Abstract
Background
As questions have been raised about the appropriateness of direct oral anticoagulant (DOAC) dosing among outpatients with atrial fibrillation, we examined this issue in patients being managed by primary care providers.
Methods and Results
This was a retrospective cohort new‐user study using electronic medical records from 744 Canadian primary care clinicians. Potentially inappropriate DOAC prescribing was defined as prescribing lower or higher doses than those recommended by guidelines for patients with nonvalvular atrial fibrillation. Of the 6658 patients with nonvalvular atrial fibrillation who were prescribed a DOAC (mean age: 74.8; 55% male), 626 (9.4%) had a CHADS 2 score of 0, and 168 (2.5%) had a CHADS‐VASc score of 0. Of the DOAC prescriptions, 527 (7.7%) were deemed potentially inappropriate: 496 (7.2%) were potentially underdosed, and 31 (0.5%) were prescribed a dose that was higher than recommended. Patients were more likely to be prescribed lower‐than‐recommended doses if they were female (adjusted odds ratio [aOR]: 1.3 [95% confidence interval (CI), 1.0–1.5]), had multiple comorbidities (aOR: 1.4 [95% CI, 1.1–1.8])—particularly heart failure (aOR: 1.6 [95% CI, 1.2–2.0]) or dementia (aOR: 1.4 [95% CI, 1.1–1.8])—or if they were also taking aspirin (aOR: 1.7 [95% CI, 1.3–2.1]) or nonsteroidal anti‐inflammatory drugs (aOR: 1.2 [95% CI, 1.02–1.5]). Potentially inappropriate DOAC dosing was more common in rural practices (aOR: 2.1 [95% CI, 1.7–2.6]) or smaller practices (aOR: 1.9 [95% CI, 1.6–2.4] for practices smaller than median).
Conclusions
The vast majority of DOAC prescriptions in our cohort of primary care–managed patients appeared to be for appropriate doses, particularly since prescribing a reduced dose of DOAC may be appropriate in frail patients or those taking other medications that predispose to bleeding.
Keywords: anticoagulation, atrial fibrillation, quality of care
Subject Categories: Atrial Fibrillation, Quality and Outcomes
Clinical Perspective
What Is New?
Although concerns have been raised about the appropriateness of direct oral anticoagulant dosing among outpatients with atrial fibrillation, examination of the electronic health records of 744 Canadian primary care clinicians revealed that only 496 (7%) of 6658 direct oral anticoagulant prescriptions were for lower‐than‐recommended doses, and 31 (0.5%) were for a higher‐than‐recommended dose, given the patient's age, weight, and renal function.
Patients prescribed lower‐than‐recommended doses had more comorbidities and were more likely to be taking other medications that predispose to bleeding.
What Are the Clinical Implications?
The vast majority of direct oral anticoagulant prescriptions in our cohort of primary care–managed patients were for recommended doses.
The appropriateness of direct oral anticoagulant prescribing may be even higher because the vast majority of prescriptions for non–guideline‐recommended doses were for lower doses in our cohort and in frail or multimorbid patients or for those taking other medications that predispose to bleeding those doses may well represent appropriate caution on the part of prescribers.
Primary care management of patients with nonvalvular atrial fibrillation is a viable means to achieve high‐quality anticoagulation care.
Introduction
Patients with atrial fibrillation (AF) are at increased risk of thromboembolic events including stroke, transient ischemic attack, and systemic emboli, and this risk can be mitigated by antiplatelet and oral anticoagulant medications.1, 2 Although the mainstay of anticoagulation has been warfarin (Coumadin; Bristol‐Myers Squibb) for >20 years, the direct oral anticoagulants (DOACs) have been shown to have similar, or in some cases even superior, efficacy:safety ratios in AF patients without valvular heart disease,3 and this has led to their approval by regulatory agencies; wide adoption in Canadian, American, and European guidelines; and increasing use in clinical practice.
Although the efficacy:safety ratio for DOACs depends on dosing, 2 recent studies have suggested that a substantial proportion of patients may be receiving inappropriate doses of DOACs. One in 8 patients in ORBIT‐AF II (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II; which included 5738 DOAC‐treated patients) were prescribed inappropriate DOAC doses.4 Given the voluntary nature of ORBIT‐AF II and the fact that 94% of patients were managed by cardiologists or electrophysiology specialists, the generalizability of their findings to a broader population is uncertain. A report5 from Quebec, Canada, noted that 30% of AF patients discharged from a single hospital with a DOAC prescription were receiving an inappropriate dose (57% underdosed and 43% overdosed); however, this finding was based on inpatients treated by specialists at a university health center, and again, the results may not be generalizable to a broader sample of outpatients. The extent to which DOAC dosing does not conform to the doses recommended by guideline panels and espoused in product monographs (based on trial evidence) is important to evaluate because overdosing of DOACs was associated with an increase in all‐cause mortality in ORBIT‐AF II and underdosing was associated with higher rates of cardiovascular hospitalizations.4
We studied DOAC prescribing patterns in Canada among patients managed in the outpatient setting by primary care providers and explored whether patient or provider factors were associated with the prescribed dose.
Methods
The data, analytic methods, and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure, given privacy constraints (each of the 744 physicians participating in the Canadian Primary Care Sentinel Surveillance Network [CPCSSN] is the legal custodian of his or her practice's data, and there is no approval for broader sharing of the data at this time). The CPCSSN received approval from the research ethics board of each host university and from the Health Canada research ethics board with a waiver of informed consent for patients, since only deidentified data were transmitted. Written consent for the collection and analysis of this anonymous electronic medical record (EMR) data was obtained from all participating CPSCCN primary care providers. Using data from CPCSSN, a national collaboration of primary care physicians from 7 provinces and 1 territory in Canada who permit their EMRs to be collated for analysis, we identified all patients with a first prescription for a DOAC at any point in 2010–2015 (DOACs were first approved for use in Canada in 2010). We restricted our retrospective cohort new‐user study to any patient with a diagnosis of nonvalvular AF—that is, those patients with an International Classification of Diseases, Ninth Revision (ICD‐9) coded diagnosis of AF (427.3, 427.31, 427.32) in the billing or health conditions tables (which are completed by attending physicians or nurse practitioners at each visit) for that patient and no ICD‐9 codes or text in the problem list or individual visit diagnoses noting valvular heart disease or mechanical valves (ie, ICD‐9 codes 394.x, 395.x, 396.x, 424.0, or V43.3).
To evaluate whether DOAC dosing was appropriate, we used the estimated glomerular filtration rate (eGFR), age, and weight recorded in the patient's EMR closest in time to the initial DOAC prescription. We estimated the GFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula, as follows:
where Scr is serum creatinine (mg/dL), k is 0.7 for women and 0.9 for men, a is −0.329 for women and −0.411 for men, min indicates the minimum of SCr/k or 1, max indicates the maximum of SCr/k or 1, and age is in years. For patients who were prescribed different DOAC doses over time, only the first prescription was used for the purposes of this analysis, with the creatinine/eGFR and weight recorded closest to the prescription (usually before or, at most, within 1 week after the prescription) used to define appropriateness of dose. Each patient was included only once in the analysis.
Using previously standardized and validated case definitions, we also extracted patient‐level comorbidity data from the CPCSSN EMR indicating the presence of chronic obstructive pulmonary disease, depression, diabetes mellitus, hypertension, osteoarthritis, epilepsy, dementia, and Parkinson disease (hereafter called CPCSSN comorbidities).6 We also included data from the health conditions and billing tables in each patient's EMR to assess the presence of other diagnoses of interest (ie, cerebrovascular disease, peripheral arterial disease, heart failure, coronary artery disease, chronic kidney disease) and classified these as non‐CPCSSN comorbidities. We also calculated the CHADS2 and CHADS‐VASc scores for each patient at the time of initial DOAC prescription using diagnoses and billing codes entered in each patient's EMR by his or her primary care physician up until the date of the prescription.
We used the guideline‐recommended and trial‐tested doses to define appropriate dosing for each DOAC, as follows:
Rivaroxaban: 20 mg daily or 15 mg daily if eGFR was <50 mL/min.
Apixaban: 5 mg BID or 2.5 mg BID if ≥2 of 3 were present: age ≥80 years, weight ≤60 kg, or serum creatinine >133 μmol/L (or on dialysis).
Dabigatran: 150 mg BID or 110 mg BID if >80 years or 75 mg BID if eGFR was 15 to 30 mL/min.
Our primary outcome of interest was the proportion of patients with a potentially inappropriate DOAC prescription, defined as either underdosing (low‐dose DOAC prescribed despite patient not being in a subgroup for which the lower dose is recommended) or overdosing (higher dose prescribed despite patient being in a subgroup that should receive a low dose).
We compared baseline characteristics using chi‐square tests for dichotomous variables and Student t tests for continuous variables and performed multivariate analysis using logistic regression with potentially inappropriate DOAC prescription as the outcome of interest and including baseline variables. We dichotomized practice size by the median for participating practices (1734 patients). SAS version 9.4 (SAS Institute) was used for analyses.
Results
Our study included 6658 patients with a DOAC prescription. Median age was 75 years: 56% of patients prescribed a DOAC were aged ≥75 years, 26% were 65 to 74 years old, and 20% were <65 years. Rivaroxaban was the most frequently used DOAC (57%), followed by dabigatran (34%) and apixaban (17%). One‐third of patients had a diagnosis of diabetes mellitus, one‐sixth had heart failure, and more than two‐thirds had hypertension (Table 1). We were able to find eGFR, age, and weight data for all patients.
Table 1.
Baseline Characteristics at Time of Initial DOAC Prescription
| Appropriate DOAC Prescription Dose (n=6131) | Potentially Inappropriate DOAC Prescription Dose (n=527) | P Value | |
|---|---|---|---|
| Age (y), mean (SD) | 74.5 (12.8) | 77.3 (12.3) | 0.03 |
| Male, % | 54.8 | 49.9 | 0.04 |
| Weight (kg), mean (SD) | 90.5 (29.7) | 67.2 (19.7) | 0.98 |
| eGFR, mean (SD) | 72.7 (23.9) | 87.1 (34.8) | 0.80 |
| History of hypertension, % | 71.9 | 73.8 | 0.36 |
| Diabetes mellitus, % | 34.1 | 35.3 | 0.60 |
| Dementia, % | 11.5 | 15.2 | 0.02 |
| Peripheral artery disease, % | 0.4 | 0.2 | 0.39 |
| History of coronary artery disease, % | 16.5 | 19.9 | 0.04 |
| History of cerebrovascular disease, % | 1.3 | 0.4 | 0.07 |
| History of heart failure, % | 14.4 | 23.2 | <0.0001 |
| CHADS2 score, median (IQR) | 2 (1–2) | 2 (1–3) | 0.008 |
| CHADS2 score 0, % | 9.5 | 7.8 | |
| CHADS2 score 1, % | 29.1 | 23.5 | |
| CHADS2 score ≥2, % | 61.4 | 68.7 | |
| CHA2DS2‐VASc score, median (IQR) | 2 (1–3) | 2 (1–3) | 0.07 |
| CHA2DS2‐VASc score 0, % | 2.6 | 1.1 | |
| CHA2DS2‐VASc score 1, % | 10.3 | 8.7 | |
| CHA2DS2‐VASc score ≥2, % | 87.1 | 90.1 | |
| Other medications, % | |||
| Aspirin | 43.3 | 50.7 | 0.003 |
| Non‐ASA antiplatelet agents | 34.1 | 31.5 | 0.16 |
| NSAIDs | 39.4 | 43.8 | 0.04 |
| ACEI or ARB | 71.5 | 79.3 | 0.0002 |
| Beta blocker agents | 66.7 | 71.7 | 0.03 |
| Diuretics | 57.9 | 63.6 | 0.02 |
| Antiarrhythmic and/or digoxin | 40.1 | 40.8 | 0.91 |
| Lipid‐modifying agent | 64.4 | 62.4 | 0.33 |
| Characteristics of practices | |||
| Provider age (mean) | 47.5 | 48.8 | <0.0001 |
| Male provider, % | 59.1 | 63 | 0.05 |
| Practice size (median number of patients) | 1753 | 1381 | |
| Family physician provider type, % | 98.3 | 99 | 0.23 |
| Rural location, % | 13.6 | 25.6 | <0.0001 |
Canadian Primary Care Sentinel Surveillance Network case definitions were used for hypertension, diabetes mellitus, and dementia.6 Non‐ASA antiplatelet agents include clopidogrel, ticlopidine, dipyridamole, and ticagrelor. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rates; IQR, interquartile range; NSAID, nonsteroidal anti‐inflammatory drug.
DOACs were potentially inappropriately dosed in 7.7% of patients (n=527), most of whom 496 (7.2%) were underdosed. Only 31 (0.5%) were prescribed too high a dose for their age (18 were ≥80 years), weight (1 weighed <60 kg), or eGFR (12 had renal function below the threshold to trigger dose adjustment: <50 mL/min for rivaroxaban, <30 mL/min for dabigatran or apixaban). Of the 496 patients prescribed a lower‐than‐recommended dose, 64% (318 patients) were aged ≥75 years (ie, within 5 years of the age cut point for apixaban or dabigatran), 20% (99 patients, 49 of whom were aged ≥75 years) weighed <65 kg (within 5 kg of the apixaban weight threshold), and 58 (12%) had eGFR within 10 mL/min of the renal function cut points. Although most patients prescribed DOACs were at increased risk of stroke, 626 (9.4%) had a CHADS2 score of 0 and 168 (2.5%) had a CHADS‐VASc score of 0 (risk profiles for which all guidelines currently recommend antiplatelet therapy alone; Figure. Nearly half of patients prescribed a DOAC also had a prescription for an antiplatelet agent or nonsteroidal anti‐inflammatory drug (Table 1), and 956 patients (13.9%) were on triple therapy (DOAC and dual antiplatelet therapy). Of the 1391 patients prescribed a DOAC and an antiplatelet agent, 718 (51.6%) had coronary artery or cerebrovascular disease.
Figure 1.
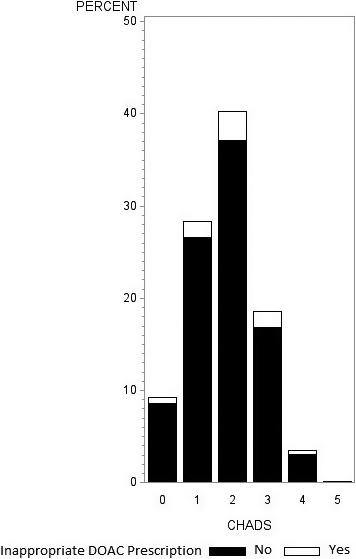
Potentially inappropriate direct oral anticoagulant (DOAC) dosing by patient CHADS 2 score.
Patients were more likely to be prescribed lower‐than‐recommended initial doses of a DOAC if they were female (adjusted odds ratio [aOR]: 1.3 [95% confidence interval (CI), 1.0–1.5]), had multiple comorbidities (aOR: 1.4 [95% CI, 1.1–1.8])—particularly heart failure (aOR: 1.6 [95% CI, 1.2–2.0]) or dementia (aOR: 1.4 [95% CI, 1.1–1.8])—or if they were also taking aspirin (aOR: 1.7 [95% CI, 1.3–2.1]) or nonsteroidal anti‐inflammatory drugs (aOR: 1.2 [95% CI, 1.02–1.5]; Tables 2 and 3. Potentially inappropriate DOAC prescribing was more common in rural practices (aOR: 2.1 [95% CI, 1.7–2.6]) or smaller practices (aOR: 1.9 [95% CI, 1.6–2.4] for practices smaller than median; Table 2.
Table 2.
Multiple Logistic Regression of Factors Associated With a Potentially Inappropriate DOAC Dose
| Variable | P Value | Adjusted OR | 95% CI |
|---|---|---|---|
| Patient sex (female) | 0.02 | 1.3 | 1.04–1.53 |
| Patient aged ≥65 y (vs <65 y) | 0.11 | 1.3 | 0.95–1. 65 |
| CPCSSN comorbidity (≥3 vs 1) | 0.006 | 1.4 | 1.1–1.79 |
| Non‐CPCSSN comorbidity (≥2 vs 0) | 0.14 | 1.3 | 0.91–1.95 |
| Non‐CPCSSN comorbidity (1 vs 0) | 0.04 | 1.3 | 1.01–1.63 |
| Polypharmacya (>5 medications vs none) | 0.16 | 2.4 | 0.83–6.96 |
| Polypharmacya (1–4 medications vs none) | 0.12 | 2.4 | 0.83–6.79 |
| Provider age (≥55 vs <35 y) | 0.06 | 1.3 | 0.99–1.69 |
| Provider age (35–54 vs ≥55 y) | 0.27 | 0.9 | 0.71–1.1 |
| Practice size (<1734 vs ≥1734 patients per clinic) | <0.0001 | 1.95 | 1.59–2.39 |
| Provider type (NP vs FP) | 0.11 | 2.1 | 0.85–5.33 |
| Location (rural vs urban) | <0.0001 | 2.1 | 1.67–2.6 |
| Provider sex (female vs male) | 0.03 | 0.8 | 0.66–0.98 |
CI indicates confidence interval; CPCSSN, Canadian Primary Care Sentinel Surveillance Network; DOAC, direct oral anticoagulant; FP, family physician; NP, nurse practitioner; OR, odds ratio.
Polypharmacy considers Anatomical Therapeutic Chemical codes: N02BA01/B01AC06, B01AC, B01AB, M01A, C01, C02, C03, C04, C05, C07, C08, C09, C10.
Table 3.
Multiple Logistic Regression of Associations Between Specific Comorbidities or Medications and Potentially Inappropriate DOAC Dose
| Variable (Yes vs No) | P Value | Adjusted OR | 95% CI |
|---|---|---|---|
| CPCSSN comorbidities | |||
| Hypertension | 0.51 | 0.93 | 0.74–1.16 |
| Diabetes mellitus | 0.98 | 0.99 | 0.82–1.21 |
| Dementia | 0.007 | 1.42 | 1.1–1.83 |
| Non‐CPCSSN comorbidities | |||
| Peripheral arterial disease | 0.44 | 0.46 | 0.06–3.39 |
| Cerebrovascular | 0.09 | 0.31 | 0.07–1.25 |
| Heart failure | 0.0002 | 1.57 | 1.24–1.98 |
| Medications | |||
| Aspirin | <0.0001 | 1.67 | 1.33–2.09 |
| Non‐ASA antiplatelet agents | <0.0001 | 0.61 | 0.48–0.77 |
| NSAIDs | 0.03 | 1.22 | 1.02–1.46 |
| ACEI or ARB | 0.001 | 1.53 | 1.19–1.98 |
| Beta blocker | 0.20 | 1.15 | 0.93–1.42 |
| Diuretic | 0.81 | 1.03 | 0.84–1.26 |
| Antiarrhythmic and/or digoxin | 0.21 | 0.88 | 0.73–1.07 |
| Lipid‐modifying agent | 0.03 | 0.80 | 0.65–0.98 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; CI, confidence interval; CPCSSN, Canadian Primary Care Sentinel Surveillance Network; DOAC, direct oral anticoagulant; NSAID, nonsteroidal anti‐inflammatory drug; OR, odds ratio.
Discussion
This study is the first to look at DOAC prescribing in a large, nationally representative7 sample of primary care providers, and the key finding is that >92% of the DOAC prescriptions in our cohort were for appropriate doses. At first blush, this appears to be slightly better than the 87.5% reported from ORBIT‐AF II.4 However, the appropriateness of prescribing may be even higher because the vast majority of prescriptions for non–guideline‐recommended doses were for lower doses in our cohort, and in frail or multimorbid patients or those taking other medications that predispose to bleeding, those doses may well represent appropriate caution on the part of prescribers. Consequently, the actual rate of potentially inappropriate DOAC dosing may be substantially lower in our results than in ORBIT‐AF II: Only 0.5% in our cohort were prescribed a higher‐than‐recommended dose compared with 7.3% of primary care–treated patients in ORBIT‐AF II (albeit those results were based on only 316 patients).4 We acknowledge that whether these dose choices represent appropriate care is uncertain in the absence of outcome data for our cohort; however, ORBIT‐AF II already established that higher‐than‐recommended dosing of DOACs is associated with an increase in all‐cause mortality and underdosing is associated with higher rates of cardiovascular hospitalizations.4
Although we found that primary care providers practicing in rural areas or within smaller practices were more likely to prescribe a potentially inappropriate DOAC dose, this is not a consistent finding in the literature. Prior investigations looking at prescribing practices have, in contrast, found either higher rates of inappropriate prescribing in urban practices or no difference after adjustment for baseline differences in patient characteristics.8 Conceivably, the provider mindset that leads to DOAC underdosing may differ from that leading to inappropriate prescribing of other medications, since it might derive from a more cautious and patient‐centered approach to prescribing in older multimorbid patients.
Two caveats are worth noting with our cohort. First, we chose to exclude AF patients with any valvular heart disease documented by their attending physician. We acknowledge that the definition of valvular disease in the setting of AF has evolved over time, and new guidelines restrict valvular AF to that associated with prosthetic mechanical valves or hemodynamically significant mitral stenosis (meaning that some patients whom we classified as having valvular heart disease would in fact meet newer definitions of nonvalvular AF).9 However, we used a broader definition in this study because the severity of valvular disease was not well documented in the EMRs, and we were collecting data from as far back as 2010, when older definitions of valvular AF were in place. Moreover, in light of emerging evidence that patients with nonprosthetic valvular AF may still derive benefits from these agents,10 we felt that DOACs may not necessarily be inappropriate in these cases. The second caveat with our findings is that we did not classify a DOAC prescription for the 9% of patients with CHADS scores of 0 as inappropriate, although some guidelines would,11, 12 because we did not know if those patients had cardioversions planned, and we were not privy to the discussions between clinicians and patients about their values and preferences with respect to stroke prevention versus bleeding risk.
The strength of this large repository is that although CPCSSN, like ORBIT‐AF II, is a voluntary registry, all participants are primary care physicians (whereas 94% of physicians in ORBIT‐AF II were specialists). CPCSSN practices provide a reasonably representative sample of the Canadian population; although CPCSSN has an overrepresentation of elderly individuals, this is beneficial for this study, given that nonvalvular AF is more common in elderly individuals.7 However, CPCSSN‐participating physicians tend to be younger than the Canadian average, and a higher proportion (19% versus 8%) are in academic practice.7 A second potential limitation is that EMR data quality in Canada is not consistent, and we found substantial variation across participating practices in terms of laboratory and clinical data available for analysis. Although the ICD‐9–based AF case definition we used has been evaluated in multiple studies and has specificity of nearly 99%,13 variables such as number of units of alcohol consumed per week are poorly documented in EMRs such that we were unable to calculate bleeding risk scores such as the HAS‐BLED score. Third, we have already acknowledged that all factors influencing decisions about DOAC dosing (including patient preferences and values and plans for imminent cardioversions) may not have been captured in the medical records of the primary care physicians participating in the CPCSSN. Fourth, we only examined the dosing at the time the DOAC was initially prescribed and did not take into account changes in eGFR, age, or weight over time or use of intercurrent medications (eg, antibiotics) or development of intercurrent conditions (eg, bleeding) which may influence prescribed doses. However, it seems unlikely that primary care physicians with an ongoing office‐based relationship with a patient would first prescribe a chronic medication such as a DOAC at the time of an intercurrent illness; we believe they would instead defer initiating that medication until the patient was stable (CPCSSN collects data from outpatient office visits only, not emergency room or hospital encounters). Fifth, we used eGFR based on the CKD‐EPI equation to define renal function, although the trials used the Cockcroft–Gault estimates of creatinine clearance. However, the eGFR is now recommended in the Kidney Disease: Improving Global Outcomes guidelines and included in current product monographs and closely mirrors the Cockcroft–Gault estimates except in those patients with more advanced degrees of renal dysfunction.14, 15
In conclusion, we found that the vast majority of DOAC prescriptions in our cohort of primary care–managed patients were for recommended doses. It is tempting to attribute the higher rate of appropriate DOAC dosing among CPCSSN primary care physicians to greater continuity of care than seen in the predominantly specialty care practices participating in ORBIT‐AF II (several studies have demonstrated associations between physician continuity and better outcomes for patients with other chronic conditions)16, 17, 18; however, this hypothesis cannot be tested in registry‐based studies. Future research needs to explore prescribing practices and outcomes for patients managed by a wide variety of providers and across a range of continuity models to determine the system most likely to optimize health outcomes (effectiveness, safety, and cost‐efficiency) for patients with nonvalvular AF.
Sources of Funding
This work was supported by the Heart and Stroke Foundation of Canada. McAlister is supported by the University of Alberta Chair in Cardiovascular Outcomes Research. The authors would like to recognize the contribution of the Public Health Agency of Canada and the College of Family Physicians of Canada for the initial funding to create the Canadian Primary Care Sentinel Surveillance Network.
Disclosures
None.
Acknowledgments
The authors of this report are grateful to Bill Peeler, data manager for MaPCReN database development. We would also like to acknowledge the support of the primary care providers who participate in the Canadian Primary Care Sentinel Surveillance Network (CPCSSN). Ezekowitz was on the steering committee for the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, supported by Bristol‐Myers Squibb/Pfizer. The interpretation and conclusions are those of the researchers and do not represent the views of the CPCSSN. The funders had no role in the design or analysis of this study, nor in the drafting or approval of this article. Singer and Kosowan have access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2018;7:e007603 DOI: 10.1161/JAHA.117.007603.)29374047
References
- 1. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 2. Verma A, Cairns JA, Mitchell LB, Macle L, Stiell IG, Gladstone D, McMurtry MS, Connolly S, Cox JL, Dorian P, Ivers N, Leblanc K, Nattel S, Healey JS. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114–1130. [DOI] [PubMed] [Google Scholar]
- 3. Ruff CT, Giuglianao RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 4. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Naccarelli G, Reiffel J, Singer DE, Peterson ED, Piccini JP; ORBIT‐AF Investigators and Patients . Off‐label dosing of non‐vitamin K antagonist oral anticoagulants and adverse outcomes. The ORBIT‐AF II Registry. J Am Coll Cardiol. 2016;68:2597–2607. [DOI] [PubMed] [Google Scholar]
- 5. Lavoie K, Turgeon MH, Brais C, Larochelle J, Blais L, Farand P, Templier G, Perreault S, Beauchesne MF. Inappropriate dosing of direct oral anticoagulants in patients with atrial fibrillation. J Atr Fibrillation. 2017;9:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson T, Green ME, Birtwhistle R, Khan S, Garies S, Wong ST, Natarajan N, Manca D, Drummond N. Validating the 8 CPCSSN case definitions for chronic disease surveillance in a primary care database of electronic health records. Ann Fam Med. 2014;12:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Queenan JA, Williamson T, Khan S, Drummond N, Garies S, Morkem R, Birtwhistle R. Representativeness of patients and providers in the Canadian Primary Care Sentinel Surveillance Network: a cross‐sectional study. CMAJ Open. 2016;4:e28–e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy GK, McAlister FA, Weir D, Tjosvold L, Eurich DT. Cardiovascular medication utilization and adherence among adults living in rural and urban areas: a systematic review and meta‐analysis. BMC Public Health. 2014;14:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Martins RP, Galand V, Colette E, Behar N, Pavin D, Leclercq C, Daubert JC, Mabo P. Defining nonvalvular atrial fibrillation: a quest for clarification. Am Heart J. 2016;178:161–167. [DOI] [PubMed] [Google Scholar]
- 10. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, Clemens A, Reilly PA, Connolly SJ, Yusuf S, Wallentin L. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease. The RE‐LY Trial (Randomized Evaluation of Long‐term anticoagulant therapY). Circulation. 2016;134:589–598. [DOI] [PubMed] [Google Scholar]
- 11. McAlister FA. Overtreatment of low‐risk patients with atrial fibrillation—the quality coin has two sides. JAMA Cardiol. 2016;1:848–849. [DOI] [PubMed] [Google Scholar]
- 12. Hsu JC, Chan PS, Tang F, Maddox TM, Marcus GM. Oral anticoagulant prescription in patients with atrial fibrillation and a low risk of thromboembolism: insights from the NCDR PINNACLE Registry. JAMA Intern Med. 2015;175:1062–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 16. Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014;174:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Van Walraven C, Oake N, Jennings A, Forster AJ. The association between continuity of care and outcomes: a systematic and critical review. J Eval Clin Pract. 2010;16:947–956. [DOI] [PubMed] [Google Scholar]
- 18. Weir DL, McAlister FA, Majumdar SR, Eurich DT. The interplay between continuity of care, multimorbidity, and health outcomes in patients with diabetes. Med Care. 2016;54:386–393. [DOI] [PubMed] [Google Scholar]


