Abstract
Background
The relationship between ideal cardiovascular health reflected in the cardiovascular health score (CVHS) and valvular heart disease is not known. The purpose of this study was to determine the association of CVHS attainment through midlife to late life with aortic stenosis prevalence and severity in late life.
Methods and Results
The following 6 ideal cardiovascular health metrics were assessed in ARIC (Atherosclerosis Risk in Communities) Study participants at 5 examination visits between 1987 and 2013 (visits 1–4 in 1987–1998 and visit 5 in 2011–2013): smoking, body mass index, total cholesterol, blood pressure, physical activity, and blood glucose. Percentage attained CVHS was calculated in 6034 participants as the sum of CVHS at each visit/the maximum possible score. Aortic stenosis was assessed by echocardiography at visit 5 on the basis of the peak aortic valve velocity. Aortic stenosis was categorized sclerosis, mild stenosis, and moderate‐to‐severe stenosis. Mean age was 76±5 years, 42% were men, and 22% were black. Mean percentage attained CVHS was 63±14%, and the prevalence of aortic stenosis stages were 15.9% for sclerosis, 4.3% for mild stenosis, and 0.7% for moderate‐to‐severe stenosis. Worse percentage attained CVHS was associated with higher prevalence of aortic sclerosis (P<0.001 for trend), mild stenosis (P<0.001), and moderate‐to‐severe stenosis (P=0.002), adjusting for age, sex, and race.
Conclusions
Greater attainment of ideal cardiovascular health in midlife to late life is associated with a lower prevalence of aortic sclerosis and stenosis in late life in a large cohort of older adults.
Keywords: aortic stenosis, echocardiography, epidemiology, primary prevention, risk factor
Subject Categories: Valvular Heart Disease, Echocardiography, Epidemiology, Risk Factors, Primary Prevention
Clinical Perspective
What Is New?
This study aimed to define the relationship between the attainment of ideal cardiovascular health, as assessed by 6 health metrics (smoking, body mass index, total cholesterol, blood pressure, physical activity, and blood glucose) assessed at midlife to late life, with the prevalence and severity of aortic stenosis in late life.
In >6000 participants in the ARIC (Atherosclerosis Risk in Communities) Study, we assigned a unique cardiovascular health score to each participant at the 5 examination study visits over a 25‐year period.
We found that consistent attainment of ideal cardiovascular health in adult life was associated with a lower prevalence and severity of aortic stenosis in late life.
What Are the Clinical Implications?
Our findings emphasize the importance of consistent attainment of ideal cardiovascular health through midlife to late life and suggest that primary prevention of calcific aortic valve disease could potentially be achieved through modification of cardiovascular health metrics.
Introduction
Calcific aortic valve (AV) disease is the most common valvular lesion among people >75 years of age, in whom the prevalence of aortic calcification has been reported to be 26%.1 Although aortic stenosis (AS) is robustly associated with adverse outcomes,2, 3 aortic sclerosis has also been associated with an increased risk of cardiovascular morbidity and mortality,4 and aortic sclerosis is a significant risk factor for progression to AS.5 More important, AV calcification and stenosis are associated with similar risk factors as atherosclerosis,1, 6, 7 and a wealth of data now point towards parallel active processes leading to AV dysfunction, including inflammation, lipid deposition, and calcification.8
In 2010, the American Heart Association Strategic Planning Task Force and Statistics Committee defined the concept of ideal cardiovascular health as the attainment of 4 health behaviors (body mass index <25 kg/m2, absent of smoking, physical activity, and healthy diet) and 3 health factors (untreated blood pressure <120/<80 mm Hg, fasting blood glucose <100 mg/dL, and untreated total cholesterol <200 mg/dL).9 Attainment of these ideal health metrics has been associated with lower incidence of cardiovascular disease, including coronary heart disease, myocardial infarction, heart failure,10, 11, 12, 13, 14 stroke,15 and retinopathy,16 in addition to noncardiovascular conditions, such as cancer.11, 17 Greater attainment of ideal cardiovascular health through midlife to late life is associated with both a lower prevalence of cardiovascular disease in late life and better cardiac structure and function in late life among people free of prevalent cardiovascular disease.18 However, the relationship between attainment of ideal cardiovascular health and valvular heart disease is unknown.
We sought to determine whether greater attainment of ideal cardiovascular health score (CVHS) in midlife to late life is associated with a lower prevalence of AV disease in late life. We relate the attainment of cardiovascular health metrics assessed serially over a 25‐year period in midlife to late life with late‐life AV function among 6034 participants in the ARIC (Atherosclerosis Risk in Communities) Study who underwent comprehensive echocardiographic evaluation of the AV at the fifth study visit (2011–2013).
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. This analysis uses data from the National Heart, Lung, and Blood Institute–sponsored ARIC Study. ARIC Study data are available for distribution to outside researchers through the ARIC Study Limited Access Data set, according to established National Heart, Lung, and Blood Institute procedures and in accordance with National Institutes of Health policies.
Study Population
The ARIC Study is a prospective community‐based cohort, whose objective and design have previously been described.19 Between 1987 and 1989, 15 792 individuals, aged 45 to 64 years, were enrolled in 4 communities in the United States: Forsyth County, North Carolina; Jackson, MS; suburban Minneapolis, MN; and Washington County, Maryland. The cohort participants underwent 4 study visits between 1987 and 1998. Between 2011 and 2013, 6538 participants (aged 67–91 years) returned for a fifth study visit that included questionnaires, laboratory testing, and a comprehensive echocardiographic examination. The study protocol was approved by institutional review boards at all field centers, and all participants provided written informed consent. For this study, we included 6034 participants with available echocardiographic measurements of AV function, no prior AV surgery as of visit 5 (n=32), and available serial data on ideal cardiovascular health metrics. We excluded participants with a bicuspid AV identified on echocardiography (n=11).
Ideal Cardiovascular Health Score
Ideal CVHS was determined, as previously described in detail.3 Briefly, total cholesterol, seated blood pressure after 5‐minute rest, body mass index (BMI), fasting glucose, physical activity, and smoking status were assessed at ARIC Study visits 1 to 5. The prevalence of missing values for each health metric at each visit is provided in Table S1. All missing values were imputed to be intermediate status, except in the event in which status was known at both the visits immediately before and after the visit in question. If poor status was observed at both neighboring visits, then poor status was imputed at the missing visit. Similarly, if ideal status was observed at both neighboring visits, then ideal status was imputed at the missing visit. Physical activity was assessed at visit 1, 3, and 5, and missing values were extrapolated as the lowest value from the 2 adjacent visits. Information about diet was not included because of inadequate serial data. Each health metric was classified as ideal (2 points), intermediate (1 point), or poor (0 points) at each visit, as defined in Table 1. These points were summed from visits 1 through 5 and then divided by the maximum number of attainable points to quantify the percentage of ideal cardiovascular health attained through adult life.18 Missing values were imputed to be intermediate status, except if the values were known at both neighboring visits. In this case, the health score was extrapolated from those 2 visits. The 6034 participants were then grouped on the basis of percentage attained CVHS: <50%, ≥50% to <60%, ≥60% to <70%, ≥70% to <80%, and ≥80%.
Table 1.
Demographics of ARIC Study Participants at Visits 1 and 5 by Severity of AS
| Variable | AS Grade at Visit 5 | Visit 1 | Visit 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Sclerotic | Mild | Moderate and < Severe | |||||||
| Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | |||
| n=4775 | n=960 | n=257 | n=42 | P Value for Trend | ||||||
| Age, y | 51.6±5.0 | 75.8±5.1 | 52.4±5.1 | 76.6±5.2 | 54.1±5.3 | 78.4±5.4 | 54.1±5.3 | 78.5±4.5 | <0.001 | <0.001 |
| Male sex | 1967 (41.2) | 412 (42.9) | 125 (48.6) | 23 (54.8) | 0.001 | |||||
| Black race | 1085 (22.7) | 194 (20.2) | 35 (13.6) | 7 (16.7) | 0.005 | |||||
| Field center | <0.001 | |||||||||
| Forsyth County | 1158 (24.3) | 181 (18.9) | 42 (16.3) | 10 (23.8) | ||||||
| Jackson | 985 (20.6) | 176 (18.3) | 32 (12.5) | 7 (16.7) | ||||||
| Minneapolis | 1429 (29.9) | 263 (27.4) | 91 (35.4) | 13 (31) | ||||||
| Washington County | 1203 (25.2) | 194 (20.2) | 92 (35.8) | 12 (26.8) | ||||||
| Cumulative CVHS | 66.8±17.3 | 64.1±14.0 | 63.0±13.5 | 60.3±13.5 | 59.1±16.7 | 58.2±13.2 | 59.7±15.9 | 56.8±11.9 | 0.001 | 0.001 |
Data are given as mean±SD or number (percentage). ARIC indicates Atherosclerosis Risk in Communities; AS, aortic stenosis; and CVHS, cardiovascular health score.
Echocardiography
Detailed methods for echocardiography at visit 5 of the ARIC Study have been previously published.20 All the examinations were performed by certified sonographers at the 4 field centers, using the uniform equipment (Philips iE33 Ultrasound systems) and in accordance with a standardized image acquisition protocol. This protocol included pulse‐wave Doppler assessment of the left ventricular outflow tract (LVOT), continuous‐wave Doppler assessment of flow velocities across the AV, and assessment of the AV and LVOT in the parasternal long‐ and short‐axis views. Analysts who were blinded to participant characteristics performed quantitative measures at a dedicated Echocardiography Reading Center. Any given measure was performed by the same analyst for all echocardiographic studies. Reproducibility metrics for key measures of cardiac structure and function have been previously published.20 Intrareader reproducibility of key AV measures was performed in 20 studies with the following results: AV peak velocity: coefficient of variation, 3%; correlation coefficient, 0.98; AV mean gradient: coefficient of variation, 7%; correlation coefficient, 0.97; LVOT diameter: coefficient of variation, 4%; correlation coefficient, 0.82; AV area (AVA): coefficient of variation, 9%; correlation coefficient, 0.82.
Classification of AV Disease
AV disease was assessed at visit 5 and was based primarily on the peak AV velocity, concordant with the 2014 American Heart Association/American College of Cardiology valvular heart disease guidelines.21 AV disease was classified as follows: (1) normal: peak AV velocity, <1.5 m/s; (2) sclerosis: peak AV velocity, ≥1.5 to <2.0 m/s; (3) mild stenosis: peak AV velocity, ≥2.0 to <3.0 m/s; (4) moderate or severe stenosis: peak AV velocity ≥3 m/s. In parallel analyses, AV disease was also classified on the basis of the AVA, calculated as π×(LVOT diameter/2)2×(LVOT Velocity Time Integral/AV Velocity Time Integral), as follows: (1) normal: peak AV velocity, <1.5 m/s; (2) sclerosis: peak AV velocity, ≥1.5 to <2.0 m/s; (3) mild stenosis: AVA, 1.5 to 2 cm2; (4) moderate or severe stenosis: AVA, <1.5 cm2.
Statistical Analysis
Peak AV velocity and AVA were visually represented by frequency distributions and scatter plots with linear regression lines. Participant characteristics at visit 1 and visit 5, adjusted for age, race, sex, and field center, were displayed by AV disease classification. Tests for trend across AV categories were performed using multivariable linear regression for continuous variables and logistic regression for binary categorical variables. The same analyses were then repeated stratified for sex and race. The prevalence of each AV category was determined among participant categories on the basis of percentage attained CVHS (<50%, 50%–60%, 60%–70%, 70%–80%, and >80%). Odds ratios for AV sclerosis, mild stenosis, and moderate‐to‐severe stenosis associated with each CVHS category relative to the <50% category were obtained from multivariable logistic regression models adjusting for age, sex, race, and field center. For AV sclerosis, participants with mild, moderate, and severe AS were excluded. For mild AS, participants with moderate and severe AS were excluded. We assessed the relationship between pattern of CVHS attainment through midlife to late life and AV disease in late life. As previously published,18 we identified trajectories of ideal cardiovascular health attainment through midlife to late life on the basis of CVHS at the 5 study visits using trajectory analysis22 with the use of the STATA macro TRAJ. We used the bayesian information criterion to decide the number of trajectories (2–5 trajectories assessed). We compared the prevalence of each AV category between the 5 identified trajectories in analyses adjusted for age, sex, race, and center. To determine the population attributable risk for AV dysfunction (sclerosis or stenosis) associated with low percentage attained CVHS (<50%, <60%, <70%, or <80%), we used the prevalence among cases and the odds ratio estimate to calculate the percentage population attributable risk using the following formulation23: population attributable risk%=pdi×[RRi−1/RRi], where pdi is the proportion of total cases in the population arising from the ith exposure category and RRi is the adjusted risk ratio for the ith exposure category. A 2‐sided P value of <0.05 was considered significant.
Results
Adequate quality echocardiograms were available in 6118 participants at ARIC Study visit 5. Of the 6034 participants in the analysis, the mean age was 52±5 years at visit 1 and 76±5 years at visit 5, 58% were women, and 22% were black (Table 1). By visit 5, the mean percentage attained CVHS was 63±14%. At visit 5, the mean AV peak velocity was 1.3±0.4 m/s (Figure 1) and the mean AVA was 2.4±0.5 cm2 (Figure S1). AV disease was absent in 4775 (79%), whereas 960 (15.9%) had aortic sclerosis, 257 (4.3%) had mild stenosis, and 42 (0.7%) had moderate or severe stenosis.
Figure 1.
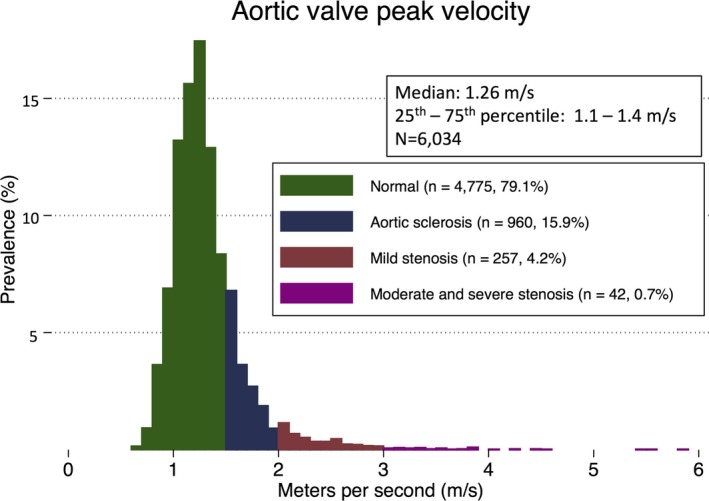
Distribution of aortic valve peak velocity among ARIC (Atherosclerosis Risk in Communities) Study participants at visit 5. Colors signify categories of aortic valve function: normal (green), aortic sclerosis (navy), mild stenosis (red), and moderate‐to‐severe stenosis (purple).
AV Disease and Cardiovascular Risk Factors
Worse AV disease category was associated with older age, male sex, and white race (Table 1). After adjusting for age, sex, race, and field center, worse AV disease category was associated with a higher prevalence of previous smoking, hypertension, and diabetes mellitus, higher BMI, higher glucose, and lower estimated glomerular filtration rate at visit 5 (Table 2). The prevalence of cardiovascular diseases, including atrial fibrillation, coronary disease, previous stroke, and heart failure, was also higher among worse AV disease categories. More advanced categories of AV disease also demonstrated higher circulating biomarkers of inflammation (high‐sensitivity C‐reactive protein), ventricular wall stress (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide]), and myocardial injury (high‐sensitivity troponin T).
Table 2.
Clinical Characteristics of ARIC Study Participants at Visits 1 and 5 by Severity of AS
| Characteristics | AS Grade at Visit 5 | Visit 1 | Visit 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | Sclerotic | Mild | Moderate and Severe | |||||||
| Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | Visit 1 | Visit 5 | |||
| n=4775 | n=960 | n=257 | n=42 | P Value for Trend | ||||||
| Hypertension, % | 22 (21–23) | 82 (81–83) | 30 (27–32) | 88 (86–90) | 35 (29–41) | 90 (86–94) | 30 (17–43) | 92 (84–100) | <0.001 | <0.001 |
| Smoking (ever), % | 33 (32–34) | 61 (60–62) | 34 (31–37) | 61 (58–64) | 38 (33–44) | 72 (67–78) | 36 (22–50) | 70 (56–84) | 0.1 | 0.005 |
| Smoking (current), % | 18 (17–19) | 6 (5–6) | 17 (15–19) | 6 (5–8) | 21 (16–26) | 6 (3–9) | 24 (11–38) | 10 (0–20) | 0.39 | 0.44 |
| AF, % | 0.01 (0–0.03) | 8 (7–9) | 0.05 (0–0.1) | 11 (9–13) | 0 | 12 (9–15) | 0 | 19 (9–21) | 0.23 | <0.001 |
| Diabetes mellitus, % | 5 (4–6) | 37 (35–38) | 6 (4–7) | 41 (38–44) | 7 (4–10) | 45 (39–51) | 12 (2–21) | 57 (43–72) | 0.027 | <0.001 |
| eGFR, <60 mL/min per 1.73 m2, % | 1 (0.7–1) | 28 (26–29) | 1 (0.7–2) | 31 (28–33) | 1 (0–2) | 31 (26–36) | 4 (1–9) | 41 (26–55) | 0.09 | 0.009 |
| Coronary disease, % | 2 (1–2) | 16 (15–17) | 2 (1–2) | 18 (16–20) | 4 (2–6) | 26 (23–32) | 2 (0–6) | 23 (12–35) | 0.047 | <0.001 |
| Previous MI, % | 1 (1–2) | 8 (7–8) | 1 (0–2) | 8 (6–9) | 4 (1–6) | 11 (7–14) | 0 | 8 (1–16) | 0.098 | 0.18 |
| Previous stroke, % | 0.5 (0.3–0.7) | 3 (3–4) | 0.9 (0.3–2) | 4 (3–6) | 1 (0–3) | 8 (5–11) | 5 (0–11) | 6 (1–12) | 0.005 | <0.001 |
| Heart failure, % | 2 (2–2) | 12 (11–13) | 3 (2–4) | 15 (13–18) | 4 (1–7) | 21 (16–26) | 0 | 33 (19–46) | 0.027 | <0.001 |
| BMI, kg/m2 | 26.7 (27–27) | 28.3 (28–29) | 28.2 (28–29) | 30 (30–30) | 28.5 (28–29) | 30.2 (30–31) | 29.2 (30–31) | 30.1 (28–32) | <0.001 | <0.001 |
| SBP, mm Hg | 116 (115–116) | 130 (130–131) | 119 (118–120) | 131 (130–133) | 120 (118–122) | 127 (125–130) | 124 (119–128) | 131 (126–136) | <0.001 | 0.77 |
| DBP, mm Hg | 73 (72–73) | 67 (67–67) | 74 (73–74) | 66 (65–66) | 74 (73–75) | 63 (62–64) | 74 (71–77) | 62 (58–65) | <0.001 | <0.001 |
| Heart rate, beats/min | 66 (65–66) | 63 (62–63) | 65 (65–66) | 62 (62–63) | 66 (65–67) | 63 (62–65) | 65 (62–68) | 62 (59–65) | 0.87 | 0.75 |
| QRS duration, ms | 97 (96–97) | 96 (95–97) | 98 (97–99) | 100 (98–101) | 98 (96–99) | 102 (99–104) | 100 (97–103) | 108 (102–114) | <0.001 | <0.001 |
| Creatinine, mg/dL | 0.72 (0.71–072) | 0.98 (0.97–0.99) | 0.72 (0.71–0.72) | 1 (0.98–1.03) | 0.70 (0.68–0.71) | 1.09 (1.04 –1.08) | 0.73 (0.69–0.77) | 1.10 (1.06–1.14) | 0.15 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 92 (93–93) | 70 (69– 70) | 93 (92–94) | 69 (68–70) | 95 (93–96) | 68 (66–70) | 92 (88–96) | 64 (59–69) | 0.13 | 0.010 |
| LDL, mg/dL | 133 (131–134) | 105 (104–106) | 136 (134–139) | 102 (100–104) | 147 (142–152) | 101 (97–105) | 134 (123–145) | 100 (89–110) | <0.001 | 0.004 |
| HDL, mg/dL | 54 (53–54) | 53 (52–53) | 52 (51–53) | 51 (50–52) | 49 (47–51) | 50 (49–52) | 47 (42–51) | 49 (45–53) | <0.001 | <0.001 |
| Glucose, mg/dL | 101 (100–102) | 113 (112–114) | 102 (100–103) | 115 (113–117) | 102 (100–105) | 117 (114–120) | 104 (97–111) | 123 (115–132) | 0.09 | <0.001 |
| Hemoglobin A1c, % | 5.94 (5.91–5.96) | 5.94 (5.89–6.00) | 6.04 (5.94–6.14) | 6.1 (5.85–6.36) | 0.07 | |||||
| hs‐CRP, mg/L | 3.9 (3.6–4.1) | 5.1 (4.6–5.6) | 4.5 (3.6–5.5) | 5 (2.6–7.6) | <0.001 | |||||
| NT‐proBNP, ng/L | 280 (258–303) | 303 (252–353) | 457 (349–545) | 1240 (998–1481) | <0.001 | |||||
| HS troponin T, ng/L | 1.4 (1.3–1.4) | 1.5 (1.4–1.6) | 1.8 (1.6–2.0) | 2.3 (1.9–2.8) | <0.001 | |||||
Data are given as means and values in parentheses are 95% confidence intervals. Values and P values for trend are adjusted for age, race, sex, and field center. AF indicates atrial fibrillation; ARIC, Atherosclerosis Risk in Communities; AS, aortic stenosis; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HS troponin T, high‐sensitivity troponin T; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and SBP, systolic blood pressure.
Categories of Percentage Attained CVHS and Severity of AS
Significant associations of modest magnitude were observed between percentage attained CVHS and both peak AV velocity and AVA (Figure 2). The percentage attained CVHS was <50% in 17% of participants, 50% to 60% in 23%, 60% to 70% in 30%, 70% to 80% in 16%, and >80% in 14%. Worse category of percentage attained CVHS was associated with a higher prevalence of aortic sclerosis and stenosis after adjusting for age, sex, and race (Figure 3, Table S2). Comparing participants in the lowest (<50%) with those in the highest (>80%) group of percentage attained CVHS, aortic sclerosis was present in 22% and 11%, respectively (odds ratio, 0.40 [95% confidence interval, 0.30–0.53]; P<0.001; P<0.001 for trend across categories), mild stenosis in 7.2% and 2.3%, respectively (odds ratio, 0.30 [95% confidence interval, 0.18–0.50]; P<0.001; P<0.001 for trend), and moderate or severe stenosis in 1.2% and 0.2%, respectively (odd ratio, 0.19 [95% confidence interval, 0.04–0.89]; P=0.035; P=0.001 for trend) (Table 3). Similar associations were noted when defining AV disease on the basis of the AVA as opposed to the AV peak velocity (Figure S2). In addition, similar associations were noted in a sensitivity analysis including 3287 participants free of atrial fibrillation, heart failure, coronary artery disease, stroke, or an estimated glomerular filtration rate <60 mL/min per 1.73 m2 (Figures S3 and S4). The population attributable fraction of AV dysfunction (sclerosis and stenosis) was 8.2% for a percentage attained CVHS <50%, 18.1% for a percentage attained CVHS <60%, 31.4% for a percentage attained CVHS <70%, and 33.6% for a percentage attained CVHS <80%, suggesting that one third of all AV sclerosis and stenosis would not occur if the percentage attained CVHS by late life were uniformly ≥80% (Figure S5). Similar findings were observed when using the percentage attained CVHS through visit 4 to predict AV dysfunction at visit 5 (Figure S6).
Figure 2.
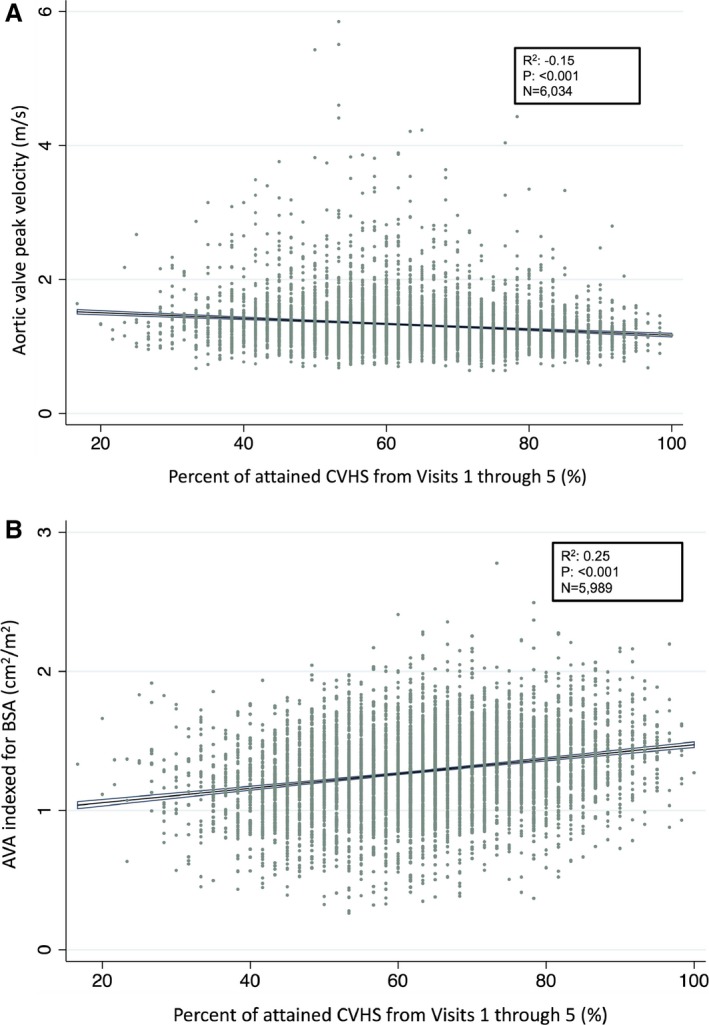
Continuous relationship between percentage attained cardiovascular health score (CVHS) from midlife to late life and late life aortic valve peak velocity (A) and aortic valve area (AVA; B). BSA indicates body surface area.
Figure 3.
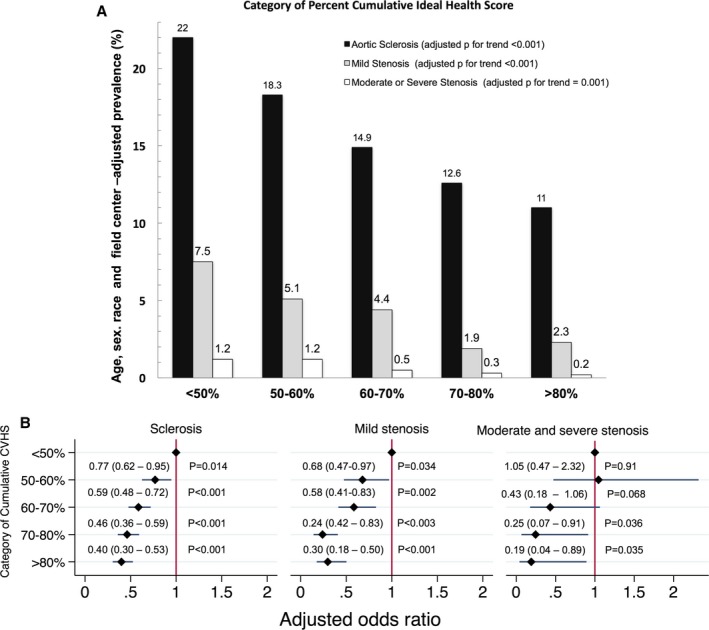
Relationship between categories on the basis of attained cardiovascular health score (CVHS) in midlife through late life and presence of aortic valve dysfunction in late life. A, Prevalence of aortic sclerosis, mild stenosis, and moderate‐to‐severe stenosis in late life (visit 5) among categories on the basis of percentage attained CVHS from midlife to late life (visits 1 through 5). B, Odds of aortic sclerosis, mild stenosis, and moderate‐to‐severe stenosis in late life (visit 5) among categories of percentage attained CVHS relative to the lowest category of attained CVHS (<50%). P for trend across categories is adjusted for age, sex, race, and field center. Odds ratios are adjusted for age, sex, race, and field center.
Table 3.
Prevalence and Odds of AV Dysfunction (Sclerosis, Mild Stenosis, or Moderate‐to‐Severe Stenosis) Associated With Each Component Metric of the CVHS
| Metric | <50% | ≥50% to <60% | ≥60% to <70% | ≥70% to <80% | ≥80% | P Value for Trend | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | Prevalence | OR (95% CI) | ||
| Smoking status | 21.3 (18.4–24.2 | Reference | 21.9 (12.8–30.9) | 0.96 (0.54–1.7) | 21.2 (15.1–27.4) | 0.86 (57 –1.32) | 20.8 (14.7–27) | 0.85 (0.56–1.31) | 20.8 (19.6–21.9) | 0.87 (0.72–1.06) | 0.17 |
| BMI | 25.9 (24.2–27.7) | Reference | 21.2 (18.7–23.8) | 0.81 (0.67–0.92) | 18.9 (15.5–22.4) | 0.70 (0.55–0.89) | 17.5 (13.6–21.5) | 0.66 (0.49–0.89) | 14.8 (13.1–16.5) | 0.55 (0.46–0.66) | <0.001 |
| Physical activity | 22.3 (20.5–24.1) | Reference | 22 (19.2 –24.7) | 1.03 (0.85–1.25) | 19.8 (16.7–23) | 0.92 (0.74–1.16) | 19.9 (17–22.8) | 0.95 (0.77–1.18) | 19.2 (17.3–21.2) | 1.01 (0.85–1.20) | 0.83 |
| Total cholesterol | 23.8 (21.8–25.8) | Reference | 18.8 (16.1 –21.4) | 0.73 (0.60–0.91) | 21.2 (18.1–24.2) | 0.87 (0.70–1.09) | 19.9 (16.8–22.9) | 0.80 (0.64–1.02) | 19.4 (17.8–21.1) | 0.81 (0.69–0.95) | 0.031 |
| Blood pressure | 26.2 (24.1–28.3) | Reference | 23.2 (20.5–25.8) | 0.82 (0.68 –0.99) | 23.1 (19.9–26.4) | 0.88 (0.71–1.10) | 18.3 (15.5–21.1) | 0.67 (0.53–0.83) | 14.8 (13.2–16.4) | 0.54 (0.45–0.65) | <0.001 |
| Fasting glucose | 25.3 (22.6–28) | Reference | 23.4 (20.5–26.3) | 0.96 (0.77–1.20) | 21.3 (18.5–24.2) | 0.89 (0.71–1.12) | 21.1 (18.4–23.9) | 0.92 (0.74–0.1.16) | 18.2 (16.7–19.7) | 0.89 (0.74–1.08) | 0.27 |
Age, sex, and race‐adjusted prevalences are given as means and values in parentheses are 95% confidence intervals. The P value for trend is calculated from a logistic regression model with CVHS category as the primary exposure variable and adjusted for age, sex, race, and percentage attainment of each of the other health metrics. Logistic regression models used to calculate the OR associated with each CVHS category adjusted for age, sex, race, and percentage attainment of each of the other health metrics. AV indicates aortic valve; BMI, body mass index; CI, confidence interval; CVHS, cardiovascular health score; and OR, odds ratio.
Percentage attained CVHS and age had additive effects on the likelihood of having AV disease (Figure 4A and 4B, respectively). For example, participants <75 years of age with a mean age of 72 years and <50% attained CVHS demonstrated similar prevalences of AV peak velocity >1.5 and >2.0 m/s as participants >80 years of age with a mean age of 83 years with >80% attained CVHS (24.5% and 19.8%, respectively, for AV peak velocity >1.5 m/s [P=0.20]; 6.2% and 4.9%, respectively, for AV peak velocity >1.5 m/s [P=0.60]). For each health metric, with the exception of smoking, greater percentage achieved ideal status was significantly associated with a lower prevalence of AV dysfunction (sclerosis and mild and moderate stenosis) in models adjusted for age, sex, and race. After further adjusting for all other health metrics, greater percentage achieved ideal status for BMI, blood pressure, and total cholesterol, each remained significantly associated with a lower prevalence of AV dysfunction (Table 3, Tables S3 through S5).
Figure 4.
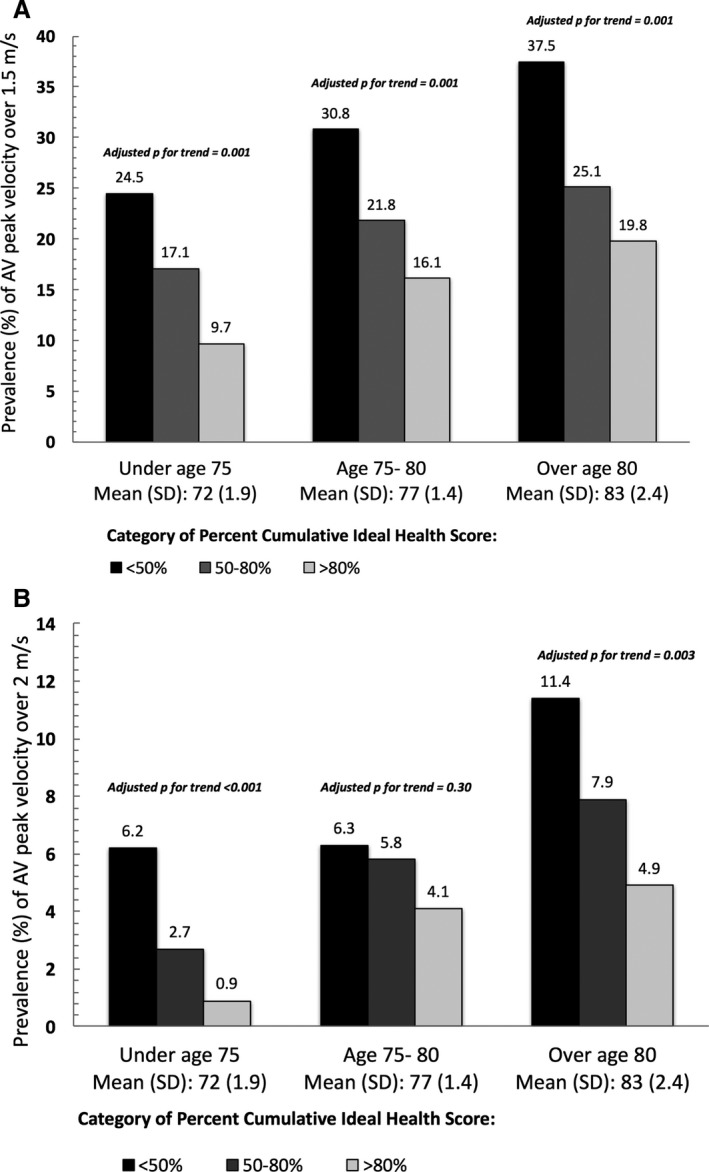
Prevalence of late‐life (visit 5) aortic valve (AV) peak velocity over 1.5 m/s (A) and 2.0 m/s (B), on the basis of category of percentage attained cardiovascular health score through midlife to late life (from visits 1 through 5) and participant age at visit 5.
Trajectories of CVHS from midlife to late life were also significantly related to the prevalence of AV dysfunction in late life (Figure 5). Five CVHS trajectories have been previously defined,18 with trajectories 1 through 4 demonstrating progressively lower baseline CVHS, and each characterized by a decline in CVHS over time (Figure 5A). As expected, the prevalence of aortic sclerosis and stenosis was lowest in trajectory 1 and increased progressively to the highest levels in trajectory 4 (Figure 5B). Trajectory 5 began with a low CVHS, similar to trajectory 3, but demonstrated improvement in score over time, with a CVHS similar to trajectory 2 at visit 5 (Figure 5A). Although the prevalence of aortic sclerosis in trajectory 5 was similar to that of trajectory 3, the prevalence of AS more closely approximated that of trajectory 2 (Figure 5B).
Figure 5.
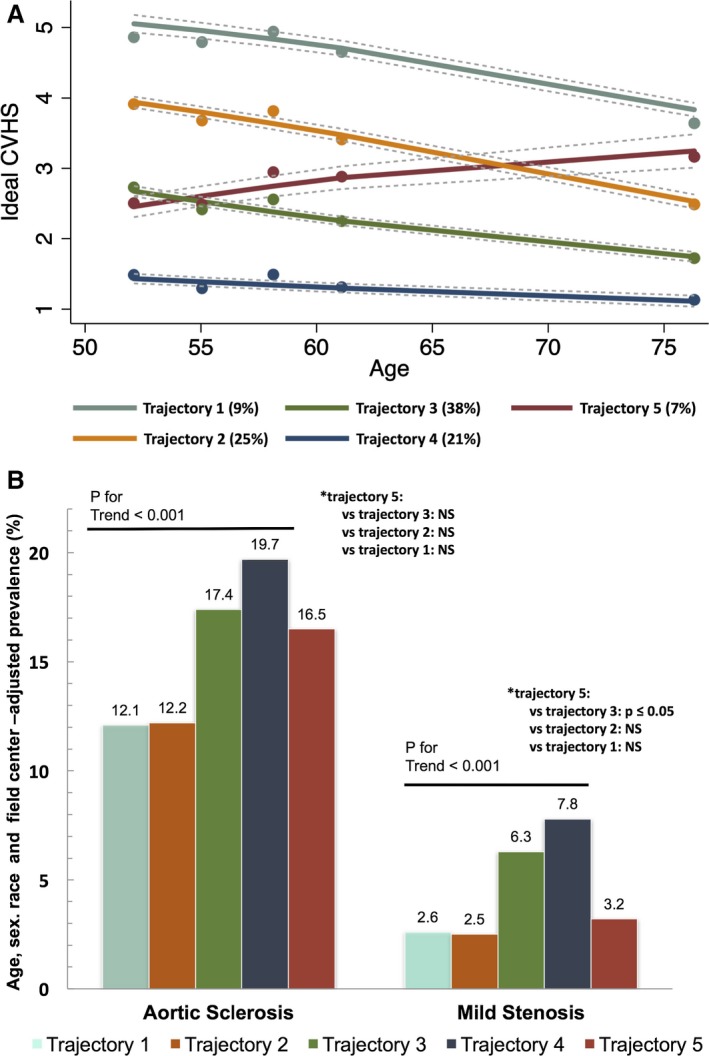
Trajectories of cardiovascular health score (CVHS) and aortic valve dysfunction. A, Trajectories of percentage cumulative CVHS in the ARIC (Atherosclerosis Risk in Communities) Study. Percentages in the figure legend refer to population prevalence. B, Prevalence of aortic sclerosis and mild or greater stenosis by trajectory of CVHS. Prevalence estimates and P values are adjusted for age, sex, race, and field center. NS indicates not significant. Reprinted from Shah et al18 with permission. Copyright© 2015, American Heart Association, Inc.
Influence of Race and Sex
Male participants demonstrated a higher prevalence of aortic sclerosis and stenosis compared with female participants (22.2% versus 19.9%; P=0.036), but sex did not modify the relationship between percentage attained CVHS and AS grade (Table S6, Figures S7A and S8A; P=0.13, 0.07, and 0.28 for sclerosis, mild, and moderate to severe stenosis, respectively, for interaction). Black participants tended to have a lower prevalence of AV disease than white participants. Again, no effect modification by race was observed on the association between percentage attained CVHS and AS grade (Table S7, Figures S7B and S8B; P=0.9, 0.2, and 0.21 for sclerosis, mild, and moderate to severe stenosis, respectively, for interaction). In addition, no interaction of sex and race was found by basing the analysis on the calculated AVA (Tables S8 and S9).
Discussion
This study is one of the first, to our knowledge, to examine the relationship of attainment of ideal cardiovascular health metrics through adult life, as summarized in the CVHS, with the prevalence and severity of AS in a large cohort of older adults from the general population. A unique strength of this analysis is the use of quantitative echocardiographic data of AV function. This allowed us to determine the association of CVHS with not only moderate and severe AS, but also lesser degrees of AV dysfunction, including sclerosis and mild stenosis. Greater attainment of ideal cardiovascular health in midlife to late life was associated with a lower prevalence of aortic sclerosis and stenosis in late life. This association was not modified by sex or race.
Moderate or severe valvular heart disease affects ≈2.5% of the US population and increases in prevalence with age to nearly 12% of people ≥75 years old.24 With the growing elderly population, the prevalence is expected to increase further. Indeed, recent projections from The OxVALVE population cohort study25 suggest that the number of elderly people with moderate or severe valvular heart disease in the United Kingdom alone will more than double by 2056, from 1.5 to 3.3 million people. AS is the most prevalent valvular disease among older adults in the United States, many of whom are asymptomatic but have a heightened risk of mortality and rapid disease progression.26, 27 Our estimate of the prevalence of aortic sclerosis or stenosis of 20.9% is similar to other cohorts in elderly individuals.28 More important, both sclerosis and stenosis in elderly individuals have been associated with greater risk of mortality and incident cardiovascular events.4
Multiple studies support the presence of shared pathologic pathways between calcific AV disease and atherosclerosis.29 Calcific AV disease and atherosclerosis share common risk factors, such as age, smoking, hypertension, hypercholesterolemia, diabetes mellitus, and metabolic syndrome.1, 30, 31 The presence of AV calcification is associated with coronary disease reflected in coronary artery calcification.32 Smoking, higher BMI, higher low‐density lipoprotein cholesterol levels, and the rate of progression in coronary artery calcification all predict the development of AV calcification.32 Traditional cardiovascular risk assessed in midlife predicts the presence and extent of AV calcification by cardiac computed tomography 3 decades later.33 These cardiovascular risk factors also predict the progression of calcific AV disease and the prevalence of AS.1, 34 Recently, results from the CANHEART (Cardiovascular Health in Ambulatory Care Research Team) study from 1.12 million individuals showed that cardiovascular risk factors, including hypertension, diabetes mellitus, and dyslipidemia, were associated with incident severe AS, with a dose‐response relationship noted between risk factors in incidence of severe AS.35 Our findings of association between worse AV disease with higher BMI, hypertension, diabetes mellitus, prior smoking, and prevalent coronary heart disease are concordant with these data, extending these findings to AV dysfunction of lesser severity, before clinical intervention is warranted. However, although the association between traditional cardiovascular risk factors and AV calcification, hemodynamic obstruction, and severe stenosis is well described, few studies have addressed the implications of achieving specific targets for cardiovascular health factors and behaviors. The CVHS integrates information about the extent to which optimal targets for key health behaviors and factors associated with atherosclerotic risk are achieved.
Beyond risk of cardiovascular disease, worse CVHS has been associated with higher circulating levels of biomarkers of inflammation, endothelial dysfunction, and neurohormonal activation,18, 36, 37, 38, 39 pathways that have also been implicated in the development and progression of AV sclerosis and calcification.8, 40, 41 Lipoproteins and inflammatory cells, such as macrophages and T cells,42 have been identified in early AV lesions,43 and inflammatory activity, visualized by positron emission tomography imaging, has been documented in patients with even mild stenosis.44 This lipid deposition has also been associated with increased angiotensin‐converting enzyme activity in the valve, with profibrotic effects.45 Furthermore, valvular interstitial cells can exhibit osteoblast‐like activity, thus leading to calcific deposition and valvular calcification over time.46 This process of lipid deposition, inflammation, and calcification results in a dynamic lifelong remodeling process,47 which motivates the hypothesis that it is amenable to influence by improving cardiovascular health risk factors.
Our finding that better achievement of ideal cardiovascular health metrics through midlife to late life predicts a lower prevalence of AS and sclerosis in late life is particularly relevant given the growing public health burden of AS related to the aging population. The emerging importance of AV stenosis at the population level is highlighted by a recent meta‐analysis and simulation study that estimated that 290 000 people >75 years of age in Europe and North America are candidates for transcatheter AV replacement, with 27 000 new candidates annually.48 These findings argue forcefully for efforts to prevent progression of AV disease to hemodynamic significance in elderly individuals. Although several studies suggested potential for treatment with hydroxymethylglutaryl–coenzyme A reductase inhibitors (statins) in this regard,49, 50 randomized clinical trials have failed to demonstrate efficacy of statin therapy to delay disease progression, assessed by either AV peak velocity or clinical outcomes, among people with mild‐to‐moderate AS. One potential explanation for the negative findings in these studies is the relatively advanced stage of disease process at the time of intervention. However, given the long time period over which calcific AV disease develops and the relatively low incidence of significant stenosis, a primary prevention intervention trial is likely not feasible. Indeed, there is no medical therapy available for primary or secondary prevention of calcific AV disease or AS.21 More important, in our analysis, AV disease prevalence was associated with CVHS assessed over ≈25 years. Our findings suggest that up to 31% of AV dysfunction (sclerosis or stenosis) could be prevented if the percentage attained CVHS through midlife to late life were uniformly >70%. Furthermore, our analysis of the relationship between trajectories of CVHS through midlife to late life and late life AV dysfunction demonstrate that worse CVHS trajectory is associated with a greater prevalence of AV dysfunction. More important, we also found that improvement in CVHS through midlife to late life (trajectory 5) was associated with a late‐life prevalence of aortic sclerosis similar to people with similar baseline CVHS who continued to decline (trajectory 3) but a significantly lower prevalence of AS, which more closely approximated the prevalence of AS in participants with a higher CVHS through most of midlife to late life (trajectory 2). These findings suggest that, although midlife CVHS is associated with progressive valve dysfunction, improvement in CVHS through late life may predict a lower prevalence of stenosis in late life. Although these findings are observational, they suggest that consistent attainment of ideal cardiovascular health metrics through midlife to late life has the potential to significantly affect the prevalence of calcific AV disease in late life, extending the importance of ideal cardiovascular health metrics to the primary prevention of AV disease.
Several limitations of this study should be considered. This was an observational cohort study and, therefore, causality cannot be established. However, we believe these observational data are particularly informative because clinical trial data on the relationship between lifetime attainment of cardiovascular health goals and late‐life valvular disease are unlikely to be forthcoming because of both ethical considerations and feasibility. In addition, the relatively few participants with moderate or severe AS limits the precision of these prevalence estimates. Missing data for cardiovascular health metrics at each visit were uncommon, but were imputed from adjacent visits, which could have resulted in misclassification of CVHS.
Because serial data on diet were not available, ideal diet was not considered in determining the CVHS, although it is included in the ideal cardiovascular health metrics, as defined by the American College of Cardiology/American Heart Association. Survivor bias may influence the observed associations, because 32% of ARIC Study participants had died by the time of visit 5. In addition, of participants alive at the start of visit 5, 38% declined to participate, possibly resulting in selection bias. However, sensitivity analyses using inverse probability attrition weighting for visit 5 nonattendance showed consistent results in the association between CVHS category and the prevalence of AS (Figure S9, Table S10). AV disease was assessed by echocardiography in this analysis, using definitions that are concordant with the American College of Cardiology/American Heart Association.21 Direct quantification of AV calcification (eg, by use of cardiac computed tomography) was not available.
Conclusions
Greater attainment of ideal cardiovascular health in midlife to late life is associated with a lower prevalence of aortic sclerosis and stenosis in late life in a large elderly cohort from the general population. These findings extend the importance of attaining ideal cardiovascular health metrics to the primary prevention of AV disease.
Sources of Funding
The ARIC (Atherosclerosis Risk in Communities) Study is performed as a collaborative study, supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The work for this manuscript was also supported by National Heart, Lung, and Blood Institute grants K08HL116792 and R01HL135008 (Shah), American Heart Association grant 14CRP20380422 (Shah), Watkins Discovery Award from the Brigham and Women's Heart and Vascular Center (Shah), Novo Nordisk Research Foundation grant number NNF15OC0017456 (Sengeløv), and in part by R01‐HL131532, R01‐HL134168, and a grant from the Ellison Foundation (Cheng).
Disclosures
None.
Supporting information
Table S1. The Prevalence of Missing Data for Each Component of the CVHS at Each ARIC Visit
Table S2. Prevalence of Aortic Stenosis, Defined Using the Peak Aortic Valve Velocity, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 Including Confidence Intervals
Table S3. Prevalence and Odds of AV Sclerosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S4. Prevalence and Odds of Mild Stenosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S5. Prevalence and Odds of Moderate and Severe Stenosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S6. Prevalence of Aortic Stenosis Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Sex
Table S7. Prevalence of Aortic Stenosis Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Race
Table S8. Prevalence of Aortic Stenosis, Defined Using the Calculated AVA, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Sex
Table S9. Prevalence of Aortic Stenosis, Defined Using the Calculated AVA, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Race
Table S10. Prevalence of Aortic Stenosis, Defined Using the AV Peak Velocity, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 Including Confidence Intervals and Utilizing Inverse Probability Attrition Weighting
Figure S1. Distribution of aortic valve area among ARIC participants at visit 5.
Figure S2. Prevalence of aortic stenosis based on AS category, defined using the calculated AVA, and category of percent attained CVHS from visit 1 through 5.
Figure S3. Prevalence of AS, defined using the AV peak velocity, based on category of percent attained CVHS from visit 1 through 5 excluding participants with coronary heart disease, atrial fibrillation, heart failure, stroke and eGFR <60 mL/min per 1.73 m2.
Figure S4. Prevalence of AS, defined using the calculated AVA, based on category of percent attained CVHS from visit 1 through 5 excluding participants with coronary heart disease, atrial fibrillation, heart failure, stroke and eGFR <60 mL/min per 1.73 m2.
Figure S5. Population attributable fraction for aortic valve dysfunction (sclerosis or stenosis) associated with a percent attained CVHS of <50%, <60%, <70%, and <80%.
Figure S6. Population attributable fraction for AV dysfunction (sclerosis or stenosis) associated with a percent attained CVHS through visit 4 of <50%, <60%, <70%, and <80%.
Figure S7. Histograms of (A) AV peak velocity and (B) AV area at ARIC visit 5 by sex and race.
Figure S8. Scatter plots of AV peak velocity and percent attained CVHS with fitted linear regression lines by (A) sex and (B) race.
Figure S9. Prevalence of AS, defined using the AV peak velocity, based on category of percent attained CVHS from visit 1 through 5 utilizing inverse probability of attrition weighting.
Acknowledgments
We thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) Study for their important contributions.
(J Am Heart Assoc. 2018;7:e007234 DOI: 10.1161/JAHA.117.007234.)29431107
References
- 1. Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. [DOI] [PubMed] [Google Scholar]
- 2. Varadarajan P, Kapoor N, Bansal R, Pai R. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: results from a cohort of 277 patients aged ≥80 years. Eur J Cardiothorac Surg. 2006;30:722–727. [DOI] [PubMed] [Google Scholar]
- 3. Kojodjojo P, Gohil N, Barker D, Youssefi P, Salukhe TV, Choong A, Koa‐Wing M, Bayliss J, Hackett DR, Khan MA. Outcomes of elderly patients aged 80 and over with symptomatic, severe aortic stenosis: impact of patient's choice of refusing aortic valve replacement on survival. QJM. 2008;101:567–573. [DOI] [PubMed] [Google Scholar]
- 4. Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic‐valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. [DOI] [PubMed] [Google Scholar]
- 5. Cosmi JE, Kort S, Tunick PA, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. The risk of the development of aortic stenosis in patients with “benign” aortic valve thickening. Arch Intern Med. 2002;162:2345–2347. [DOI] [PubMed] [Google Scholar]
- 6. Agmon Y, Khandheria BK, Meissner I, Sicks JD, O'Fallon WM, Wiebers DO, Whisnant JP, Seward JB, Tajik AJ. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? J Am Coll Cardiol. 2001;38:827–834. [DOI] [PubMed] [Google Scholar]
- 7. Pohle K, Mäffert R, Ropers D, Moshage W, Stilianakis N, Daniel WG, Achenbach S. Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation. 2001;104:1927–1932. [DOI] [PubMed] [Google Scholar]
- 8. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis. J Am Coll Cardiol. 2015;66:561–577. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 10. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, Zhao H, Ruan C, Wu Y, Xin A, Li K, Jin C, Cai J. Prevalence of ideal cardiovascular health and its relationship with the 4‐year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes. 2012;5:487–493. [DOI] [PubMed] [Google Scholar]
- 14. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ogagarue ER, Lutsey PL, Klein R, Klein BE, Folsom AR. Association of ideal cardiovascular health metrics and retinal microvascular findings: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2013;2:e000430 DOI: 10.1161/JAHA.113.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rasmussen‐Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk in Communities study. Circulation. 2013;127:1270–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 20. Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community‐dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 22. Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR, Liu K, Lloyd‐Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health. 1998;88:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet. 2006;368:1005–1011. [DOI] [PubMed] [Google Scholar]
- 25. Coffey S, d'Arcy JL, Loudon MA, Mant D, Farmer AJ, Prendergast BD; OxVALVE‐PCS Group . The OxVALVE population cohort study (OxVALVE‐PCS)‐population screening for undiagnosed valvular heart disease in the elderly: study design and objectives. Open Heart. 2014;1:e000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenhek R. Mild and moderate aortic stenosis: natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. [DOI] [PubMed] [Google Scholar]
- 27. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. [DOI] [PubMed] [Google Scholar]
- 28. Aronow WS, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am J Cardiol. 1991;67:776–777. [DOI] [PubMed] [Google Scholar]
- 29. Prasad Y, Bhalodkar NC. Aortic sclerosis: a marker of coronary atherosclerosis. Clin Cardiol. 2004;27:671–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ngo MV, Gottdiener JS, Fletcher RD, Fernicola DJ, Gersh BJ. Smoking and obesity are associated with the progression of aortic stenosis. Am J Geriatr Cardiol. 2001;10:86–90. [DOI] [PubMed] [Google Scholar]
- 31. Kamalesh M, Ng C, El Masry H, Eckert G, Sawada S. Does diabetes accelerate progression of calcific aortic stenosis? Eur J Echocardiogr. 2009;10:723–725. [DOI] [PubMed] [Google Scholar]
- 32. Messika‐Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez‐Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. [DOI] [PubMed] [Google Scholar]
- 33. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O'Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg‐Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang S‐J, Liu Y, Sjogren M, van der Pals J, Kälsch H, Mühleisen TW, Nöthen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O'Donnell CJ, Post WS. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Cardiol. 2010;105:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan AT, Koh M, Chan KK, Guo H, Alter DA, Austin PC, Tu JV, Wijeysundera HC, Ko DT. Association between cardiovascular risk factors and aortic stenosis. J Am Coll Cardiol. 2017;69:1523–1532. [DOI] [PubMed] [Google Scholar]
- 36. Pahkala K, Hietalampi H, Laitinen TT, Viikari JSA, Ronnemaa T, Niinikoski H, Lagstrom H, Talvia S, Jula A, Heinonen OJ, Juonala M, Simell O, Raitakari OT. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima‐media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation. 2013;127:2088–2096. [DOI] [PubMed] [Google Scholar]
- 37. Spring B, Moller AC, Colangelo LA, Siddique J, Roehrig M, Daviglus ML, Polak JF, Reis JP, Sidney S, Liu K. Healthy lifestyle change and subclinical atherosclerosis in young adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2014;130:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 39. Loprinzi PD, Branscum A, Hanks J, Smit E. Healthy lifestyle characteristics and their joint association with cardiovascular disease biomarkers in US adults. Mayo Clin Proc. 2016;91:432–442. [DOI] [PubMed] [Google Scholar]
- 40. Bossé Y, Mathieu P, Pibarot P. Genomics: the next step to elucidate the etiology of calcific aortic valve stenosis. J Am Coll Cardiol. 2008;51:1327–1336. [DOI] [PubMed] [Google Scholar]
- 41. New SEP, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res. 2011;108:1381–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Otto CM, Kuusisto J, Reichenbach DD, Gown AM, O'Brien KD. Characterization of the early lesion of “degenerative” valvular aortic stenosis: histological and immunohistochemical studies. Circulation. 1994;90:844–853. [DOI] [PubMed] [Google Scholar]
- 43. O'Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of “degenerative” valvular aortic stenosis. Arterioscler Thromb Vasc Biol. 1996;16:523–532. [DOI] [PubMed] [Google Scholar]
- 44. Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, Marsden M, Pessotto R, Clark JC, Wallace WA, Salter DM, McKillop G, van Beek EJR, Boon NA, Rudd JHF, Newby DE. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation. 2012;125:76–86. [DOI] [PubMed] [Google Scholar]
- 45. O'Brien KD. Association of angiotensin‐converting enzyme with low‐density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. [DOI] [PubMed] [Google Scholar]
- 46. Merryman WD, Schoen FJ. Mechanisms of calcification in aortic valve disease: role of mechanokinetics and mechanodynamics. Curr Cardiol Rep. 2013;15:355 http://link.springer.com/10.1007/s11886-013-0355-5. Accessed April 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rajamannan NM, Evans FJ, Aikawa E, Grande‐Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group * executive summary: calcific aortic valve disease—2011 update. Circulation. 2011;124:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Osnabrugge RLJ, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJJC, Piazza N, Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta‐analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 49. Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation. 2001;104:2205–2209. [DOI] [PubMed] [Google Scholar]
- 50. Rosenhek R. Statins but not angiotensin‐converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The Prevalence of Missing Data for Each Component of the CVHS at Each ARIC Visit
Table S2. Prevalence of Aortic Stenosis, Defined Using the Peak Aortic Valve Velocity, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 Including Confidence Intervals
Table S3. Prevalence and Odds of AV Sclerosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S4. Prevalence and Odds of Mild Stenosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S5. Prevalence and Odds of Moderate and Severe Stenosis, Defined Using the AV Peak Velocity, Associated With Each Component Metric of the CVHS
Table S6. Prevalence of Aortic Stenosis Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Sex
Table S7. Prevalence of Aortic Stenosis Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Race
Table S8. Prevalence of Aortic Stenosis, Defined Using the Calculated AVA, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Sex
Table S9. Prevalence of Aortic Stenosis, Defined Using the Calculated AVA, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 for Race
Table S10. Prevalence of Aortic Stenosis, Defined Using the AV Peak Velocity, Based on Category of Percent Cumulative CVHS Attained From Visit 1 Through 5 Including Confidence Intervals and Utilizing Inverse Probability Attrition Weighting
Figure S1. Distribution of aortic valve area among ARIC participants at visit 5.
Figure S2. Prevalence of aortic stenosis based on AS category, defined using the calculated AVA, and category of percent attained CVHS from visit 1 through 5.
Figure S3. Prevalence of AS, defined using the AV peak velocity, based on category of percent attained CVHS from visit 1 through 5 excluding participants with coronary heart disease, atrial fibrillation, heart failure, stroke and eGFR <60 mL/min per 1.73 m2.
Figure S4. Prevalence of AS, defined using the calculated AVA, based on category of percent attained CVHS from visit 1 through 5 excluding participants with coronary heart disease, atrial fibrillation, heart failure, stroke and eGFR <60 mL/min per 1.73 m2.
Figure S5. Population attributable fraction for aortic valve dysfunction (sclerosis or stenosis) associated with a percent attained CVHS of <50%, <60%, <70%, and <80%.
Figure S6. Population attributable fraction for AV dysfunction (sclerosis or stenosis) associated with a percent attained CVHS through visit 4 of <50%, <60%, <70%, and <80%.
Figure S7. Histograms of (A) AV peak velocity and (B) AV area at ARIC visit 5 by sex and race.
Figure S8. Scatter plots of AV peak velocity and percent attained CVHS with fitted linear regression lines by (A) sex and (B) race.
Figure S9. Prevalence of AS, defined using the AV peak velocity, based on category of percent attained CVHS from visit 1 through 5 utilizing inverse probability of attrition weighting.


