Abstract
Background
Dyslipidemia is a major risk factor for cardiovascular events. The prognostic importance of lipoproteins in patients with atrial fibrillation is not well understood. We aimed to explore the association between apolipoprotein A1 (ApoA1) and B (ApoB) and cardiovascular events in patients with atrial fibrillation receiving oral anticoagulation.
Methods and Results
Using data from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, ApoA1 and ApoB plasma levels were measured at baseline in 14 884 atrial fibrillation patients. Median length of follow‐up was 1.9 years. Relationships between continuous levels of ApoA1 and ApoB and clinical outcomes were evaluated using Cox models adjusted for cardiovascular risk factors, medication including statins, and cardiovascular biomarkers. A composite ischemic outcome (ischemic stroke, systemic embolism, myocardial infarction, and cardiovascular death) was used as the primary end point. Median (25th, 75th) ApoA1 and ApoB levels were 1.10 (0.93, 1.30) and 0.70 g/L (0.55, 0.85), respectively. In adjusted analyses, higher levels of ApoA1 were independently associated with a lower risk of the composite ischemic outcome (hazard ratio, 0.81; P<0.0001). Similar results were observed for the individual components of the composite outcome. ApoB was not significantly associated with the composite ischemic outcome (P=0.8240). Neither apolipoprotein was significantly associated with major bleeding. There was no interaction between lipoproteins and randomized treatment for the primary outcome (both P values ≥0.2448).
Conclusions
In patients with atrial fibrillation on oral anticoagulation, higher levels of ApoA1 were independently associated with lower risk of ischemic cardiovascular outcomes. Investigating therapies targeting dyslipidemia may thus be useful to improve cardiovascular outcomes in patients with atrial fibrillation.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00412984.
Keywords: atrial fibrillation, biomarkers, cardiovascular disease, cerebrovascular disease/stroke
Subject Categories: Atrial Fibrillation, Biomarkers, Cardiovascular Disease, Cerebrovascular Disease/Stroke
Clinical Perspective
What Is New?
In patients with atrial fibrillation treated with oral anticoagulation, higher levels of apolipoprotein A1 were independently associated with lower risk of ischemic cardiovascular outcomes, including stroke/systemic embolic event and mortality, and higher apolipoprotein B levels were associated with higher rates of myocardial infarction.
What Are the Clinical Implications?
These data provide a better understanding of the risks associated with dyslipidemia in patients with atrial fibrillation and suggest that investigating therapies targeting dyslipidemia may play a role in improving cardiovascular outcomes in atrial fibrillation.
Introduction
Atrial fibrillation (AF) is associated with an increased risk of stroke, mortality, and health costs worldwide.1, 2 Biomarkers have shown increasing promise in improving risk prediction in AF.3, 4 Elevated levels of natriuretic peptides and troponins, signifying myocardial damage and stress, have each shown to more than double the risk of stroke and all‐cause mortality.5, 6 Other biomarkers, such as the marker for oxidative stress and inflammation, growth differentiation factor 15, have been reported to double the risk for major bleeding and death by approximately the same amount when elevated.7 However, the predictive role of more‐traditional biomarkers, such as those that are components of dyslipidemia, is less clear.
Dyslipidemia is known to promote atherosclerosis. It is a complex disease and is a major risk factor for adverse cardiovascular events.8, 9, 10 High levels of low‐density lipoprotein (LDL) and low levels of high‐density lipoprotein (HDL) are associated with myocardial infarction (MI) and stroke.11, 12, 13 The relation between dyslipidemia and cardiovascular outcomes and its role as a risk factor in patients with AF treated with oral anticoagulation therapy have not been previously examined.
The aim of this substudy from the biomarker population of the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial was therefore to assess the association between the concentration of apolipoprotein A1 (ApoA1), the main protein component of high‐density lipoprotein (HDL), and apolipoprotein B (ApoB) the main protein component of LDL, at baseline and clinical outcomes after adjusting for cardiovascular risk factors as well as other relevant biomarkers that have shown prognostic value for adverse events in AF.5, 6, 7, 14, 15
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Population and Trial Design
The present study population consisted of participants from the ARISTOTLE trial; a multicenter, double‐blind, double‐dummy, randomized, clinical trial which enrolled 18 201 patients with AF and at least 1 additional risk factor for stroke or systemic embolism (systemic embolic event; SEE). The details and outcomes of the ARISTOTLE trial have been described and published previously.16, 17 All patients were randomized to receive either warfarin or apixaban for stroke prevention in a 1:1 fashion. The apolipoprotein biomarker substudy cohort comprised of the first included 14 884 patients, and the median length of follow‐up was 1.9 years. Overall, the ARISTOTLE biomarker cohort was representative of the full study cohort and has been described in detail previously.18 Approval by the appropriate ethics committees was obtained at all sites. All patients provided written informed consent.
End Points
The primary outcome of this biomarker analysis was a composite of ischemic stroke, SEE, MI, and cardiovascular death. Other evaluated outcomes were the individual constituents of the composite ischemic outcome, all‐cause mortality and major bleeding, according to the International Society on Thrombosis and Haemostasis criteria.16 All outcomes were centrally adjudicated as previously described.16, 17
Biomarker Collection, Storage, and Laboratory Methods
Venous blood samples were obtained before study drug administration from enrolled patients in the biomarker study of the ARISTOTLE trial. Samples were stored in aliquots at −70°C and subsequently transferred to the Clinical Chemistry Laboratory at Uppsala University Hospital (Uppsala, Sweden) for analysis. Apolipoproteins were analyzed using a particle‐enhanced immunoturbidimetric assay (Abbott, Abbott Park, IL).
Analysis of high‐sensitivity cardiac troponin T, N‐terminal pro‐B‐type natriuretic peptide, growth‐differentiation factor‐15, cystatin C, interleukin 6 (IL‐6), and C‐reactive protein have been described in detail previously.5, 6, 7, 14, 15
Statistical Analysis
In total, 14 884 patients in the ARISTOTLE study had apolipoproteins measured at baseline and were included in our analysis. Demographics and other baseline characteristics were summarized using frequencies for categorical variables and median and 25th and 75th percentiles for continuous variables. For tests of differences among groups, the χ2 test was used for categorical variables and the Kruskal–Wallis test was used for continuous variables.
The association between baseline apolipoprotein levels and adverse outcomes was studied using multivariable Cox proportional‐hazards models with apolipoprotein as continuous variables. Patients were followed until the respective event occurred or, if the event did not occur, were censored at end of study or at death (for nonfatal outcomes). Results are presented showing hazard ratio per interquartile change, that is, identical to comparing the 75th with the 25th percentile, of the respective apolipoprotein sample distribution, or, in other words, a difference in apolipoprotein levels that contains the inner half of the sample values.19 The first model (model 1) was adjusted for baseline characteristics and clinical risk factors; age, sex, body mass index, smoking status, systolic blood pressure, AF type, creatinine clearance, diabetes mellitus, heart failure, previous stroke/systemic embolism (systemic embolic event; SEE)/transient ischemic attack, hypertension, use of warfarin within 7 days before randomization, randomized treatment (apixaban/warfarin), use of statin medication within 30 days before randomization, treatment at randomization with aspirin, treatment with angiotensin‐converting enzyme inhibitors, or angiotensin II receptor blocker. The second Cox model (model 2) was further adjusted for other prognostic biomarkers, all log‐transformed: C‐reactive protein, IL‐6, high‐sensitivity cardiac troponin T, cystatin C, and N‐terminal pro‐B‐type natriuretic peptide. For the major bleeding outcome, additional adjustments were made for past bleeding and hemoglobin in model 2, and log‐transformed growth differentiation factor 15 level in model 3. Kaplan–Meier estimates of the cumulative risk to the first occurrence of an event were plotted. All statistical tests were 2‐tailed and performed at the 0.05 significance level. Interaction between study treatment (apixaban or warfarin) and apolipoprotein level was analyzed using Cox proportional‐hazards models including study treatment group, apolipoprotein, and treatment by apolipoprotein interaction as covariates. Given that statin therapy may affect ApoB levels substantially, sensitivity analyses were performed for the association of ApoB to outcomes in patients without any statin therapy at baseline (n=8420). Because all analyses were exploratory, there were no adjustments for multiple comparisons. The Biostatistics section at Uppsala Clinical Research Center conducted the statistical analyses.
Results
Baseline demographics and clinical characteristics of the study population in relation to apolipoprotein levels are summarized in Tables 1 and 2. In summary, the median age was 70 years and ≈64% were male. The median (25th, 75th) ApoA1 concentration was 1.10 g/L (0.94, 1.30). For ApoB, the median (25th, 75th) was 0.70 g/L (0.55, 0.85). Most clinical characteristics, treatment, and risk factors for stroke were associated with both ApoA1 and ApoB. In multivariable models (Table 3), low levels of ApoA1 were most strongly associated with male sex, higher IL‐6 levels, and permanent or persistent AF (P<0.0001 for all). High levels of ApoA1 were most strongly associated with a better renal function, older age, and higher hemoglobin levels. For ApoB, low levels were more strongly associated with statin therapy, male sex, higher growth differentiation factor 15 levels, and higher IL‐6 levels (P<0.0001 for all; Table 4). High levels of ApoB were most strongly associated with a better renal function and higher hemoglobin levels (P<0.0001 for both).
Table 1.
Baseline Characteristics of Participants in Relation to ApoA1 Levels
| ApoA1 Level, g/L | P Valuea | ||||
|---|---|---|---|---|---|
| ≤0.94 | >0.94 to 1.1 | >1.1 to 1.3 | >1.3 | ||
| n | 3823 | 4521 | 3728 | 2812 | |
| Age, y median (Q1, Q3) | 70.0 (62.0, 76.0) | 69.0 (62.0, 76.0) | 70.0 (63.0, 76.0) | 71.0 (64.0, 76.0) | <0.0001 |
| Male | 2823 (73.8%) | 3085 (68.2%) | 2329 (62.5%) | 1347 (47.9%) | <0.0001 |
| Weight, kg, median (Q1, Q3) | 83.5 (70.4, 97.5) | 84.0 (71.0, 98.0) | 82.0 (70.0, 95.0) | 78.5 (67.1, 90.0) | <0.0001 |
| Permanent or persistent AF | 3387 (88.6%) | 3848 (85.2%) | 3109 (83.4%) | 2287 (81.3%) | <0.0001 |
| Heart failure | 1588 (41.5%) | 1661 (36.7%) | 1257 (33.7%) | 833 (29.6%) | <0.0001 |
| Hypertension | 3327 (87.0%) | 3957 (87.5%) | 3277 (87.9%) | 2467 (87.7%) | 0.6897 |
| Age ≥75 y | 1142 (29.9%) | 1329 (29.4%) | 1169 (31.4%) | 922 (32.8%) | <0.0001 |
| Diabetes mellitus | 1098 (28.7%) | 1244 (27.5%) | 806 (21.6%) | 532 (18.9%) | <0.0001 |
| Previous stroke or TIA | 728 (19.0%) | 860 (19.0%) | 691 (18.5%) | 514 (18.3%) | 0.8122 |
| MI | 617 (16.1%) | 575 (12.7%) | 452 (12.1%) | 269 (9.6%) | <0.0001 |
| Previous PCI/CABG | 594 (15.5%) | 621 (13.7%) | 490 (13.1%) | 314 (11.2%) | <0.0001 |
| Peripheral arterial disease | 200 (5.2%) | 227 (5.0%) | 182 (4.9%) | 115 (4.1%) | 0.1714 |
| Age 65 to 75 y | 1467 (38.4%) | 1767 (39.1%) | 1448 (38.8%) | 1153 (41.0%) | 0.1606 |
| CHA2DS2VASc‐score, median (Q1, Q3) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.0138 |
| Aspirin | 1289 (33.7%) | 1441 (31.9%) | 1087 (29.2%) | 782 (27.8%) | <0.0001 |
| ACEi or ARB | 2766 (75.1%) | 3224 (74.0%) | 2636 (73.9%) | 1941 (71.9%) | <0.0001 |
| Beta‐blocker | 2558 (69.4%) | 2935 (67.4%) | 2384 (66.9%) | 1678 (62.2%) | <0.0001 |
| Calcium‐channel blocker | 1085 (29.4%) | 1378 (31.6%) | 1122 (31.5%) | 962 (35.7%) | <0.0001 |
| Digoxin | 1374 (37.3%) | 1494 (34.3%) | 1105 (31.0%) | 784 (29.1%) | <0.0001 |
| Statin treatment | 1644 (43.0%) | 1941 (42.9%) | 1633 (43.8%) | 1246 (44.3%) | 0.6069 |
| Creatinine clearance (mL/min), median (Q1, Q3) | 74.8 (57.2, 97.1) | 75.3 (57.3, 98.8) | 74.4 (57.3, 95.3) | 71.1 (54.4, 89.4) | <0.0001 |
| CRP (mg/L), median (Q1, Q3) | 2.2 (1.0, 5.2) | 2.4 (1.1, 5.2) | 2.1 (1.0, 4.4) | 2.1 (1.0, 4.3) | <0.0001 |
| Cystatin C (mg/L), median (Q1, Q3) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | <0.0001 |
| GDF‐15 (ng/L), median (Q1, Q3) | 1477.0 (996.5, 2266.5) | 1414.0 (985.5, 2155.5) | 1310.0 (957.0, 1901.0) | 1331.5 (978.0, 1875.2) | <0.0001 |
| IL‐6 (ng/L), median (Q1, Q3) | 2.8 (1.8, 5.0) | 2.4 (1.6, 4.1) | 2.1 (1.4, 3.5) | 2.0 (1.3, 3.1) | <0.0001 |
| NT‐proBNP (ng/L), median (Q1, Q3) | 695.0 (361.0, 1258.5) | 728.0 (367.0, 1293.0) | 716.0 (364.0, 1237.2) | 711.5 (356.0, 1204.2) | 0.5783 |
| cTnT‐hs (ng/L), median (Q1, Q3) | 11.5 (7.7, 17.9) | 11.2 (7.6, 16.8) | 10.7 (7.4, 16.1) | 10.4 (7.4, 15.4) | <0.0001 |
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ApoA1, apolipoprotein A1; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft; CHD, congestive heart disease; CrCL, creatinine clearance; CRP, C‐reactive protein; cTnT‐hs, high‐sensitivity cardiac troponin T; GDF‐15, growth differentiation factor 15; IL‐6, interleukin 6; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; Q, quartile; TIA, transient ischemic attack.
Tests used: Pearson's χ2 test for the CHA2DS2‐VASc score and for statin treatment, all other by the Kruskal–Wallis test.
Table 2.
Baseline Characteristics of Participants in Relation to ApoB Levels
| ApoB Level, g/L | P Valuea | ||||
|---|---|---|---|---|---|
| ≤0.55 | >0.55 to 0.7 | >0.7 to 0.85 | >0.85 | ||
| n | 3747 | 3951 | 3504 | 3682 | |
| Age, y, median (Q1, Q3) | 72.0 (65.0, 78.0) | 70.0 (64.0, 76.0) | 69.0 (62.0, 75.0) | 68.0 (60.0, 74.0) | <0.0001 |
| Male | 2511 (67.0%) | 2583 (65.4%) | 2195 (62.6%) | 2294 (62.3%) | <0.0001 |
| Weight, kg, median (Q1, Q3) | 80.5 (68.0, 94.5) | 82.0 (70.0, 95.3) | 82.0 (70.0, 95.3) | 83.5 (71.6, 97.0) | <0.0001 |
| Permanent or persistent AF | 3212 (85.7%) | 3408 (86.3%) | 2971 (84.8%) | 3040 (82.6%) | <0.0001 |
| Heart failure | 1281 (34.2%) | 1346 (34.1%) | 1241 (35.4%) | 1471 (40.0%) | <0.0001 |
| Hypertension | 3269 (87.2%) | 3461 (87.6%) | 3033 (86.6%) | 3265 (88.7%) | 0.0515 |
| Age ≥75 y | 1463 (39.0%) | 1290 (32.6%) | 977 (27.9%) | 833 (22.6%) | <0.0001 |
| Diabetes mellitus | 1097 (29.3%) | 1027 (26.0%) | 823 (23.5%) | 733 (19.9%) | <0.0001 |
| Previous stroke or TIA | 774 (20.7%) | 775 (19.6%) | 633 (18.1%) | 613 (16.6%) | <0.0001 |
| MI | 647 (17.3%) | 544 (13.8%) | 354 (10.1%) | 369 (10.0%) | <0.0001 |
| Previous PCI/CABG | 774 (20.7%) | 636 (16.1%) | 357 (10.2%) | 252 (6.8%) | <0.0001 |
| Peripheral arterial disease | 211 (5.6%) | 220 (5.6%) | 140 (4.0%) | 152 (4.1%) | 0.0003 |
| Age 65 to 75 y | 1451 (38.7%) | 1576 (39.9%) | 1406 (40.1%) | 1402 (38.1%) | <0.0001 |
| CHA2DS2VASc‐score, median (Q1, Q3) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | <0.0001 |
| Aspirin | 1657 (33.0%) | 906 (30.7%) | 1298 (29.9%) | 733 (28.6%) | 0.0003 |
| ACEi or ARB | 3577 (74.3%) | 2060 (73.1%) | 3087 (73.7%) | 1831 (74.2%) | <0.0001 |
| Beta‐blocker | 3105 (64.5%) | 1866 (66.2%) | 2851 (68.0%) | 1721 (69.8%) | <0.0001 |
| Calcium‐channel blocker | 1765 (36.7%) | 896 (31.8%) | 1242 (29.6%) | 641 (26.0%) | <0.0001 |
| Digoxin | 1300 (27.0%) | 909 (32.2%) | 1503 (35.9%) | 1043 (42.3%) | <0.0001 |
| Statin treatment | 2323 (62.0%) | 1965 (49.7%) | 1278 (36.5%) | 898 (24.4%) | <0.0001 |
| Creatinine clearance (mL/min), median (Q1, Q3) | 70.0 (53.3, 90.0) | 73.2 (56.3, 92.9) | 75.4 (58.5, 96.7) | 78.5 (60.1, 101.5) | <0.0001 |
| CRP (mg/L), median (Q1, Q3) | 1.6 (0.8, 3.8) | 2.1 (1.0, 4.6) | 2.4 (1.2, 5.2) | 2.8 (1.4, 5.6) | <0.0001 |
| Cystatin C (mg/L), median (Q1, Q3) | 0.9 (0.7, 1.1) | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | <0.0001 |
| GDF‐15 (ng/L), median (Q1, Q3) | 1520.0 (1050.0, 2319.0) | 1457.0 (995.0, 2118.0) | 1330.0 (966.8, 1965.0) | 1256.0 (915.0, 1836.8) | <0.0001 |
| IL‐6 (ng/L), median (Q1, Q3) | 2.5 (1.6, 4.1) | 2.3 (1.5, 3.9) | 2.3 (1.5, 3.8) | 2.3 (1.5, 3.8) | <0.0001 |
| NT‐proBNP (ng/L), median (Q1, Q3) | 740.0 (381.0, 1299.0) | 739.0 (382.0, 1290.8) | 701.0 (365.5, 1221.0) | 672.5 (331.0, 1183.0) | <0.0001 |
| cTnT‐hs (ng/L), median (Q1, Q3) | 11.6 (7.9, 17.7) | 11.2 (7.6, 17.0) | 10.6 (7.3, 15.9) | 10.4 (7.3, 15.7) | <0.0001 |
ACEi indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ApoB, apolipoprotein B; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft; CHD, congestive heart disease; CrCL, creatinine clearance; CRP, C‐reactive protein; cTnT‐hs, high‐sensitivity cardiac troponin T; GDF‐15, growth differentiation factor 15; IL‐6, interleukin 6; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PCI, percutaneous coronary intervention; Q, quartile; TIA, transient ischemic attack.
Tests used: Pearson's χ2 test for the CHA2DS2‐VASc score and for statin treatment, all other by the Kruskal–Wallis test.
Table 3.
Baseline Characteristics With the Strongest Association on ApoA1 Level
| Variable | Comment | Ratio of Geometric Means (95% CI) | P Value |
|---|---|---|---|
| Age, y | 10‐y increase | 1.039 (1.034, 1.045) | <0.0001 |
| AF | Permanent vs persistent | 0.962 (0.952, 0.973) | <0.0001 |
| Creatinine clearance | 100% increase | 1.172 (1.158, 1.186) | <0.0001 |
| Hemoglobin, g/dL | Per 1‐g/dL increase | 1.024 (1.022, 1.027) | <0.0001 |
| IL‐6 | 100% increase | 0.954 (0.950, 0.958) | <0.0001 |
| Sex | Male vs female | 0.857 (0.850, 0.865) | <0.0001 |
The analysis is based on a model including all variables shown in Table 1. AF indicates atrial fibrillation; ApoA1, apolipoprotein A1; CI, confidence interval; IL‐6, interleukin 6.
Table 4.
Baseline Characteristics With the Strongest Association on ApoB Level
| Variable | Comment | Ratio of Geometric Means (95% CI) | P Value |
|---|---|---|---|
| Creatinine clearance | 100% increase | 1.105 (1.088, 1.123) | <0.0001 |
| GDF‐15 | 100% increase | 0.952 (0.945, 0.959) | <0.0001 |
| Hemoglobin, g/dL | Per 1‐g/dL increase | 1.051 (1.048, 1.055) | <0.0001 |
| IL‐6 | 100% increase | 0.959 (0.954, 0.964) | <0.0001 |
| Sex | Male vs female | 0.906 (0.896, 0.917) | <0.0001 |
| Statin treatment | Yes vs no | 0.856 (0.848, 0.865) | <0.0001 |
The analysis is based on a model including all variables shown in Table 1. ApoB indicates apolipoprotein B; CI, confidence interval; GDF‐15, growth‐differentiation factor‐15; IL‐6, interleukin 6.
Dyslipidemia in Relation to Composite Ischemic Outcome
There were a total of 883 events of the composite ischemic outcome consisting of ischemic stroke, SEE, MI, and cardiovascular death. Risk was substantially lower with higher baseline levels of ApoA1. In the fully adjusted analyses, ApoA1 was independently associated with a lower risk in the composite ischemic outcome with a hazard ratio (HR) of 0.81 (95% confidence interval [CI], 0.73–0.90; P<0.0001) per interquartile change (Table 5).
Table 5.
Association of ApoA1 at Baseline With Outcomes According to Continuous Levels of ApoA1
| n | Events | Unadjusted | Adjusted Clinical Risk Factors | Adjusted Clinical+Biomarkers | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Ischemic composite outcome | 14 884 | 883 (3.13) | 0.75 (0.69–0.82) | <0.0001 | 0.80 (0.72–0.87) | <0.0001 | 0.81 (0.73–0.90) | <0.0001 |
| Stroke or systemic embolism | 14 884 | 397 (1.41) | 0.83 (0.73–0.95) | 0.0080 | 0.83 (0.73–0.96) | 0.0094 | 0.84 (0.72–0.98) | 0.0248 |
| MI | 14 884 | 149 (0.52) | 0.85 (0.68–1.06) | 0.1522 | 0.89 (0.71–1.12) | 0.3200 | 0.86 (0.67–1.10) | 0.2356 |
| Major bleeding | 14 853 | 702 (2.74) | 0.87 (0.78–0.96) | 0.0065 | 0.91 (0.82–1.01) | 0.0768 | 0.90 (0.80–1.01) | 0.0724 |
| Cardiovascular death | 14 884 | 543 (1.88) | 0.69 (0.61–0.77) | <0.0001 | 0.75 (0.66–0.84) | <0.0001 | 0.78 (0.68–0.89) | 0.0002 |
| Death | 14 884 | 1068 (3.69) | 0.69 (0.64–0.75) | <0.0001 | 0.73 (0.67–0.80) | <0.0001 | 0.77 (0.70–0.85) | <0.0001 |
Three different proportional hazards model have been used, 1 without any adjustment, 1 adjusted for randomized treatment, demographic, and clinical risk factors, and 1 adjusted for randomized treatment, demographic, and clinical risk factors plus biomarkers. The demographic and clinical risk factors used were: age, sex, body mass index, smoking status, systolic blood pressure, atrial fibrillation type, creatinine clearance, diabetes mellitus, heart failure, previous stroke/systemic embolic event/transient ischemic attack, hypertension, randomized treatment, use of warfarin within 7 days of randomization and use of statin medication within 30 days before randomization, treatment at randomization with aspirin, and treatment with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blocker. The used biomarkers markers were high‐sensitivity cardiac troponin T, N‐terminal pro‐B‐type natriuretic peptide, cystatin C, C‐reactive protein, and interleukin 6. For major bleeding, past bleeding and hemoglobin were added to model 1, and growth differentiation factor 15 to model 2. Cox models based on continuous biomarker levels showing hazard ratio per interquartile change (eg, Q3 vs Q1). ApoA1 indicates apolipoprotein A1; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
ApoB was not statistically significantly associated with the risk of the composite ischemic outcome with an HR of 1.01 (95% CI, 0.92–1.12; P=0.8240; Table 6).
Table 6.
Association of ApoB at Baseline With Outcomes According to Continuous Levels of ApoB
| n | Events | Unadjusted | Adjusted Clinical Risk Factors | Adjusted Clinical+Biomarkers | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Ischemic composite outcome | 14 884 | 883 (3.13) | 0.94 (0.86–1.03) | 0.1609 | 1.00 (0.91–1.10) | 0.9841 | 1.01 (0.92–1.12) | 0.8240 |
| Stroke or systemic embolism | 14 884 | 397 (1.41) | 0.94 (0.83–1.07) | 0.3722 | 1.02 (0.89–1.18) | 0.7798 | 1.05 (0.90–1.21) | 0.5564 |
| MI | 14 884 | 149 (0.52) | 1.10 (0.89–1.35) | 0.3685 | 1.37 (1.10–1.71) | 0.0055 | 1.33 (1.06–1.68) | 0.0144 |
| Major bleeding | 14 853 | 702 (2.74) | 0.80 (0.72–0.88) | <0.0001 | 0.98 (0.87–1.10) | 0.7562 | 0.98 (0.86–1.11) | 0.7100 |
| Cardiovascular death | 14 884 | 543 (1.88) | 0.87 (0.78–0.98) | 0.0170 | 0.88 (0.78–0.99) | 0.0357 | 0.89 (0.79–1.02) | 0.0839 |
| Death | 14 884 | 1068 (3.69) | 0.81 (0.74–0.88) | <0.0001 | 0.84 (0.77–0.92) | 0.0002 | 0.84 (0.76–0.92) | 0.0002 |
Three different proportional hazards model have been used, 1 without any adjustment, 1 adjusted for randomized treatment, demographic, and clinical risk factors, and 1 adjusted for randomized treatment, demographic, and clinical risk factors plus biomarkers. The demographic and clinical risk factors used were: age, sex, body mass index, smoking status, systolic blood pressure, atrial fibrillation type, creatinine clearance, diabetes mellitus, heart failure, previous stroke/systemic embolic event/transient ischemic attack, hypertension, randomized treatment, use of warfarin within 7 days of randomization and use of statin medication within 30 days before randomization, treatment at randomization with aspirin, and treatment with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blocker. The used biomarkers markers were high‐sensitivity cardiac troponin T, N‐terminal pro‐B‐type natriuretic peptide, cystatin C, C‐reactive protein, and interleukin 6. For major bleeding, past bleeding and hemoglobin were added to model 1 and growth differentiation factor 15 to model 2. Cox models based on continuous biomarker levels showing hazard ratio per interquartile change (eg, Q3 vs Q1). ApoB indicates apolipoprotein B; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Kaplan–Meier plots illustrating the associations between the apolipoproteins and the composite ischemic outcome are shown in Figures 1 and 2.
Figure 1.
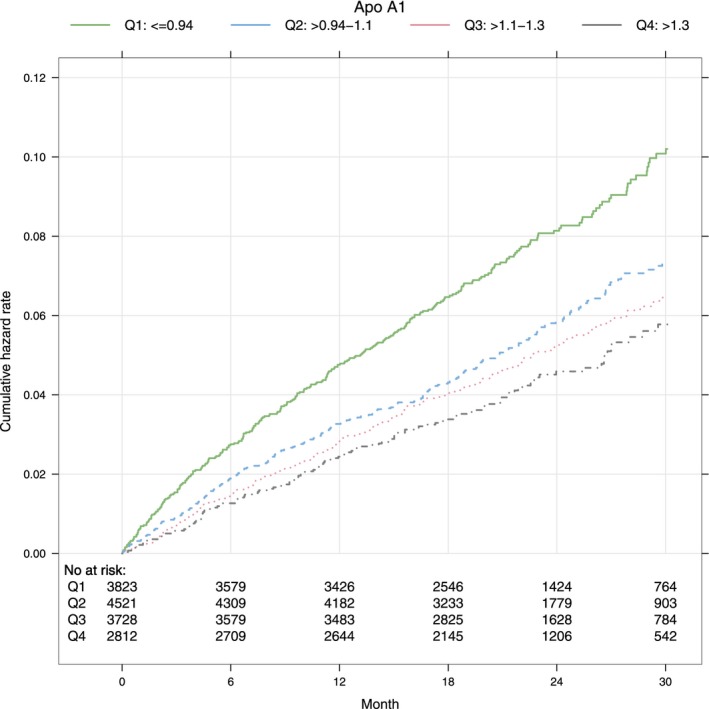
Cumulative hazard rates for the composite ischemic outcome by quartiles of ApoA1. ApoA1 indicates apolipoprotein A1; Q, quartile.
Figure 2.

Cumulative hazard rates for the composite ischemic outcome by quartiles of ApoB. ApoB indicates apolipoprotein B; Q, quartile.
Dyslipidemia and the Risk of Stroke or Systemic Embolism
In total, there were 397 occurrences of stroke or SEE during the trial follow‐up. In the fully adjusted analyses, ApoA1 was independently associated with a lower risk of stroke or SEE with a HR of 0.84 (95% CI, 0.72–0.98; P=0.0248) per interquartile change (Table 5). ApoB was not statistically significantly associated with stroke or SEE in any model (Table 6).
Dyslipidemia and the Risk of MI
A total of 149 MI events were observed during follow‐up. There was a lower risk of MI with higher baseline levels of ApoA1, however not statistically significant in any model (Table 5).
In the fully adjusted analyses, higher ApoB was independently associated with an increased risk of MI with an HR of 1.33 (95% CI, 1.06–1.68; P=0.0144) per interquartile change.
Dyslipidemia and the Risk of Mortality
During follow‐up, a total of 1068 patients died from all causes, of which 543 died from cardiovascular causes. Higher levels of ApoA1 were statistically significantly associated with lower risk of all‐cause and cardiovascular mortality, respectively. This association remained statistically significant in the model also adjusting for cardiovascular biomarkers with an HR of 0.77 (95% CI, 0.70–0.85; P<0.0001) for all‐cause mortality, and an HR of 0.78 (95% CI, 0.68–0.89; P=0.0002), for cardiovascular mortality, per interquartile change (Table 5).
In fully adjusted models, ApoB was associated with all‐cause mortality with a higher risk in those with lower ApoB levels with an HR of 0.84 (95% CI, 0.76–0.92; P=0.0002) hazard ratio per interquartile change. A similar association was observed for ApoB with cardiovascular death; however, this did not remain statistically significant in fully adjusted analyses (Table 6).
Dyslipidemia and the Risk of Major Bleeding
A total of 702 major bleeding events were observed in this biomarker cohort. There was lower risk of major bleeding with higher ApoA1 levels. However, none of the apolipoproteins remained significantly associated with major bleeding in fully adjusted models (Tables 5 and 6).
Sensitivity Analysis
Sensitivity analyses for the association of ApoB with cardiovascular outcomes in patients without statin therapy (n=8420) overall showed similar results (Table 7). Additional sensitivity analyses for ApoB showed that the association with all‐cause mortality was primarily driven by noncardiovascular death (Table 8), of which malignancy and infection were the most common causes of death.
Table 7.
Association of ApoB at Baseline With Outcomes According to Continuous Levels of ApoB Showing the Hazard Ratio Per Interquartile Change in Patients Without Statin Treatment
| n | Events | Unadjusted | Adjusted Clinical Risk Factors | Adjusted Clinical+Biomarkers | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| Ischemic composite outcome | 8420 | 515 (3.22) | 0.88 (0.78–0.99) | 0.0327 | 0.95 (0.85–1.09) | 0.4161 | 0.96 (0.85–1.09) | 0.5601 |
| Stroke or systemic embolism | 8420 | 220 (1.37) | 0.87 (0.73–1.04) | 0.1178 | 0.95 (0.79–1.14) | 0.5757 | 0.98 (0.81–1.19) | 0.8310 |
| MI | 8420 | 70 (0.43) | 1.29 (0.96–1.73) | 0.0908 | 1.36 (1.01–1.84) | 0.0447 | 1.29 (0.94–1.78) | 0.1161 |
| Major bleeding | 8409 | 365 (2.52) | 0.82 (0.71–0.94) | 0.0051 | 0.99 (0.86–1.15) | 0.9290 | 0.98 (0.84–1.16) | 0.8403 |
| Cardiovascular death | 8420 | 343 (2.09) | 0.79 (0.68–0.91) | 0.0015 | 0.86 (0.74–0.99) | 0.0372 | 0.87 (0.74–1.02) | 0.0783 |
| Death | 8420 | 655 (3.99) | 0.74 (0.67–0.82) | <0.0001 | 0.81 (0.73–0.91) | 0.0002 | 0.81 (0.72–0.90) | 0.0002 |
Three different proportional hazards model have been used, 1 without any adjustment, 1 adjusted for randomized treatment, demographic, and clinical risk factors, and 1 adjusted for randomized treatment, demographic, and clinical risk factors plus biomarkers. The demographic and clinical risk factors used were: age, sex, body mass index, smoking status, systolic blood pressure, atrial fibrillation type, creatinine clearance, diabetes mellitus, heart failure, previous stroke/systemic embolic event/transient ischemic attack, hypertension, randomized treatment, use of warfarin within 7 days of randomization and use of statin medication within 30 days before randomization, treatment at randomization with aspirin, and treatment with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blocker. The used biomarkers markers were high‐sensitivity cardiac troponin T, N‐terminal pro‐B‐type natriuretic peptide, cystatin C, C‐reactive protein, and interleukin 6. For major bleeding, past bleeding and hemoglobin were added to model 1 and growth differentiation factor 15 to model 2. Cox models based on continuous biomarker levels showing hazard ratio per interquartile change (eg, Q3 vs Q1). ApoB indicates apolipoprotein B; CI, confidence interval; HR, hazard ratio.
Table 8.
Association of ApoB at Baseline With Noncardiovascular Death According to Continuous Levels of ApoB Showing the Hazard Ratio Per Interquartile Change in All Patients and in Patients Without Statin Treatment
| n | Events | Unadjusted | Adjusted Clinical Risk Factors | Adjusted Clinical+Biomarkers | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| All | 14 884 | 525 (1.82) | 0.74 (0.66–0.84) | <0.0001 | 0.81 (0.71–0.92) | 0.0015 | 0.79 (0.69–0.90) | 0.0005 |
| Without statin medication | 8420 | 312 (1.90) | 0.69 (0.59–0.80) | <0.0001 | 0.77 (0.66–0.91) | 0.0015 | 0.75 (0.63–0.88) | 0.0006 |
ApoB indicates apolipoprotein B; CI, confidence interval; HR, hazard ratio.
Outcomes According to Dyslipidemia and Randomized Treatment
The study treatment (apixaban or warfarin) interaction by apolipoprotein levels for the composite ischemic outcome was not statistically significant. For major bleeding, apixaban showed a greater relative risk reduction in those with high ApoB levels (P=0.0234), with a similar result for ApoA1 (P=0.0584; Figures 3 and 4).
Figure 3.

One‐year event rates for continuous level of ApoA1 according to randomized treatment. ApoA1 indicates apolipoprotein A1; Cardiac dth, cardiac death; MI, myocardial infarction; SEE, systemic embolic event.
Figure 4.
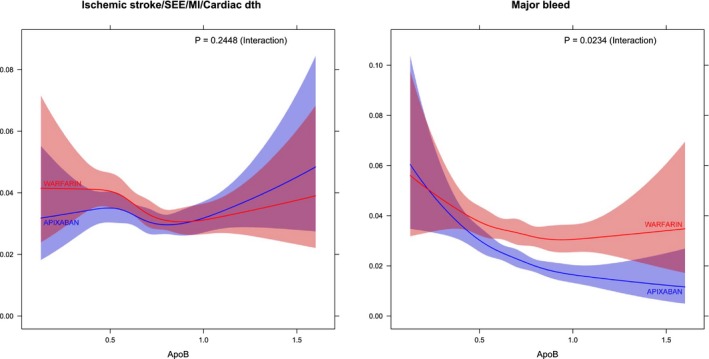
One‐year event rates for continuous level of ApoB according to randomized treatment. ApoB indicates apolipoprotein B; Cardiac dth, cardiac death; MI, myocardial infarction; SEE, systemic embolic event.
Discussion
In patients with AF on oral anticoagulation, higher levels of ApoA1 were associated with lower risk of composite ischemic outcomes, stroke, and death even after adjustment for baseline characteristics, comorbidities, other biomarkers, and medications. Higher levels of ApoB were associated with higher rates of MI only, but not with the other cardiovascular outcomes. In contrast, lower levels of ApoB were associated with an increased risk of all‐cause death. Importantly, both apolipoproteins remained statistically significantly associated with these outcomes even after adjustment for other prognostic biomarkers (high‐sensitivity cardiac troponin T, N‐terminal pro‐B‐type natriuretic peptide, cystatin C, C‐reactive protein, and IL‐6). Furthermore, among those with higher apolipoprotein levels, apixaban showed an even greater relative risk reduction of major bleeding than in patients with lower levels of these apolipoproteins.
Dyslipidemia is a complex disease and a traditional risk factor for adverse cardiovascular events. Other than a possible association between dyslipidemia and incidence of AF, its relationship with AF has not been well described before.20, 21, 22, 23, 24, 25, 26 To our knowledge, this is the first study showing that plasma levels of apolipoproteins were independently associated with major cardiovascular events in anticoagulated patients with AF. In this study, high baseline ApoA1 levels were associated with ≈15% lower risk of stroke or SEE, 20% reduced risk for cardiac and all‐cause mortality, and similar association to composite ischemic outcomes. High levels of ApoB, on the other hand, were associated with 30% increased the risk of MI. These associations, similar to that of dyslipidemia in coronary artery disease, might be explained by the atherogenic properties of dyslipidemia.27, 28, 29, 30
An important finding in this study is the association between dyslipidemia and mortality for both ApoA1 and ApoB. Similar to low HDL levels, low ApoA1 conferred higher risk of death. However, for ApoB, the all‐cause mortality rate was paradoxically higher in patients with lower ApoB concentrations. Similar findings have been presented in other cohorts as well.31, 32 In a large, prospective, observational study of patients following an acute MI, an increased risk of all‐cause mortality was shown in the group with the lowest levels of LDL cholesterol.31 Increased rate of all‐cause mortality has also been associated with low levels of LDL cholesterol in the elderly.32 Plasma cholesterol levels decline with age, malnutrition, chronic disease, and even with inflammation.32, 33, 34, 35 This “lipid paradox” in which patients with the lowest levels of LDL cholesterol, or in this case ApoB, are at an increased risk for all‐cause mortality could thus possibly be explained, at least in part, by a higher disease burden and frailty. Patients in this study in the lowest ApoB quartile were, in fact, more comorbid, perhaps best illustrated by higher CHA2DS2‐VASc scores, and were therefore likely at an increased risk for death. Similar patterns were also observed in the multivariable models and in sensitivity analyses in patients not on statin therapy.
Women in this study had higher levels of ApoA1 and lower levels of ApoB (Tables 1 and 2). These findings are consistent with previous studies.36 However, in the adjusted Cox analyses, the associations between the studied apolipoproteins with outcomes are adjusted for sex apolipoprotein differences. Thus, sex should not influence the apolipoprotein association with outcomes.
The relationship between dyslipidemia and stroke is complex because the association seems to vary dependent on stroke subtype as well as lipid parameter studied.13, 37 Most observational studies have shown an association between higher levels of LDL cholesterol and lower levels of HDL cholesterol with increased ischemic stroke risk.13 Few studies have, however, evaluated dyslipidemia in relation to stroke risk in an AF population on oral anticoagulation. Recently, in a small cohort of AF patients without anticoagulant therapy, high LDL cholesterol was found to be an independent predictor of ischemic stroke.38 In the present study, no such findings were observed, which may be attributable to a relatively lower event rate which in turn is attributed to the fact that all patients received oral anticoagulants. Low ApoA1 levels were, on the other hand, associated with higher stroke/SEE rates and even more so to the composite ischemic outcome. This could indicate that ApoA1 indeed may play a role in the pathophysiology of ischemic outcomes in AF. Furthermore, a greater relative risk reduction of major bleedings was observed with apixaban compared with warfarin in patients with higher levels of apolipoprotein. In these individuals, apixaban may be an even more‐attractive choice than warfarin. The underlying mechanism for the treatment interaction is, however, unclear and warrants further investigation.
Several biomarkers have, in recent studies, shown to significantly improve the prognostication for stroke in AF.39, 40, 41 They could therefore lead to a better understanding of AF and its associated adverse outcomes, improve risk stratification, and, potentially, create new therapeutic approaches to reduce morbidity and mortality.
The findings from the present study indicate that dyslipidemia, a traditional cardiovascular risk factor, may play a role in AF‐related adverse outcomes. Therapeutic interventions for dyslipidemia could therefore prove beneficial effects in reducing the risk of these complications. In clinical practice, however, most drugs that increase ApoA1/HDL cholesterol (such as niacin and fibrates) have so far not been able to show further reduction in cardiovascular events.42, 43 It is possible that newer agents, such as cholesteryl ester transfer protein inhibitors, may be more favorable.44 The medications, however, have not been studied specifically in patients with AF, and any potential beneficial effect of these agents on AF burden and associated outcomes need to be tested in future prospective trials and may possibly also need stratification according to genetic variances.45 Another issue that warrants mentioning is that only half (51.6%) of the patients with AF and a traditional indication for statin therapy (eg, established vascular disease or diabetes mellitus) was on statin treatment in the study cohort. Better adherence to existing guidelines for management of dyslipidemia may thus also be an important factor in the efforts to improve outcomes in AF.
Even though the statistical analyses were adjusted for a variety of cardiovascular risk factors, patient background characteristics, and cardiovascular biomarkers, residual confounding cannot be excluded. Information of pre‐existing hyperlipidemia per se was not collected within the ARISTOTLE trial, neither were traditional markers of hyperlipidemia (cholesterol or triglyceride levels). Furthermore, the observational nature of this study shows associations and does not permit any deductions concerning causal relationships between dyslipidemia and AF.
Conclusions
In patients with AF treated with oral anticoagulation, higher levels of ApoA1 were independently associated with lower risk of ischemic cardiovascular outcomes, including stroke/SEE and mortality. Higher ApoB levels were associated with higher rates of MI, but, paradoxically, lower risk of all‐cause mortality. The benefits of apixaban over warfarin were consistent, regardless of the levels of ApoA1 and ApoB. Our findings provide unique insights to the interaction between AF and lipoproteins, and suggest that investigating therapies targeting dyslipidemia may play a role in improving cardiovascular outcomes in patients with AF.
Sources of Funding
The ARISTOTLE trial was funded by Bristol‐Myers Squibb, Co (Princeton, NJ) and Pfizer Inc. (New York, NY), and coordinated by the Duke Clinical Research Institute (Durham, NC) and Uppsala Clinical Research Center (Uppsala, Sweden). The analyses were supported by Bristol‐Myers Squibb, Pfizer, and grants from the Swedish Heart‐Lung Foundation (20090183). The funding sources were given the opportunity to review and comment on the final version of the article. The first (Pol) and senior authors (Hijazi) and the statisticians (Westerbergh and Lindbäck) were responsible for, and accordingly had full access to, the database. The decision on submission was made by all co‐authors.
Disclosures
Dr Held reports an institutional research grant and speaker's bureau from AstraZeneca; institutional research grants from Bristol‐Myers Squibb, GlaxoSmithKline, Merck & Co, and Roche and consulting fees from Boehringer Ingelheim and Bayer. Mr Westerbergh reports institutional research grants from Bristol‐Myers Squibb/Pfizer. Mr Lindbäck reports institutional research grants from Boehringer Ingelheim and Bristol‐Myers Squibb/Pfizer. Dr Alexander reports institutional research grants and consulting fee/honoraria from Bristol‐Myers Squibb, Regado Biosciences, and Merck and consulting fee/honoraria from Pfizer, AstraZeneca, Boehringer Ingelheim, Ortho‐McNeil‐Janssen, Polymedix, and Bayer. Dr Alings reports consulting fees from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, Milestone, Pfizer, and Sanofi‐Aventis. Dr Granger reports grants and personal fees from GlaxoSmithKline, Boehringer Ingelheim, Bristol‐Myers Squibb, Pfizer, Sanofi‐Aventis, Takeda, The Medicines Company, Janssen, Bayer, and Hoffmann‐La Roche; grants from Medtronics Foundation, Merck & Co., and Armetheon; personal fees from Lilly, AstraZeneca, Daiichi Sankyo, Ross Medical Corporation, Salix Pharmaceuticals, and Gilead. Dr Goto reports research grants from Sanofi, Pfizer, and Ono; lecture fees from Bayer and AstraZeneca; and DSMB member for Daiichi‐Sankyo and Bayer. Dr Halvorsen reports speaker fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Merck, Pfizer, and Sanofi. Dr Hanna reports being an employee of Bristol‐Myers Squibb and receiving stock as a part of compensation during the conduct of the ARISTOTLE trial. Dr Lopes reports an institutional research grant and consulting fees from Bristol‐Myers Squibb; institutional research grant from GlaxoSmithKline; and consulting fees from Bayer, Boehringer Ingelheim, Pfizer, Merck, and Portola. Dr Hijazi reports lecture fees from Boehringer Ingelheim, Roche, Bristol‐Myers Squibb, and Pfizer; consulting fees from Merck Sharp & Dohme, Roche, Bristol‐Myers Squibb, and Pfizer. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e007444 DOI: 10.1161/JAHA.117.007444.)29419390
An abstract containing a part of the findings has been presented at the ESC Congress, August 26 to 30, 2017 in Barcelona, Spain.
References
- 1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 4. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63:152–164. [DOI] [PubMed] [Google Scholar]
- 5. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz JD, Husted S, Hylek EM, Lopes RD, McMurray JJ, Granger CB. High‐sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol. 2014;63:52–61. [DOI] [PubMed] [Google Scholar]
- 6. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation. J Am Coll Cardiol. 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 7. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S, Granger CB, Siegbahn A. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 8. Wong N D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. [DOI] [PubMed] [Google Scholar]
- 9. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 10. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. [DOI] [PubMed] [Google Scholar]
- 11. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. [DOI] [PubMed] [Google Scholar]
- 12. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen MR, Tokgözoglu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL. Low‐density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yaghi S, Elkind MS. Lipids and cerebrovascular disease. Stroke. 2015;46:3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hijazi Z, Aulin J, Andersson U, Alexander JH, Gersh B, Granger CB, Hanna M, Horowitz J, Hylek EM, Lopes RD, Siegbahn A, Wallentin L; ARISTOTLE Investigators . Biomarkers of inflammation and risk of cardiovascular events in anticoagulated patients with atrial fibrillation. Heart. 2016;102:508–517. [DOI] [PubMed] [Google Scholar]
- 15. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez‐Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821–2830. [DOI] [PubMed] [Google Scholar]
- 16. Lopes RD, Alexander JH, Al‐Khatib SM, Ansell J, Diaz R, Easton JD, Gersh BJ, Granger CB, Hanna M, Horowitz J, Hylek EM, McMurray JJ, Verheugt FW, Wallentin L; ARISTOTLE Investigators . Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–339. [DOI] [PubMed] [Google Scholar]
- 17. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 18. Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L; ARISTOTLE Investigators . High‐sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. [DOI] [PubMed] [Google Scholar]
- 19. Harrell FE Jr. Regression Modeling Strategies, With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd ed New York, NY: Springer; 2015. [Google Scholar]
- 20. Kim SM, Kim JM, Shin DG, Kim JR, Cho KH. Relation of atrial fibrillation (AF) and change of lipoproteins: male patients with AF exhibited severe pro‐inflammatory and pro‐atherogenic properties in lipoproteins. Clin Biochem. 2014;47:869–875. [DOI] [PubMed] [Google Scholar]
- 21. Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, Chen LY, Lubitz SA, McClelland RL, McManus DD, Soliman EZ, Huxley RR, Nazarian S, Szklo M, Heckbert SR, Benjamin EJ. Blood lipids and the incidence of atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3:e001211 DOI: 10.1161/JAHA.114.001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR, Konety S, Ballantyne CM, Alonso A. Blood lipid levels, lipid‐lowering medications, and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities study. Circ Arrhythm Electrophysiol. 2012;5:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Watanabe H, Tanabe N, Yagihara N, Watanabe T, Aizawa Y, Kodama M. Association between lipid profile and risk of atrial fibrillation. Circulation. 2011;75:2767–2774. [DOI] [PubMed] [Google Scholar]
- 24. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 25. Mora S, Akinkuolie AO, Sandhu RK, Conen D, Albert CM. Paradoxical association of lipoprotein measures with incident atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Norby FL, Eryd SA, Niemeijer MN, Rose LM, Smith AV, Yin X, Agarwal SK, Arking DE, Chasman DL, Chen LY, Eijgelsheim M, Engstrom G, Franco OH, Heeringa J, Hindy G, Hofman A, Lutsey PL, Magnani JW, McManus DD, Orho‐Melander M, Pankow JS, Rukh G, Schulz CA, Uitterlinden AG, Albert CM, Benjamin EJ, Gudnason V, Smith JG, Stricker BH, Alonso A. Association of lipid‐related genetic variants with the incidence of atrial fibrillation: the AFGen consortium. PLoS One. 2016;11:e0151932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krauss RM. Triglycerides and atherogenic lipoproteins: rationale for lipid management. Am J Med. 1998;105:58S–62S. [DOI] [PubMed] [Google Scholar]
- 28. Grundy SM, Small LDL. Atherogenic dyslipidemia, and the metabolic syndrome. Circulation. 1997;95:1–4. [DOI] [PubMed] [Google Scholar]
- 29. Francis GA, Perry RJ. Targeting HDL‐mediated cellular cholesterol efflux for the treatment and prevention of atherosclerosis. Clin Chim Acta. 1999;286:219–230. [DOI] [PubMed] [Google Scholar]
- 30. Mackness MI, Durrington PN. HDL, its enzymes and its potential to influence lipid peroxidation. Atherosclerosis. 1995;115:243–253. [DOI] [PubMed] [Google Scholar]
- 31. Reddy VS, Bui QT, Jacobs JR, Begelman SM, Miller DP, French WJ. Relationship between serum low‐density lipoprotein cholesterol and in‐hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol. 2015;115:557–562. [DOI] [PubMed] [Google Scholar]
- 32. Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all‐cause mortality in nondemented elderly. J Am Geriatr Soc. 2005;53:219–226. [DOI] [PubMed] [Google Scholar]
- 33. Rosenson RS. Myocardial injury: the acute phase response and lipoprotein metabolism. J Am Coll Cardiol. 1993;22:933–940. [DOI] [PubMed] [Google Scholar]
- 34. Weverling‐Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119–1123. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ. Association between cholesterol level and mortality in dialysis patients. JAMA. 2004;291:451–459. [DOI] [PubMed] [Google Scholar]
- 36. Jungner I, Marcovina SM, Walldius G, Holme I, Kolar W, Steiner E. Apolipoprotein B and A‐I values in 147576 Swedish males and females, standardized according to the World Health Organization‐International Federation of Clinical Chemistry First International Reference Materials. Clin Chem. 1998;44:1641–1649. [PubMed] [Google Scholar]
- 37. Wang X, Dong Y, Qi X, Huang C, Hou L. Cholesterol levels and risk of hemorrhagic stroke: a systematic review and meta‐analysis. Stroke. 2013;44:1833–1839. [DOI] [PubMed] [Google Scholar]
- 38. Qi Z, Chen H, Wen Z, Yuan F, Ni H, Gao W, Shen J, Li J, Lin Y, Shan Y, Jin B, Yan P, Shi H, Luo X. Relation of low‐density lipoprotein cholesterol to ischemic stroke in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2017;119:1224–1228. [DOI] [PubMed] [Google Scholar]
- 39. Ruff CT, Giugliano RP, Braunwald E, Murphy SA, Brown K, Jarolim P, Mercuri M, Antman EM, Morrow DA. Cardiovascular biomarker score and clinical outcomes in patients with atrial fibrillation: a subanalysis of the ENGAGE AF‐TIMI 48 randomized clinical trial. JAMA Cardiol. 2016;1:999–1006. [DOI] [PubMed] [Google Scholar]
- 40. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L. The novel biomarker‐based ABC (age, biomarkers, clinical history)‐bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
- 41. Hijazi Z, Lindbäck J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RA, White HD, Granger CB, Wallentin L. The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker‐based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keene D, Price C, Shun‐Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta‐analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaur N, Pandey A, Negi H, Shafiq N, Reddy S, Kaur H, Chadha N, Malhotra S. Effect of HDL‐raising drugs on cardiovascular outcomes: a systematic review and meta‐regression. PLoS One. 2014;9:e94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Group HTRC , Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217–1227. [DOI] [PubMed] [Google Scholar]
- 45. Tardif JC, Rhéaume E, Lemieux Perreault LP, Grégoire JC, Feroz Zada Y, Asselin G, Provost S, Barhdadi A, Rhainds D, L'Allier PL, Ibrahim R, Upmanyu R, Niesor EJ, Benghozi R, Suchankova G, Laghrissi‐Thode F, Guertin MC, Olsson AG, Mongrain I, Schwartz GG, Dubé MP. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ Cardiovasc Genet. 2015;8:372–382. [DOI] [PubMed] [Google Scholar]


