5, 42.0) | 24.0 (17.0, 41.0) | 24.0 (16.0, 40.0) | 0.70 |
ECMO indicates extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; IQR, interquartile range; MBTS, modified Blalock‐Taussig shunt; RVPAS, right ventricle to pulmonary artery shunt; SES, socioeconomic status.
P values are from χ2 tests for categorical variables and ANOVA or Kruskal–Wallis tests for continuous variables.
Thirty‐Day and 1‐Year Mortality
As SES tertile increased, crude 30‐day and 1‐year mortality or transplantation decreased (Table 1). There was no association between SES and outcome in the first 30 days (log‐rank P=0.146) (Figure 1A). However, SES was associated with 1‐year death or transplantation (log‐rank P=0.023) (Figure 1B). At 1 year, patients in the highest SES tertile had a 42% lower unadjusted hazard of death than patients in the lowest SES tertile (hazard ratio [HR] 0.58, 95% confidence interval [CI], 0.39–0.62) and a 27% lower unadjusted hazard of death than patients in the middle SES tertile (HR 0.73 [95% CI, 0.49–1.09]) (Table 2). The difference between the highest and lowest SES tertiles persisted after adjustment for patient demographics, birth characteristics, and anatomy (HR 0.62 [95% CI, 0.40–0.96]) (Figure 2A).
Figure 1.

Kaplan–Meier curves for (A) 30‐day mortality or cardiac transplantation, (B) 1‐year mortality or cardiac transplantation, and (C) 1‐year mortality or cardiac transplantation among 30‐day transplant‐free survivors by neighborhood socioeconomic status (SES) tertiles.
Table 2.
Unadjusted and Adjusted HR for 30‐Day and 1‐Year Mortality/Transplantation Composite by Neighborhood SES Tertile
| Outcome | No. Events/N | Unadjusted | Adjusted | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Valuea | HR (95% CI) | P Valuea | ||
| 30‐d mortality or transplantationb | 59/525 | 0.16 | 0.18 | ||
| Middle vs lowest tertile | 0.68 (0.37, 1.24) | 0.72 (0.38, 1.37) | |||
| Highest vs lowest tertile | 0.55 (0.29, 1.04) | 0.51 (0.25, 1.05) | |||
| Highest vs middle tertile | 0.81 (0.41, 1.60) | 0.71 (0.35, 1.45) | |||
| 1‐y mortality or transplantationb | 160/525 | 0.022 | 0.09 | ||
| Middle vs lowest tertile | 0.80 (0.56, 1.15) | 0.82 (0.56, 1.20) | |||
| Highest vs lowest tertile | 0.58 (0.39, 0.62) | 0.62 (0.40, 0.96) | |||
| Highest vs middle tertile | 0.73 (0.49, 1.09) | 0.76 (0.50, 1.15) | |||
| 1‐y mortality or transplantation among hospital transplant‐free survivorsc | 76/441 | 0.38 | 0.86 | ||
| Middle vs lowest tertile | 0.93 (0.54, 1.58) | 0.89 (0.47, 1.66) | |||
| Highest vs lowest tertile | 0.69 (0.39, 1.20) | 0.91 (0.46, 1.77) | |||
| Highest vs middle tertile | 0.74 (0.42, 1.30) | 1.02 (0.53, 1.96) | |||
CI indicates confidence interval; HR, hazard ratio; SES, socioeconomic status.
Global P values for SES variable (2 df) significance in model.
Multivariable models adjusted for patient age, sex, race/ethnicity, gestational age, birth weight, prenatal diagnosis, and hypoplastic left heart syndrome.
Multivariable models for 30‐d survivors adjusted for patient age, sex, race/ethnicity, gestational age, birth weight, prenatal diagnosis, hypoplastic left heart syndrome, treatment group (modified Blalock‐Taussig shunt vs right ventricle‐to‐pulmonary‐artery shunt), extracorporeal membrane oxygenation, open sternum, number of post‐Norwood inpatient complications, weight at discharge, and tube feeding requirement at discharge.
Figure 2.
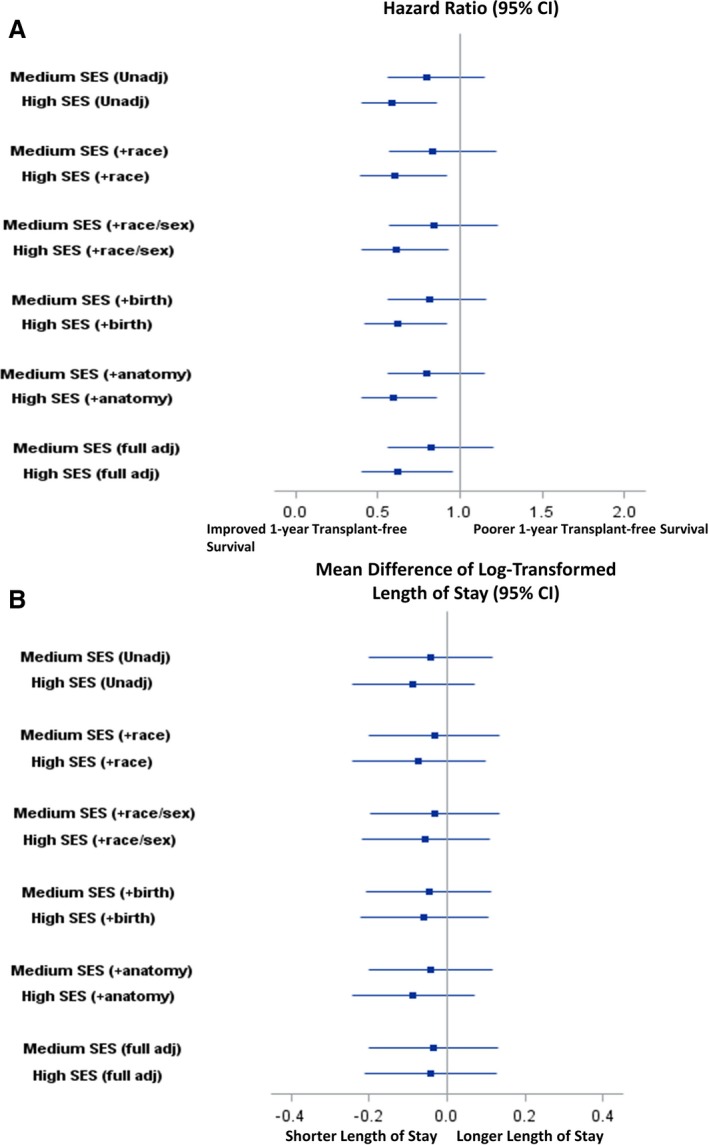
Association between neighborhood socioeconomic status (SES) tertile with (A) 1‐year mortality or transplantation, and (B) log‐transformed Norwood length of stay (days) sequentially adjusted for patient demographic and clinical characteristics. CI indicates confidence interval.
When SES was examined as a continuous variable, the hazard of 1‐year mortality or transplantation decreased steadily with increasing neighborhood SES (Figure 3). The highest risk of death was observed in patients living in very low SES neighborhoods. For example, the hazard of death or transplant for a patient with an SES score of 2.5 (0.5 SD below the average) was 9% higher than that of a patient with average SES (score of 0). Analyses modeling SES as a continuous variable for 30‐day mortality or transplantation are also provided in Figure 3.
Figure 3.
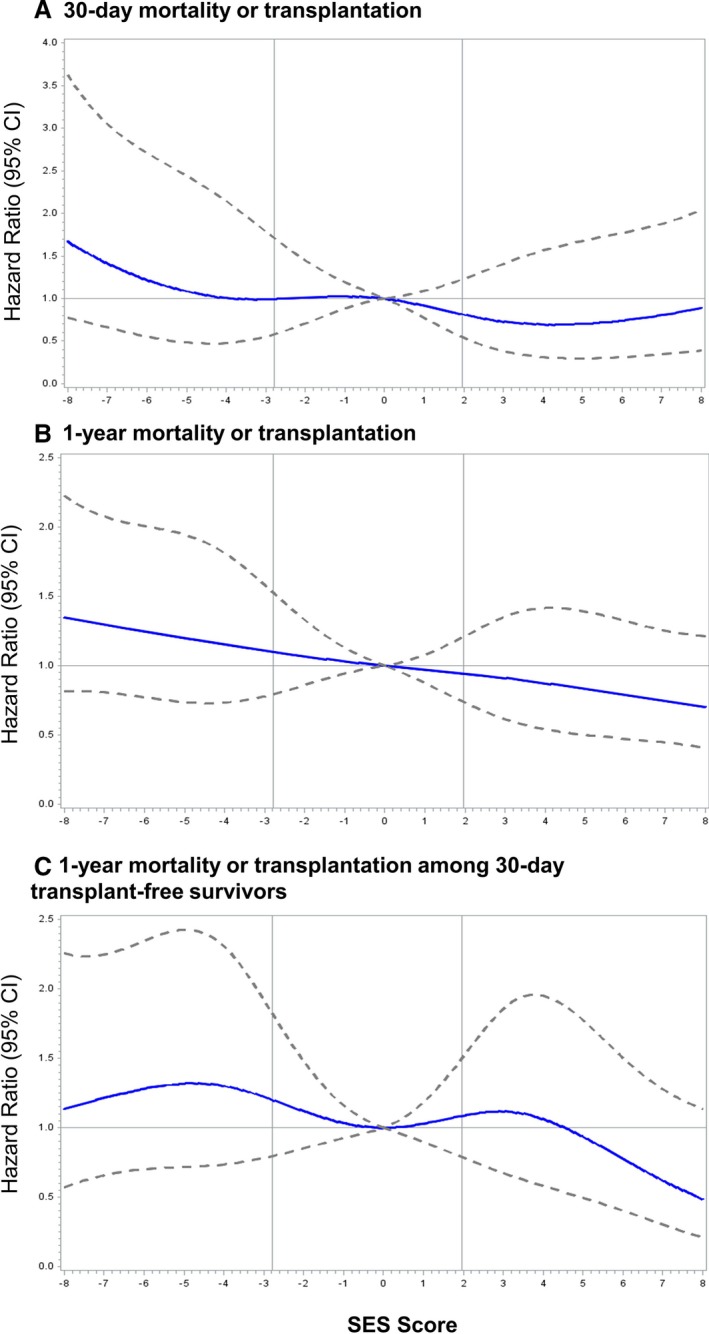
Unadjusted hazard ratios (95% confidence intervals [CI]) for (A) 30‐day mortality or cardiac transplantation among all patients, (B) 1‐year mortality or cardiac transplantation among all patients, and (C) 1‐year mortality or cardiac transplantation among 30‐day transplant‐free survivors with a flexible cubic spline fit to neighborhood socioeconomic status (SES) modeled. Hazard ratios were estimated using Cox proportional hazards models. Vertical lines correspond to cutoff values for SES tertiles.
We hypothesized that SES may have a stronger association with transplant‐free survival after a patient is discharged (ie, after ≈30 days) (Figure 1). To test this hypothesis, we evaluated the interaction between SES tertile and time (ie, first 30 days versus >30 days to 1 year) and calculated unadjusted and adjusted HRs for transplant‐free survival up to 1 year among transplant‐free survivors (Table 2). The P value for the SES*time interaction was nonsignificant (P=0.80) and the HRs among survivors were nonsignificant, suggesting that the effect of SES on transplant‐free survival did not vary with time.
Norwood Length of Stay
Median Norwood length of stay was similar across SES tertiles (Table 1 and Figure 4). There was also no significant difference in mean log‐transformed length of stay across tertiles before or after adjustment for other patient characteristics (Figure 2B).
Figure 4.

Distribution of Norwood hospitalization length of stay by neighborhood socioeconomic status (SES) tertile.
Interactions With Race and Gestational Age
The interaction of high SES with race was borderline significant for 1‐year mortality (P=0.055). Among white patients, high SES patients had a significant survival advantage over low SES patients (HR 0.52 [95% CI, 0.32–0.83]). However, among black patients, there was no significant difference in mortality or transplantation in high SES patients relative to low SES patients (HR 1.44 [95% CI, 0.57–3.68]). All other interactions between SES with race or gestational age were not significant.
Discussion
Using a comprehensive measure of neighborhood SES, we found that high SES was associated with improved 1‐year transplant‐free survival after the Norwood procedure but was not associated with Norwood length of stay. These differences in transplant‐free survival between the highest versus lowest SES tertiles persisted after adjustment for patient demographics, birth characteristics, and anatomy. The relationship between neighborhood SES and 1‐year mortality or transplant appeared to be largely linear with the lowest rate of mortality or transplantation observed in patients with the highest SES.
Although the current article provides the most extensive evaluation of the role of SES in infants undergoing the Norwood procedure, prior studies have reported a similar relationship between SES and mortality. Tweddell et al evaluated the role of neighborhood SES as one of the preoperative risk factors for 1‐year death and found that lower SES was associated with higher early mortality within the first year but not late phase mortality over 3 years.5 In contrast, Ghanayem et al used a census‐based neighborhood poverty level and found an inverted U‐shaped relationship between poverty level and interstage mortality.8 Like prior studies, we found that higher SES was associated with improved 1‐year transplant‐free survival after the Norwood procedure, an effect that was not explained by patient demographic, birth characteristics, or anatomy. Our findings extend those of prior studies by evaluating the effect of SES as a continuous variable at different follow‐up time points while adjusting sequentially for patient demographics, birth characteristics, and anatomy to determine whether any set of variables might explain the effect of SES.
There are several possible explanations for the differences in mortality observed in patients with high versus low neighborhood SES. Because infants with SV physiology require early and continued surgical and medical interventions, access to high‐quality specialty care and follow‐up would be expected to improve the likelihood of survival. Infants living in low SES communities, who are also more likely to have low family SES, may experience more barriers to accessing these services or obtaining necessary follow‐up. Although there are limited data on the impact of low SES on healthcare access in children with CHD, data from general pediatric populations suggest that children of families with low income or education have significantly more barriers to accessing primary care.28, 29, 30 Low SES may be associated with inability to purchase needed healthcare services, lack of insurance leading to greater use of emergency departments for medical care, or reduced likelihood of seeking help for symptoms of illness because of poor education.24 Accessing the frequent follow‐up appointments and cardiac rehabilitation required by these children may be particularly difficult for low SES families living far from the primary treatment center relative to families with low SES who live closer and to families with higher SES. Unfortunately, the SVR public use data set does not include precalculated distance of a family's residence from treatment center, limiting our ability to analyze the influence of this factor on outcomes. Additionally, families living in low SES neighborhoods may have less community social support, greater job‐related stress or unstable employment, and less access to health insurance, paid vacations, or sick days, making it difficult for parents to advocate on behalf of their child.25, 31, 32, 33
Outside of factors affecting healthcare access, lower quality nutrition and environmental exposures may explain part of the relationship between low SES and higher mortality in children with chronic conditions. Prior studies have reported higher rates of prematurity, low birth weight, and birth‐related complications in mothers living in poverty.34, 35 Although we adjusted for gestational age and birth weight, we did not adjust for other birth defects or comorbid conditions, which may have further attenuated the relationship between SES and outcomes. Additionally, children living in low SES areas have higher rates of respiratory illnesses,36, 37 iron‐deficiency anemia,38 and hospital admissions,39 which may reflect inadequate nutrition or poor living conditions.40 Indeed, Burch et al showed that higher neighborhood SES was associated with a greater weight gain from Norwood to Stage II procedure.23
The association of SES with transplant‐free survival was not statistically significant when the analyses were limited to 30‐day mortality or to 1‐year mortality among hospital transplant‐free survivors. In addition, the interaction analysis suggested no differential effect of SES before and after 30 days. Compared to the overall 1‐year outcome, however, these analyses had lower statistical power. Of note, the effect of SES on mortality may be greatest in the first 30 days as evidenced by a HR of 0.51 for the highest versus lowest SES tertile, in contrast to the HR of nearly 1 for highest versus lowest SES tertile among 1‐year transplant‐free survivors. These disparities in early 30‐day outcome may be attributable to differences between hospitals or surgeon quality, in utero exposures, or coexisting congenital anomalies.
We found that the effect of race modifies the role of neighborhood SES on mortality. This observation has been observed in older populations of cardiac patients and has been attributed to the diminishing returns hypothesis.41 This theory, proposed by Farmer and Ferraro, hypothesizes that blacks do not receive the same health benefits as whites with increasing SES.42 Indeed, we found that white patients living in the highest SES neighborhood had a significant survival advantage relative to white patients living in low SES areas. The same was not true for black patients. Several theories have been proposed to explain this phenomenon including lower social support for black patients living in wealthier, commonly white, neighborhoods and greater racial discrimination in high SES neighborhoods.43, 44
Our study has important implications for efforts to understand and eliminate socioeconomic disparities in pediatric cardiac outcomes. Given the strong, inverse relationship between neighborhood SES and mortality after the Norwood procedure, postoperative care should focus not only on operative management strategies but also socioeconomic and environmental factors, which may be equally important determinants of patient outcomes. To date, nearly all studies aimed at improving outcomes in children with CHD have focused on screening or operative management. Few studies have examined other strategies such as feeding protocols, parental empowerment, or financial support programs.45, 46, 47 There is a clear need for such studies, particularly after discharge, to globally improve the quality of care delivered and reduce disparities in patient outcomes.
This study has its limitations. First, we had limited power to detect subtle differences in outcomes or interactions between SES and other variables, including time. Second, the data used in this study antedate the widespread use of interstage monitoring programs for home monitoring and facilitating communication between families and providers. While it is possible that the association of SES with outcomes may be somewhat diminished in a contemporary cohort due to home monitoring programs, there are many factors not addressed by these programs such as community social support, financial stress, living conditions, or parental accessibility that likely contribute to the poorer outcomes of children living in low SES neighborhoods. Therefore, the results are likely to be applicable to current populations of children with SV heart disease.
In summary, we found that infants with SV physiology from low SES neighborhoods are at significantly higher risk of death or transplant within the first year after the Norwood procedure. Although the effect was modest, it persisted after adjustment for patient characteristics. Norwood length of stay did not differ significantly by neighborhood SES. These findings highlight the need for additional research to better understand the relationship between neighborhood SES and outcomes in children with CHD and to identify new strategies to address these socioeconomic factors.
Sources of Funding
This work was supported in part by the Farb Family Fund and the Kostin Family Innovation Fund.
Disclosures
None.
Acknowledgments
The NIH/NHLBI Pediatric Heart Network Single Ventricle Reconstruction Trial data set was used in preparation of this work, and the authors acknowledge the many contributors to this trial. The authors especially thank Victor Zak, PhD, at New England Research Institutes, for his help in working with the Pediatric Heart Network SVR Public Use Dataset. Data were downloaded from http://www.pediatricheartnetwork.org/ForResearchers/PHNPublicUseDatasets/SingleVentricleReconstructionTrial.aspx on September 20, 2016. Dr Bucholz had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses.
(J Am Heart Assoc. 2018;7:e007065 DOI: 10.1161/JAHA.117.007065.)29420218
References
- 1. Sistino JJ, Bonilha HS. Improvements in survival and neurodevelopmental outcomes in surgical treatment of hypoplastic left heart syndrome: a meta‐analytic review. J Extra Corpor Technol. 2012;44:216–223. [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs JP, Mayer JE Jr, Mavroudis C, O'Brien SM, Austin EH III, Pasquali SK, Hill KD, Overman DM, St Louis JD, Karamlou T, Pizarro C, Hirsch‐Romano JC, McDonald D, Han JM, Becker S, Tchervenkov CI, Lacour‐Gayet F, Backer CL, Fraser CD, Tweddell JS, Elliott MJ, Walters H III, Jonas RA, Prager RL, Shahian DM, Jacobs ML. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 update on outcomes and quality. Ann Thorac Surg. 2017;103:699–709. [DOI] [PubMed] [Google Scholar]
- 3. Chowdhury SM, Graham EM, Atz AM, Bradley SM, Kavarana MN, Butts RJ. Validation of a simple score to determine risk of hospital mortality after the Norwood procedure. Semin Thorac Cardiovasc Surg. 2016;28:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alsoufi B, Mori M, Gillespie S, Schlosser B, Slesnick T, Kogon B, Kim D, Sachdeva R, Kanter K. Impact of patient characteristics and anatomy on results of Norwood operation for hypoplastic left heart syndrome. Ann Thorac Surg. 2015;100:591–598. [DOI] [PubMed] [Google Scholar]
- 5. Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, Pemberton VL, Frommelt PC, Bradley SM, Cnota JF, Hirsch J, Kirshbom PM, Li JS, Pike N, Puchalski M, Ravishankar C, Jacobs JP, Laussen PC, McCrindle BW; Pediatric Heart Network Investigators . Intermediate‐term mortality and cardiac transplantation in infants with single‐ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, Lu M, Pizarro C, Frommelt P, Goldberg CS, Graham EM, Krawczeski CD, Lai WW, Lewis A, Kirsh JA, Mahony L, Ohye RG, Simsic J, Lodge AJ, Spurrier E, Stylianou M, Laussen P; Pediatric Heart Network Investigators . Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121:644–650. [DOI] [PubMed] [Google Scholar]
- 8. Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS; Pediatric Heart Network Investigators . Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor LC, Burke B, Donohue JE, Yu S, Hirsch‐Romano JC, Ohye RG, Goldberg CS. Risk factors for interstage mortality following the Norwood procedure: impact of sociodemographic factors. Pediatr Cardiol. 2016;37:68–75. [DOI] [PubMed] [Google Scholar]
- 10. Garcia Guerra G, Robertson CM, Alton GY, Joffe AR, Dinu IA, Nicholas D, Ross DB, Rebeyka IM; Western Canadian Complex Pediatric Therapies Follow‐up Group . Quality of life 4 years after complex heart surgery in infancy. J Thorac Cardiovasc Surg. 2013;145:482–488.e2. [DOI] [PubMed] [Google Scholar]
- 11. Kucik JE, Nembhard WN, Donohue P, Devine O, Wang Y, Minkovitz CS, Burke T. Community socioeconomic disadvantage and the survival of infants with congenital heart defects. Am J Public Health. 2014;104:e150–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Davies RR, Russo MJ, Reinhartz O, Maeda K, Rosenthal DN, Chin C, Bernstein D, Mallidi HR. Lower socioeconomic status is associated with worse outcomes after both listing and transplanting children with heart failure. Pediatr Transplant. 2013;17:573–581. [DOI] [PubMed] [Google Scholar]
- 13. Cassedy A, Drotar D, Ittenbach R, Hottinger S, Wray J, Wernovsky G, Newburger JW, Mahony L, Mussatto K, Cohen MI, Marino BS. The impact of socio‐economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. 2013;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mackie AS, Gauvreau K, Newburger JW, Mayer JE, Erickson LC. Risk factors for readmission after neonatal cardiac surgery. Ann Thorac Surg. 2004;78:1972–1978. [DOI] [PubMed] [Google Scholar]
- 15. Tahirovic E, Begic H, Sutovic A, Tahirovic H. Impact of the family socioeconomic status on health related quality of life in children operated on for congenital heart defects. Acta Med Croatica. 2010;64:9–16. [PubMed] [Google Scholar]
- 16. Werner H, Latal B, Valsangiacomo Buechel E, Beck I, Landolt MA. Health‐related quality of life after open‐heart surgery. J Pediatr. 2014;164:254–258.e1. [DOI] [PubMed] [Google Scholar]
- 17. Sistino JJ, Ellis C Jr. Effects of health disparities on survival after neonatal heart surgery: why should racial, ethnic, gender, and socioeconomic status be included in the risk analysis? J Extra Corpor Technol. 2011;43:232–235. [PMC free article] [PubMed] [Google Scholar]
- 18. Connor JA, Kline NE, Mott S, Harris SK, Jenkins KJ. The meaning of cost for families of children with congenital heart disease. J Pediatr Health Care. 2010;24:318–325. [DOI] [PubMed] [Google Scholar]
- 19. Single Ventricle Reconstruction Trial. Pediatric Heart Network; 2017. Available at: http://www.pediatricheartnetwork.org/ForResearchers/PHNPublicUseDatasets/SingleVentricleReconstructionTrial.aspx. Accessed December 18, 2017. [Google Scholar]
- 20. Ohye RG, Gaynor JW, Ghanayem NS, Goldberg CS, Laussen PC, Frommelt PC, Newburger JW, Pearson GD, Tabbutt S, Wernovsky G, Wruck LM, Atz AM, Colan SD, Jaggers J, McCrindle BW, Prakash A, Puchalski MD, Sleeper LA, Stylianou MP, Mahony L; Pediatric Heart Network Investigators . Design and rationale of a randomized trial comparing the Blalock‐Taussig and right ventricle‐pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 22. Goldberg CS, Lu M, Sleeper LA, Mahle WT, Gaynor JW, Williams IA, Mussatto KA, Ohye RG, Graham EM, Frank DU, Jacobs JP, Krawczeski C, Lambert L, Lewis A, Pemberton VL, Sananes R, Sood E, Wechsler SB, Bellinger DC, Newburger JW; Pediatric Heart Network Investigators . Factors associated with neurodevelopment for children with single ventricle lesions. J Pediatr. 2014;165:490–496.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burch PT, Gerstenberger E, Ravishankar C, Hehir DA, Davies RR, Colan SD, Sleeper LA, Newburger JW, Clabby ML, Williams IA, Li JS, Uzark K, Cooper DS, Lambert LM, Pemberton VL, Pike NA, Anderson JB, Dunbar‐Masterson C, Khaikin S, Zyblewski SC, Minich LL; Pediatric Heart Network Investigators . Longitudinal assessment of growth in hypoplastic left heart syndrome: results from the Single Ventricle Reconstruction trial. JAMA. 2014;3:e000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ravishankar C, Gerstenberger E, Sleeper LA, Atz AM, Affolter JT, Bradley TJ, Gaynor JW, Goldstein BH, Henderson HT, Jacobs JP, Lewis AB, Dunbar‐Masterson C, Menon SC, Pemberton VL, Petit CJ, Pike NA, Pizarro C, Schumacher KR, Williams IA, Newburger JW; Pediatric Heart Network Investigators . Factors affecting Fontan length of stay: results from the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2016;151:669–675.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. [DOI] [PubMed] [Google Scholar]
- 26. Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–208. [DOI] [PubMed] [Google Scholar]
- 27. RCS: gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Available at: http://cemsiis.meduniwien.ac.at. Accessed January 3, 2017. [DOI] [PubMed]
- 28. Heck KE, Parker JD. Family structure, socioeconomic status, and access to health care for children. Health Serv Res. 2002;37:173–186. [PubMed] [Google Scholar]
- 29. Weinick RM, Krauss NA. Racial/ethnic differences in children's access to care. Am J Public Health. 2000;90:1771–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newacheck PW, Hughes DC, Stoddard JJ. Children's access to primary care: differences by race, income, and insurance status. Pediatrics. 1996;97:26–32. [PubMed] [Google Scholar]
- 31. Richman A, Johnson A, Buxbaum L. Workplace Flexibility for Lower‐Wage Workers. Washington, DC: Corporate Voices for Working Families; 2006. [Google Scholar]
- 32. McLoyd VC. Socioeconomic disadvantage and child development. Am Psychol. 1998;53:185–204. [DOI] [PubMed] [Google Scholar]
- 33. Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress. Does stress account for SES effects on health? Ann N Y Acad Sci. 1999;896:131–144. [DOI] [PubMed] [Google Scholar]
- 34. Starfield B, Shapiro S, Weiss J, Liang KY, Ra K, Paige D, Wang XB. Race, family income, and low birth weight. Am J Epidemiol. 1991;134:1167–1174. [DOI] [PubMed] [Google Scholar]
- 35. Parker JD, Schoendorf KC, Kiely JL. Associations between measures of socioeconomic status and low birth weight, small for gestational age, and premature delivery in the United States. Ann Epidemiol. 1994;4:271–278. [DOI] [PubMed] [Google Scholar]
- 36. Cohen S. Social status and susceptibility to respiratory infections. Ann N Y Acad Sci. 1999;896:246–253. [DOI] [PubMed] [Google Scholar]
- 37. Johnston‐Brooks CH, Lewis MA, Evans GW, Whalen CK. Chronic stress and illness in children: the role of allostatic load. Psychosom Med. 1998;60:597–603. [DOI] [PubMed] [Google Scholar]
- 38. Oski FA. Iron deficiency in infancy and childhood. N Engl J Med. 1993;329:190–193. [DOI] [PubMed] [Google Scholar]
- 39. Brooks‐Gunn J, Duncan GJ. The effects of poverty on children. Future Child. 1997;7:55–71. [PubMed] [Google Scholar]
- 40. Evans GW, Kantrowitz E. Socioeconomic status and health: the potential role of environmental risk exposure. Annu Rev Public Health. 2002;23:303–331. [DOI] [PubMed] [Google Scholar]
- 41. Bucholz EM, Ma S, Normand SL, Krumholz HM. Race, socioeconomic status, and life expectancy after acute myocardial infarction. Circulation. 2015;132:1338–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med. 2005;60:191–204. DOI: 10.1016/j.socscimed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 43. Pickett KE, Wilkinson RG. People like us: ethnic group density effects on health. Ethn Health. 2008;13:321–334. [DOI] [PubMed] [Google Scholar]
- 44. Jurcik T, Ahmed R, Yakobov E, Solopieieva‐Jurcikova I, Ryder AG. Understanding the role of the ethnic density effect: issues of acculturation, discrimination, and social support. J Community Psychol. 2013;41:662–678. [Google Scholar]
- 45. Newcombe J, Fry‐Bowers E. A post‐operative feeding protocol to improve outcomes for neonates with critical congenital heart disease. J Pediatr Nurs. 2017;35:139–143. [DOI] [PubMed] [Google Scholar]
- 46. Edraki M, Kamali M, Beheshtipour N, Amoozgar H, Zare N, Montaseri S. The effect of educational program on the quality of life and self‐efficacy of the mothers of the infants with congenital heart disease: a randomized controlled trial. Int J Community Based Nurs Midwifery. 2014;2:51–59. [PMC free article] [PubMed] [Google Scholar]
- 47. Staveski SL, Zhelva B, Paul R, Conway R, Carlson A, Soma G, Kools S, Franck LS. Pediatric cardiac surgery Parent Education Discharge Instruction (PEDI) program: a pilot study. World J Pediatr Congenit Heart Surg. 2015;6:18–25. [DOI] [PubMed] [Google Scholar]


