Abstract
Background
Ischemic stroke is a complex disease with multiple etiologies and clinical manifestations. Paired immunoglobulin‐like receptor B (PirB), which is originally thought to function exclusively in the immune system, is now also known to be expressed by neurons. A growing number of studies indicate that PirB can inhibit neurite outgrowth and restrict neuronal plasticity. The aim of the study is to investigate whether PirB can be an attractive theranostic target for ischemic stroke.
Methods and Results
First, we investigated the spatial‐temporal expression of PirB in multiple ischemic stroke models, including transient middle cerebral artery occlusion, photothrombotic cerebral cortex ischemia, and the neuronal oxygen glucose deprivation model. Then, anti‐PirB immunoliposome nanoprobe was developed by thin‐film hydration method and investigated its specific targeting in vitro and in vivo. Finally, soluble PirB ectodomain (sPirB) protein delivered by polyethylene glycol–modified nanoliposome was used as a therapeutic reagent for ischemic stroke by blocking PirB binding to its endogenous ligands. These results showed that PirB was significantly upregulated after cerebral ischemic injury in ischemic stroke models. Anti‐PirB immunoliposome nanoprobe was successfully developed and specifically bound to PirB in vitro. There was accumulation of anti‐PirB immunoliposome nanoprobe in the ischemic hemisphere in vivo. Soluble PirB ectodomains remarkably improved ischemic stroke model recovery by liposomal delivery system.
Conclusions
These data indicated that PirB was a significant element in the pathological process of cerebral ischemia. Therefore, PirB may act as a novel theranostic target for ischemic stroke.
Keywords: PirB, imaging, ischemic stroke, liposome, therapy
Subject Categories: Imaging, Ischemic Stroke, Neuroprotectants
Clinical Perspective
What Is New?
The study demonstrated that paired immunoglobulin‐like receptor B was a significant element in the pathological process of cerebral ischemia.
We constructed anti‐paired immunoglobulin‐like receptor B immunoliposome probe with a near‐infrared fluorophore, which could be applied to in vivo imaging for upregulated paired immunoglobulin‐like receptor B molecules in a cerebral ischemic stroke model.
Soluble paired immunoglobulin‐like receptor B ectodomain, which was delivered by polyethylene glycol–modified liposome, had better therapeutic effects.
What Are the Clinical Implications?
Our work provides a new potential therapeutic target for real‐time monitoring and treatment of ischemic stroke.
Introduction
Cerebral ischemic stroke, which is characterized by the sudden loss of blood circulation to an area of the brain and is caused by thrombotic and embolic occlusion of a cerebral artery, resulting in a corresponding loss of neurological function.1 Acute ischemic stroke is more common than hemorrhagic stroke.2 It is one of the most common causes of death and disability worldwide, as well as being a significant socioeconomic burden.3 With an ever‐increasing aging population, ischemic stroke is projected to become more common in the near future. Therefore, it is urgent to investigate more targets for adjutant diagnosis, real‐time monitoring, and treatment of ischemic stroke.
Mouse paired immunoglobulin‐like receptor B (PirB) is a type I transmembrane glycoprotein consisting of 6 extracellular immunoglobulin‐like domains, a hydrophobic transmembrane segment and an intracellular polypeptide with 4 immunoreceptor tyrosine‐based inhibitory motif sequences.4, 5, 6 PirB, which is originally thought to function exclusively in the immune system,7 is now also found in neurons.8 In immune cells and neurons, PirB binding with ligand recruits small heterodimer partner 1 and small heterodimer partner 2 phosphatases.9 It is reported that PirB plays an important role in neurite outgrowth and restricts neuronal plasticity.8, 10, 11, 12, 13 Germline PirB−/− mice have enhanced ocular dominance plasticity not only during the critical period, but also beyond it,8, 11 and they recover more rapidly in a stroke model than wild‐type mice.14 However, it remains unknown whether the enhanced stroke recovery in germline PirB−/− mice are because of early developmental changes, or whether PirB could be an attractive therapeutic target for ischemic stroke in wild‐type mice. Because PirB is a receptor, signaling can be modulated by interfering with ligand binding. Soluble PirB ectodomain (sPirB) protein, containing the first 6 immunoglobulin G‐like domains and tags used for purification, has been demonstrated to block the binding of PirB with endogenous ligands and reduce downstream signaling.13 So, this study investigated the therapeutic effect of sPirB protein in ischemic stroke.
The blood–brain barrier helps to maintain brain homeostasis by restricting the transfer of molecules into the brain. However, the integrity of the blood–brain barrier is compromised and cerebral vascular permeability increases after cerebral ischemia. This change permits drugs, which cannot penetrate into the blood–brain barrier under the normal condition, to accumulate in cerebral parenchyma.15, 16 It has been reported that liposome nanoparticles injected intravenously into a cerebral ischemic stroke model can pass through the disrupted blood–brain barrier and accumulate in the ischemic core and penumbra region owing to the enhanced permeability and retention effect.17, 18 Furthermore, polyethylene glycol (PEG)‐modified liposomes possess the qualification of a long circulating property in the bloodstream by decreasing interaction with opsonins and the cells of the mononuclear phagocytic system.19, 20 To enable specifically targeted molecular imaging or therapy, PEGylated immunoliposomes surface can be labeled with functional molecules, including antibodies, ligands, and receptors.21 Therefore, in the present study, we developed anti‐PirB vectorized stealth immunoliposome nanoprobe containing near‐infrared fluorophore DiR to target PirB and used liposome‐delivered soluble PirB ectodomains to block the binding of PirB with endogenous ligands in ischemic hemisphere.
The purpose of this study was to investigate the expression and roles of neuronal PirB in the pathogenesis of multiple ischemic stroke models, including transient middle cerebral artery occlusion, photothrombotic cerebral cortex ischemia, and the neuronal oxygen glucose deprivation model, to develop anti‐PirB vectorized stealth immunoliposomes containing imaging probes for real‐time monitoring of ischemic stroke, and to deliver soluble PirB ectodomains by nanoliposomes to improve stroke model recovery.
Materials and Methods
The authors declare that the data, analytical methods, and study materials are available within the article.
Animal Preparation
The 8‐week‐old male C57BL/6 mice were purchased from the College of Veterinary Medicine Yangzhou University. All animal experiments were conducted in accord with the protocols evaluated and approved by the Institutional Animal Use and Care Committee of the Medical School of Southeast University.
Transient middle cerebral artery occlusion (MCAO) was induced in male mice by using the intraluminal suture technique, as previously described, with slight modifications.22, 23 Briefly, mice were anesthetized with isoflurane, and the right common carotid artery, right external carotid artery, and right internal carotid artery were isolated under microscope. A 6‐0 nylon monofilament suture (Doccol Co., Redlands, CA) was inserted from the common carotid artery into the internal carotid artery to occlude the right middle cerebral artery at its origin. The suture was withdrawn after 1 hour for reperfusion, the common carotid artery ligated, and the wound closed. The sham‐operated mice were treated with the same method, but the monofilament was inserted into the internal carotid artery to half the insertion depth described above and removed after 1 hour.
Cerebral cortex ischemia was induced in male mice by photothrombosis, as previously described.24, 25 Mice were anesthetized with isoflurane, then their hair was removed. Rose bengal (Sigma‐Aldrich, St. Louis, MO) was injected intraperitoneally at a dose of 100 mg/kg. Brains were illuminated by a 4‐mm‐diameter fiber of a cold light source (Zeiss, Heidenheim, Germany) centered 2 mm to the right of the bregma for 15 minutes. Sham‐operated mice were injected only with rose bengal and were not illuminated by a cold light source.
Two ischemic mouse models were verified by T2‐weighted imaging 6 hours postsurgery.
Real‐Time Quantitive Polymerase Chain Reaction
Hemispheres were dissected by cutting brains at midline. The olfactory bulbs, brainstem, and cerebellum were discarded. Samples from the ischemic hemispheres and contralateral hemispheres from MCAO models were used. For cerebral cortex ischemia models, ischemic cortex and contralateral cortex were dissected. Total RNA from hemispheres or cortices was extracted using the Trizol protocol (Invitrogen, San Diego, CA). One microgram of total RNA was reverse transcribed using the PrimeScript RT reagent kit with gDNA Eraser (Takara, Otsu, Japan), according to the manufacturer's instructions. Quantitative PCR analysis was performed with an automated sequence detection system (StepOnePlus Real‐time PCR System; Applied Biosystems, Foster City, CA). Expression of mouse genes βIIItubulin, PirB, H2‐Db, and H2‐Kb were analyzed using Power SYBR Green PCR Master Mix (Roche, Mannheim, Germany). βIIItubulin was used as an internal control gene. The primers used in this study are shown in Table. Data were analyzed using the 2−ΔΔCT relative quantitation method.
Table 1.
Primer Sequences of Genes Used in This Study
| Genes | Primer Sequence (5′‐3′) | Length |
|---|---|---|
| PirB |
F:5′‐TGCCACCCAGGAAGAAAGCCTA‐3′ R:5′‐GCCCTCTTCTGCTTGTTCATATTC‐3′ |
214 bp |
| H2‐Kb |
F:5′‐CGGCGCTGATCACCAAACA‐3′ R:5′‐AGCGTCGCGTTCCCGTT‐3′ |
121 bp |
| H2‐Db |
F:5′‐GTAAAGCGTGAAGACAGCTGC‐3′ R:5′‐CTGAACCCAAGCTCACAGG‐3′ |
132 bp |
| βIII‐tubulin (Tuj1) |
F:5′‐CGAGACCTACTGCATCGACA‐3′ R:5′‐CATTGAGCTGACCAGGGAAT‐3′ |
152 bp |
PirB indicates paired immunoglobulin‐like receptor B.
Immunoprecipitation and Western Blots
Tissue samples from hemispheres or cortices were homogenized in protein lysis buffer (50 mmol/L of Tris‐HCl, 1 mmol/L of Na2EDTA.H2O, 200 mmol/L of NaCl, 1% NP‐40, 0.5% C24H39O4Na, and 0.1% SDS) with protease inhibitors and phosphatase inhibitors (Roche). The concentration of solubilized protein was quantified by the BCA protein assay kit (Pierce, Rockford, IL) using BSA as the standard. Tyrosine phosphorylated PirB was detected by immunoprecipitation with anti‐P‐Tyr‐100 antibody (Cell Signaling Technology, Danvers, MA). After heating at 100°C for 5 minutes, samples were separated by 10% to 12% SDS‐PAGE and then electrophoretically transferred onto a PVDF membrane (Millipore, Bedford, MA). Immunoblot analysis was carried out with anti‐PirB antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and then HRP‐conjugated rabbit anti‐goat secondary antibodies (Sigma‐Aldrich, St. Louis, MO), and detected with the enhanced chemiluminescence kits (Pierce). Signals were imaged with a luminoimage analyzer (Tanon, Shanghai, China), and quantification was performed by densitometry using ImageJ software (NIH, Bethesda, MD).
Immunohistochemistry
Frozen sections (16 μm) were rinsed in PBS for 5 minutes. Microwave antigen retrieval was performed in 0.01 mol/L of sodium citrate buffer (pH 6.0) for 15 minutes. Incubation with 3% H2O2 for 20 minutes was used to quench endogenous peroxidase activity. Then, sections were incubated with 10% normal rabbit serum at 37°C for 30 minutes to reduce background nonspecific binding. Subsequently, sections were incubated with a goat anti‐mouse primary polyclonal antibody against, PirB (1:200; Santa Cruz Biotechnology) overnight at 4°C. Rabbit anti‐goat IgG/biotin (1:200) was used as a secondary antibody and incubated for 1 hour at room temperature. Tertiary antibody streptavidin/HRP (1:300) was incubated for 40 minutes at room temperature. Sections were stained with DAB and counterstained with hematoxylin. The DAB staining in every section was performed under the same condition. All dilutions were made in PBS. All sections were rinsed with PBS supplemented with 0.05% (v/v) Tween‐20.
Immunofluorescence
Antibodies used in immunofluorescence experiments were specific for PirB (1:100; Santa Cruz Biotechnology), neuronal nuclei (1:1000, Millipore), glial fibrillary acidic protein (1:500, Millipore), donkey anti‐mouse IgG (H+L), donkey anti‐goat IgG (H+L), and donkey anti‐rabbit IgG (H+L; 1:500; Invitrogen). Sections were rinsed in PBS for 5 minutes and then permeabilized with 0.01 mol/L of PBS containing Triton X‐100 for 30 minutes. Nonspecific antibody binding sites were blocked with 5% BSA in PBS containing Triton X‐100 for 2 hours at room temperature. Sections were then incubated with primary antibodies overnight at 4°C in a humid chamber. After rinsing 3 times for 5 minutes each in PBS containing Triton X‐100, sections were incubated with a secondary antibody for 2 hours in a humid chamber at room temperature. Sections were then stained nuclei with DAPI, rinsed in PBS, mounted in a mixture of glycerol/PBS (3:1), and observed with a laser scanning confocal microscope (Olympus Fluoview FV 1000; Olympus, Tokyo, Japan).
Oxygen Glucose Deprivation Model
The oxygen glucose deprivation (OGD) model was used in this study, as previously described, with slight modifications.26 Cortex neurons were cultured in neurobasal medium without glucose in a humidified incubator filled with an anoxic gas mixture (5% CO2 and 93.5% N2) at 37°C for 2 hours. Then, neurons were cultured in normal medium at 37°C in a humidified atmosphere with 5% CO2 and 95% air.
Preparation of Liposomes
PEG/LP/DiR
PEG liposomes were prepared using the thin‐film hydration method. PEG/LP was composed of distearoylphosphatidylcholine, cholesterol, and mPEG2000/distearoylphosphatidylethanolamine at a molar ratio of 20:10:1. The compounds dissolved in chloroform were evaporated to form a thin lipid film in a rotary evaporator. The lipid film was dried for at least 1 hour in a vacuum environment. Then, the lipid film was hydrated with PBS and incubated 65°C for 1 hour. Liposomes were sonicated and serially passed through 0.45‐ and 0.22‐μm‐pore‐size filters. For preparing near‐infrared fluorescence (NIRF)‐labeled liposomes (PEG/LP/DiR), DiR was added to the initial lipid solution.
6C1/PEG/LP/DiR
6C1/PEG/LP/DiR nanoprobes were prepared as previously described using postinsertion, with slight modifications.27, 28 Sulfhydryl was introduced to purified anti‐PirB antibody (clone 6C1; BD Biosciences, San Jose, CA) by incubating with Traut's reagent (Pierce) using an antibody/Traut's reagent molar ratio of 1:20. Free Traut's reagent was removed by 3 successive centrifugations in centrifugal filter devices. Anti‐PirB‐SH was incubated with liposomes of Mal/PEG2000/distearoylphosphatidylethanolamine and mPEG2000/distearoylphosphatidylethanolamine at a molar ratio of 1:4 overnight at 4°C. Uncoupled antibodies were removed by centrifugation at 12 000g for 20 minutes 3 times. Anti‐PirB‐PEG2000‐DSPE was then incubated with PEG/LP/DiR for 60 minutes at 65°C in PBS buffer. The resulting 6C1/PEG/LP/DiR nanoprobes were suspended in PBS and stored at 4°C to avoid exposure to light and air until use.
sPirB/PEG/LP
Soluble PirB ectodomains (sPirB; R&D systems, Minneapolis, MN) were labeled on the liposomes through a PEG linker using the same method as anti‐PirB antibodies labeled.
Characterization of the Nanoprobes
The Micro BCA Protein Assay Kit (Pierce, Rockford, IL) was used to detect labeled antibodies or protein content, according to the manufacturer's instructions, as previously described. Morphology and particle size of liposomes were determined by transmission electron microscope (Hitachi High‐Technologies Corporation, Tokyo, Japan) and Zetasizer Nano (Malvern Instruments Ltd, Worcestershire, UK).
Experimental Groups
Mmouse models without an ischemic lesion on T2‐weighted images (3 mice) or dead mice (4 mice died within 1 day postischemia) were excluded from the experiment.
To investigate the targeting specificity of nanoprobes in vivo, cerebral ischemic stroke mice were randomly assigned to 1 of 3 groups on day 2 postischemia and intravenously injected by tail vein with the following treatments: (1) PirB‐targeted nanoprobe 6C1/PEG/LP/DiR (4 mmol/L, 0.2 mL/mouse; n=9); (2) control nanoprobe IgG/PEG/LP/DiR (4 mmol/L, 0.2 mL/mouse; n=9); and (3) PBS (n=7). Then, NIRF imaging was performed at different time points (1, 4, 8, 12, and 24 hours, respectively).
For therapy, mouse models were randomly assigned to receive sPirB/PEG/LP (100 μg/kg as sPirB dose; ≈0.7 or 1 μmol of phospholipid/mouse), sPirB (100 μg/kg), or PEG/LP (1 μmol of phospholipid/mouse) every day by tail vein after ischemia until day 7 (n=6 per group).
Magnetic Resonance Imaging
In vivo magnetic resonance imaging was performed using a 7.0‐Tesla small animal magnetic resonance scanner (Bruker PharmaScan, Ettlingen, Germany). Mice were anesthetized by a continuous supplying of 1% isoflurane. Respiratory rate and body temperature were monitored by a physiology monitor (Model 1025; SA Instruments Inc, Stony Brook, NY). T2‐weighted images were acquired using a turbo‐rapid acquisition with refocused echo/T2 sequence, and the parameters were as follows: repetition time, 2500 ms; echo time, 50 ms; field of view, 2.0×2.0 cm; flip angle, 180 degrees; matrix size, 256×256; and slice thickness, 1 mm.
NIRF Imaging
In vivo and ex vivo NIRF imaging was performed using a fluorescence imaging system (IVIS Spectrum System; Caliper Life Sciences, Inc, Waltham, MA) equipped with filter sets (excitation/emission, 748/780 nm) fluorescence. Region of interest was manually drawn in the regions of the ischemic ipsilateral and contralateral hemisphere on the NIRF images. Target‐to‐background ratio was calculated as follows: (region of interest value from ipsilateral hemisphere)/(region of interest value from contralateral hemisphere).
Behavioral Tests
Foot fault and Modified Neurological Severity Score were examined on day 7 after ischemia by 2 investigators who were blinded to the experimental groups, in an approach that Bai et al25 previously reported on.
The Modified Neurological Severity Score includes motor, sensory, reflex, and balance tests. Neurological function is graded on a scale of 0 to 18 (normal score, 0; maximal deficit score, 18). In the severity scores of injury, 1 score point is awarded for the inability to perform the test or for the lack of a tested reflex. Therefore, the higher score represents the greater severity of the injury.
For foot‐fault test, mice were tested for a placement dysfunction of the forelimbs. Each mouse was individually placed on the top of an elevated wire grid and was allowed to freely walk for a period of 5 minutes. A video was located beneath the apparatus to assess the stepping errors of the animals. With each weight‐bearing step, the paw may fall or slip between the wire. This fall was recorded as a foot fault. The percentage of foot‐faults of the left paw to total steps was determined.
Statistical Analysis
All experiments were repeated at least 3 times. Quantitative data are presented as the mean±SEM. Statistical differences between treatments were analyzed using either 1‐way ANOVA for multiple group comparisons or t test for 2 group comparisons. For the data that were not appropriate for a parametric test, the Kruskal–Wallis test followed by a Dunn post‐hoc comparison was performed. P<0.05 was considered as statistical significance. Quantitative data plotting was performed in Prism software (version 5; GraphPad Software Inc, La Jolla, CA), and all statistical calculations were performed using SPSS software (version 22.0; IBM Corp, Armonk, NY).
Results
Expression of PirB in Neurons After MCAO
Real‐time quantitative PCR analysis revealed highly increased PirB mRNA in the ischemic hemisphere compared with the undamaged contralateral side, sham‐operated hemisphere, and control group before MCAO both at 24 hours and 4 days post‐MCAO. Cortex, hippocampus, striatum, and thalamus were dissected to compare PirB mRNA expression in different parts between the ischemic hemisphere and contralateral hemisphere. Enhanced PirB mRNA expression in cortex, hippocampus, and striatum from the ischemic hemisphere was observed (Figure 1A). Results of immunohistochemistry showed that PirB protein levels were markedly increased in the damaged hemisphere 24 hours post‐MCAO, compared with the undamaged contralateral side (Figure 1B). The majority of PirB immunostaining colocalized with neuronal marker neuronal nuclei in the cortical penumbra after 24 hours post‐MCAO (Figure 1C).
Figure 1.
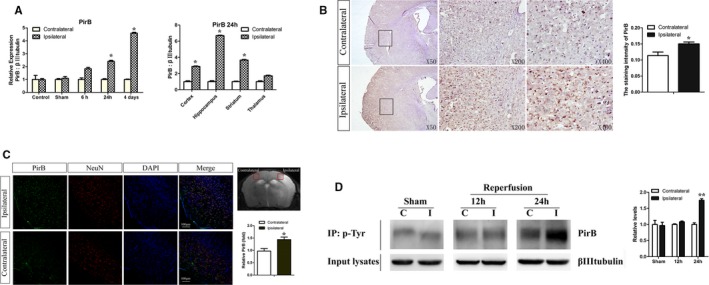
A through D, Enhanced PirB expression and PirB signaling after transient middle cerebral artery occlusion (MCAO). A, Real‐time quantitative PCR analysis revealed highly increased PirB mRNA in the ischemic hemisphere compared with the undamaged contralateral side, sham‐operated hemisphere, and control group before MCAO both at 24 hours and at 4 days post‐MCAO (n=5). B, Representative images of immunohistochemical staining showed that PirB protein levels were markedly increased in the damaged hemisphere 24 hours post‐MCAO, compared with the undamaged contralateral side. Staining intensity of molecule was quantified by the simple PCI image analysis software (n=4). C, Majority of PirB immunostaining (green) colocalized with neuronal marker NeuN (red) in the cortical penumbra 24 hours after ischemia. Rectangle in T2‐weighted image indicates cortical area used for cell staining. Quantification of PirB staining in tMCAO brain slices was by ImageJ software (NIH, Bethesda, MD). Scale bar=100 μm (n=4). D, Protein extracts from hemispheres of post‐MCAO brains were subjected to immunoprecipitation for phosphotyrosine to assess levels of tyrosine phosphorylation of PirB. Expression of βIIItubulin was detected in input lysates. Phosphorylation of PirB was quantified as the ratio of band density to that of βIIItubulin. C indicates contralateral to ischemia; I, ipsilateral to ischemia; Sham, sham‐operated mice (n=4). Data are the mean±SEM. Statistical significance is indicated by: *P<0.05; **P<0.01. NeuN indicates neuronal nuclei; PirB, paired immunoglobulin‐like receptor B.
Tyrosine phosphorylation of PirB on cytoplasmic motifs increased significantly in the ischemic hemisphere compared with the contralateral side at 24 hours post‐MCAO (Figure 1D). It meant that downstream signaling of PirB was significantly increased in ischemia.
Expression of PirB in Neurons After Cerebral Cortex Ischemia
In cerebral cortex ischemic stroke by photothrombosis, expression of PirB at the mRNA level in ischemic cortices was upregulated at 24 hours poststroke. The peak of upregulation of PirB in the cerebral cortex with ischemia was observed at 3 days (Figure 2A). Expression of PirB protein also remarkably increased in the ischemic ipsilateral side, reached a peak at 3 days and lasted for 7 days (Figure 2B).
Figure 2.
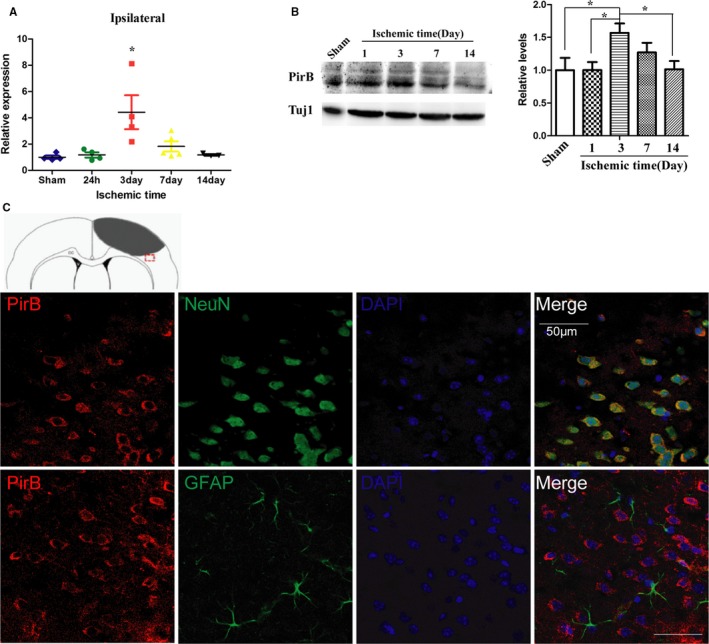
A through C, Expression and cellular location of PirB after cerebral cortex ischemia. Expression of PirB mRNA (A) and protein level (B) in the cortex with ischemia at different time points were detected by real‐time quantitative PCR and western blot analysis, respectively (n=4). Data are the mean±SEM. Statistical significance is indicated by *P<0.05, the expression level of ischemic cortex at different time points post‐injury compared with sham‐operated cortex. C, Representative images of PirB (red) and NeuN (green), or PirB (red) and GFAP (astrocytes, green) double immunofluorescene staining in the ischemic border zone of brain sections are presented. Scale bar=50 μm. GFAP indicates glial fibrillary acidic protein; NeuN, neuronal nuclei; PirB, paired immunoglobulin‐like receptor B; Tuj1, neuron‐specific Class III β‐tubulin.
PirB immunostaining is primarily associated with neurons in the ischemic border zone of brain sections from 3 days poststroke. PirB staining is not detected in astrocytes, as assessed by double staining with marker of astrocyte glial fibrillary acidic protein (Figure 2C).
Synthesis and Characterization of the Anti‐PirB Immunoliposome Nanoprobe
In order to make expression of PirB traceable by fluorescence, we successfully developed anti‐PirB vectorized stealth immunoliposomes containing near‐infrared fluorophore DiR. In addition, transmission electron microscopy of the immunoliposomes verified the formation of homogeneous unilamellar liposomes of a mean diameter of around 100 nm (Figure 3A). Hydrodynamic size and polydispersity index of the 3 kinds of liposomes were determined and suggested a good control of the nanoprobes dispersion (Figure 3B). Binding specificity of the anti‐PirB immunoliposome nanoprobe (6C1/PEG/LP/DiR) was first demonstrated in the PirB‐positive spleen cells. Signal intensities of cells on NIRF images were significantly higher in the 6C1/PEG/LP/DiR group compared with control groups. The majority of the signal intensity in the 6C1/PEG/LP/DiR group was blocked by pretreatment with free anti‐PirB antibody (6C1; Figure 3C and 3D).
Figure 3.
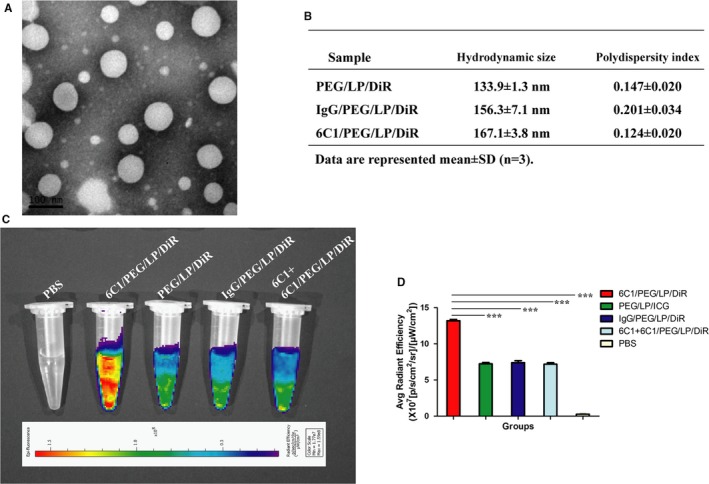
A, Transmission electron microscopy image of 6C1/PEG/LP/DiR. B, Hydrodynamic size and polydispersity index of PEG/LP/DiR, IgG/PEG/LP/DiR, and 6C1/PEG/LP/DiR were determined by dynamic light scattering. C, Representative near‐infrared fluorescence (NIRF) images of spleen cells in different groups showed a remarkable fluorescence signal in the 6C1/PEG/LP/DiR group, which indicated a higher cellular binding efficacy of 6C1/PEG/LP/DiR compared with the control nanoprobes in cells. D, Quantitative data of (C) is shown (n=3): ***P<0.01. PEG indicates polyethylene glycol.
Anti‐PirB Immunoliposome Nanoprobe Targeting Ischemic Cortex in Cerebral Cortex Ischemia Model
To investigate the targeting specificity of nanoprobes in vivo, 6C1/PEG/LP/DiR or control probe were administered intravenously by tail vein in cerebral cortex ischemic stroke mice. Our results showed that target‐to‐background ratio values of both the 6C1/PEG/LP/DiR and control groups were increased with probes’ circulating time extended. However, signal intensities of ischemic hemispheres on NIRF images were significantly higher in the 6C1/PEG/LP/DiR group when compared with the control group at 4 hours and lasted for 1 day postinjection (Figure 4A through 4C).
Figure 4.
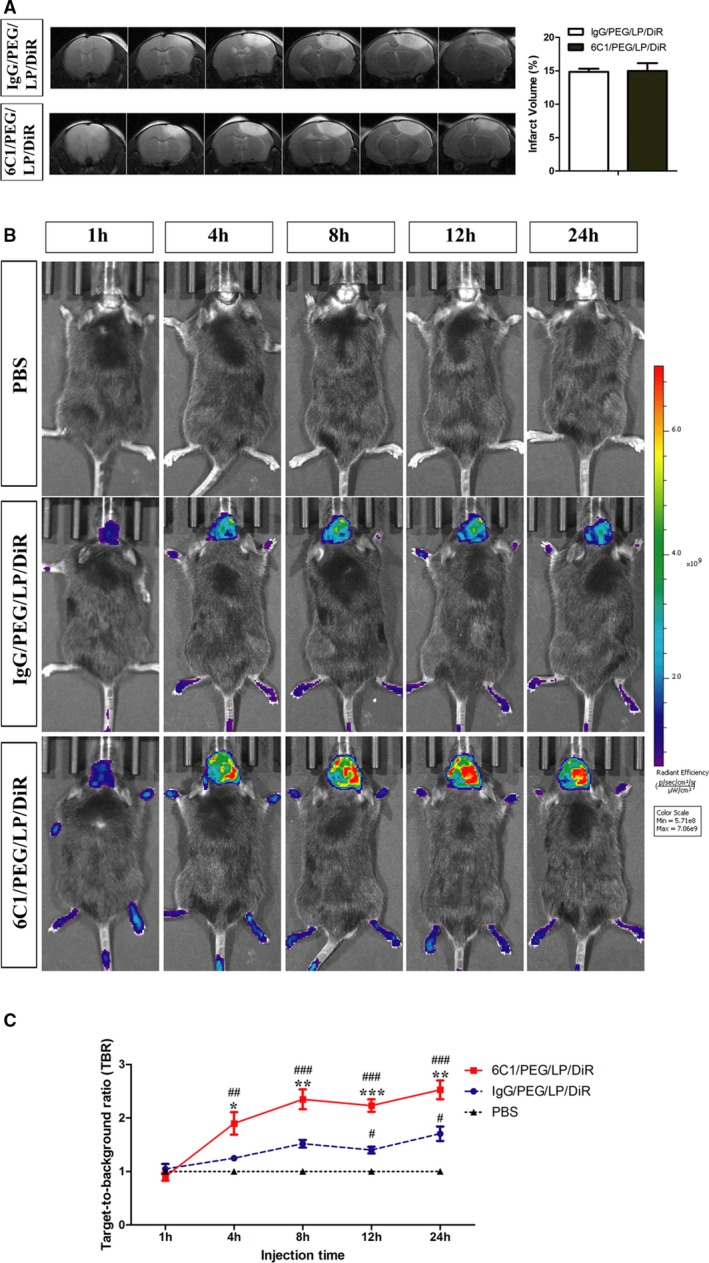
A, Representative T2‐weighted images and quantification of infarct volume in ischemic mice on the day receiving 6C1/PEG/LP/DiR or IgG/PEG/LP/DiR probes. In vivo near‐infrared fluorescence (NIRF) images (B) and quantification of target‐to‐background (TBR) values (C) of ischemic mice after injection of immunoliposome nanoprobes at different time points. Data are the mean±SEM (n=5 in PBS group or n=6 in nanoprobe groups). Statistical significance is indicated by: # P<0.05; ## P<0.01; ### P<0.001 compared with PBS group; *P<0.05; **P<0.01; ***P<0.001 compared with IgG/PEG/LP/DiR group. PEG indicates polyethylene glycol.
In vivo and ex vivo NIRF images and quantification of target‐to‐background ratio values of ischemic brain 24 hours after immunoliposome nanoprobes injection are presented (Figure 5A through 5D). NIRF signals and target‐to‐background ratio values were significantly increased in the 6C1/PEG/LP/DiR group when compared with the control group. Presence of anti‐PirB immunoliposome nanoprobes in peri‐infarct tissue in the ischemic hemisphere was confirmed by ex vivo fluorescence microscopy of brain sections obtained at 24 hours after injection probes (Figure 5E).
Figure 5.
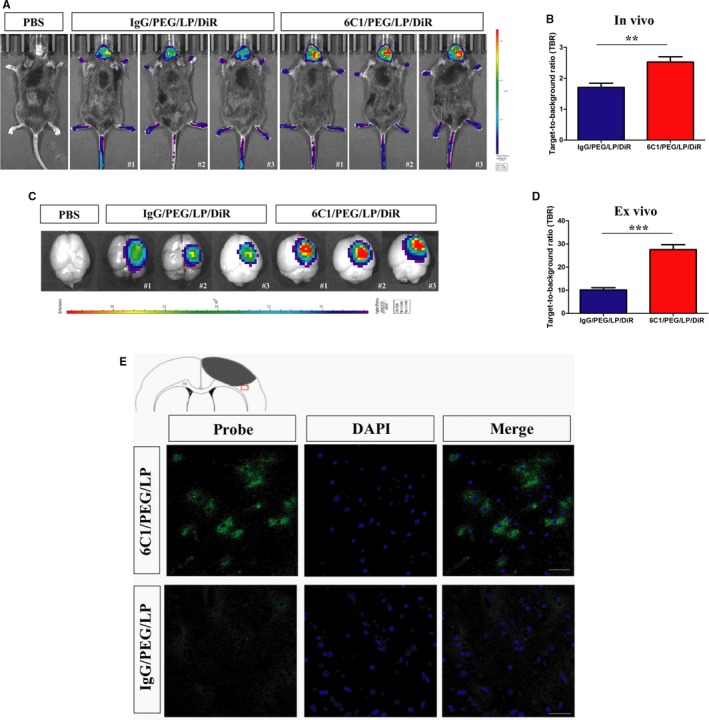
Representive in vivo NIRF images (A) and quantification of target‐to‐background (TBR) values (B) of ischemic mice after injection of immunoliposome nanoprobes at 24 hours. Brain tissues were dissected and acquired NIRF imaging at 24 hours after injection (C). Quantification of TBR values of brain tissues (D). Data are the mean±SEM (n=5 in PBS group or n=6 in nanoprobe groups). Statistical significance was indicated by: **P<0.01: ***P<0.001. E, Representive ex vivo fluorescence microscopy images of the peri‐infarct region of cerebral cortex ischemic mice obtained 24 hours after intravenous injection of anti‐PirB vectorized liposomes or control liposomes (cell nuclei stained in blue with DAPI; scale bar=50 μm). PirB, paired immunoglobulin‐like receptor B.
Soluble PirB Ectodomains Improved Cerebral Cortex Ischemic Stroke Model Recovery by Liposomal Delivery System
sPirB or sPirB/PEG/LP were administered daily to cerebral cortex ischemic stroke. Vehicle‐treated mice were used as the control. The results of T2‐weighted magnetic resonance imaging demonstrated that infarct volume after sPirB or sPirB/PEG/LP treatment was reduced when compared with the vehicle group from days 3 to 7. The difference in infarct volume between the 3 groups was statistically significant by 1‐way ANOVA. Although treatment with sPirB did not show a significant effect, it exhibited a good trend. When cerebral cortex ischemic stroke mice were treated with sPirB/PEG/LP, infarct volume decreased significantly (Figure 6A and 6B). To examine motor recovery post stroke, 2 behavior tests were performed, including foot fault and Modified Neurological Severity Score. The percentage of foot fault was remarkably lower in mice administered sPirB/PEG/LP on day 7 compared with the control group (Figure 6C). Reduction of Modified Neurological Severity Score was significant on day 7 after sPirB/PEG/LP treatment compared with the vehicle group (Figure 6D). The results indicated that soluble PirB ectodomains delivered by liposomal system could promote functional recovery after ischemic injury.
Figure 6.
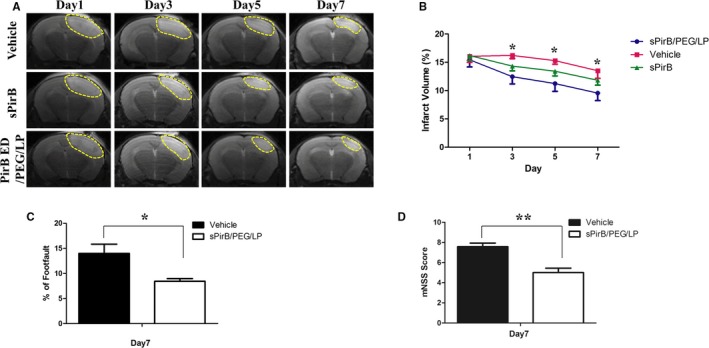
A, T2‐weighted images and (B) quantification of infarct volume in the ischemic brain after vehicle, sPirB, or sPirB/PEG/LP treatment. C, Foot fault and (D) Modified Neurological Severity Score analyses in cerebral cortex ischemic stroke mice. Data are the mean±SEM (n=6). Statistical significance is indicated by: *P<0.05. PirB indicates paired immunoglobulin‐like receptor B.
Blockade of PirB Ligands Binding by PirB Ectodomain Ameliorated Neuronal Apoptosis Following OGD Injury
The OGD model was used to study ischemic injury in vitro. The results showed that after OGD injury, expression of PirB and its ligands in the OGD group was remarkably higher than that in the control in a time‐dependent manner (Figure 7). It suggested that downstream signaling of PirB was significantly increased in vitro model. OGD exposure induced cleavage of procaspase to the active form of caspase‐3. At 24 and 48 hours after OGD injury, activation of caspase‐3 in the OGD group was significantly higher than activation in the control group. However, OGD‐induced caspase‐3 activation was alleviated by sPirB treatment (Figure 8). Therefore, blockade of PirB ligand binding significantly alleviated neuronal apoptosis after exposed to OGD.
Figure 7.
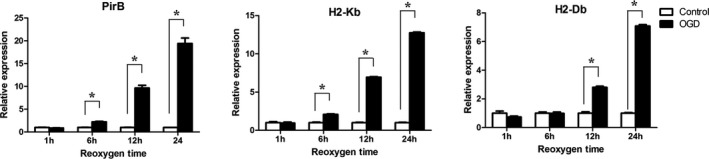
mRNA expression of PirB, H2‐Kb, and H2‐Db in neurons were examined in OGD group or control group at different reoxygen time points. Data are the mean±SEM (n=3). Statistical significance is indicated by: *P<0.05. OGD indicates oxygen glucose deprivation; PirB indicates paired immunoglobulin‐like receptor B.
Figure 8.
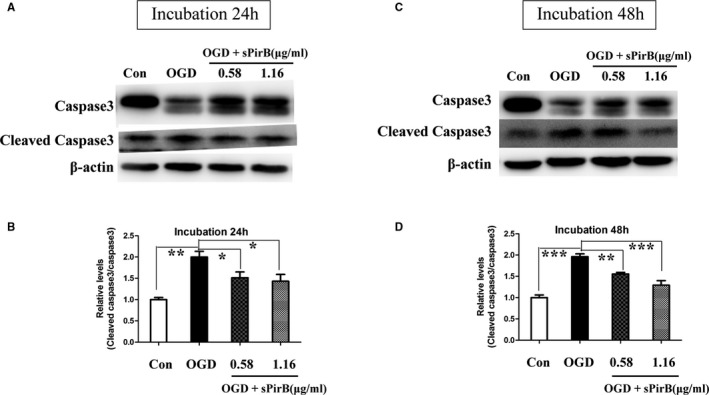
Effect of soluble PirB ectodomains (sPirB) on cleaved caspase‐3 in neurons exposed to OGD. Neurons were exposed to OGD for 2 hours and then cultured in normal condition and incubated with sPirB for 24 (A) or 48 hours (C). B and D, Quantification of cleaved caspase3/caspase 3 represented in (A and C). Data are the mean±SEM (n=3). Statistical significance is indicated by: *P<0.05; **P<0.01; ***P<0.001. OGD indicates oxygen glucose deprivation; PirB indicates paired immunoglobulin‐like receptor B.
Discussion
Ischemic stroke is a complex disease with multiple etiologies and clinical manifestations.29, 30 There are several subtypes within ischemic stroke. So, multiple rodent models have been developed to investigate the underlying etiology of different subtypes of ischemic stroke. However, most animal models of disease, including ischemic stroke, cannot model the whole disease process.31, 32 Some molecules may exhibit different expression pattern in different ischemic stroke model. For example, heat shock protein 70 was induced following the transient focal cerebral ischemia model,33, 34, 35 but was equally expressed in both peri‐infarct and healthy tissues in the permanent focal cerebral ischemia model36; sirtuin 1 expression was increased in neurons of the ipsilesional mouse brain in transient MCAO model,37 but was decreased in the ischemic hemisphere in the permanent MCAO model.38 To study a candidate for molecular therapeutic or imaging target in a variety of cerebral ischemic stroke may provide adequate information for its application.
A previous study showed that PirB expression began to increase at 2 hours, peaked at 24 hours, and lasted for 7 days after reperfusion in the ischemic penumbra.39 We investigated the expression of PirB in a transient MCAO mice model, cerebral cortex ischemic model, and in vitro OGD model. The results showed that PirB was significantly upregulated after ischemic injury in multiple ischemic stroke models. It is suggested that PirB is a significant molecule in the pathological process of cerebral ischemia. Therefore, we developed anti‐PirB vectorized stealth immunoliposome nanoprobe containing near‐infrared fluorophore DiR to make them traceable. The binding specificity of anti‐PirB immunoliposome nanoprobe was demonstrated in PirB‐positive spleen cells. Signal intensities of the ischemic hemispheres in in vivo NIRF images or ex vivo NIRF images were significantly higher in the 6C1/PEG/LP/DiR group when compared with the control group at 24 hours after the probes’ intravenous injection. These results indicated that the anti‐PirB immunoliposome nanoprobe targeted PirB in the cerebral cortex ischemia model. A liposomal delivery system may be used as a theranostic nanocarrier that specifically targets the peri‐infarct tissue in cerebral ischemia.
In the nervous system, PirB binds several ligands, including major histocompatibility complex class I proteins,8 myelin inhibitors (Nogo, MAG, and OMgp),10 and beta‐amyloid.12 The greater recovery in PirB versus H2‐Db/H2‐Kb knockout mice fits well with a model in PirB that binds multiple ligands in the central nervous system.14 Furthermore, mutant mice lacking Nogo‐AB recover complex motor function poststroke more completely than do control animals.40 In our study, sPirB protein was used as a therapeutic reagent to improve cerebral ischemic stroke model recovery by blocking PirB binding to endogenous ligands. sPirB was delivered by a PEG‐modified nanoliposome, which possesses the qualification of a long circulating property in the bloodstream and accumulation in the cerebral ischemic region. Our results indicated that the sPirB immunoliposome could significantly promote functional recovery after ischemic injury. Blockade of PirB ligand binding by sPirB significantly alleviated apoptosis of neurons after being exposed to OGD. Thus, it suggested that the amelioration of neuron apoptosis by PirB ectodomains might result in the improvement of motor abilities of cerebral ischemic model mice.
Sources of Funding
This study was supported by a grant from the National Basic Research Program of China (2013CB733801) and National High Technology Research and Development Program of China (863 Program: 2015AA020502).
Disclosures
None.
Acknowledgments
We thank Cheng Qian, Rui Yang, Yanli An, and Qiusha Tang for technical assistance.
(J Am Heart Assoc. 2018;7:e007197 DOI: 10.1161/JAHA.117.007197.)29378731
References
- 1. Margaritescu O, Mogoanta L, Pirici I, Pirici D, Cernea D, Margaritescu C. Histopathological changes in acute ischemic stroke. Rom J Morphol Embryol. 2009;50:327–339. [PubMed] [Google Scholar]
- 2. Hasan N, McColgan P, Bentley P, Edwards RJ, Sharma P. Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol. 2012;74:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. [DOI] [PubMed] [Google Scholar]
- 4. Hayami K, Fukuta D, Nishikawa Y, Yamashita Y, Inui M, Ohyama Y, Hikida M, Ohmori H, Takai T. Molecular cloning of a novel murine cell‐surface glycoprotein homologous to killer cell inhibitory receptors. J Biol Chem. 1997;272:7320–7327. [DOI] [PubMed] [Google Scholar]
- 5. Kubagawa H, Burrows PD, Cooper MD. A novel pair of immunoglobulin‐like receptors expressed by B cells and myeloid cells. Proc Natl Acad Sci USA. 1997;94:5261–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kubagawa H, Chen CC, Ho LH, Shimada TS, Gartland L, Mashburn C, Uehara T, Ravetch JV, Cooper MD. Biochemical nature and cellular distribution of the paired immunoglobulin‐like receptors, PIR‐A and PIR‐B. J Exp Med. 1999;189:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takai T. Paired immunoglobulin‐like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular‐dominance plasticity in visual cortex. Science. 2006;313:1795–1800. [DOI] [PubMed] [Google Scholar]
- 9. Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2‐containing protein tyrosine phosphatases SHP‐1 and SHP‐2 for paired immunoglobulin‐like receptor B (PIR‐B)‐mediated inhibitory signal. J Exp Med. 1998;187:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Atwal JK, Pinkston‐Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier‐Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. [DOI] [PubMed] [Google Scholar]
- 11. Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hubener M, Shatz CJ. PirB regulates a structural substrate for cortical plasticity. Proc Natl Acad Sci USA. 2013;110:20771–20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a beta‐amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341:1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, Syken J, Dan Y, Shatz CJ. Blocking PirB up‐regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Transl Med. 2014;6:258ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giffard RG. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang ZG, Zhang L, Tsang W, Soltanian‐Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood‐brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. [DOI] [PubMed] [Google Scholar]
- 16. Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase‐mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. [DOI] [PubMed] [Google Scholar]
- 17. Ishii T, Asai T, Oyama D, Fukuta T, Yasuda N, Shimizu K, Minamino T, Oku N. Amelioration of cerebral ischemia‐reperfusion injury based on liposomal drug delivery system with asialo‐erythropoietin. J Control Release. 2012;160:81–87. [DOI] [PubMed] [Google Scholar]
- 18. Fukuta T, Ishii T, Asai T, Sato A, Kikuchi T, Shimizu K, Minamino T, Oku N. Treatment of stroke with liposomal neuroprotective agents under cerebral ischemia conditions. Eur J Pharm Biopharm. 2015;97:1–7. [DOI] [PubMed] [Google Scholar]
- 19. Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochem Biophys Acta. 1991;1068:133–141. [DOI] [PubMed] [Google Scholar]
- 20. Ishii T, Asai T, Oyama D, Agato Y, Yasuda N, Fukuta T, Shimizu K, Minamino T, Oku N. Treatment of cerebral ischemia‐reperfusion injury with PEGylated liposomes encapsulating FK506. FASEB J. 2013;27:1362–1370. [DOI] [PubMed] [Google Scholar]
- 21. Sofou S, Sgouros G. Antibody‐targeted liposomes in cancer therapy and imaging. Expert Opin Drug Deliv. 2008;5:189–204. [DOI] [PubMed] [Google Scholar]
- 22. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. [DOI] [PubMed] [Google Scholar]
- 23. Hoyte LC, Brooks KJ, Nagel S, Akhtar A, Chen R, Mardiguian S, McAteer MA, Anthony DC, Choudhury RP, Buchan AM, Sibson NR. Molecular magnetic resonance imaging of acute vascular cell adhesion molecule‐1 expression in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985;17:497–504. [DOI] [PubMed] [Google Scholar]
- 25. Bai YY, Gao X, Wang YC, Peng XG, Chang D, Zheng S, Li C, Ju S. Image‐guided pro‐angiogenic therapy in diabetic stroke mouse models using a multi‐modal nanoprobe. Theranostics. 2014;4:787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng B, Gou X, Chen H, Li L, Zhong H, Xu H, Jiang F, Zhao Z, Wang Q, Xu L. Targeted delivery of neurogenin‐2 protein in the treatment for cerebral ischemia‐reperfusion injury. Biomaterials. 2013;34:8786–8797. [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Tang Y, Li S, Liao C, Guo X. Targeting of the B‐lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J Drug Target. 2010;18:675–687. [DOI] [PubMed] [Google Scholar]
- 28. Yang R, An LY, Miao QF, Li FM, Han Y, Wang HX, Liu DP, Chen R, Tang SQ. Effective elimination of liver cancer stem‐like cells by CD90 antibody targeted thermosensitive magnetoliposomes. Oncotarget. 2016;7:35894–35916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas J, Fitzpatrick L. Acute ischemic stroke review. J Neurosci Nurs. 2008;40:69; author reply, 69. [PubMed] [Google Scholar]
- 30. Kim BJ, Kim JS. Ischemic stroke subtype classification: an Asian viewpoint. J Stroke. 2014;16:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krafft PR, Bailey EL, Lekic T, Rolland WB, Altay O, Tang J, Wardlaw JM, Zhang JH, Sudlow CL. Etiology of stroke and choice of models. Int J Stroke. 2012;7:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fluri F, Schuhmann MK, Kleinschnitz C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 2015;9:3445–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kinouchi H, Sharp FR, Hill MP, Koistinaho J, Sagar SM, Chan PH. Induction of 70‐kDa heat shock protein and hsp70 mRNA following transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1993;13:105–115. [DOI] [PubMed] [Google Scholar]
- 34. Planas AM, Soriano MA, Estrada A, Sanz O, Martin F, Ferrer I. The heat shock stress response after brain lesions: induction of 72 kDa heat shock protein (cell types involved, axonal transport, transcriptional regulation) and protein synthesis inhibition. Prog Neurobiol. 1997;51:607–636. [DOI] [PubMed] [Google Scholar]
- 35. de la Rosa X, Santalucia T, Fortin PY, Purroy J, Calvo M, Salas‐Perdomo A, Justicia C, Couillaud F, Planas AM. In vivo imaging of induction of heat‐shock protein‐70 gene expression with fluorescence reflectance imaging and intravital confocal microscopy following brain ischaemia in reporter mice. Eur J Nucl Med Mol Imaging. 2013;40:426–438. [DOI] [PubMed] [Google Scholar]
- 36. Agulla J, Brea D, Campos F, Sobrino T, Argibay B, Al‐Soufi W, Blanco M, Castillo J, Ramos‐Cabrer P. In vivo theranostics at the peri‐infarct region in cerebral ischemia. Theranostics. 2013;4:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernandez‐Jimenez M, Hurtado O, Cuartero MI, Ballesteros I, Moraga A, Pradillo JM, McBurney MW, Lizasoain I, Moro MA. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke. 2013;44:2333–2337. [DOI] [PubMed] [Google Scholar]
- 38. Kalaivani P, Ganesh M, Sathiya S, Ranju V, Gayathiri V, Saravana Babu C. Alteration in bioenergetic regulators, SirT1 and Parp1 expression precedes oxidative stress in rats subjected to transient cerebral focal ischemia: molecular and histopathologic evidences. J Stroke Cerebrovasc Dis. 2014;23:2753–2766. [DOI] [PubMed] [Google Scholar]
- 39. Gou X, Zhang Q, Xu N, Deng B, Wang H, Xu L, Wang Q. Spatio‐temporal expression of paired immunoglobulin‐like receptor‐B in the adult mouse brain after focal cerebral ischaemia. Brain Inj. 2013;27:1311–1315. [DOI] [PubMed] [Google Scholar]
- 40. Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]


