Abstract
Background
Exposure to preeclampsia or gestational hypertension is associated with higher offspring systolic blood pressure (SBP), but less is known about associations with other cardiometabolic risk markers.
Methods and Results
We studied 1097 children from the Project Viva cohort born 1999‐2002. Exposures were preeclampsia or gestational hypertension and mean maternal SBP in each trimester from prenatal records. Outcomes were research measures in midchildhood (mean 8.0 years) of SBP, overall adiposity, and a global cardiometabolic risk score comprising measures of SBP, waist circumference, glycemia, and lipids. We conducted linear regression analyses adjusted for maternal characteristics and offspring sex and age. In adjusted models, maternal preeclampsia or gestational hypertension (n=98, 9.1%) versus normal blood pressure was associated with slightly higher offspring SBP z‐score (0.15 units; 95% confidence interval [CI] −0.03, 0.32) but otherwise predicted better cardiometabolic health markers including metabolic risk z‐score (−0.23 units; −95% CI 0.44, −0.03) and several of its components as well as lower body mass index z‐score (−0.27 units; 95% CI −0.48, −0.06) and lower fat mass index (−0.91 kg/m2; 95% CI −1.35, −0.47). Similarly, higher mean third‐trimester maternal SBP was associated with higher offspring SBP z‐score (0.09 units per 10 mm Hg; 95% CI 0.02, 0.16) and lower overall and central adiposity but not with biomarkers of metabolic risk. Results for second‐trimester SBP were generally similar. First‐trimester blood pressure was associated with higher offspring blood pressure but not with other outcomes.
Conclusions
Higher maternal late‐pregnancy SBP and hypertensive disorders of pregnancy were associated with higher offspring SBP but otherwise better cardiometabolic health.
Keywords: high blood pressure, hypertension, preeclampsia/pregnancy
Subject Categories: Epidemiology, Pregnancy, Pediatrics, Risk Factors
Clinical Perspective
What Is New?
Higher maternal blood pressure in late pregnancy and hypertensive disorders of pregnancy predict lower offspring adiposity and better cardiometabolic health overall.
What Are the Clinical Implications?
Children born following a pregnancy complicated by hypertension should themselves be monitored for elevated blood pressure.
Introduction
Hypertensive disorders of pregnancy (HDP), including preeclampsia (PE) and gestational hypertension (GH), affect 5% to 10% of all pregnancies in the United States.1 According to current definitions, GH (formerly called pregnancy‐induced hypertension) is de novo hypertension (systolic blood pressure [SBP] ≥140 mm Hg and/or diastolic blood pressure [DBP] ≥90 mm Hg) at ≥20 weeks of gestation in the absence of end‐organ dysfunction, whereas PE is the combination of GH or chronic hypertension with evidence of end‐organ dysfunction such as proteinuria.2
Preeclampsia is a multisystem disorder of pregnancy characterized by abnormal vascular response to placentation. Although its precise pathophysiology remains unknown, alterations in vasoactive hormones and endothelial function have been proposed as possible pathogenetic mechanisms3, and a genetic contribution has been identified.4 It is a major contributor to maternal morbidity and mortality, preterm birth, intrauterine growth restriction, and perinatal death.5 Recent studies have consistently supported the role of hypertension in pregnancy as a risk factor for maternal cardiovascular disease later in life. A meta‐analysis including ≈3.5 million women found that a history of PE was associated with a higher subsequent lifetime risk of cardiovascular disease, including an almost 4‐fold increased risk of hypertension and an ≈2‐fold increased risk of fatal and nonfatal ischemic heart disease, stroke, and venous thromboembolism in later life.6 Although GH alone is comparatively benign, in roughly half of the cases, the disorder progresses to PE.7
Exposure to PE or GH may also predict cardiovascular health outcomes for the offspring of affected pregnancies. In utero exposure to PE is also associated with higher SBP throughout childhood and into adolescence. A long‐term follow‐up of the Helsinki Birth Cohort study reported that the offspring of preeclamptic pregnancies had a 1.9‐fold (95% confidence interval [CI] 1.2, 3.0) higher risk of stroke mortality as adults.8 In a 2011 systematic review and meta‐analysis of 10 studies (all undertaken in developed, high‐income, and predominantly white populations), PE was associated with a 2.39 mm Hg (95% CI 1.74, 3.05) higher SBP and a 1.35 mm Hg (95% CI 0.90, 1.80) higher DBP during childhood and young adulthood.9 These associations might result from long‐term programming by in utero exposure to HDP or from shared familial genetics and/or behavioral factors. If the former pathway were operational, we would expect to see associations of HDP with accompanying cardiometabolic markers and not solely blood pressure (BP). It has been reported that HDP promotes the release of vasculotoxic factors into maternal circulation, which subsequently traverse the placenta to adversely impact fetal development by causing impaired vascular function and chronic inflammation that may persist throughout the offspring's life course.10 Relatively few studies have investigated whether offspring of pregnancies completed with hypertensive disorders have higher cardiometabolic risk generally. In the present article we build on previously published literature by examining the associations of maternal third‐trimester blood pressure and PE or GH with offspring midchildhood cardiometabolic risk markers (including BP, lipid levels, adiposity, and global metabolic risk) among 6‐ to 10‐year‐old participants of Project Viva.
Methods
Population and Study Design
Project Viva is a prospective prebirth cohort study that recruited pregnant women at their initial prenatal visit from 1 of 8 obstetric offices of Atrius Harvard Vanguard Medical Associates in urban and suburban eastern Massachusetts between 1999 and 2002. Further detail on recruitment, exclusion criteria, and study design have been published.11 All study instruments are publicly available online (https://www.hms.harvard.edu/viva/). In accordance with Project Viva policies, the data, analytic methods, and study materials for this analysis will be made available to other researchers for purposes of reproducing the results on request to and approval from the Project Viva decision‐making body.
Of the 2128 live singleton infants, 1122 children attended an in‐person visit at midchildhood (mean age 8.0±0.9 years old) and provided data on any of the midchildhood outcomes of interest. Among these 1122, we excluded 8 mothers with pregestational type 1 and or type 2 pregestational diabetes mellitus, 11 on antihypertensive medications during pregnancy, and an additional 6 without any information on hypertensive disorders of pregnancy or data on prenatal blood pressures. The final sample therefore included 1097 mother‐child pairs. We compared characteristics of the 1097 included in our current analyses with the 1031 excluded pairs. Overall, we found that compared with mothers excluded from the analyses, the analyzed sample included a higher proportion of women with a college degree (68% versus 61%) and higher annual household income (64% versus 58% reported >$70 000/y at enrollment) but were less likely to report smoking during pregnancy (10% versus 16%). The 2 groups were relatively similar in terms of mean maternal age at enrollment (32.1 [standard deviation {SD} 5.4] versus 31.5 [SD 5.1] years), mean prepregnancy body mass index (BMI) (24.6 [SD 5.1] versus 25.2 [SD 5.9] kg/m2), parity (52% versus 52% parous), and prevalence of chronic hypertension (CHTN) (1% versus 2%), GH (6% versus 7%) and PE (3% versus 4%).
All participating pregnant women provided written informed consent at recruitment and at outcome assessments, and all children provided verbal assent at the midchildhood visit. Harvard Pilgrim Health Care's institutional review board reviewed and approved study protocols. All procedures were in accordance with the ethical standards for human experimentation established by the Declaration of Helsinki.
Exposures: Maternal Blood Pressure and Hypertensive Disorders of Pregnancy
We extracted clinical measures of maternal prenatal blood pressure from electronic medical records. To analyze maternal blood pressure as a continuous exposure, we calculated average systolic and diastolic blood pressure values within each trimester for each participant. We defined first trimester as last menstrual period to 91 days, second trimester as 92 to 182 days, and third trimester as 183 days to delivery.12 The median number of blood pressure values within each trimester was 2 in the first trimester, 3 in the second, and 9 in the third.
To ascertain the presence of HDPs, we evaluated prenatal records for blood pressure and urine protein results. We also reviewed inpatient hospital charts among women who had a diagnosis or discharge code designating PE or GH and who did not already fulfill criteria for the same diagnosis based on our reviews of outpatient blood pressure and urine values. We created a 4‐level exposure variable with the categories normotensive, GH, PE, and CHTN in line with guidelines from the 2000 National High Blood Pressure Working Group on High Blood Pressure in Pregnancy.13, 14 We categorized women as having CHTN if they were taking antihypertensive medications or if they had 2 elevated clinically measured blood pressure values (systolic ≥140 mm Hg or diastolic ≥90 mm Hg) before 20 weeks of gestation. We categorized women as having GH if they did not have chronic hypertension and developed elevated systolic (≥140 mm Hg) or diastolic (≥90 mm Hg) blood pressure on 2 or more occasions after 20 weeks of gestation. We categorized women as having PE if they did not have chronic hypertension but developed increased blood pressure (as above) and proteinuria (dipstick value of ≥2+ once or 1+ on 2 or more occasions >4 hours but ≤7 days apart), or if they had CHTN and developed proteinuria after 20 weeks of gestation. Otherwise, we categorized women as normotensive.
Outcomes: Midchildhood Metabolic Biomarkers and Anthropometry
We assessed offspring cardiometabolic risk outcomes including blood pressure, lipid levels, overall and central adiposity, markers of inflammation, and global metabolic risk as described below.
Blood Pressure
Trained research assistants measured BP in children using biannually calibrated automated oscillometric monitors (Dinamap Pro100, Tampa, Florida). The research assistants assessed BP on the child's upper arm up to 5 times at 1‐minute intervals. Although the first measurement tended to be higher than the second through fifth measurements, the intraclass coefficient was high (0.74). We included all 5 in the analysis to improve precision when measuring between‐person differences rather than absolute levels.15 We used the average of the 5 measurements to derive blood pressure z‐scores standardized for age, sex, and height, based on a national reference.16
Metabolic and Inflammatory Biomarkers
Experienced phlebotomists collected blood specimens via venipuncture, which we processed within 24 hours and stored at −80°C until the time of analysis. We assayed glucose, fasting insulin, total cholesterol, high‐density lipoprotein (HDL), and triglycerides from fasting blood samples only. Plasma glucose was measured enzymatically; insulin was measured using an electrochemilumiscence immunoassay (both Roche Diagnostics, Indianapolis, IN). We calculated insulin resistance using the homeostasis model assessment (as fasting insulin [μU/mL]×fasting glucose [mg/dL]/405). Lipids were measured enzymatically with correction for endogenous glycerol. We calculated low‐density lipoprotein cholesterol levels from total cholesterol, HDL, and triglycerides. Plasma leptin and adiponectin were measured via radioimmunoassay (Linco Research Inc, St. Charles, MO). Plasma interleukin (IL‐6) was measured by enzyme‐linked immunosorbent assay. Concentration of high‐sensitivity C‐reactive protein was measured using an immunoturbidimetric high‐sensitivity assay on a Hitachi 911 analyzer (Roche Diagnostics, Indianapolis, IN).
We calculated a sex‐specific metabolic risk z‐score as the mean of sex‐specific internal z‐scores for SBP, waist circumference, log‐transformed insulin resistance, log‐transformed triglycerides, and inverted HDL (HDL×−1); higher scores indicate higher risk.17 Although there is no universal agreement on the optimal definition of metabolic syndrome in children, prior research has incorporated analogous metabolic risk scores.18, 19, 20 Metabolic syndrome cluster scores are more powerful estimates of predicting children at risk of developing early cardiovascular disease and type 2 diabetes mellitus than single measures.21
Child Anthropometry and Adiposity Indices
Trained research assistants measured child weight (TBF‐300 A; Tanita, Arlington Heights, IL) and height (calibrated stadiometer; Shorr Productions, Olney, MD) using standardized protocols. Using the Centers for Disease Control and Prevention 2000 growth reference, we calculated age‐ and sex‐specific BMI percentiles and BMI z‐scores.22 Using standard protocols, research assistants measured subscapular and triceps skinfold thicknesses using Holtain calipers (Holtain, Crosswell, UK) and waist circumference above the iliac crest using nonstretchable measuring tape (Hoechstmass Balzer, Sulzbach, Germany). We used the sum of subscapular+triceps skinfolds to represent overall adiposity and the skinfold ratio (subscapular/triceps) as well as waist circumference as measures of central adiposity.
Research assistants also administered whole‐body dual x‐ray absorptiometry (DXA) scans with the Hologic model Discovery A (Hologic, Bedford, MA). DXA scans provided data on total fat mass, trunk fat mass, and fat‐free mass. We calculated fat mass index, trunk fat mass index, and fat‐free mass index as kg/(height [m]2). A single trained research assistant checked all scans for positioning, movement, and artifacts and defined body regions for analysis; intrarater reliability was high (r=0.99).
Covariates
Mothers provided information on their age, race/ethnicity, education, household income, smoking status during pregnancy, medical history, history of pregnancy complications, and prepregnancy weight and height via a combination of interviews and self‐administered questionnaires administered at enrollment, during midpregnancy, and shortly after delivery. We categorized these characteristics as presented in Table 1. We calculated maternal prepregnancy BMI using self‐reported prepregnancy weight and height. We obtained infant birth weight and date of delivery from hospital medical records and calculated sex‐specific birth weight‐for‐gestational age z‐scores using national reference data.23
Table 1.
Characteristics of 1097 Pregnant Women and Their Infants in Project Viva Overall and According to Hypertensive Disorder of Pregnancy Status
| Maternal Characteristics | All | Normal | PE or GH | CHTN |
|---|---|---|---|---|
| N=1097 | n=964 | n=98 | n=10 | |
| Maternal age at enrollment, y | 32.1 (5.4) | 32.1 (5.3) | 31.2 (5.6) | 34.3 (4.5) |
| Prepregnancy BMI, kg/m2 | 24.6 (5.1) | 24.4 (5.1) | 26.3 (5.0) | 28.6 (5.7) |
| Height, m | 1.7 (0.1) | 1.6 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Race/ethnicity | ||||
| Black | 16.1% | 16.1% | 15.3% | 10.0% |
| Hispanic | 6.4% | 6.7% | 4.1% | 10.0% |
| Asian | 5.2% | 5.3% | 2.0% | 20.0% |
| White | 67.7% | 67.5% | 73.5% | 60.0% |
| Other | 4.5% | 4.4% | 5.1% | 0% |
| Nullliparous | 47.6% | 45.3% | 73.5% | 60% |
| Married or cohabiting | 91.2% | 91.3% | 88.8% | 100% |
| College graduate | 68.2% | 68.0% | 72.4% | 60% |
| Annual household income >$70 000 | 64.1% | 63.2% | 67.8% | 80.0% |
| Smoking during pregnancy status | ||||
| Never | 70.8% | 71.9% | 66.3% | 40.0% |
| Former | 19.4% | 18.4% | 23.5% | 50.0% |
| During pregnancy | 9.8% | 9.7% | 10.2% | 10.0% |
| Mean blood pressure by trimester of pregnancy, mm Hg | ||||
| SBP 1st trim | 111.0 (8.9) | 110.2 (8.6) | 116.9 (8.1) | 125.6 (8.7) |
| SBP 2nd trim | 110.3 (7.6) | 109.5 (7.1) | 116.4 (7.1) | 126.6 (6.7) |
| SBP 3rd trim | 112.6 (7.6) | 111.2 (6.4) | 124.2 (7.9) | 127.1 (4.5) |
| DBP 1st trim | 69.2 (6.6) | 68.6 (6.4) | 73.5 (6.1) | 79.6 (3.8) |
| DBP 2nd trim | 67.5 (5.6) | 67.0 (5.4) | 71.7 (4.9) | 75.3 (5.0) |
| DBP 3rd trim | 69.8 (5.5) | 68.9 (4.7) | 78.1 (5.0) | 79.0 (7.0) |
| Child characteristics at birth | ||||
| Female | 49.5% | 49.4% | 53.1% | 40.0% |
| Birth weight for gestational age z‐score | 22.2% | 0.22 (0.97) | −0.00 (0.96) | 0.08 (0.54) |
| Cesarean delivery | 1.6% | 21.0% | 36.7% | 20.0% |
| Gestation length <34 wks | 0.19 (0.97) | 1.1% | 4.1% | 0% |
| Child outcomes at midchildhood visit | ||||
| Age, y | 8.0 (0.9) | 7.9 (0.9) | 8.1 (0.9) | 8.0 (0.9) |
| Blood pressure | ||||
| SBP, z‐score | −0.42 (0.77) | −0.45 (0.77) | −0.26 (0.74) | 0.04 (0.93) |
| DBP, z‐score | −0.41 (0.51) | −0.42 (0.51) | −0.31 (0.45) | −0.40 (0.51) |
| SBP, mm Hg | 94.6 (8.7) | 94.3 (8.5) | 96.6 (8.5) | 100.6 (11.2) |
| DBP, mm Hg | 54.4 (5.7) | 54.2 (5.7) | 55.7 (5.2) | 55.1 (5.9) |
| Lipids and inflammation | ||||
| HDL cholesterol, mg/dL | 57.1 (13.7) | 56.7 (13.4) | 60.8 (16.6) | 59.6 (13.2) |
| LDL cholesterol, mg/dL | 91.6 (23.1) | 92.0 (23.2) | 89.3 (23.0) | 98.1 (19.3) |
| Triglyceride, mg/dL | 57.9 (25.5) | 58.4 (26.0) | 54.6 (22.2) | 55.3 (12.9) |
| IL‐6, pg/mL | 1.01 (1.41) | 1.01 (1.36) | 1.07 (1.89) | 1.10 (1.68) |
| hsCRP, mg/L | 0.91 (2.67) | 0.88 (2.70) | 0.90 (2.34) | 0.71 (0.93) |
| Glycemia | ||||
| HOMA‐IR | 1.9 (1.8) | 1.9 (1.9) | 1.6 (0.9) | 1.9 (0.6) |
| Adiponectin, μg/mL | 15.6 (8.8) | 15.8 (9.1) | 14.9 (6.0) | 10.8 (5.1) |
| Overall size and adiposity | ||||
| BMI z‐score | 0.38 (1.00) | 0.38 (1.02) | 0.29 (0.84) | 0.87 (0.78) |
| BMI percentile | ||||
| <5 | 2.4% | 2.6% | 1.0% | 0.0% |
| 5th to <85th | 72.1% | 71.5% | 81.4% | 50.0% |
| 85th to <95th | 13.5% | 13.4% | 12.4% | 30.0% |
| ≥95th | 12.0% | 12.4% | 5.2% | 20.0% |
| DXA fat mass index, kg/m2 | 4.4 (2.0) | 4.5 (2.0) | 3.9 (1.5) | 4.8 (0.8) |
| DXA fat free mass index, kg/m2 | 13.0 (1.5) | 13.0 (1.5) | 12.9 (1.1) | 13.3 (1.3) |
| Percent fat, % | 24.5 (6.3) | 24.7 (6.4) | 22.6 (5.5) | 26.6 (2.4) |
| SS+TR, mm | 19.8 (9.8) | 19.9 (9.9) | 18.6 (8.2) | 21.4 (5.7) |
| Leptin, ng/mL | 6.1 (7.5) | 6.2 (7.6) | 4.6 (5.8) | 4.5 (1.4) |
| Central adiposity | ||||
| DXA trunk fat mass index, kg/m2 | 1.5 (0.9) | 1.5 (0.9) | 1.3 (0.6) | 1.7 (0.3) |
| Waist circumference, cm | 60.0 (8.3) | 60.0 (8.4) | 59.2 (6.9) | 62.4 (4.9) |
| SS:TR ratio | 0.7 (0.2) | 0.7 (0.2) | 0.7 (0.2) | 0.8 (0.2) |
| Global metabolic risk z‐score | 0.00 (0.63) | 0.01 (0.64) | −0.11 (0.61) | 0.09 (0.37) |
Data are shown as mean (SD) or as percentage. BMI indicates body mass index; CHTN, chronic hypertension diagnosed prior to pregnancy; DBP, diastolic blood pressure; DXA, dual‐energy X‐ray absorptiometry scan; GH, gestational hypertension; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C‐reactive protein; IL‐6, interleukin 6; LDL, low‐density lipoprotein cholesterol; PE, preeclampsia; SBP, systolic blood pressure; SS:TR, ratio of subscapular (SS) and triceps skinfold thicknesses (TR); SS+TR, sum of the subscapular and triceps skinfold thicknesses; trim, trimester.
Statistical Analyses
The primary exposure of interest was maternal prenatal blood pressure, which we examined both continuously (as average systolic blood pressure within the first, second, and third trimesters) and categorically (as CHTN, PE, GH, or normal). We initially examined GH (n=65) and PE (n=33) as distinct exposures, but because their relatively small sample sizes resulted in wide CIs, we combined them for our primary analyses, and as a secondary analysis we present selected estimates for each exposure separately. We used normal blood pressure as the reference group for all analyses of categorical exposures. We examined all continuous outcome variables for normality. High‐sensitivity C‐reactive protein was positively skewed, so we performed a natural‐log transformation for this outcome to produce approximately normal distributions and normal residuals in the regression models.
Additionally we conducted separate linear regression analyses examining associations of mean maternal SBP in each trimester with midchildhood outcomes. To ensure that the different number of measured BP values in each trimester would not affect the precision of estimates differentially in the 3 trimesters, we also ran a sensitivity analysis in which we randomly selected 1 BP per trimester and got similar results. We selected 10 mm Hg as the effect size because this was the approximate SD of SBP in each trimester in our cohort.
Model 1 was adjusted for child sex and age at the midchildhood assessment, except in the models predicting child SBP z‐score, DBP z‐score, and BMI z‐score, which already accounted for age and sex. Model 2 was adjusted for maternal characteristics at the time of study enrollment: race/ethnicity, age, prepregnancy BMI, height, education, household income, parity, and smoking status. In an additional “mediator” model, Model 3, we adjusted for birth weight for gestational age z‐score to examine the extent to which size at birth could be on the pathway between maternal blood pressure and child cardiometabolic outcomes. Because we examined 21 outcomes, we additionally considered whether multiple testing might have resulted in a higher rate of type 1 error. We calculated false discovery rate–adjusted P values for each exposure/outcome combination, accounting for the 21 tests. There were no differences in statistical significance at a standard threshold of α=0.05 or interpretation, so we present the native 95% CIs as originally planned. We performed all analyses using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
Among the 1097 mothers included in this study, 964 (89.9%) were normotensive, 98 (9.1%) developed PE or GH, and 10 (0.9%) had CHTN (Table 1). Compared with women with normal BP, women with PE or GH had higher mean prepregnancy BMI (26.3 [SD 5.0] versus 24.4 [SD 5.1] kg/m2), were more likely to be nulliparous (73.5% versus 45.3%), less likely to be married or cohabitating (88.8% versus 91.3%), and less likely to be never smokers (66.3% versus 71.9%) (Table 1). At the midchildhood visit mean (SD) age was 8.0 years (0.9), SBP z‐score was −0.42 (0.77), and DBP z‐score −0.41 (0.51). Offspring of mothers who had PE or GH had slightly higher mean HDL but lower mean metabolic risk z‐score and measures of adiposity including BMI z‐score, DXA fat mass index, fat‐free mass index, trunk fat mass index, percentage fat, and subscapular+triceps skinfolds compared to offspring of normotensive mothers (Table 1). Insulin resistance, leptin, and adiponectin were also lower, whereas inflammatory biomarkers were similar.
In linear regression analyses (Table 2), models adjusted for child sex and age (Model 1) showed that prenatal exposure to PE or GH (versus normal BP) was associated with higher offspring blood pressure (eg, SBP z‐score 0.19 units; 95% CI 0.03, 0.35), DBP z‐score 0.11 units; 95% CI 0.01, 0.22). However, after adjustment for maternal race/ethnicity, age at enrollment, height, prepregnancy BMI, education, household income, parity, and smoking during pregnancy (Model 2), associations of PE or GH (versus normal BP) with higher offspring BP were attenuated and included the null (SBP z‐score 0.15 units; 95% CI −0.03, 0.32, DBP z‐score 0.05 units; 95% CI −0.06, 0.17). In both Models (1 and 2), PE or GH predicted better cardiometabolic health markers (Figure) including metabolic risk z‐score (−0.23 units; 95% CI −0.44, −0.03) and many of its components including higher HDL cholesterol (4.66 mg/dL; 95% CI 0.43, 8.88), lower waist circumference (−2.59 cm; 95% CI −4.19, −0.99), as well as nonsignificant improvements in triglycerides (−3.36 mg/dL; 95% CI −11.3, 4.57) and insulin resistance (−0.52; 95% CI −1.07, 0.03). PE or GH was also associated with lower overall size reflected by BMI z‐score (−0.27 units; 95% CI −0.48, −0.06) as well as lower overall adiposity reflected by DXA fat mass index (−0.91; 95% CI −1.35, −0.47). Further adjustment for birth weight for gestational age did not appreciably alter the associations (Table 2, Model 3).
Table 2.
Multivariable Associations of Hypertensive Disorders of Pregnancy (PE or GH) Versus Normal Maternal Blood Pressure During Pregnancy With Offspring Outcomes at Midchildhood
| Outcomes | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Blood pressure | |||
| SBP, z‐score | 0.19 (0.03, 0.35) | 0.15 (−0.03, 0.32) | 0.13 (−0.04, 0.31) |
| DBP, z‐score | 0.11 (0.01, 0.22) | 0.05 (−0.06, 0.17) | 0.04 (−0.07, 0.15) |
| SBP, mm Hg | 2.05 (0.29, 3.80) | 1.29 (−0.58, 3.16) | 1.31 (−0.56, 3.19) |
| DBP, mm Hg | 1.30 (0.11, 2.48) | 0.50 (−0.75, 1.76) | 0.44 (−0.82, 1.69) |
| Lipids and inflammation | |||
| HDL cholesterol, mg/dL | 4.24 (0.35, 8.12) | 4.66 (0.43, 8.88) | 4.72 (0.47, 8.98) |
| LDL cholesterol, mg/dL | −3.11 (−9.67, 3.45) | −0.74 (−7.77, 6.30) | −1.38 (−8.43, 5.68) |
| Triglyceride, mg/dL | −3.94 (−11.3, 3.38) | −3.36 (−11.3, 4.57) | −4.23 (−12.2, 3.71) |
| IL‐6, pg/mL | 0.05 (−0.34, 0.44) | 0.13 (−0.31, 0.57) | 0.16 (−0.28, 0.60) |
| hsCRP, mg/L (log transformed) | −0.05 (−0.50, 0.40) | −0.16 (−0.65, 0.32) | −0.14 (−0.63, 0.35) |
| Glycemia | |||
| HOMA‐IR | −0.41 (−0.94, 0.11) | −0.52 (−1.07, 0.03) | −0.54 (−1.10, 0.01) |
| Adiponectin, μg/mL | −0.90 (−3.31, 1.51) | −0.50 (−3.15, 2.15) | −0.32 (−2.97, 2.33) |
| Overall size and adiposity | |||
| BMI z‐score | −0.10 (−0.31, 0.11) | −0.27 (−0.48, −0.06) | −0.25 (−0.45, −0.04) |
| DXA fat mass index, kg/m2 | −0.63 (−1.06, −0.19) | −0.91 (−1.35, −0.47) | −0.91 (−1.35, −0.47) |
| DXA fat‐free mass index, kg/m2 | −0.09 (−0.41, 0.22) | −0.29 (−0.61, 0.02) | −0.28 (−0.59, 0.04) |
| Percentage fat, % | −2.24 (−3.60, −0.88) | −3.02 (−4.42, −1.62) | −3.03 (−4.44, −1.63) |
| SS+TR, mm | −1.73 (−3.67, 0.21) | −2.79 (−4.70, −0.88) | −2.78 (−4.70, −0.86) |
| Leptin, ng/mL | −1.75 (−3.69, 0.20) | −2.64 (−4.64, −0.65) | −2.72 (−4.72, −0.71) |
| Central adiposity | |||
| DXA trunk fat mass index, kg/m2 | −0.26 (−0.45, −0.06) | −0.38 (−0.58, −0.18) | −0.39 (−0.59, −0.19) |
| Waist circumference, cm | −1.17 (−2.79, 0.44) | −2.59 (−4.19, −0.99) | −2.42 (−4.02, −0.82) |
| SS:TR ratio | −0.03 (−0.07, 0.01) | −0.04 (−0.08, 0.00) | −0.04 (−0.08, 0.00) |
| Global metabolic risk, z‐score | −0.14 (−0.33, 0.05) | −0.23 (−0.44, −0.03) | −0.24 (−0.44, −0.04) |
Model 1: Adjusted for child age at outcome and sex, except SBP‐z, DPB‐z, and BMI‐z scores, which already account for age and sex, and thus these models are not additionally adjusted. Model 2: Model 1+maternal race/ethnicity, age at enrollment, height and prepregnancy BMI, education, household income, parity, smoking during pregnancy. Model 3: Model 2+birth weight for gestational age z‐score. BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; DXA, dual‐energy x‐ray absorptiometry scan; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C‐reactive protein; IL‐6, interleukin 6; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SS:TR, ratio of subscapular (SS) and triceps skinfold thicknesses (TR); SS+TR, sum of the subscapular and triceps skinfold thicknesses.
Figure 1.
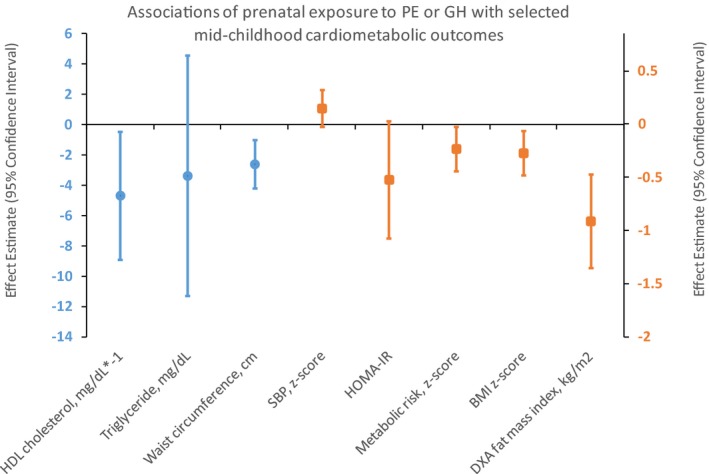
Associations of preeclampsia (PE) or gestational hypertension (GH) in pregnancy, vs normal maternal blood pressure, with selected offspring cardiometabolic outcomes in midchildhood among 1097 children in the Project Viva cohort. Data points are β coefficients (with error bars displaying 95% confidence intervals) from multivariable linear regression models adjusted for child age at outcome and sex (except SBP z‐score and BMI z‐score, which already account for age and sex), and maternal race/ethnicity, age at enrollment, height, prepregnancy BMI, education, household income, parity, and smoking during pregnancy. Circles are plotted on the primary Y axis (left side), and squares on the secondary Y axis (right side). BMI indicates body mass index; DXA, dual x‐ray absorptiometry; GH, gestational hypertension; HDL, high‐density lipoprotein; HOMA‐IR, homeostatic models assessment of insulin resistance; PE, preeclampsia; SBP, systolic blood pressure.
In covariate‐adjusted models with GH and PE as distinct exposures, some associations were stronger for GH alone (eg, SBP z‐score 0.21 units; 95% CI 0.00, 0.42) than for PE alone (0.03 units; 95% CI −0.24, 0.31), but the association of PE with HDL (10.13 μg/mL; 95% CI 2.75, 17.51) was stronger than that of GH with HDL (2.36 μg/mL; 95% CI −2.57, 7.28). Generally, associations with PE had substantially wider CIs given smaller sample sizes.
In additional analyses (Table 3), we found that chronic hypertension was associated with higher offspring SBP z‐score (0.49 mm Hg; 0.01, 0.97), but associations were attenuated after adjustment, and all CIs were quite wide given the very small number of women with chronic hypertension (n=10).
Table 3.
Multivariable Associations of Chronic Hypertension During Pregnancy With Offspring Outcomes at Midchildhood
| Outcomes | β (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Blood pressure | |||
| SBP, z‐score | 0.49 (0.01, 0.97) | 0.38 (−0.10, 0.87) | 0.38 (−0.11, 0.86) |
| DBP, z‐score | 0.02 (−0.30, 0.33) | −0.06 (−0.38, 0.25) | −0.07 (−0.38, 0.25) |
| SBP, mm Hg | 6.11 (0.89, 11.32) | 4.59 (−0.65, 9.82) | 4.60 (−0.64, 9.84) |
| DBP, mm Hg | 0.85 (−2.66, 4.37) | −0.22 (−3.72, 3.28) | −0.26 (−3.76, 3.25) |
| Lipids and inflammation | |||
| HDL cholesterol, mg/dL | 3.18 (−7.00, 13.37) | 2.91 (−7.41, 13.22) | 2.96 (−7.38, 13.29) |
| LDL cholesterol, mg/dL | 5.82 (−11.4, 23.00) | 6.27 (−10.9, 23.44) | 5.73 (−11.4, 22.87) |
| Triglyceride, mg/dL | −3.35 (−22.5, 15.82) | −3.76 (−23.1, 15.60) | −4.49 (−23.8, 14.81) |
| IL‐6, pg/mL | 0.08 (−0.98, 1.14) | 0.20 (−0.92, 1.33) | 0.24 (−0.89, 1.36) |
| hsCRP, mg/L (log transformed) | 0.33 (−0.98, 1.65) | 0.22 (−1.09, 1.52) | 0.25 (−1.06, 1.56) |
| Glycemia | |||
| HOMA‐IR | −0.06 (−1.35, 1.23) | −0.35 (−1.64, 0.93) | −0.37 (−1.65, 0.92) |
| Adiponectin, μg/mL | −4.93 (−11.5, 1.66) | −3.02 (−9.78, 3.75) | −2.82 (−9.57, 3.93) |
| Overall size and adiposity | |||
| BMI z‐score | 0.49 (−0.14, 1.11) | 0.18 (−0.40, 0.77) | 0.20 (−0.39, 0.78) |
| DXA fat mass index, kg/m2 | 0.40 (−0.89, 1.68) | −0.13 (−1.35, 1.08) | −0.14 (−1.35, 1.08) |
| DXA fat‐free mass index, kg/m2 | 0.27 (−0.66, 1.20) | 0.04 (−0.83, 0.91) | 0.06 (−0.81, 0.93) |
| Percentage fat, % | 1.95 (−2.07, 5.97) | 0.35 (−3.52, 4.23) | 0.34 (−3.54, 4.22) |
| SS+TR, mm | 1.65 (−4.11, 7.40) | −1.59 (−6.95, 3.76) | −1.59 (−6.95, 3.77) |
| Leptin, ng/mL | −1.74 (−7.03, 3.56) | −2.85 (−7.95, 2.25) | −2.93 (−8.03, 2.18) |
| Central adiposity | |||
| DXA trunk fat mass index, kg/m2 | 0.19 (−0.38, 0.77) | −0.05 (−0.60, 0.49) | −0.06 (−0.61, 0.49) |
| Waist circumference, cm | 2.11 (−2.70, 6.93) | −0.29 (−4.80, 4.22) | −0.19 (−4.69, 4.30) |
| SS:TR ratio | 0.06 (−0.06, 0.17) | 0.01 (−0.10, 0.13) | 0.01 (−0.10, 0.13) |
| Global metabolic risk, z‐score | 0.08 (−0.38, 0.53) | −0.07 (−0.52, 0.39) | −0.07 (−0.53, 0.39) |
Model 1: Adjusted for child age at outcome and sex, except for SBP, DPB, and BMI z‐scores, which already incorporate age and sex and thus were not additionally adjusted. Model 2: Model 1+maternal race/ethnicity, age at enrollment, height and prepregnancy BMI, education, household income, parity, and smoking during pregnancy. Model 3: Model 2+birth weight for gestational age z‐score. BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; DXA, dual‐energy x‐ray absorptiometry scan; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C‐reactive protein; IL‐6, interleukin 6; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SS:TR, ratio of subscapular (SS) and triceps skinfold thicknesses (TR); SS+TR, sum of subscapular and triceps skinfold thicknesses.
In similar adjusted models (Table 4, Model 2) we found that maternal systolic blood pressure in each of the 3 trimesters was directly associated with offspring SBP z‐score. In an unadjusted model, mean third‐trimester maternal SBP was associated with higher BMI z‐score (0.09 units per 10 mm Hg; 0.01, 0.17). But after adjusting for prepregnancy BMI and other covariates (Model 2), mean third‐trimester maternal SBP was associated with lower BMI z‐score (−0.10 units per 10 mm Hg; −0.18, −0.02). Mean third‐trimester maternal SBP was also associated with lower DXA fat mass index (−0.20 kg/m2; −0.39, −0.02), DXA trunk fat mass index (−0.08 kg/m2; −0.16, 0.00), and body fat (−0.70%; −1.29, −0.12). Results for second‐trimester SBP were generally similar. Maternal first‐trimester SBP was associated with higher offspring BP but not adiposity. Additional adjustment for size at birth did not alter observed associations (data not shown).
Table 4.
Multivariable Associationsa of Mean Maternal Blood Pressure in Each Trimester With Offspring Outcomes at Midchildhood
| Outcomes | β (95% CI) Per 10 mm Hg | ||
|---|---|---|---|
| First Trim SBP | Second Trim SBP | Third Trim SBP | |
| Blood pressure | |||
| SBP, z‐score | 0.10 (0.03, 0.16) | 0.09 (0.02, 0.16) | 0.09 (0.02, 0.16) |
| DBP, z‐score | 0.01 (−0.03, 0.05) | 0.01 (−0.04, 0.05) | 0.01 (−0.04, 0.05) |
| SBP, mm Hg | 1.10 (0.43, 1.77) | 0.89 (0.11, 1.66) | 0.93 (0.16, 1.70) |
| DBP, mm Hg | 0.15 (−0.30, 0.59) | 0.07 (−0.45, 0.59) | 0.08 (−0.43, 0.60) |
| Lipids and inflammation | |||
| HDL cholesterol, mg/dL | −0.49 (−1.93, 0.95) | 0.24 (−1.50, 1.97) | 0.78 (−0.97, 2.54) |
| LDL cholesterol, mg/dL | 0.74 (−1.63, 3.12) | 1.36 (−1.53, 4.24) | −1.55 (−4.47, 1.37) |
| Triglyceride, mg/dL | 0.80 (−1.92, 3.52) | −1.21 (−4.44, 2.02) | −1.64 (−4.93, 1.64) |
| IL‐6, pg/mL | −0.01 (−0.17, 0.15) | −0.08 (−0.27, 0.10) | 0.06 (−0.13, 0.25) |
| hsCRP, mg/L (log‐transformed) | 0.02 (−0.15, 0.19) | 0.03 (−0.17, 0.23) | −0.03 (−0.24, 0.18) |
| Glycemia | |||
| HOMA‐IR | 0.05 (−0.14, 0.23) | −0.13 (−0.35, 0.10) | −0.05 (−0.28, 0.18) |
| Adiponectin, μg/mL | −0.40 (−1.36, 0.55) | −0.17 (−1.29, 0.96) | −0.83 (−1.96, 0.31) |
| Overall size and adiposity | |||
| BMI z‐score | −0.01 (−0.08, 0.07) | −0.14 (−0.23, −0.06) | −0.10 (−0.18, −0.02) |
| DXA fat mass index, kg/m2 | 0.01 (−0.14, 0.16) | −0.21 (−0.40, −0.03) | −0.20 (−0.39, −0.02) |
| DXA fat free mass index, kg/m2 | −0.02 (−0.13, 0.09) | −0.08 (−0.21, 0.05) | −0.08 (−0.21, 0.05) |
| Percentage fat, % | 0.05 (−0.44, 0.53) | −0.73 (−1.33, −0.14) | −0.70 (−1.29, −0.12) |
| SS+TR, mm | 0.20 (−0.47, 0.87) | −1.03 (−1.82, −0.24) | −0.57 (−1.35, 0.21) |
| Leptin, ng/mL | 0.24 (−0.48, 0.95) | −0.40 (−1.26, 0.45) | −0.73 (−1.61, 0.14) |
| Central adiposity | |||
| DXA trunk fat mass index, kg/m2 | 0.02 (−0.05, 0.08) | −0.07 (−0.16, 0.01) | −0.08 (−0.16, 0.00) |
| Waist circumference, cm | 0.25 (−0.31, 0.82) | −0.87 (−1.54, −0.21) | −0.54 (−1.19, 0.12) |
| SS:TR ratio | 0.01 (−0.01, 0.02) | −0.01 (−0.02, 0.01) | 0.00 (−0.02, 0.01) |
| Global metabolic risk, z‐score | 0.06 (−0.01, 0.13) | −0.02 (−0.10, 0.06) | −0.02 (−0.10, 0.07) |
BMI indicates body mass index; CI, confidence interval; DBP, diastolic blood pressure; DXA, dual‐energy x‐ray absorptiometry scan; HDL, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment of insulin resistance; hsCRP, high sensitivity C‐reactive protein; IL‐6, interleukin 6; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SS:TR, ratio of subscapular (SS) and triceps skinfold thicknesses (TR); SS+TR, sum of subscapular and triceps skinfold thicknesses; Trim, trimester.
All estimates adjusted for child age at outcome and sex, maternal race/ethnicity, age at enrollment, height, prepregnancy BMI, education, household income, parity, and smoking during pregnancy, except for models predicting SBP, DPB, and BMI z‐scores, which already incorporate age and sex and thus were not additionally adjusted for child sex and age at outcome.
Discussion
In this prospective study we found evidence that prenatal exposure to PE or GH was associated with generally better cardiometabolic health including higher HDL, lower leptin, overall adiposity, central adiposity, and lower global metabolic risk z‐score. Mean maternal BP across all 3 trimesters was associated with higher offspring SBP. Second‐ and third‐trimester maternal BPs were each associated with lower overall and central adiposity.
Our findings corroborate several studies in which GH (in participants ranging from 9 to 69 years of age) was associated with higher offspring BP; these were studies in which GH was examined both separately from and in conjunction with PE. In the ALSPAC (Avon Longitudinal Study of Parents and Children) cohort from the United Kingdom, for example, SBP and DBP were both higher in the offspring of mothers with GH (mean difference 2.06 mm Hg; 95% CI 1.28, 2.84 and 1.11 mg; 95% CI: 0.54, 1.69) and PE (1.12 mm Hg; 95% CI −0.89, 3.12 mg and 1.71 mm Hg; 95% CI 0.23, 3.17) compared to mothers without HDP. The proposed physiological bases of the association between HDP and offspring health include abnormal placental implantation, inflammatory biomarkers during pregnancy, maternal glucocorticoid metabolism, and exogenous glucocorticoid exposure.24 These associations are likely at least in part attributable to common genetic background, and shared familial behaviors and environmental exposures that could influence both mother and offspring.24
Contrary to previous literature, we found evidence of a long‐term association of HDP with higher offspring HDL cholesterol, lower leptin, and lower global metabolic risk z‐score. These results were unexpected in that we had hypothesized that HDP would be predictive of a worse overall metabolic profile, not a healthier one.10 We identified 7 studies that provided data on blood biomarkers such as total cholesterol, triglycerides, high‐density and low‐density lipoprotein, glucose, insulin, insulin resistance, C‐reactive protein, interleukin‐6, and apolipoprotein A1 and B in participants up to 20 years of age.25, 26, 27, 28, 29, 30, 31 These studies showed no evidence of PE or GH being associated with HDL. Similarly, in the 4 studies that assessed fasting glucose, there were no observable differences in serum insulin, glucose, or glucose‐to‐insulin ratio.29, 30, 32, 33 There is currently limited evidence that PE or GH exposure leads to higher risk of metabolic syndrome in the offspring or differences in circulating lipids or glucose tolerance, beyond, acute transient changes in cord blood samples.9, 34 Authors of these studies suggested that a possible reason for the overall null associations for the metabolic outcomes studied is that their study populations were relatively young (ages 12 to 20), and metabolic derangements may not manifest until later in the life course.
A handful of studies have examined the association between HDP and offspring body composition at midchildhood.30, 31, 35, 36 The results among these studies are heterogeneous. Two of these articles did not detect an association between intrauterine exposure to PE and anthropometric measurements including BMI, height, weight, waist circumference, hip circumference, and waist‐to‐hip ratio in children 5 to 13 years of age.30, 36 Another examined the association between GH and anthropometric measurements and also had null findings.30 In a systematic review and meta‐analysis of 8 studies comprising 39 611 participants, Davis et al report that among offspring born to mothers with PE, BMI was 0.62 kg/m2 higher (95% CI 0.41, 0.84); however, no difference was evident in studies that included only children aged <10 years (0.15; 95% CI −0.3 to 0.64). Our findings, however, mirror those of the Geelhoed et al examination of the ALSPAC cohort in which they uncovered inverse associations of PE (but not GH) with BMI z‐score, waist circumference z‐score, fat mass z‐score, and lean mass z‐score.26 Similar to our findings, the initially null associations of PE with all measures of offspring adiposity were rendered inverse following adjustment for potential confounding factors; the main covariate attributable to this change was maternal prepregnancy BMI. Similar observations were uncovered with the association between second‐ and third‐trimester maternal BP and offspring body composition measures. These findings suggest that maternal gestational BP may program smaller offspring body size, corroborated by findings from the Mendelian Randomization study in which genetically elevated maternal SBP was related to lower birth weight,37 suggesting that this relationship is likely causal rather than confounded by shared behaviors or environment.
A recent publication from the HUNT study in Norway that measured outcomes at a mean age of 29 years found that HDP exposure (assessed by collection of administrative data from the Medical Birth Registry of Norway) was associated with more adverse levels for a number of offspring cardiovascular risk factors.38 Intrauterine exposure to maternal gestational hypertension or term preeclampsia was associated with higher systolic and diastolic blood pressure, BMI, and waist circumference, and in the term preeclampsia group, non‐HDL cholesterol and triglyceride concentrations were slightly higher. However, the HUNT study did not have information on maternal early‐pregnancy BP, BMI, or other factors during pregnancy. In an analysis of a subset with information on these maternal characteristics collected before or after pregnancy, effect estimates for BMI and waist circumference were attenuated by 80% to 90% and no longer significant, and effect estimates for blood pressure were attenuated by 60% to 70%. Thus, it is likely that maternal prenatal health is a major confounder of this association, and our study offers the advantage of contemporaneous assessment of these important factors.
Our study has some limitations. First, there is likely to be some unmeasured confounding, although we accounted for many key sociodemographic and perinatal characteristics. As with many longitudinal cohort studies, there may be attrition bias, as participants who remained in the study through midchildhood follow‐up were more likely to be white and to include a larger proportion of mothers who were college graduates and nonsmokers with higher annual income. Our findings may have limited generalizability because all Project Viva participants lived in Massachusetts, had health insurance, and were generally of higher socioeconomic status. Another limitation of this study is that we did not consider the severity of preeclampsia or timeline of the onset of disease. There is literature that suggests that early‐ and late‐onset PE may represent distinct pathogenic conditions that may result in differential offspring outcomes.39 The definition of PE has become more comprehensive since our cohort participants were pregnant; we defined PE as elevated blood pressure with proteinuria, whereas the definition now also includes other signs of end‐organ dysfunction including renal insufficiency, liver disease, neurological problems, hematological disturbances, or fetal growth restriction. In addition, we cannot discount the possibility of chance findings given the large numbers of models tested. However, many of the midchildhood outcomes are measurements of the same biological indicator (DXA total fat mass, fat mass index, and BMI z‐scores are measures of overall adiposity), and the fact that we observed similar associations across many outcomes supports the validity of our findings. We intentionally did not adjust for multiple comparisons, as the outcome domains we studied are not distinct.
An advantage of this study is the longitudinal design. We used research standard outcome measures including direct assessment of adiposity with DXA and research‐quality blood pressure and biomarker measurements, we considered a large number of covariates, and we included not only GH and PE but also maternal BP in each trimester of pregnancy.
Conclusion
The findings of this study suggest that offspring of mothers experiencing HDP, as well as those with higher second‐ and third‐trimester blood pressures, have somewhat higher SBP but otherwise better cardiometabolic health in midchildhood, including higher HDL, lower leptin and global metabolic risk, as well as lower overall measures of central and overall adiposity. Associations with higher offspring BP were attenuated with adjustment for maternal characteristics. Ongoing follow‐up of children into adulthood will help us to further understand long‐term outcomes among offspring prenatally exposed to hypertensive disorders during pregnancy.
Sources of Funding
Project Viva is supported by funding from the US National Institutes of Health (R01 HD034568, K24 HD069408, UG3OD023286). Ms Tripathi was supported by the Yale School of Public Health Weinerman Fellowship.
Disclosures
None.
Acknowledgments
We are indebted to the mothers and children of Project Viva for their generous participation and appreciate the invaluable assistance of past and present Project Viva staff. Ms Rifas‐Shiman and Ms Tripathi had full access to all the data in the study and take responsibility for its integrity and the data analysis.
(J Am Heart Assoc. 2018;7:e007426 DOI: 10.1161/JAHA.117.007426.)29382664
References
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 2. Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30:S1–S48.18817592 [Google Scholar]
- 3. Davis EF, Newton L, Lewandowski AJ, Lazdam M, Kelly BA, Kyriakou T, Leeson P. Pre‐eclampsia and offspring cardiovascular health: mechanistic insights from experimental studies. Clin Sci (Lond). 2012;123:53–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper DW, Brennecke SP, Wilton AN. Genetics of pre‐eclampsia. Hypertens Pregnancy. 1993;12:1–23. [Google Scholar]
- 5. Wagner SJ, Barac S, Garovic VD. Hypertensive pregnancy disorders: current concepts. J Clin Hypertens (Greenwich). 2007;9:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barton JR, O'Brien JM, Bergauer NK, Jacques DL, Sibai BM. Mild gestational hypertension remote from term: progression and outcome. Am J Obstet Gynecol. 2001;184:979–983. [DOI] [PubMed] [Google Scholar]
- 8. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre‐eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40:1176–1180. [DOI] [PubMed] [Google Scholar]
- 9. Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I, Redman C, Leeson P. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e1561. [DOI] [PubMed] [Google Scholar]
- 10. Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori‐Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation. 2010;122:488–494. [DOI] [PubMed] [Google Scholar]
- 11. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich‐Edwards JW, Rifas‐Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA Jr, Huh SY, Mantzoros C, Parker MG, Gillman MW. Cohort profile: Project Viva. Int J Epidemiol. 2015;44:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hivert MF, Rifas‐Shiman SL, Gillman MW, Oken E. Greater early and mid‐pregnancy gestational weight gains are associated with excess adiposity in mid‐childhood. Obesity (Silver Spring). 2016;24:1546–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 14. Oken E, Ning Y, Rifas‐Shiman SL, Rich‐Edwards JW, Olsen SF, Gillman MW. Diet during pregnancy and risk of preeclampsia or gestational hypertension. Ann Epidemiol. 2007;17:663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–1057. [DOI] [PubMed] [Google Scholar]
- 16. Falkner B, Daniels SR. Summary of the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Hypertension. 2004;44:387–388. [DOI] [PubMed] [Google Scholar]
- 17. Cespedes EM, Rifas‐Shiman SL, Redline S, Gillman MW, Pena MM, Taveras EM. Longitudinal associations of sleep curtailment with metabolic risk in mid‐childhood. Obesity (Silver Spring). 2014;22:2586–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viitasalo A, Lakka TA, Laaksonen DE, Savonen K, Lakka HM, Hassinen M, Komulainen P, Tompuri T, Kurl S, Laukkanen JA, Rauramaa R. Validation of metabolic syndrome score by confirmatory factor analysis in children and adults and prediction of cardiometabolic outcomes in adults. Diabetologia. 2014;57:940–949. [DOI] [PubMed] [Google Scholar]
- 19. De Ferranti SD, Osganian SK. Epidemiology of paediatric metabolic syndrome and type 2 diabetes mellitus. Diab Vasc Dis Res. 2007;4:285–296. [DOI] [PubMed] [Google Scholar]
- 20. Eisenmann JC, Laurson KR, DuBose KD, Smith BK, Donnelly JE. Construct validity of a continuous metabolic syndrome score in children. Diabetol Metab Syndr. 2010;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly AS, Steinberger J, Jacobs DR, Hong CP, Moran A, Sinaiko AR. Predicting cardiovascular risk in young adulthood from the metabolic syndrome, its component risk factors, and a cluster score in childhood. Int J Pediatr Obes. 2011;6:e283–e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuczmarski RJ, Ogden CL, Guo SS, Grummer‐Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002; 11:1–190. [PubMed] [Google Scholar]
- 23. Oken E, Kleinman KP, Rich‐Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinheiro TV, Brunetto S, Ramos JG, Bernardi JR, Goldani MZ. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis. 2016;7:391–407. [DOI] [PubMed] [Google Scholar]
- 25. Oglaend B, Forman MR, Romundstad PR, Nilsen ST, Vatten LJ. Blood pressure in early adolescence in the offspring of preeclamptic and normotensive pregnancies. J Hypertens. 2009;27:2051–2054. [DOI] [PubMed] [Google Scholar]
- 26. Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, Nelson SM, Lawlor DA. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. 2011;58:63–69. [DOI] [PubMed] [Google Scholar]
- 28. Lawlor DA, Macdonald‐Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, Sattar N, Deanfield J. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fraser A, Nelson SM, Macdonald‐Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miettola S, Hartikainen AL, Vaarasmaki M, Bloigu A, Ruokonen A, Jarvelin MR, Pouta A. Offspring's blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol. 2013;28:87–98. [DOI] [PubMed] [Google Scholar]
- 31. Miettola S, Hovi P, Andersson S, Strang‐Karlsson S, Pouta A, Laivuori H, Jarvenpaa AL, Eriksson JG, Makitie O, Kajantie E. Maternal preeclampsia and bone mineral density of the adult offspring. Am J Obstet Gynecol. 2013;209:443.e1–443.e10. [DOI] [PubMed] [Google Scholar]
- 32. Alsnes IV, Janszky I, Forman MR, Vatten LJ, Okland I. A population‐based study of associations between preeclampsia and later cardiovascular risk factors. Am J Obstet Gynecol. 2014;211:657.e1– 657.e7. [DOI] [PubMed] [Google Scholar]
- 33. Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. [DOI] [PubMed] [Google Scholar]
- 34. Libby G, Murphy DJ, McEwan NF, Greene SA, Forsyth JS, Chien PW, Morris AD. Pre‐eclampsia and the later development of type 2 diabetes in mothers and their children: an intergenerational study from the Walker cohort. Diabetologia. 2007;50:523–530. [DOI] [PubMed] [Google Scholar]
- 35. Lazdam M, de la Horra A, Diesch J, Kenworthy Y, Davis E, Lewandowski AJ, Szmigielski C, Shore A, Mackillop L, Kharbanda R, Alp N, Redman C, Kelly B, Leeson P. Unique blood pressure characteristics in mother and offspring after early onset preeclampsia. Hypertension. 2012;60:1338–1345. [DOI] [PubMed] [Google Scholar]
- 36. Kvehaugen AS, Andersen LF, Staff AC. Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstet Gynecol Scand. 2010;89:1478–1485. [DOI] [PubMed] [Google Scholar]
- 37. Tyrrell J, Richmond RC, Palmer TM, Feenstra B, Rangarajan J, Metrustry S, Cavadino A, Paternoster L, Armstrong LL, De Silva NM, Wood AR, Horikoshi M, Geller F, Myhre R, Bradfield JP, Kreiner‐Moller E, Huikari V, Painter JN, Hottenga JJ, Allard C, Berry DJ, Bouchard L, Das S, Evans DM, Hakonarson H, Hayes MG, Heikkinen J, Hofman A, Knight B, Lind PA, McCarthy MI, McMahon G, Medland SE, Melbye M, Morris AP, Nodzenski M, Reichetzeder C, Ring SM, Sebert S, Sengpiel V, Sorensen TI, Willemsen G, de Geus EJ, Martin NG, Spector TD, Power C, Jarvelin MR, Bisgaard H, Grant SF, Nohr EA, Jaddoe VW, Jacobsson B, Murray JC, Hocher B, Hattersley AT, Scholtens DM, Davey Smith G, Hivert MF, Felix JF, Hypponen E, Lowe WL Jr, Frayling TM, Lawlor DA, Freathy RM; Early Growth Genetics (EGG) Consortium . Genetic evidence for causal relationships between maternal obesity‐related traits and birth weight. JAMA. 2016;315:1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alsnes IV, Vatten LJ, Fraser A, Bjorngaard JH, Rich‐Edwards J, Romundstad PR, Asvold BO. Hypertension in pregnancy and offspring cardiovascular risk in young adulthood: prospective and sibling studies in the HUNT study (Nord‐Trondelag Health Study) in Norway. Hypertension. 2017;69:591–598. [DOI] [PubMed] [Google Scholar]
- 39. Vatten LJ, Skjaerven R. Is pre‐eclampsia more than one disease? BJOG. 2004;111:298–302. [DOI] [PubMed] [Google Scholar]


