Abstract
Background
We examined the cardiovascular risk of abatacept compared with tumor necrosis factor (TNF) inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus (DM).
Methods and Results
We conducted a cohort study of patients with rheumatoid arthritis who newly started abatacept or TNF inhibitors using claims data from Medicare and MarketScan. The primary outcome was a composite cardiovascular end point of myocardial infarction (MI), stroke/transient ischemic attack, and coronary revascularization. To account for >60 baseline characteristics, abatacept initiators were 1:1 propensity score (PS) matched to TNF initiators in each database. Cox proportional hazards models estimated hazard ratio (HR) and 95% confidence interval (CI) in the PS‐matched cohort per database. A fixed‐effects meta‐analysis pooled database‐specific HRs. We included a total of 13 039 PS‐matched pairs of abatacept and TNF inhibitor initiators (6103 pairs in Medicare and 6936 pairs in MarketScan). A total of 34.7% in Medicare and 19.8% in MarketScan had baseline DM. The HR (95% CI) for the primary outcome associated with abatacept use versus TNF inhibitor was 0.81 (0.66–0.99) in Medicare and 0.95 (0.74–1.23) in MarketScan, with a pooled HR of 0.86 (95% CI, 0.73–1.01; P=0.3 for heterogeneity). The risk of the primary outcome was lower in abatacept initiators versus TNF inhibitors in the DM subgroup, with a pooled HR of 0.74 (95% CI, 0.57–0.96; P=0.7 for heterogeneity), but not in the non‐DM subgroup, with a pooled HR of 0.94 (95% CI, 0.77–1.14; P=0.4 for heterogeneity).
Conclusions
In this large population‐based cohort of patients with rheumatoid arthritis, abatacept use appeared to be associated with a modestly reduced cardiovascular risk when compared with TNF inhibitor use, particularly in patients with DM.
Keywords: cardiovascular disease, comparative effectiveness research, diabetes mellitus, rheumatoid arthritis, treatment
Subject Categories: Clinical Studies, Cardiovascular Disease, Pharmacology
Clinical Perspective
What Is New?
Despite comparable efficacy to control rheumatoid arthritis, use of abatacept was associated with a reduced cardiovascular risk compared with use of tumor necrosis factor inhibitors, particularly in diabetic patients.
What Are the Clinical Implications?
Certain subsets of patients with rheumatoid arthritis with underlying cardiovascular risk factors might benefit from the use of abatacept over tumor necrosis factor inhibitor with regard to their cardiovascular risk.
Patients with rheumatoid arthritis (RA) are at high risk of developing cardiovascular disease (CVD).1, 2, 3, 4, 5, 6, 7, 8, 9 The magnitude of cardiovascular risk in patients with RA is reported to be at least comparable to that in diabetic patients, both doubling the risk as compared with the general population.4 This increased risk in patients with RA is not fully explained by baseline traditional CVD risk factors. Several studies report that systemic inflammatory burden is a major independent risk factor for CVD in patients with RA.5, 6, 7, 8, 9 Epidemiologic studies report that the prevalence of insulin resistance is associated with RA activity or severity.10, 11, 12 Biological evidence further shows that the key inflammatory cytokines in RA provide the link between inflammation and intermediary metabolic outcomes, such insulin resistance or dyslipidemia.13 Consequently, patients with RA with sustained disease activity are prone to develop metabolic syndrome, which ultimately leads to premature atherosclerosis.8, 10, 11 Patients with RA with coexisting diabetes mellitus (DM), therefore, would constitute a high cardiovascular risk subset calling for particular attention.
Disease‐modifying antirheumatic drugs (DMARDs) that control systemic inflammation may reduce the cardiovascular risk in patients with RA. In particular, the effect of biologic DMARDs on cardiovascular risk has been a clinically important topic of interest on the basis of their potent anti‐inflammatory effect in RA. The use of tumor necrosis factor (TNF) inhibitors is currently thought to be associated with a reduced risk of CVD compared with use of nonbiologic DMARD treatment in RA.14, 15 Few studies have conducted a head‐to‐head comparison of different biologic DMARDs. In a recent cohort study of elderly patients with RA, use of abatacept was associated with fewer coronary heart disease events compared with use of TNF inhibitors.16 However, to date, little is known with regard to comparative cardiovascular safety of abatacept and TNF inhibitors in different patient populations, such as younger or healthier populations, or in high cardiovascular risk subsets, such as patients with RA with underlying DM.
The objective of the present study was to compare cardiovascular risk in patients with RA who were newly initiated on abatacept versus TNF inhibitors in a population‐representative cohort using data from both public and commercial insurance claims databases in the United States. We also aimed to study the comparative cardiovascular safety of these biologic agents in patients with RA stratified by the presence of DM at baseline. Abatacept and TNF inhibitors are interchangeably used to treat active RA,17, 18 which strengthens study validity by minimizing confounding by indication associated with disease duration or severity.
Methods
Data Sources
Using longitudinal data from 2 large US healthcare claims databases (Medicare [Parts A/B/D 2008–2013] and Truven “MarketScan” [January 2006–June 30, 2015]), we conducted a cohort study of patients with RA who had been initiated on either abatacept or TNF inhibitors. Medicare is a federally funded health plan that provides healthcare coverage for nearly all adults aged ≥65 years and some disabled patients aged <65 years in the United States. Medicare Part A is generally for inpatient care; Part B is for outpatient medical services, including some drugs given in a physician's office or clinic; and Part D is for outpatient prescription drug coverage.19 The MarketScan database contains longitudinal medical and pharmacy claims from several different managed care plans, representing a national commercially insured population in the United States. The Institutional Review Board of the Brigham and Women's Hospital approved the study protocol and privacy precautions. Because of the data use agreement with the Centers for Medicare and Medicaid Services and with Truven Health, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Cohort
Eligible patients were those aged 18 years and older, having at least 2 International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes of RA (code 714.x) given on separate visits ≥7 days apart.20 Of these patients, we identified new users of either abatacept or TNF inhibitors (ie, adalimumab, certolizumab, etanercept, golimumab, and infliximab). The new users were defined as those who had no prior dispensing of abatacept or TNF inhibitors for at least 12 months before the index date. The index date was defined as the date of first dispensing abatacept or a TNF inhibitor. Past or current use of nonbiologic DMARDs was allowed, which included methotrexate, hydroxychloroquine, leflunomide, azathioprine, cyclophosphamide, cyclosporine, d‐penicillamine, gold, and sulfasalazine. Patients who used anakinra, rituximab, tocilizumab, or tofacitinib in the 1 year before the index period were excluded. Patients were allowed to contribute multiple episodes to the study cohort as long as each episode met the study enrollment criteria.
For the primary as‐treated analysis, follow‐up time started the day after the index date and continued until the earliest date among the following censoring events: discontinuation of TNF inhibitors for those in the TNF inhibitor group (switching to a different TNF inhibitor was allowed), discontinuation of abatacept for those in the abatacept group, outcome occurrence, insurance disenrollment, end of study database, or death. Censoring attributable to discontinuation of the study medication occurred at the last drug available date, defined as the last dispensing date plus days’ supply of a given drug regardless of the gap between exhaustion of the days’ supply of the previous dispensing and the next dispensing. To estimate the drug adherence in the 2 treatment groups, we calculated proportions of days covered, defined as the sum of days’ supply during follow‐up time/the follow‐up time of a given patient.
Outcome Definition
The primary outcome of interest was a composite cardiovascular end point of MI, coronary revascularization, and stroke/transient ischemic attack (TIA). The date of composite cardiovascular outcome was defined by the first occurrence of any of the 3 components: MI, stroke/TIA, or coronary revascularization. MI and stroke/TIA were identified using an inpatient ICD‐9 diagnosis code of MI (codes 410.x0 and 410.x1) in any position and stroke/TIA (codes 430, 431, 433.x1, 434.x1, 435, 436, and 362.3) in primary position. Coronary revascularization was identified using ICD‐9 procedure codes, Current Procedural Terminology‐5 codes, or diagnosis‐related group codes.
The secondary outcomes included each component of the composite cardiovascular end point, heart failure (HF), and venous thromboembolism (VTE), consisting of deep venous thrombosis and pulmonary embolism. Any inpatient ICD‐9 diagnosis code (code 428.xx) was used to identify HF. To identify VTE, we used any inpatient ICD‐9 diagnosis code for deep vein thrombosis (code 451.1x) or pulmonary embolism (code 415.1x) combined with at least 1 outpatient pharmacy claims for anticoagulants. In prior studies, the positive predictive values of these algorithms to identify each CVD outcome were at least 80%.21, 22, 23, 24, 25
Baseline Covariates
We assessed variables potentially associated with RA severity and risk of CVD, HF, or VTE, on the basis of the data from the 12‐month period before the index date. These variables were cohort entry year, demographics, traditional risk factors for CVD (ie, hypertension, dyslipidemia, chronic kidney disease, peripheral vascular disease, smoking, and obesity), comorbidities, RA‐related medications (eg, DMARDs, NSAIDs, and steroids), other medications, markers of healthcare use intensity, and the use of laboratory or other diagnostic tests (Table 1). Existing CVD conditions (including coronary heart disease encompassing acute and old MI, acute coronary syndrome, stable angina, and other chronic ischemic heart disease and stroke/TIA), HF, and VTE were also included as baseline covariates. To further assess potential differences in comorbidities between the 2 treatment groups, we used a Charlson and Deyo comorbidity score on the basis of 17 comorbidity categories.26
Table 1.
Baseline Characteristics of Study Cohort Before PS Matching
| Characteristics | DM Subgroup | Non‐DM Subgroup | ||||||
|---|---|---|---|---|---|---|---|---|
| Medicare | MarketScan | Medicare | MarketScan | |||||
| Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | |
| (n=2122) | (n=9142) | (n=1377) | (n=11 057) | (n=3985) | (n=16 650) | (n=5565) | (n=54 407) | |
| Demographics | ||||||||
| Age, y | 73.6±6.3 | 72.5±6.1 | 60.3±11.4 | 56.8±11.4 | 73.9±6.4 | 72.7±6.2 | 56.1±13.2 | 51.9±13.0 |
| Female sex | 80.4 | 77.6 | 80.1 | 71.7 | 84.5 | 79.3 | 82.6 | 75.8 |
| Race | ||||||||
| Black | 9.9 | 11.6 | ··· | ··· | 4.8 | 6.4 | ··· | ··· |
| White | 79.5 | 76.3 | ··· | ··· | 89.1 | 87.0 | ··· | ··· |
| Others | 10.7 | 12.1 | ··· | ··· | 6.1 | 6.6 | ··· | ··· |
| RA medications | ||||||||
| Hydroxychloroquine | 27.2 | 22.2 | 22.4 | 23.1 | 29.1 | 24.3 | 21.2 | 24.1 |
| Methotrexate | 52.1 | 59.1 | 37.8 | 56.5 | 55.1 | 62.6 | 35.8 | 56.7 |
| Leflunomide | 19.5 | 15.5 | 14.7 | 13.0 | 20.4 | 15.9 | 13.6 | 11.6 |
| Other DMARD | 19.6 | 15.4 | 11.3 | 14.1 | 18.6 | 16.4 | 13.0 | 14.3 |
| No. of DMARDs | 1.2±0.9 | 1.1±0.8 | 0.9±0.9 | 1.1±0.8 | 1.2±0.8 | 1.2±0.8 | 0.8±0.9 | 1.1±0.8 |
| Glucocorticoids | ||||||||
| Inhaled glucocorticoids | 24.9 | 27.0 | 20.0 | 20.9 | 21.5 | 22.0 | 14.1 | 17.2 |
| Oral glucocorticoids (≤30 d) | 34.4 | 30.7 | 21.1 | 24.9 | 34.8 | 32.6 | 20.4 | 25.8 |
| Oral glucocorticoid (≤1 y) | 71.9 | 68.5 | 53.2 | 62.2 | 74.0 | 71.7 | 49.1 | 62.4 |
| Cumulative dose, mga | 1264±1468 | 1139±1522 | 1113±3564 | 1198±7597 | 1152±1316 | 1118±1356 | 932±3550 | 1126±15 071 |
| Analgesics | ||||||||
| NSAIDs | 40.3 | 45.5 | 33.1 | 47.8 | 37.2 | 41.5 | 31.2 | 48.0 |
| Celecoxib | 11.3 | 12.1 | 8.8 | 10.7 | 10.4 | 11.2 | 7.9 | 9.8 |
| Opioids | 39.0 | 36.5 | 24.8 | 28.3 | 32.3 | 30.0 | 19.3 | 21.7 |
| Baseline CVD | ||||||||
| Coronary heart disease | 42.5 | 37.8 | 21.9 | 15.2 | 25.8 | 22.9 | 9.9 | 5.7 |
| Stroke | 9.1 | 8.3 | 3.9 | 3.2 | 6.2 | 5.8 | 2.4 | 1.3 |
| PVD | 22.3 | 20.4 | 7.0 | 5.1 | 13.0 | 11.1 | 3.0 | 1.7 |
| Traditional CVD risk factors | ||||||||
| Smoking | 17.2 | 16.2 | 9.3 | 11.1 | 13.9 | 15.5 | 9.4 | 10.0 |
| Hypertension | 89.6 | 89.6 | 67.0 | 59.9 | 75.4 | 71.9 | 38.6 | 31.8 |
| Hyperlipidemia | 81.5 | 80.3 | 56.3 | 50.9 | 62.8 | 60.2 | 28.8 | 25.6 |
| Obesity | 31.3 | 31.9 | 19.6 | 17.9 | 16.4 | 15.4 | 9.2 | 7.8 |
| Chronic kidney disease | 22.0 | 18.0 | 9.9 | 6.6 | 10.79 | 8.7 | 3.4 | 1.9 |
| DM complications | ||||||||
| DM nephropathy | 7.5 | 6.4 | 4.0 | 3.4 | ··· | ··· | ··· | ··· |
| DM neuropathy | 19.8 | 19.4 | 14.2 | 9.5 | ··· | ··· | ··· | ··· |
| DM retinopathy | 11.2 | 11.4 | 7.7 | 7.1 | ··· | ··· | ··· | ··· |
| Diabetic foot | 10.4 | 8.0 | 4.8 | 3.3 | ··· | ··· | ··· | ··· |
| Comorbidities | ||||||||
| VTE | 8.8 | 6.0 | 5.1 | 2.9 | 5.9 | 4.1 | 2.9 | 1.6 |
| Atrial fibrillation | 15.3 | 13.0 | 6.8 | 4.0 | 13.4 | 8.9 | 3.8 | 1.9 |
| Heart failure | 26.0 | 19.5 | 11.0 | 5.2 | 12.8 | 9.0 | 3.8 | 1.3 |
| Asthma | 17.0 | 16.8 | 15.3 | 11.2 | 12.5 | 11.7 | 9.0 | 7.6 |
| COPD | 26.7 | 26.9 | 11.8 | 9.2 | 20.6 | 20.5 | 7.9 | 5.2 |
| Chronic liver disease | 12.6 | 12.1 | 9.4 | 7.9 | 7.9 | 7.5 | 5.1 | 4.3 |
| Hepatitis | 1.9 | 2.2 | 1.1 | 1.2 | 1.1 | 1.6 | 0.9 | 1.1 |
| Hypothyroidism | 38.3 | 33.8 | 22.2 | 20.7 | 31.9 | 27.9 | 16.3 | 13.7 |
| Depression | 24.1 | 22.5 | 15.3 | 13.5 | 18.7 | 17.9 | 13.2 | 11.8 |
| Fracture | 15.1 | 12.9 | 9.3 | 6.9 | 13.9 | 11.4 | 6.5 | 4.5 |
| Malignancy | 18.1 | 14.9 | 11.1 | 7.3 | 16.3 | 14.5 | 8.8 | 5.2 |
| Medications | ||||||||
| Cardiovascular drugs | ||||||||
| ACEIs/ARBs | 61.6 | 62.8 | 41.2 | 49.7 | 43.5 | 41.7 | 19.3 | 20.5 |
| β‐Blockers | 43.0 | 40.8 | 25.8 | 24.5 | 36.1 | 32.7 | 14.7 | 13.4 |
| Calcium channel blockers | 33.2 | 34.6 | 20.0 | 20.1 | 26.1 | 25.7 | 10.5 | 10.1 |
| Nitrates | 12.3 | 11.2 | 6.7 | 4.3 | 6.7 | 5.2 | 1.6 | 1.6 |
| Antiarrhythmics | 3.7 | 2.3 | 1.0 | 0.9 | 3.0 | 1.8 | 0.9 | 0.5 |
| Anticoagulants | 14.5 | 11.4 | 8.6 | 6.3 | 11.8 | 8.9 | 4.8 | 3.2 |
| Antiplatelets | 15.7 | 14.0 | 6.8 | 6.3 | 8.7 | 7.4 | 3.0 | 2.5 |
| Statins | 57.7 | 57.1 | 38.4 | 42.9 | 37.1 | 37.0 | 14.6 | 16.3 |
| Other antilipid drugs | 12.4 | 12.4 | 12.6 | 12.2 | 7.2 | 7.1 | 4.1 | 4.3 |
| Loop diuretics | 32.9 | 29.3 | 17.7 | 14.1 | 19.8 | 15.7 | 7.0 | 5.1 |
| Thiazide | 34.5 | 35.1 | 23.0 | 29.5 | 27.3 | 29.6 | 14.3 | 16.3 |
| Other diuretics | 11.3 | 9.6 | 7.9 | 7.8 | 8.7 | 8.0 | 5.0 | 4.7 |
| DM medication | ||||||||
| Insulin | 28.6 | 29.0 | 26.8 | 32.5 | ··· | ··· | ··· | ··· |
| Metformin | 31.6 | 37.0 | 30.1 | 41.2 | ··· | ··· | ··· | ··· |
| Sulfonylureas | 21.0 | 24.1 | 16.6 | 18.1 | ··· | ··· | ··· | ··· |
| Thiazolidinediones | 5.7 | 7.6 | 5.5 | 8.3 | ··· | ··· | ··· | ··· |
| DPP4 inhibitors | 7.0 | 8.4 | 7.2 | 8.8 | ··· | ··· | ··· | ··· |
| Bronchodilators | ||||||||
| Inhaled LABA | 10.4 | 11.7 | 6.4 | 7.1 | 8.2 | 8.1 | 4.5 | 4.8 |
| Inhaled SABA | 17.3 | 18.6 | 15.0 | 15.3 | 12.9 | 13.8 | 9.8 | 11.0 |
| Others | ||||||||
| Benzodiazepines | 5.9 | 6.1 | 19.9 | 22.1 | 6.6 | 5.4 | 16.1 | 17.8 |
| Bisphosphonates | 21.3 | 21.5 | 9.2 | 8.0 | 24.0 | 23.1 | 10.0 | 7.8 |
| PPIs | 52.6 | 51.2 | 33.3 | 35.9 | 43.1 | 41.6 | 24.5 | 25.8 |
| H1 blocker | 15.7 | 16.3 | 17.4 | 20.7 | 11.8 | 12.1 | 12.9 | 16.7 |
| H2 blocker | 7.5 | 10.3 | 3.0 | 4.9 | 6.9 | 7.2 | 3.2 | 3.2 |
| No. of unique generics | 17.7±7.3 | 17.4±7.7 | 13.9±10.7 | 15.5±8.6 | 13.2±6.0 | 12.6±6.2 | 9.0±8.4 | 10.3±7.0 |
| Healthcare use during preindex period | ||||||||
| Tests ever ordered | ||||||||
| Hemoglobin A1C | 58.9 | 59.5 | 57.6 | 62.2 | 8.0 | 7.4 | 8.8 | 9.1 |
| ESR | 71.1 | 64.8 | 63.3 | 69.7 | 67.0 | 61.8 | 65.4 | 71.4 |
| C‐reactive protein | 61.3 | 55.8 | 53.2 | 61.0 | 56.9 | 52.9 | 56.5 | 62.7 |
| Serum creatinine | 25.5 | 22.5 | 25.1 | 27.0 | 27.4 | 24.2 | 25.8 | 26.7 |
| Lipid/cholesterol panel | 59.1 | 58.8 | 52.9 | 59.7 | 44.5 | 42.2 | 35.4 | 38.5 |
| ECG | 63.7 | 59.3 | 50.5 | 43.3 | 51.3 | 47.2 | 35.3 | 29.0 |
| Echocardiogram | 37.3 | 33.0 | 26.1 | 19.2 | 27.3 | 23.2 | 16.3 | 10.6 |
| Pulmonary function test | 21.1 | 19.8 | 22.7 | 17.7 | 17.1 | 14.8 | 15.9 | 12.2 |
| No. of physician visits | 19.8±10.7 | 18.2±10.5 | 17.5±10.2 | 15.2±8.7 | 16.3±9.4 | 14.8±9.0 | 13.2±8.3 | 11.5±7.3 |
| No. of ED visits | 1.5±4.6 | 1.2±2.8 | 1.1±2.1 | 0.8±2.0 | 0.8±1.5 | 0.8±2.0 | 0.6±1.4 | 0.5±1.4 |
| Any hospitalization | 37.2 | 32.2 | 26.1 | 20.3 | 26.2 | 22.6 | 16.7 | 12.3 |
| Recent hospitalization | 2.0 | 2.7 | 1.2 | 1.6 | 1.3 | 1.5 | 0.8 | 0.8 |
Variables showing the frequency of <5% are not shown: alcohol, GLP, glucagon‐like peptide; 1 receptor agonists, α‐glucosidase inhibitors, theophylline, and inhaled anticholinergics. Continuous variables are presented as mean±SD, and binary variables are presented as percentages. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; DMARD, disease‐modifying antirheumatic drug; DPP4, dipeptidyl peptidase 4; ED, emergency department; ESR, erythrocyte sediment rate; GLP, glucagon‐like peptide; LABA, long‐acting β2 agonist; PPI, proton pump inhibitor; PS, propensity score; PVD, peripheral vascular disease; RA, rheumatoid arthritis; SABA, short‐acting β2 agonist; TNF, tumor necrosis factor; and VTE, venous thromboembolism.
Cumulative dose during the 365 days before the index date was calculated by summing up the prednisone equivalent doses of glucocorticoid compounds used.
Statistical Analysis
For each database, we compared baseline characteristics of the abatacept and TNF inhibitor groups. PS matching was used to control for >60 potential confounders between the 2 groups. To estimate the PS, we used multivariable logistic regression analysis that included all of baseline variables listed in Table 1 plus the index year. Because of the anticipated differences between the Medicare and commercial insurance populations, we estimated the PS per database and performed nearest neighbor matching at 1:1 ratio using a caliper of 0.025 on the PS scale within each subgroup (DM and non‐DM subgroups) per database. The DM and non‐DM subgroups were then merged together to create the main PS‐matched cohort in each database (Figure 1). The achieved PS balance within each database was inspected by tabulating baseline patient characteristics by treatment status and by examining the standardized differences.
Figure 1.
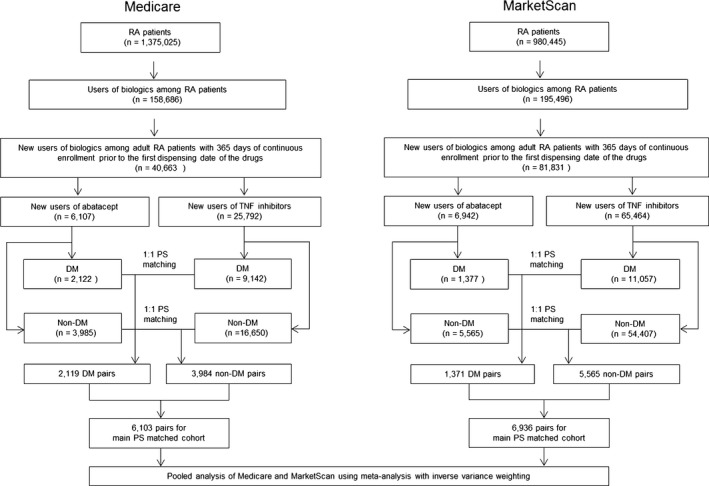
Study cohort selection process. In each of the 2 databases, the propensity score (PS) matching was done in the diabetes mellitus (DM) and non‐DM subgroups separately first, and the 2 subgroups were merged to create the main PS‐matched cohort. RA indicates rheumatoid arthritis; and TNF, tumor necrosis factor.
After PS matching, incidence rates of primary and secondary outcomes were calculated in the main cohort and in the subgroups with or without DM in each database. Cox proportional hazards models estimated the database‐specific hazard ratio (HR) with 95% confidence interval (CI) for the primary and secondary outcomes associated with initiation of abatacept versus TNF inhibitors. We tested the proportional hazards assumption using the interaction term between exposure and follow‐up time, and the assumption was not violated in any of the models.27 Kaplan‐Meier plots were also used to inspect proportionality of hazards, and the follow‐up time between treatment groups was compared in each database. HRs from the 2 databases were then pooled by an inverse variance–weighted fixed‐effects model. All analyses were completed using SAS 9.4 (SAS Institute) software.
Results
Cohort Selection and Patient Characteristics
Figure 1 illustrates the study cohort selection process. After applying the inclusion and exclusion criteria, we identified a total of 104 305 patients with RA who had been newly initiated on either abatacept or TNF inhibitors from the 2 databases. Approximately 35% of Medicare and 17% of MarketScan enrollees had DM at baseline. Before PS matching, the Medicare cohort showed higher rates for most comorbidities associated with high cardiovascular risk compared with the MarketScan cohort in both subgroups. Similarly, DM subgroups showed higher rates for these comorbidities than non‐DM subgroups in both databases (Table 1).
After 1:1 PS matching, we identified a total of 13 039 pairs of abatacept and TNF inhibitor initiators from the 2 databases (6103 pairs in Medicare and 6936 pairs in MarketScan). Of those pairs, 2119 (34.7%) in Medicare and 1371 (19.8%) in MarketScan were identified as the DM subgroup, whereas 3984 (65.3%) from Medicare and 5565 (80.2%) from MarketScan were included in the non‐DM subgroup. All baseline covariates, including cardiovascular risk factors (eg, hypertension, hyperlipidemia, and previous cardiovascular events), were well balanced in the PS matched cohorts, with the standardized difference of covariate prevalence <0.1 (Table 2). During the follow‐up, mean proportions of days covered were significantly higher in abatacept users than TNF inhibitor users in the PS‐matched cohorts across the 2 databases (Table 3).
Table 2.
Baseline Characteristics of 1:1 PS‐Matched Cohorts
| Characteristics | DM Subgroup | Non‐DM Subgroup | ||||||
|---|---|---|---|---|---|---|---|---|
| Medicare | MarketScan | Medicare | MarketScan | |||||
| Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | |
| (n=2119) | (n=2119) | (n=1371) | (n=1371) | (n=3984) | (n=3984) | (n=5565) | (n=5565) | |
| Demographics | ||||||||
| Age, y | 73.5±6.3 | 73.4±6.3 | 60.2±11.4 | 60.0±11.8 | 73.9±6.4 | 73.9±6.5 | 56.1±13.2 | 56.1±13.6 |
| Female sex | 80.4 | 79.9 | 80.0 | 79.1 | 84.5 | 84.4 | 82.6 | 81.9 |
| Race | ||||||||
| Black | 9.9 | 10.4 | ··· | ··· | 4.8 | 4.9 | ··· | ··· |
| White | 79.5 | 79.0 | ··· | ··· | 89.1 | 88.7 | ··· | ··· |
| Others | 10.7 | 10.6 | ··· | ··· | 6.1 | 6.4 | ··· | ··· |
| Nonbiological DMARDs | ||||||||
| Hydroxychloroquine | 27.2 | 21.7 | 22.5 | 19.8 | 29.1 | 24.5 | 21.2 | 20.5 |
| Methotrexate | 52.2 | 58.6 | 37.9 | 50.0 | 55.2 | 61.0 | 35.8 | 49.0 |
| Leflunomide | 19.4 | 15.6 | 14.7 | 12.8 | 20.4 | 17.1 | 13.6 | 10.9 |
| Other DMARDs | 19.6 | 14.4 | 11.4 | 11.5 | 18.6 | 16.9 | 13.0 | 12.4 |
| No. of DMARDs | 1.2±0.9 | 1.1±0.8 | 0.9±0.8 | 0.9±0.9 | 1.2±0.8 | 1.2±0.8 | 0.9±0.9 | 0.8±0.9 |
| Glucocorticoids | ||||||||
| Inhaled glucocorticoids | 24.9 | 27.8 | 20.1 | 17.9 | 21.5 | 23.2 | 14.1 | 16.0 |
| Oral glucocorticoids (≤30 d) | 34.4 | 30.2 | 21.2 | 20.6 | 34.8 | 32.6 | 20.4 | 22.5 |
| Oral glucocorticoids (≤1 y) | 71.9 | 68.9 | 53.5 | 53.8 | 74.0 | 73.4 | 49.1 | 53.8 |
| Cumulative dose, mga | 1264±1469 | 1156±1529 | 1118±3571 | 920±2077 | 1151±1315 | 1168±1431 | 932±3550 | 947±3298 |
| Analgesics | ||||||||
| NSAIDs | 40.4 | 41.1 | 33.3 | 32.1 | 37.2 | 36.7 | 31.2 | 30.4 |
| Celecoxib | 11.3 | 10.5 | 8.8 | 9.3 | 10.4 | 10.7 | 7.9 | 7.4 |
| Opioids | 39.0 | 38.6 | 25.0 | 24.9 | 32.3 | 31.9 | 19.3 | 17.9 |
| Baseline CVD | ||||||||
| Coronary heart disease | 42.4 | 43.6 | 21.6 | 21.3 | 25.8 | 25.9 | 9.9 | 10.6 |
| Stroke | 9.0 | 8.6 | 3.7 | 3.7 | 6.2 | 6.7 | 2.4 | 2.7 |
| PVD | 22.3 | 21.4 | 6.9 | 6.4 | 12.9 | 12.5 | 3.0 | 3.3 |
| Traditional CVD risk factors | ||||||||
| Smoking | 17.1 | 17.4 | 9.3 | 9.3 | 13.9 | 14.5 | 9.4 | 9.0 |
| Hypertension | 89.6 | 90.7 | 66.8 | 66.0 | 75.4 | 75.4 | 38.6 | 38.5 |
| Hyperlipidemia | 81.5 | 81.6 | 56.1 | 54.3 | 62.8 | 62.9 | 28.8 | 28.3 |
| Obesity | 31.3 | 34.4 | 19.5 | 18.4 | 16.4 | 15.6 | 9.2 | 8.9 |
| Chronic kidney disease | 21.9 | 22.8 | 9.7 | 10.5 | 10.8 | 10.0 | 3.4 | 3.5 |
| DM complications | ||||||||
| DM nephropathy | 7.5 | 7.5 | 4.0 | 3.9 | ··· | ··· | ··· | ··· |
| DM neuropathy | 19.9 | 18.3 | 13.9 | 15.0 | ··· | ··· | ··· | ··· |
| DM retinopathy | 11.2 | 11.0 | 7.7 | 7.6 | ··· | ··· | ··· | ··· |
| Diabetic foot | 10.0 | 10.2 | 4.8 | 4.8 | ··· | ··· | ··· | ··· |
| Comorbidities | ||||||||
| VTE | 8.7 | 8.8 | 5.0 | 5.3 | 5.9 | 6.2 | 2.9 | 2.9 |
| Atrial fibrillation | 15.2 | 14.4 | 6.7 | 7.1 | 13.4 | 13.1 | 3.8 | 3.7 |
| Heart failure | 25.9 | 25.6 | 10.7 | 11.2 | 12.8 | 12.4 | 3.8 | 3.8 |
| Asthma | 16.9 | 19.5 | 15.1 | 14.2 | 12.5 | 11.8 | 9.0 | 9.3 |
| COPD | 26.7 | 30.3 | 11.7 | 13.8 | 20.6 | 21.8 | 7.9 | 7.7 |
| Chronic liver disease | 12.6 | 12.8 | 9.4 | 8.8 | 7.9 | 8.0 | 5.1 | 4.8 |
| Hypothyroidism | 38.3 | 36.7 | 22.3 | 23.0 | 31.9 | 29.5 | 16.3 | 15.3 |
| Depression | 24.0 | 23.5 | 15.3 | 14.7 | 18.7 | 19.2 | 13.2 | 11.8 |
| Fracture | 15.1 | 15.4 | 9.3 | 8.2 | 13.9 | 12.3 | 6.5 | 6.2 |
| Malignancy | 18.1 | 17.0 | 11.1 | 9.8 | 16.3 | 16.8 | 8.8 | 9.2 |
| Medications | ||||||||
| Cardiovascular drugs | ||||||||
| ACEIs/ARBs | 61.6 | 62.2 | 41.4 | 39.8 | 43.5 | 43.2 | 19.3 | 18.9 |
| β‐Blockers | 43.0 | 42.5 | 25.8 | 26.2 | 36.1 | 36.6 | 14.7 | 15.2 |
| Calcium channel blockers | 33.1 | 33.7 | 20.1 | 20.4 | 26.1 | 25.8 | 10.5 | 10.6 |
| Nitrates | 12.3 | 13.3 | 6.6 | 6.7 | 6.7 | 6.7 | 1.6 | 1.8 |
| Antiarrhythmics | 3.6 | 3.8 | 1.0 | 1.1 | 3.0 | 3.3 | 0.9 | 1.0 |
| Anticoagulants | 14.4 | 15.1 | 8.5 | 9.1 | 11.8 | 11.9 | 4.8 | 5.0 |
| Antiplatelets | 15.6 | 16.5 | 6.8 | 6.1 | 8.7 | 9.1 | 3.0 | 3.3 |
| Statins | 57.6 | 58.2 | 38.5 | 38.2 | 37.1 | 37.6 | 14.6 | 15.4 |
| Other antilipid drugs | 12.4 | 12.7 | 12.6 | 13.2 | 7.2 | 6.9 | 4.1 | 4.1 |
| Loop diuretics | 32.8 | 32.6 | 17.7 | 18.2 | 19.8 | 18.7 | 7.0 | 7.6 |
| Thiazide | 34.5 | 33.9 | 23.1 | 21.9 | 27.3 | 26.7 | 14.3 | 14.5 |
| Other diuretics | 11.3 | 11.0 | 8.0 | 6.3 | 8.7 | 8.6 | 5.0 | 5.1 |
| DM medication | ||||||||
| Insulin | 28.7 | 27.8 | 26.8 | 26.7 | ··· | ··· | ··· | ··· |
| Metformin | 31.6 | 30.3 | 30.3 | 31.5 | ··· | ··· | ··· | ··· |
| Sulfonylureas | 21.0 | 20.2 | 16.6 | 17.7 | ··· | ··· | ··· | ··· |
| Thiazolidinediones | 5.7 | 5.0 | 5.5 | 6.0 | ··· | ··· | ··· | ··· |
| DPP4 inhibitors | 7.0 | 7.4 | 7.2 | 7.1 | ··· | ··· | ··· | ··· |
| Bronchodilators | ||||||||
| Inhaled LABA | 10.4 | 12.3 | 6.4 | 6.6 | 8.2 | 8.8 | 4.5 | 5.1 |
| Inhaled SABA | 17.3 | 17.8 | 15.1 | 14.5 | 12.9 | 14.1 | 9.8 | 9.8 |
| Others | ||||||||
| Benzodiazepines | 6.0 | 5.4 | 19.9 | 20.5 | 6.6 | 6.7 | 16.1 | 16.7 |
| Bisphosphonates | 21.2 | 20.9 | 9.2 | 8.7 | 24.0 | 24.1 | 10.0 | 10.6 |
| PPIs | 52.6 | 52.5 | 33.4 | 31.5 | 43.1 | 43.4 | 24.5 | 24.9 |
| H1 blocker | 15.7 | 15.6 | 17.4 | 18.5 | 11.9 | 10.9 | 12.9 | 13.2 |
| H2 blocker | 7.5 | 8.4 | 3.0 | 3.2 | 6.9 | 7.3 | 3.2 | 3.5 |
| No. of unique generics | 17.7±7.3 | 17.6±7.5 | 13.9±10.7 | 13.8±9.5 | 13.2±6.0 | 13.2±6.4 | 9.0±8.4 | 9.1±7.3 |
| Healthcare use | ||||||||
| Tests ever ordered | ||||||||
| Hemoglobin A1C | 58.9 | 59.4 | 57.6 | 57.2 | 8.0 | 7.6 | 8.8 | 8.8 |
| ESR | 71.1 | 67.6 | 63.4 | 64.4 | 67.0 | 64.9 | 65.4 | 65.2 |
| C‐reactive protein | 61.2 | 61.2 | 53.4 | 54.2 | 56.9 | 57.2 | 56.5 | 56.4 |
| Serum creatinine | 25.5 | 24.7 | 25.2 | 24.0 | 27.4 | 27.4 | 25.8 | 25.3 |
| Lipid/cholesterol panel | 59.1 | 59.7 | 53.1 | 52.4 | 44.6 | 45.1 | 35.4 | 34.3 |
| ECG ever ordered | 63.6 | 64.2 | 50.3 | 48.7 | 51.3 | 51.1 | 35.3 | 35.3 |
| Echocardiogram | 37.3 | 37.9 | 25.9 | 25.6 | 27.2 | 26.3 | 16.3 | 16.5 |
| Pulmonary function test | 21.1 | 21.1 | 22.5 | 21.1 | 17.0 | 15.7 | 15.9 | 15.0 |
| No. of physician visits | 19.8±10.6 | 19.9±11.3 | 17.4±10.0 | 17.6±11.2 | 16.3±9.3 | 16.3±9.8 | 13.2±8.3 | 13.1±8.9 |
| No. of ED visits | 1.5±4.6 | 1.4±2.8 | 1.0±2.1 | 1.1±2.3 | 0.8±1.5 | 0.8±1.8 | 0.6±1.4 | 0.6±1.6 |
| Any hospitalization | 37.1 | 37.6 | 25.8 | 27.1 | 26.2 | 26.5 | 16.7 | 16.4 |
| Recent hospitalization | 2.0 | 2.0 | 1.2 | 1.8 | 1.3 | 1.5 | 0.8 | 1.0 |
Variables showing the frequency of <5% are not shown: alcohol, GLP, glucagon‐like peptide; 1 receptor agonists, α‐glucosidase inhibitors, theophylline, and inhaled anticholinergics. Continuous variables are presented as mean±SD, and binary variables are presented as percentages. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; DMARD, disease‐modifying antirheumatic drug; DPP4, dipeptidyl peptidase 4; ED, emergency department; ESR, erythrocyte sediment rate; GLP, glucagon‐like peptide; LABA, long‐acting β2 agonist; PPI, proton pump inhibitor; PS, propensity score; PVD, peripheral vascular disease; SABA, short‐acting β2 agonist; TNF, tumor necrosis factor; and VTE, venous thromboembolism.
Cumulative dose during the 365 days before the index date was calculated by summing up the prednisone equivalent doses of glucocorticoid compounds used.
Table 3.
Follow‐Up Times and PDCs of PS‐Matched Cohorts by Baseline DM Status and Database
| Variable | DM Subgroup | Non‐DM Subgroup | ||||||
|---|---|---|---|---|---|---|---|---|
| Medicare | MarketScan | Medicare | MarketScan | |||||
| Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | Abatacept | TNF Inhibitor | |
| (n=2119) | (n=2119) | (n=1371) | (n=1371) | (n=3984) | (n=3984) | (n=5565) | (n=5565) | |
| Follow‐up time, d | 423±409 | 393±409 | 415±488 | 412±487 | 483±456 | 424±432 | 438±501 | 478±540 |
| PDCa | 0.73±0.26 | 0.58±0.32 | 0.66±0.29 | 0.48±0.32 | 0.78±0.22 | 0.62±0.33 | 0.65±0.30 | 0.46±0.32 |
Data are given as mean±SD. DM indicates diabetes mellitus; PDC, proportion of days covered; PS, propensity score; and TNF, tumor necrosis factor.
Each comparison of mean PDCs between abatacept and TNF inhibitor users was statistically significant.
Cardiovascular Risk Associated With the Use of Abatacept
During the total of 31 733 person‐years of follow‐up in the PS‐matched cohorts from the 2 databases, 299 abatacept initiators and 333 TNF inhibitor initiators had the composite cardiovascular events. The incidence rate of the composite cardiovascular outcome was 2.38 per 100 person‐years for abatacept initiators and 2.97 per 100 person‐years for TNF inhibitors in Medicare. In MarketScan, the incidence rate of the composite cardiovascular outcome was 1.38 per 100 person‐years for abatacept initiators and 1.45 per 100 person‐years for TNF inhibitor initiators (Table 4). The risk of the composite cardiovascular outcome was lower in abatacept initiators versus TNF inhibitor in Medicare, with the HR (95% CI) of 0.81 (0.66–0.99), but not in MarketScan, with the HR (95% CI) of 0.95 (0.74–1.23). When pooling the 2 database‐specific HRs, the risk of the composite cardiovascular events in abatacept initiators was numerically, not statistically significantly, lower compared with TNF inhibitors, with the pooled HR (95% CI) of 0.86 (0.73–1.01; P=0.34 for heterogeneity). Similarly, composite cardiovascular event‐free survival curves diverged from the beginning of follow‐up (Figure 2A) in the Medicare database, whereas there was no curve separation between the 2 treatments in the MarketScan database (Figure 2B).
Table 4.
Incidence Rates and HRs of Composite Cardiovascular End Point in Abatacept Initiators Versus TNF Inhibitor Initiators: PS‐Matched Analysis
| Subgroup | Database | After PS Matching | |||
|---|---|---|---|---|---|
| Events | Incidence Ratea | HR (95% CI) | Pooled HR (95% CI) | ||
| Main cohort | Medicare | 185 | 2.38 | 0.81 (0.66–0.99) | 0.86 (0.73–1.01) |
| 205 | 2.97 | 1 | ··· | ||
| MarketScan | 114 | 1.38 | 0.95 (0.74–1.23) | ··· | |
| 128 | 1.45 | 1 | ··· | ||
| DM subgroup | Medicare | 71 | 2.85 | 0.72 (0.53–0.99) | 0.74 (0.57–0.96) |
| 90 | 3.95 | 1 | ··· | ||
| MarketScan | 35 | 2.24 | 0.79 (0.50–1.25) | ··· | |
| 44 | 2.85 | 1 | ··· | ||
| Non‐DM subgroup | Medicare | 114 | 2.16 | 0.88 (0.67–1.14) | 0.94 (0.77–1.15) |
| 115 | 2.49 | 1 | ··· | ||
| MarketScan | 79 | 1.18 | 1.03 (0.76–1.40) | ··· | |
| 84 | 1.15 | 1 | ··· | ||
CI indicates confidence interval; DM, diabetes mellitus; HR, hazard ratio; PS, propensity score; and TNF, tumor necrosis factor.
Per 100 person‐years.
Figure 2.
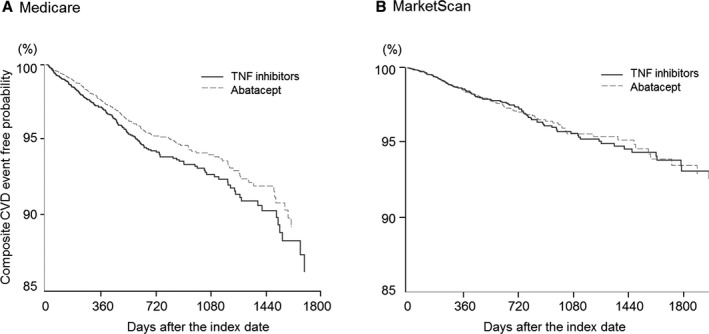
Composite cardiovascular disease (CVD) event‐free survival curve in the main propensity score–matched cohort of the 2 data sources (Medicare [A] and MarketScan [B]). TNF indicates tumor necrosis factor.
Subgroup Analysis by Baseline DM
In the subgroup with DM (Table 4), the HR (95% CI) for composite cardiovascular end point comparing abatacept versus TNF inhibitor initiators was 0.72 (0.53–0.99) in Medicare and 0.79 (0.50–1.25) in MarketScan, with a pooled HR (95% CI) of 0.74 (0.57–0.96; P=0.7 for heterogeneity). In the subgroup without DM (Table 4), the HR (95% CI) was 0.88 (0.67–1.14) in Medicare and 1.03 (0.76–1.40) in MarketScan, with a pooled HR (95% CI) of 0.94 (0.77–1.14; P=0.4 for heterogeneity).
Stratified Analysis by DM and Age
To further delineate the age and DM effect on the cardiovascular risk associated with abatacept versus TNF inhibitors, we stratified patients in MarketScan by their age (Table 5) (the old [≥65 years] and the young [<65 years]) and their baseline DM status. The old/DM subset showed the highest incidence rate of CVD events, and the PS‐matched HR (95% CI) was 0.74 (0.39–1.40). In the young/DM subset, the HR (95% CI) was 1.42 (0.70–2.86). In the non‐DM subgroup, the HR (95% CI) was 1.09 (0.71–1.67) in the old and 1.02 (0.65–1.61) in the young.
Table 5.
Incidence Rates and HRs of Composite Cardiovascular End Point in Abatacept Initiators Versus TNF Inhibitor Initiators in Elderly and Younger Age Groups in the MarketScan Database
| Subgroup | Database | After PS Matching | |||
|---|---|---|---|---|---|
| Exposure | Events | Incidence Ratea | HR (95% CI) | ||
| DM subgroup | ≥65 y (n=439 for each group) | Abatacept | 16 | 3.18 | 0.74 (0.39–1.40) |
| TNF inhibitor | 22 | 4.31 | 1 | ||
| <65 y (n=930 for each group) | Abatacept | 19 | 1.80 | 1.42 (0.70–2.86) | |
| TNF inhibitor | 14 | 1.30 | 1 | ||
| Non‐DM subgroup | ≥65 y (n=1295 for each group) | Abatacept | 42 | 2.71 | 1.09 (0.71–1.67) |
| TNF inhibitor | 44 | 2.47 | 1 | ||
| <65 y (n=4267 for each group) | Abatacept | 36 | 0.70 | 1.02 (0.65–1.61) | |
| TNF inhibitor | 39 | 0.68 | 1 | ||
CI indicates confidence interval; DM, diabetes mellitus; HR, hazard ratio; PS, propensity score; and TNF, tumor necrosis factor.
Per 100 person‐years.
We also performed a Cox regression analysis in the main cohort of each database to test interaction between abatacept treatment and the presence of DM but did not find any statistically significant interaction (P>0.05 for the interaction term) in either database.
Secondary Analysis
Our secondary analyses showed similar findings. There was an association between a lower risk for MI (pooled HR [95% CI]=0.77 [0.59–1.00]; P=0.3 for heterogeneity) and coronary revascularization (pooled HR [95% CI]=0.74 [0.57–0.97]; P=1.0 for heterogeneity) among abatacept initiators compared with TNF inhibitors in the main cohort (Figure 3A). The risk for the other secondary outcomes (stroke/TIA, HF, and VTE) was not different between the 2 groups in the main cohort and DM and non‐DM subgroups (Figure 3A through 3C). However, there was a trend toward an increased risk for VTE associated with abatacept versus TNF inhibitors in the MarketScan cohort.
Figure 3.
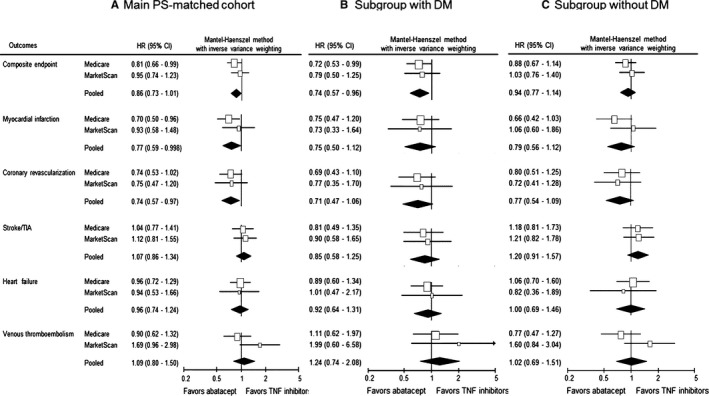
Comparative risk of secondary cardiovascular outcomes between abatacept vs tumor necrosis factor (TNF) inhibitor initiators. A, The main propensity score (PS)–matched cohort, which combines subgroups with diabetes mellitus (DM; B) and without DM (C). CI indicates confidence interval; HR, hazard ratio; and TIA, transient ischemic attack.
Discussion
In this large cohort study pooling data from 2 large national public and commercial health plans in the United States, abatacept use appears to be associated with a modestly reduced risk of composite cardiovascular event, albeit statistically not significant compared with TNF inhibitor use. There was a decreased risk for MI and coronary revascularization in abatacept initiators versus TNF inhibitors. For other secondary end points, including stroke/TIA, HF, and VTE, the risk was similar between the 2 groups. In the stratified analysis by DM, we found that the risk of composite cardiovascular end point was 26% lower with abatacept than TNF inhibitors among the high cardiovascular risk subset (ie, the DM subgroup). However, in the non‐DM subgroup, the risk of composite cardiovascular event was similar between the 2 drug groups.
When we looked at each database separately, the DM subgroup from the Medicare database showed a significant association between the use of abatacept and a reduced cardiovascular risk, whereas the non‐DM subgroup showed only such a trend. In the MarketScan database, we noted a trend toward an association between the use of abatacept and decreased cardiovascular risk only in the DM subgroup. Because the MarketScan cohort generally consists of younger and healthier patients than those in Medicare (Tables 1 and 2), it is possible that a certain subset of patients with RA (eg, with DM and/or age ≥65 years) would potentially benefit from the use of abatacept over TNF inhibitors with regard to their cardiovascular risk. To further assess whether older age and DM status affects the cardiovascular risk differently related to use of abatacept versus TNF inhibitors, we stratified our MarketScan cohort by DM status and age (≥65 and <65 years). In this analysis, we observed a trend toward a decreased cardiovascular risk only in the highest cardiovascular risk subset (age ≥65 years and with DM), but we did not find such a trend in the other subsets. In addition, in either data set, we found no significant interaction between abatacept treatment and the presence of DM on cardiovascular risk. Therefore, although our findings may suggest that benefit associated with abatacept use compared with TNF inhibitors could be greater in those with higher underlying cardiovascular risk, caution is needed in interpreting our results. Furthermore, future research should follow to confirm this hypothesis.
Because abatacept and TNF inhibitors have comparable anti‐inflammatory efficacy, observed in several randomized trials,28, 29, 30, 31, 32 it is unlikely that the potential cardiovascular benefits seen in the abatacept group are attributable to better control of RA activity. Abatacept has been reported to improve insulin sensitivity and restore lipid profile in patients with RA.33, 34 These favorable metabolic effects of abatacept may explain our findings of reduced risks of CVD in patients with RA with underlying DM. Furthermore, it is possible that the costimulation blockade affecting diverse downstream anti‐inflammatory pathways beyond cytokine‐mediated pathways, a unique mechanism of action of abatacept, could have provided a beneficial cardiovascular effect among those at high risk of accelerated atherosclerosis, such as patients with DM or elderly patients. In previous experimental studies, the critical roles of local CD4 T cells and regulatory T cells have been shown in animal models of atherosclerosis,35, 36, 37 with their functions being dependent on costimulation by interaction between B7 and CD28 or cytotoxic T‐lymphocyte‐associated protein 4 (CTLA4).38, 39, 40, 41 Abatacept use has also been shown to benefit the progression of atherosclerosis in animal models.39, 40 Because both RA and DM are important risk factors for atherosclerotic CVD,6, 12 our study will help guide treatment decision making in routine rheumatology practice.
In addition to atherosclerotic CVD, we have assessed other cardiovascular outcomes in the secondary analysis. We did not see a significant difference in the HF risk between abatacept and TNF inhibitor initiators regardless of baseline DM. To date, no apparently increased or decreased risk of HF with use of TNF inhibitors in the RA population has been reported.42, 43 With regard to the risk of VTE, overall, there was no significant difference between abatacept and TNF inhibitors. However, the VTE risk was numerically higher in abatacept initiators versus TNF inhibitors in both patients with DM and without DM in MarketScan. Because of the low event rate of the VTE outcome, our results may highlight the need for future research on this topic.
Other strengths of this study include use of rigorous pharmacoepidemiologic methods, including the new‐user design with active comparators.44 Both abatacept and TNF inhibitors are shown to be effective in treating active RA in clinical trials,28, 29, 30, 31, 32 and they are recommended equivalently by the current international guidelines against refractory RA in patients in whom nonbiological DMARD treatment has failed or in those who have poor prognostic factors.17, 18 Because the clinical circumstances for them to be used in patients with RA are similar, we expect that confounding by indication or bias by disease severity to be minimal. In support of the effectiveness by active comparator design in achieving comparability between comparison groups, all the baseline covariates were well balanced, even before PS matching (Table 1). In particular, RA severity–related covariates, such as nonbiological DMARD and glucocorticoid use, traditional CVD risk factors, and cardiovascular drug use were similar between the 2 groups, even before PS matching. Because TNF inhibitors were available earlier than abatacept, we accounted for the calendar year of the index date. Furthermore, to control for potential difference in their RA duration between the 2 groups, we excluded all patients who had ever dispensed any biological DMARD during 1 year before the index date and included the number of DMARDs that they had used in the PS model. In addition, we also performed extensive covariate adjustment using 1:1 PS matching for each database. By examining 2 large data sets with different population characteristics and including a subgroup of patients at high risk for CVD, our results provide generalizable and clinically important real‐world based evidence on the comparative cardiovascular safety of biologics. Last, we performed several prespecified secondary and subgroup analyses that showed consistent findings.
There are also limitations in this study. First, as inherent in any observational studies, our study is subject to residual or unmeasured confounding. In particular, we did not have information on RA activity and duration, physical activity, family history of CVD, diet pattern, or body mass index. However, we tried to minimize such unmeasured confounding by use of the aforementioned new user and active comparator design44 and PS matching methods. Second, even though our cohort size is large, it may be possible that we did not have an adequate statistical power for subgroup or sensitivity analysis. Third, this study may be subject to residual confounding, surveillance bias, and misclassification bias. We tried to minimize residual confounding and surveillance bias by PS matching against as many relevant covariates as possible, including healthcare use frequencies (Table 2). We also used previously validated claims‐based algorithms to define CVD outcomes.21, 22, 23, 24, 25 Last, mean proportions of days covered during follow‐up times were different between the 2 treatment groups, suggesting a better drug adherence in abatacept users. However, whether such differences in medication adherence would result in a clinically significant difference in RA activity control or CVD risk is unknown.
In conclusion, we found that in patients with RA with DM, those initiating abatacept may have a reduced risk of cardiovascular outcomes compared with those initiating TNF inhibitors. There was a trend that this benefit was greater in Medicare enrollees than MarketScan enrollees. This finding is in line with previous knowledge on the metabolic effects of abatacept in patients with RA and its protective effect in animal models of atherosclerosis. However, despite our rigorous study design and methods, including PS matching, there may be unmeasured confounding that accounts for the differential findings between 2 databases. Furthermore, future research should examine a potential cardioprotective mechanism of abatacept not shared by TNF inhibitor therapy in an RA subset at high cardiovascular risk.
Sources of Funding
This study was supported by an investigator‐sponsored grant from Bristol‐Myers Squibb (IM101‐589). However, the study was conducted by the authors independent of the sponsor. The sponsor was given the opportunity to make nonbinding comments on a draft of the article, but the authors retained the right of publication and to determine the final wording.
Disclosures
All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: Kim receives research grants to the Brigham and Women's Hospital from Lilly, Genentech, Pfizer, Merck, and AstraZeneca for unrelated studies. Kang, Jin, Brill, and Lewey have nothing to disclose for financial support or conflict of interest. Desai receives research support from Merck for unrelated studies. Patorno receives research support from Boehringer Ingelheim for unrelated studies.
(J Am Heart Assoc. 2018;7:e007393 DOI: 10.1161/JAHA.117.007393.)29367417
References
- 1. Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. [DOI] [PubMed] [Google Scholar]
- 2. Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. [DOI] [PubMed] [Google Scholar]
- 3. Maradit‐Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, Gabriel SE. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population, based cohort study. Arthritis Rheum. 2005;52:402–411. [DOI] [PubMed] [Google Scholar]
- 4. Peters MJ, van Halm VP, Voskuyl AE, Smulders YM, Boers M, Lems WF, Visser M, Stehouwer CD, Dekker JM, Nijpels G, Heine R, Dijkmans BA, Nurmohamed MT. Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum. 2009;61:1571–1579. [DOI] [PubMed] [Google Scholar]
- 5. del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, O'Fallon WM, Gabriel SE. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non‐rheumatoid arthritis patients? Ann Rheum Dis. 2008;67:64–69. [DOI] [PubMed] [Google Scholar]
- 7. Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, Setoguchi S, Greenberg JD. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karvounaris SA, Sidiropoulos PI, Papadakis JA, Spanakis EK, Bertsias GK, Kritikos HD, Ganotakis ES, Boumpas DT. Metabolic syndrome is common among middle, to, older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross, sectional, controlled, study. Ann Rheum Dis. 2007;66:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Innala L, Möller B, Ljung L, Magnusson S, Smedby T, Södergren A, Öhman ML, Rantapää‐Dahlqvist S, Wållberg‐Jonsson S. Cardiovascular events in early RA are a result of inflammatory burden and traditional risk factors: a five year prospective study. Arthritis Res Ther. 2011;13:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, Raggi P, Sokka T, Pincus T, Stein CM. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196:756–763. [DOI] [PubMed] [Google Scholar]
- 11. Dessein PH, Tobias M, Veller MG. Metabolic syndrome and subclinical atherosclerosis in rheumatoid arthritis. J Rheumatol. 2006;33:2425–2432. [PubMed] [Google Scholar]
- 12. Kerekes G, Nurmohamed MT, González‐Gay MA, Seres I, Paragh G, Kardos Z, Baráth Z, Tamási L, Soltész P, Szekanecz Z. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10:691–696. [DOI] [PubMed] [Google Scholar]
- 13. Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF, alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–762. [DOI] [PubMed] [Google Scholar]
- 14. Solomon DH, Curtis JR, Saag KG, Lii J, Chen L, Harrold LR, Herrinton LJ, Graham DJ, Kowal MK, Kuriya B, Liu L, Griffin MR, Lewis JD, Rassen JA. Cardiovascular risk in rheumatoid arthritis: comparing TNF‐α blockade with nonbiologic DMARDs. Am J Med. 2013;126:730.e9–730.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greenberg JD, Furer V, Farkouh ME. Cardiovascular safety of biologic therapies for the treatment of RA. Nat Rev Rheumatol. 2011;8:13–21. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Xie F, Yun H, Chen L, Muntner P, Levitan EB, Safford MM, Kent ST, Osterman MT, Lewis JD, Saag K, Singh JA, Curtis JR. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75:1813–1818. [DOI] [PubMed] [Google Scholar]
- 17. Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, Emery P, Gaujoux‐Viala C, Gossec L, Nam J, Ramiro S, Winthrop K, de Wit M, Aletaha D, Betteridge N, Bijlsma JW, Boers M, Buttgereit F, Combe B, Cutolo M, Damjanov N, Hazes JM, Kouloumas M, Kvien TK, Mariette X, Pavelka K, van Riel PL, Rubbert‐Roth A, Scholte‐Voshaar M, Scott DL, Sokka‐Isler T, Wong JB, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, Curtis JR, Furst DE, Parks D, Kavanaugh A, O'Dell J, King C, Leong A, Matteson EL, Schousboe JT, Drevlow B, Ginsberg S, Grober J, St Clair EW, Tindall E, Miller AS, McAlindon T; American College of Rheumatology . 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64:625–639. [DOI] [PubMed] [Google Scholar]
- 19. Hennessy S, Freeman C, Cunningham F. US Government claims databases In: Strom B, Kimmel S, Hennessy S, eds. Pharmacoepidemiology. 5th ed Philadelphia, PA: Wiley‐Blackwell; 2012:209–223. [Google Scholar]
- 20. Kim SY, Servi A, Polinski JM, Mogun H, Weinblatt ME, Katz JN, Solomon DH. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims‐based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. [DOI] [PubMed] [Google Scholar]
- 22. Choma NN, Griffin MR, Huang RL, Mitchel EF Jr, Kaltenbach LA, Gideon P, Stratton SM, Roumie CL. An algorithm to identify incident myocardial infarction using Medicaid data. Pharmacoepidemiol Drug Saf. 2009;18:1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willey VJ, Bullano MF, Hauch O, Reynolds M, Wygant G, Hoffman L, Mayzell G, Spyropoulos AC. Management patterns and outcomes of patients with venous thromboembolism in the usual community practice setting. Clin Ther. 2004;26:1149–1159. [DOI] [PubMed] [Google Scholar]
- 26. Needham DM, Scales DC, Laupacis A, Pronovost PJ. A systematic review of the Charlson comorbidity index using Canadian administrative databases: a perspective on risk adjustment in critical care research. J Crit Care. 2005;20:12–19. [DOI] [PubMed] [Google Scholar]
- 27. Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption In: Gail M, Krickberg K, Samet J, Tsiatis A, Wong W, eds. Survival Analysis: A Self, Learning Text. 3rd ed New York, NY: Springer; 2012:P131–P172. [Google Scholar]
- 28. Weinblatt ME, Schiff M, Valente R, van der Heijde D, Citera G, Zhao C, Maldonado M, Fleischmann R. Head‐to‐head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum. 2013;65:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guyot P, Taylor PC, Christensen R, Pericleous L, Drost P, Eijgelshoven I, Bergman G, Lebmeier M. Indirect treatment comparison of abatacept with methotrexate versus other biologic agents for active rheumatoid arthritis despite methotrexate therapy in the United Kingdom. J Rheumatol. 2012;39:1198–1206. [DOI] [PubMed] [Google Scholar]
- 30. Schiff M, Weinblatt ME, Valente R, van der Heijde D, Citera G, Elegbe A, Maldonado M, Fleischmann R. Head‐to‐head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two‐year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schiff M, Keiserman M, Codding C, Songcharoen S, Berman A, Nayiager S, Saldate C, Li T, Aranda R, Becker JC, Lin C, Cornet PL, Dougados M. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi‐centre, randomised, double‐blind, placebo‐controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis. 2008;67:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guyot P, Taylor P, Christensen R, Pericleous L, Poncet C, Lebmeier M, Drost P, Bergman G. Abatacept with methotrexate versus other biologic agents in treatment of patients with active rheumatoid arthritis despite methotrexate: a network meta‐analysis. Arthritis Res Ther. 2011;13:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ursini F, Russo E, Letizia Hribal M, Mauro D, Savarino F, Bruno C, Tripolino C, Rubino M, Naty S, Grembiale RD. Abatacept improves whole‐body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore). 2015;94:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mathieu S, Couderc M, Glace B, Pereira B, Tournadre A, Dubost JJ, Soubrier M. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics. 2013;7:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. [DOI] [PubMed] [Google Scholar]
- 36. Ait‐Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. [DOI] [PubMed] [Google Scholar]
- 37. Spitz C, Winkels H, Bürger C, Weber C, Lutgens E, Hansson GK, Gerdes N. Regulatory T cells in atherosclerosis: critical immune regulatory function and therapeutic potential. Cell Mol Life Sci. 2016;73:901–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ewing MM, Karper JC, Abdul S, de Jong RC, Peters HA, de Vries MR, Redeker A, Kuiper J, Toes RE, Arens R, Jukema JW, Quax PH. T‐cell co‐stimulation by CD28‐CD80/86 and its negative regulator CTLA‐4 strongly influence accelerated atherosclerosis development. Int J Cardiol. 2013;168:1965–1974. [DOI] [PubMed] [Google Scholar]
- 39. Ma K, Lv S, Liu B, Liu Z, Luo Y, Kong W, Xu Q, Feng J, Wang X. CTLA4‐IgG ameliorates homocysteine, accelerated atherosclerosis by inhibiting T‐cell overactivation in apoE(‐/‐) mice. Cardiovasc Res. 2013;97:349–359. [DOI] [PubMed] [Google Scholar]
- 40. Matsumoto T, Sasaki N, Yamashita T, Emoto T, Kasahara K, Mizoguchi T, Hayashi T, Yodoi K, Kitano N, Saito T, Yamaguchi T, Hirata K. Overexpression of cytotoxic T‐lymphocyte, associated antigen‐4 prevents atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1141–1151. [DOI] [PubMed] [Google Scholar]
- 41. Gerdes N, Zirlik A. Co‐stimulatory molecules in and beyond co‐stimulation—tipping the balance in atherosclerosis? Thromb Haemost. 2011;106:804–813. [DOI] [PubMed] [Google Scholar]
- 42. Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, Zink A. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58:667–677. [DOI] [PubMed] [Google Scholar]
- 43. Solomon DH, Rassen JA, Kuriya B, Chen L, Harrold LR, Graham DJ, Lewis JD, Lii J, Liu L, Griffin MR, Curtis JR. Heart failure risk among patients with rheumatoid arthritis starting a TNF antagonist. Ann Rheum Dis. 2013;72:1813–1818. [DOI] [PubMed] [Google Scholar]
- 44. Yoshida K, Solomon DH, Kim SC. Active‐comparator design and new‐user design in observational studies. Nat Rev Rheumatol. 2015;11:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]


