Abstract
Background
Recent studies using stress‐rest perfusion cardiovascular magnetic resonance (CMR) demonstrated a close correlation between myocardial ischemia and reduced fractional flow reserve (FFR). However, its diagnostic concordance may be reduced in patients with multivessel disease. We sought to evaluate the concordance of adenosine stress‐rest perfusion CMR for predicting reduced FFR, and to determine the additive value of measuring global coronary flow reserve (CFR) in the coronary sinus in multivessel disease.
Methods and Results
Ninety‐six patients with angiographic luminal narrowing >50% underwent comprehensive CMR study and FFR measurements in 139 coronary vessels. FFR <0.80 was considered hemodynamically significant. Global CFR was quantified as the ratio of stress‐rest coronary sinus flow measured by phase‐contrast cine CMR. In 25 patients with single‐vessel disease, visual assessment of perfusion CMR yielded high diagnostic concordance for predicting flow‐limiting stenosis, with the area under receiver operating characteristic curve of 0.93 on a per‐patient basis. However, in 71 patients with multivessel disease, perfusion CMR underestimated flow‐limiting stenosis, resulting in the reduced area under receiver operating characteristic curve of 0.74. When CFR of <2.0 measured in the coronary sinus was considered as global myocardial ischemia, combined assessment provided correct reclassifications in 7 patients with false‐negative myocardial ischemia, and improved the diagnostic concordance to 92% sensitivity and 73% specificity with the area under receiver operating characteristic curve of 0.88 (95% confidence interval, 0.80%–0.97%, P=0.002).
Conclusions
Visual analysis of stress‐rest perfusion CMR has limited concordance with FFR in patients with multivessel disease. Multiparametric CMR integrating stress‐rest perfusion CMR and flow measurement in the coronary sinus is useful for detecting reduced FFR in multivessel disease.
Keywords: coronary sinus blood flow, fractional flow reserve, multivessel coronary artery disease, perfusion imaging, phase‐contrast cine cardiovascular magnetic resonance, stress myocardial perfusion cardiovascular magnetic resonance
Subject Categories: Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
Visual assessment of stress‐rest perfusion cardiovascular magnetic resonance (CMR) alone showed limited diagnostic performance in predicting reduced fractional flow reserve in multivessel disease.
Multiparametric CMR integrating stress‐rest perfusion CMR and flow measurement in the coronary sinus was useful for detecting reduced fractional flow reserve in multivessel disease.
What Are the Clinical Implications?
Multiparametric CMR integrating stress‐rest perfusion CMR and flow measurement in the coronary sinus can be used in patients with prior coronary stents, while coronary MR angiography cannot assess stenosis and patency of coronary stents.
The blood flow assessment in the coronary sinus is a clinically feasible approach because it only requires additional imaging time of 2 breath‐holds.
Introduction
Stress myocardial perfusion cardiovascular magnetic resonance (CMR) allows for accurate assessment of flow‐limiting coronary artery disease (CAD) invasively determined by fractional flow reserve (FFR).1, 2, 3, 4, 5, 6, 7 In addition, multicenter studies have demonstrated noninferiority of stress myocardial perfusion CMR for the assessment of myocardial ischemia in comparison to single‐photon emission computed tomography.8, 9, 10 However, Hussain et al demonstrated there was some discrepancy in the identification of myocardial ischemia between perfusion CMR and FFR in patients with multivessel CAD.11 Therefore, further identification of noninvasive detection tools for multivessel disease are needed.
The high spatial and temporal resolution, absence of ionizing radiation and integrated assessment of ventricular function, myocardial viability, and coronary artery anatomy are unequivocal advantages of the CMR approach. Furthermore, coronary flow reserve (CFR) calculated by MR flow measurement in the coronary sinus (CS) using phase‐contrast cine CMR, which represents ≈96% of the total myocardial blood flow of the left ventricle,12 has been shown to be useful as a surrogate for diffuse myocardial ischemia.13, 14, 15 If global CFR measured by stress‐rest CS blood flow is added to the stress myocardial perfusion CMR approach, it may improve detection of functional coronary stenosis, allowing for more accurate identification of patients with multivessel CAD who will most benefit from revascularization. We hypothesized that multiparametric analysis with MR blood flow measurement in the CS can provide incremental diagnostic value over stress‐rest perfusion CMR alone to identify those who require revascularization in the abovementioned patient population. Accordingly, the aim of this study was to assess the concordance of stress myocardial perfusion CMR for predicting reduced FFR in patients with multivessel disease, and to determine the value of CMR study combining stress‐rest myocardial perfusion CMR and global CFR measured by blood flow quantification in the CS.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We retrospectively studied a total of 96 consecutive patients who had known or suspected CAD and who underwent a comprehensive CMR before invasive angiography and who had angiographic luminal narrowing >50% in the proximal or mid part of a major coronary artery and underwent FFR assessment. Subjects were identified by querying the Mie University Hospital clinical cardiac CMR and catheter laboratory database from March 2011 to September 2013. Exclusion criteria were cardiac arrhythmias (atrial fibrillation, uncontrolled tachycardia, and second‐ or third‐degree atrioventricular block); dilated, hypertrophic inflammatory, and infiltrative cardiomyopathies; previous coronary artery bypass grafting; unstable angina, congestive heart failure (New York Heart Association functional class III or IV), renal insufficiency (defined as a glomerular filtration rate <30 mL/min), known allergy to gadolinium contrast, and pregnancy (Figure 1). The study was carried out with Mie University Hospital Institutional Review Board approval and all patients gave their opt‐out informed consent.
Figure 1.
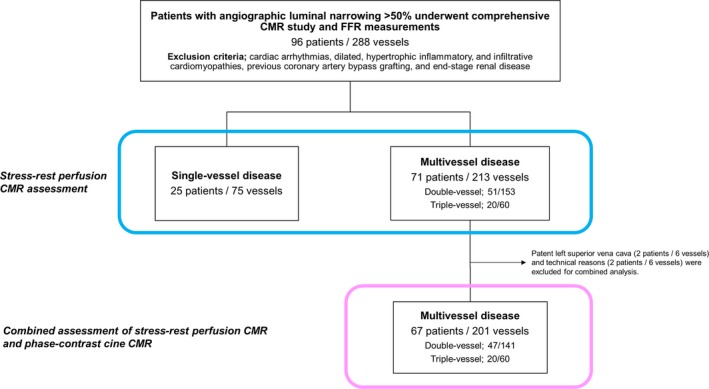
Flow chart of the study patients. CMR indicates cardiovascular magnetic resonance; FFR, fractional flow reserve.
Cardiac Magnetic Resonance Protocol
Before arrival, subjects were asked to refrain from caffeine for 12 hours before CMR. Intravenous access was obtained in the right and left antecubital veins for the administration of gadolinium contrast and adenosine, respectively. All CMR images were acquired with a 1.5‐ or 3‐T scanner (Achieva 1.5 T, Ingenia 3 T; Philips Medical Systems, The Netherlands) equipped with a 32‐element cardiac‐surface coil. The CMR protocol included (1) cine CMR, (2) stress perfusion CMR and phase‐contrast cine CMR in the CS during the intravenous infusion of adenosine (140 μg/kg per minute), (3) rest perfusion CMR and phase‐contrast cine CMR at 10 minutes following stress imaging, and (4) late gadolinium enhanced (LGE) CMR. To assess left ventricular (LV) myocardial function, geometry, and mass, 10 to 12 short‐axis stack images and 4‐chamber long‐axis images were acquired using a cine balanced steady‐state free precession sequence (1.5‐T scanner; repetition time [TR]=2.8 ms, echo time [TE]=1.4 ms, flip angle=55°, field‐of‐view=35×35 cm, acquisition matrix size=192×192, slice thickness, 10 mm, and 20 phase per cardiac cycle, 3‐T scanner; TR=3.1 ms, TE=1.5 ms, flip angle=55°, field‐of‐view=35×35 cm, acquisition matrix size=176×281, slice thickness, 10 mm, and 20 phase per cardiac cycle). First‐pass contrast‐enhanced myocardial perfusion CMR images during a continuous 3‐minute intravenous infusion of adenosine and at rest were obtained in short‐axis imaging planes of the LV with a saturation‐recovery balanced turbo field‐echo (TFE) sequence (1.5‐T scanner; TR=3.0 ms, TE=1.5 ms, flip angle=40°, field‐of‐view=36×32 cm, section thickness=8 mm, acquisition matrix size=192×154, SENSE factor=2, time between saturation preparation pulse and center of k‐space acquisition=200 ms, and duration of image data acquisition=210 ms, 3‐T scanner; TR=2.7 ms, TE=1.3 ms, flip angle=20°, field‐of‐view=34×31 cm, section thickness=10 mm, acquisition matrix size=224×134, SENSE factor=2.8, time between saturation preparation pulse and center of k‐space acquisition=110 ms, and duration of image data acquisition=117 ms). The k‐space was filled by using a sequential linear order. Dynamic MR acquisition in 4 short‐axis imaging planes of the LV was repeated continuously every 2 cardiac cycles. Thirty dynamic MR images were acquired at each slice position. The subjects were instructed to begin holding their breath at the start of image acquisition and to maintain breath‐holding for as long as possible. For both stress and rest perfusion CMR, gadolinium contrast medium (Gd‐DTPA or Gd‐DOTA) was injected into the right antecubital vein at a dose of 0.05 mmol/kg and a flow rate of 4 mL/s, followed by a 20‐mL saline flush. CS blood flow during adenosine stress and at rest was determined by breath‐hold phase‐contrast cine CMR (1.5‐T scanner; TR=8.6 ms, TE=5.6 ms, flip angle=15°, acquisition matrix size=192×112, field‐of‐view=36×26 cm, 20 phase per cardiac cycle, and velocity‐encoding=±50 cm/s, 3‐T scanner; TR=7.3 ms, TE=4.4 ms, flip angle=10°, acquisition matrix size=128×128, field‐of‐view=24×20 cm, 20 phase per cardiac cycle, and velocity‐encoding=±50 cm/s). Stress and rest phase‐contrast cine CMR images were acquired during breath‐holding with shallow inspiration (20–25 s) on an imaging plane that was perpendicular to the CS immediately after stress and rest perfusion CMR, respectively. The imaging position was carefully chosen so that blood flow in the CS was measured on the slice as close to its orifice to the right atrium as possible and the CS was visible throughout the cardiac cycle.
Immediately after rest perfusion CMR, an additional gadolinium dose was injected to reach a cumulative dose of 0.15 mmol/kg. Then, 10 to 15 minutes later, LGE CMR was obtained on short‐ and long‐axis imaging planes of the LV by using an inversion recovery 3D TFE sequence, with the following imaging parameters: 1.5‐T scanner; TR=3.8 ms, TE=1.2 ms, flip angle=15°, field‐of‐view=40×36×5 cm, acquisition matrix size=224×156×5, reconstructed matrix size=256×256×10, SENSE factor=2, TFE‐factor=24, 3‐T scanner; TR=4.6 ms, TE=2.2 ms, flip angle=15°, field‐of‐view=38×35×5 cm, acquisition matrix size=240×192×5, reconstructed matrix size=384×384×10, SENSE factor=4, TFE‐factor=28. Inversion time was adjusted in each patient to null signal from the normal myocardium by using a look‐locker sequence.
Image Analysis
CMR images were analyzed by 2 independent blinded observers using commercial workstations (CMR42; Circle Cardiovascular Imaging Inc, Calgary, Canada). At end‐diastole, epi‐ and endocardial LV borders were manually traced in contiguous short‐axis cine images covering the apex to mitral valve plane to calculate LV end‐diastolic volume and end‐systolic volume, mass, and ejection fraction. LV mass was calculated as the sum of the myocardial volume multiplied by the specific gravity (1.05 g/mL) of myocardial tissue. The presence or absence of stress‐induced perfusion defects was assigned to 3 coronary artery territories according to well‐established criteria.16 In cases where the coronary arterial anatomy varied from the above criteria, the coronary MR angiography was used to reassign segments to the appropriate vessel territory. Vessel territories were defined as positive for myocardial ischemia when the contrast signal was reduced to >6 heartbeats (ie, >3 consecutive image frames) in comparison to nonischemic myocardial segments, and worsened when compared with rest perfusion CMR. In cases with positive LGE, myocardial ischemia was defined as a perfusion defect reaching beyond scarred tissue in the corresponding LGE images, and a match between the perfusion defect and the LGE was considered chronic myocardial infarction without myocardial ischemia.
The contour of the CS was manually traced on the magnitude images at each cine frame. The traced region of interest was applied to the corresponding phase image, and the cross‐sectional area and mean velocity were recorded. Volumetric blood flow in the CS (mL/min) was calculated by integrating the product of cross‐sectional area and mean velocity in the CS from the 16 images acquired across the cardiac cycle. Global CFR was calculated as stress CS blood flow divided by rest CS blood flow (Figure 2). To evaluate inter‐ and intraobserver reproducibility, measurements of CFR in the CS from a random sample of 10 patients were independently assessed by 2 observers, and 1 observer measured CFR twice on 2 separate days with a washout period of at least 1 month. For the combined assessment of perfusion CMR and CFR in the CS, a patient or a vessel territory was considered positive if perfusion CMR showed abovementioned ischemic signs or if CFR was less than the cut‐off value of 2.0 based on previous published data,17, 18, 19 regardless of the presence or absence of myocardial ischemia on perfusion CMR. In addition, for receiver‐operator characteristic curve analysis, stress perfusion images in each vessel territory were scored on a 4‐point Likert scale (1: normal, 2: probably normal, 3: probably abnormal or subendocardial defect, 4: abnormal or transmural defect). On LGE images, the presence or absence of LGE was visually assessed in 16 segments.
Figure 2.
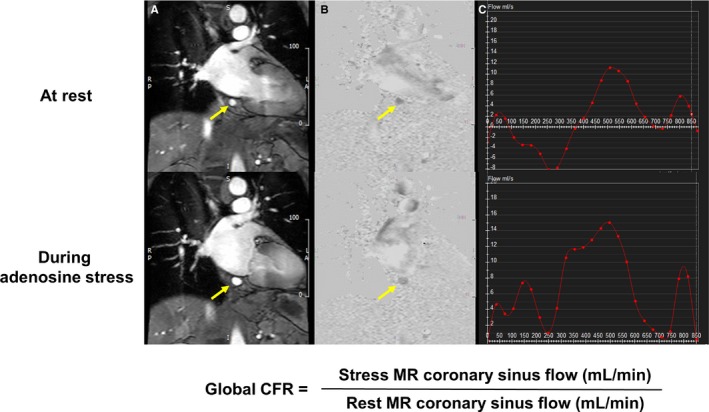
Measurement of blood flow in the coronary sinus. Phase‐contrast cine magnitude image (A), velocity map (B), and blood flow curve in the coronary sinus (arrows) at 1 cardiac cycle (C). CFR indicates coronary flow reserve; MR, magnetic resonance.
Invasive Coronary Angiography and FFR
Similarly, all subjects were asked to refrain from caffeine for 12 hours before invasive coronary angiography. Invasive angiography was performed according to standard techniques, with a catheter inserted via the radial or brachial artery by using a 5‐ or 6‐F guiding catheter. All angiograms were analyzed by 2 experienced cardiologists without knowledge of CMR findings for the presence of a significant coronary artery stenosis >50%. FFR was measured with a sensor‐tipped 0.014‐inch guidewire (Pressure Wire; Radi Medical Systems, Uppsala, Sweden) in every lesion with a luminal narrowing between 50% and 90%. After positioning the pressure sensor distal of the stenosis, maximal myocardial hyperemia was induced with a continuous intravenous infusion of adenosine (140 μg/kg per minute) for a minimum of 2 minutes. During maximum hyperemia, FFR was calculated as the ratio of the mean distal pressure, measured by the pressure wire, divided by the mean proximal pressure measured by the guiding catheter. A coronary artery stenosis of >50% with an FFR value of <0.8 or luminal narrowing of >90% was considered functionally significant.
Statistical Analysis
Continuous variables are expressed as mean±SD or median (quartiles) if not normally distributed, and compared using an unpaired Student t test. Categorical variables were expressed as numbers and proportions, and compared using a χ2 test. Significance of difference of CFR among 3 groups was evaluated by 1‐way ANOVA with Bonferroni's post hoc test. All tests were 2 sided and a P<0.05 was considered statistically significant. Analysis was performed on both a patient and coronary territory basis by calculating the sensitivity, specificity, positive predictive value, and negative predictive value to detect hemodynamically significant stenosis. The area under the receiver‐operator characteristic curve (AUC) was calculated and compared for all diagnostic testing strategies for FFR <0.80. Intra‐ and interobserver measurements of CFR were assessed with intraclass correlation coefficient. A P<0.05 was considered statistically significant. All analyses were performed using the SPSS software version 19.0 (SPSS Inc, Chicago, IL) and R version 3.2.3.
Results
The patient demographics are summarized in Table 1. No recruited patients were excluded because of side effects because of adenosine or gadolinium contrast. A total of 288 coronary territories in 96 patients (28 women, mean age 70 years) were thus available for analysis. Visual analysis of invasive coronary angiography revealed 36 vessels with ≥90% stenosis and 151 vessels with 50% to 90% stenosis. There were 25 patients with single‐vessel disease, 51 patients with double‐vessel disease, and 20 patients with triple‐vessel disease, which was defined as luminal narrowing >50%. FFR evaluation was performed in 139 vessels (92%), and measured FFR ranged from 0.38 to 1.0 with 66 vessels presenting with FFR <0.80. Twelve vessels in which FFR measurement was considered as unsafe (4) or failed because of technical reason (6) and luminal diameter was <2 mm (2) were considered functionally significant.
Table 1.
Baseline Characteristics
| Characteristic | Value |
|---|---|
| Age, y | 70±9 |
| Sex, n (%) | Women, 28 (29%) |
| Coronary risk factor | |
| Hypertension | 68 (71%) |
| Dyslipidemia | 61 (64%) |
| Smoking | |
| Current smoker | 19 (20%) |
| Ex‐smoker | 12 (13%) |
| Diabetes mellitus | 36 (38%) |
| Family history of CAD | 14 (15%) |
| Symptoms | |
| Typical angina | 50 (52%) |
| Atypical angina | 19 (20%) |
| Dyspnea on effort | 9 (9%) |
| ECG abnormality | 18 (19%) |
| Prior percutaneous coronary intervention | 29 (30%) |
| Prior myocardial infarction | 20 (21%) |
| Days between CMR and ICA | 16 (5–28) |
| Angiographic data | |
| Single‐vessel disease | 25 (26%) |
| Double‐vessel disease | 51 (53%) |
| Triple‐vessel disease | 20 (21%) |
| Vessels with luminal narrowing of >90% | 36/288 (13%) |
| Vessels with luminal narrowing with 50% to 90% | 151/288 (52%) |
| FFR measurement | 139/151 (92%) |
| Unsafe/technical failure/luminal diameter <2 mm | 4/6/2 (8%) |
| Vessels with FFR >0.80 | 73/139 (53%) |
| Vessels with FFR <0.80 | 66/139 (47%) |
| CMR data | |
| LVEDV, mL | 126±32 |
| LVESV, mL | 54±27 |
| LVEF, % | 59±11 |
| LV mass, g | 90±22 |
| Coronary flow reserve | 2.9±1.2 |
| The presence of LGE | 21/96 (22%) |
Values are mean±SD, n (%), or median (interquartile range). CAD indicates coronary artery disease; CMR, cardiovascular magnetic resonance; FFR, fractional flow reserve; ICA, invasive coronary angiography; LGE, late gadolinium enhancement; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume.
Distribution of FFR According to the Presence or Absence of Myocardial Ischemia
Figure 3 shows FFR values in the corresponding vessel territories with and without myocardial ischemia by visual assessment of stress‐rest perfusion CMR. The mean FFR in vessels without myocardial ischemia on stress perfusion CMR was 0.82±0.09, while the mean FFR in vessels with myocardial ischemia was 0.77±0.10 (P<0.01). Although the presence of myocardial ischemia was associated with higher likelihood of functional stenosis defined as FFR <0.8, we found a substantial overlap between the 2 groups. Visual assessment of stress CMR had a sensitivity of 65% (74/114), a specificity of 86% (149/174), a negative predictive value of 79% (149/189), and a positive predictive value of 75% (74/99) with AUC of 0.83 (95% confidence interval [CI], 0.78%–0.87%) for detecting hemodynamically significant coronary artery stenosis in the vessel‐based analysis and 88% (60/68), 75% (21/28), 58% (21/36), and 88% (53/60) with AUC of 0.82 (95% CI, 0.72%–0.89%) in the patient‐based analysis.
Figure 3.
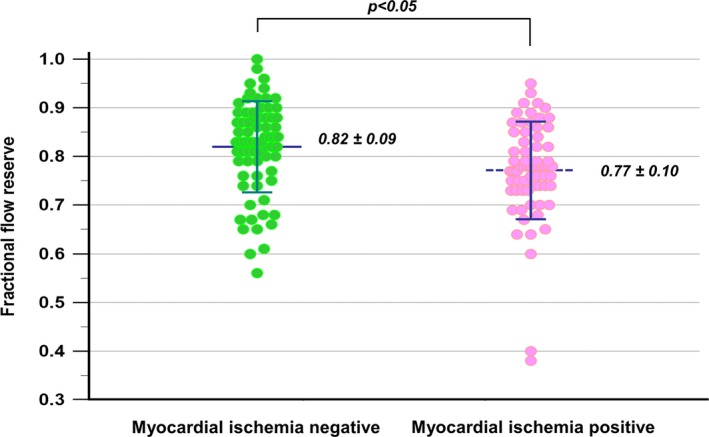
Distribution of fractional flow reserve value according to vessel territories with and without myocardial ischemia. There is a substantial overlap in the whole study population, including many subjects with multivessel disease, although statistical significance is observed between both groups.
Diagnostic Concordance of Stress‐Rest Perfusion CMR in Single‐Vessel Disease and Multivessel Disease
Figure 4 shows representative stress perfusion CMR and angiographic images from patients with single‐vessel and multivessel disease. In 71 patients with multivessel disease, the concordance of stress‐rest CMR decreased to a sensitivity of 64% (65/102) and a specificity of 79% (88/111) with AUC of 0.78 (95% CI, 0.71%–0.84%), for per‐vessel level. In contrast, 25 patients with single‐vessel disease exhibited a sensitivity of 83% (10/12) and a specificity of 95% (60/63) with AUC of 0.95 (95% CI, 0.87%–0.99%). A per‐patient level analysis showed a similar trend, with a sensitivity of 79% (44/56) and a specificity of 67% (10/15) with AUC of 0.74 (95% CI, 0.60%–0.88%) in multivessel disease, versus 83% (10/12) and 85% (11/13), respectively, with AUC of 0.93 (95% CI, 0.76%–0.99%) in single‐vessel disease.
Figure 4.
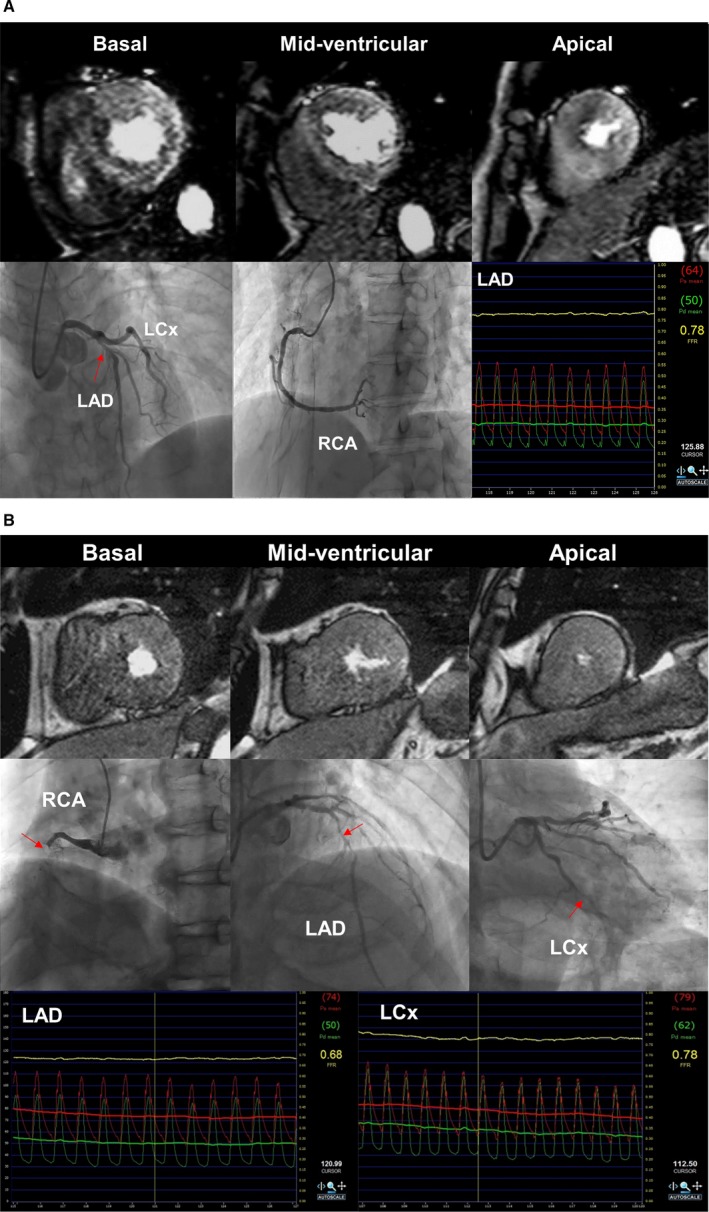
Representative cases. A, Sixty‐one‐year‐old woman with no prior myocardial infarction (MI) and single‐vessel disease. Stress myocardial perfusion cardiovascular magnetic resonance (CMR) images demonstrated a mild perfusion defect in the basal anterior and severe perfusion defects in the mid‐apex anterior and anteroseptal walls. The proximal left anterior descending (LAD) artery revealed an intermediate stenosis by coronary angiography (arrow). Fractional flow reserve (FFR) measured in the LAD artery was 0.78, confirming flow‐limiting stenosis. In this case, global CFR was 3.1 by MR blood flow quantification in the coronary sinus. B, Seventy‐eight‐year‐old man with no prior MI and multivessel disease. Coronary angiography revealed triple‐vessel disease with right coronary artery (RCA) total occlusion (arrows). FFR values in the LAD and left circumflex artery (LCx) were reduced to 0.68 and 0.78, suggesting flow‐limiting lesions. However, on stress myocardial perfusion CMR, regional myocardial ischemia was not detected by qualitative assessment. By MR coronary sinus blood flow measurements, global coronary flow reserve was severely reduced to 1.9.
Combined Assessment of Perfusion CMR and Phase‐Contrast Cine CMR During Stress and at Rest
CMR blood flow assessment in the CS was unsuitable for analysis in 4 patients with multivessel disease because of technical reasons (2) or a patent left superior vena cava (2). The mean CFR measured in the CS in patients in the entire study was 2.9±1.2. The mean CFR in patients with single‐vessel disease was 3.8±0.9, and no patients with single‐vessel disease had a CFR value of <2.5. In 67 patients with multivessel disease, the mean CFR was 2.6±1.1, which was significantly lower than that in single‐vessel disease. After patients with the multivessel disease were subclassified into double‐vessel and triple‐vessel diseases, the mean CFR was significantly lower in both groups when compared with that in single‐vessel (2.3±1.2 in triple, 2.7±1.1 in double, and 3.8±0.9 in single‐vessel disease, P<0.05 after Bonferroni correction, respectively) (Figure 5). The intraclass correlation coefficient for interobserver and intraobserver measurements of CFR were 0.83 (95% CI, 0.46%–0.96%) and 0.88 (95% CI, 0.60%–0.97%).
Figure 5.
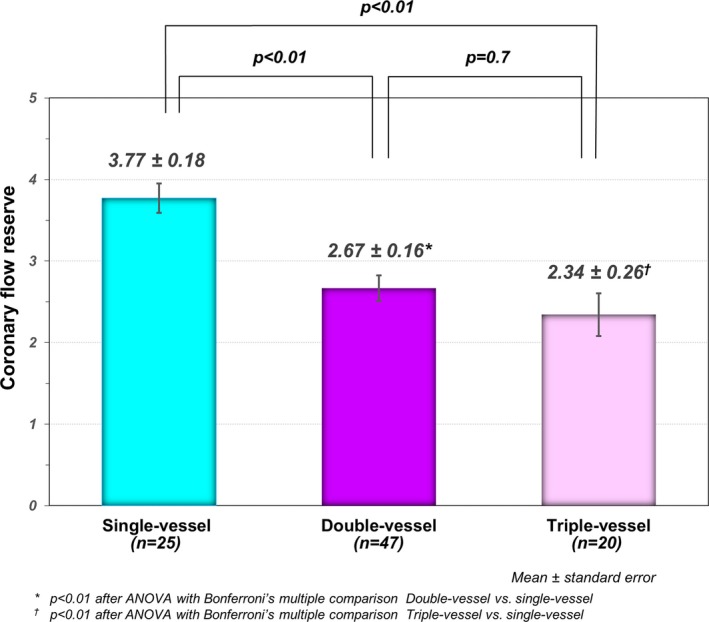
Comparison of coronary flow reserve. The mean coronary flow reserve (CFR) was significantly lower in patients with multivessel disease in comparison to subjects with single‐vessel disease. There was no significant difference in CFR value between double‐vessel vs triple‐vessel disease.
Given the cut‐off CFR value of 2.0 in predicting flow‐limiting stenosis, 21 (62%) of 34 vessel territories with false‐negative result on stress‐rest perfusion CMR were reclassified to the correct diagnoses. On the other hand, 9 (11%) of 85 vessel territories with true negative result on stress‐rest perfusion CMR resulted in incorrect reclassification. In the multivessel group, combined assessment of stress perfusion CMR and global CFR provided sensitivity, specificity, negative predictive value, and positive predictive value, 86% (82/95), 73% (77/106), 86% (77/90), and 74% (82/111), respectively, on a per‐vessel basis with AUC of 0.84 (95% CI, 0.79%–0.90%). Overall, the addition of blood flow assessment in the CS showed a greater AUC than that of stress‐rest CMR alone (AUC=0.84, 0.80, respectively, Delong test; P=0.04). Furthermore, integration of CS flow and visual assessment of stress perfusion CMR yield 7 correct (up) reclassifications and no incorrect (down) reclassifications in patients with negative myocardial ischemia and provided better diagnostic concordance with a sensitivity of 92% (48/52) and a specificity of 73% (11/15) with AUC of 0.88 (95% CI, 0.80%–0.97%) at the per‐patient level in the multivessel group (Delong test; P=0.002) (Table 2 and Figure 6).
Table 2.
Comparison of Diagnostic Performance for Predicting Hemodynamically Significant Stenosis in Single‐Vessel Versus Multivessel Disease
| Measure | Per‐Patient Level | Per‐Vessel Level | ||||
|---|---|---|---|---|---|---|
| Single‐Vessel (n=25) | Multivessel (n=67) | Single‐Vessel (n=75) | Multivessel (n=201) | |||
| Perfusion CMR (95% CI) | Perfusion CMR (95% CI) | Combined Perfusion CMR/CS Blood Flow (95% CI) | Perfusion CMR (95% CI) | Perfusion CMR (95% CI) | Combined Perfusion CMR/CS Blood Flow (95% CI) | |
| Functional CAD, % | 48.0 | 77.6 | 77.6 | 16.0 | 47.3 | 47.3 |
| Sensitivity, % |
83 (52–98) 10/12 |
79 (65–89) 41/52 |
92 (82–98) 48/52 |
83 (52–98) 10/12 |
64 (54–74) 61/95 |
86 (78–93) 82/95 |
| Specificity, % |
85 (55–98) 11/13 |
73 (45–92) 11/15 |
73 (45–92) 11/15 |
95 (87–99) 60/63 |
81 (72–88) 85/106 |
73 (63–81) 77/106 |
| NPV, % |
85 (55–98) 11/13 |
50 (28–72) 11/22 |
73 (44–93) 11/15 |
97 (89–100) 60/62 |
72 (63–80) 85/119 |
86 (77–92) 77/90 |
| PPV, % |
83 (52–98) 10/12 |
91 (79–98) 41/45 |
92 (82–98) 48/52 |
77 (46–95) 10/13 |
75 (65–84) 61/82 |
74 (65–82) 82/111 |
| LR (+) | 5.42 | 2.96 | 3.46 | 17.5 | 3.4 | 3.15 |
| LR (−) | 0.20 | 0.29 | 0.10 | 0.18 | 0.44 | 0.19 |
| AUC | 0.93 (0.76–0.99) | 0.76 (0.64–0.86) | 0.88 (0.80–0.97) | 0.95 (0.87–0.99) | 0.80 (0.73–0.85) | 0.84 (0.70–0.90) |
Values for sensitivity, specificity, PPV, NPV, and AUC are presented with 95% CI. AUC indicates area under the receiver operator characteristic curve; CAD, coronary artery disease; CI, confidence interval; CMR, cardiovascular magnetic resonance; CS, coronary sinus; LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Figure 6.
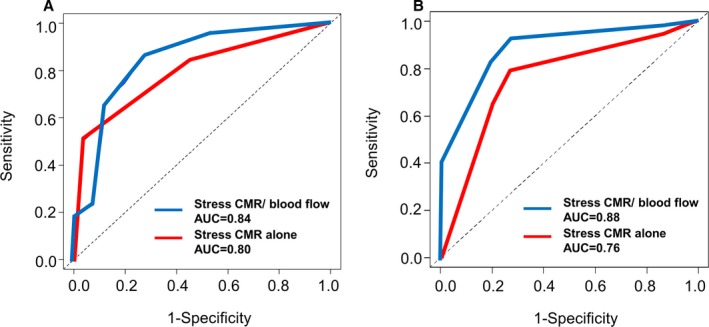
Comparison of the receiver operating characteristic (ROC) curves. ROC curve and corresponding area under the curve (AUC) describing the diagnostic concordance of stress‐rest perfusion cardiovascular magnetic resonance (CMR) alone (red line) and combined assessment of stress‐rest perfusion CMR and blood flow in the coronary sinus (blue line) to identify flow‐limiting stenosis at vessel level (A) and patient level (B).
We also examined diagnostic concordance of perfusion CMR in patients with prior myocardial infarction (MI) versus those without prior MI. The diagnostic concordance of stress CMR alone at vessel level did not differ between those with and without MI, with a sensitivity of 69% (18/26) and a specificity of 82% (28/34) with AUC of 0.79 (95% CI, 0.68%–0.91%) in patients with prior MI, versus 62% (43/69), 81% (58/72), respectively, with AUC of 0.80 (95% CI, 0.73–0.87) in non‐MI patients. In both groups, integration of CS flow assessment improved diagnostic concordance for detection of flow‐limiting stenosis to AUC of 0.84 (95% CI, 0.74–0.94) in patients with prior MI, and to 0.85 (95% CI, 0.78–0.91) in non‐MI patients.
Discussion
In this study, we demonstrate that the mean FFR value in territories without myocardial ischemia on stress perfusion CMR was significantly higher than the mean FFR in territories with myocardial ischemia. Visual assessment of stress perfusion CMR provided high diagnostic concordance in predicting reduced FFR in single vessel. In multivessel disease, however, visual assessment of stress perfusion CMR alone showed limited diagnostic concordance in predicting reduced FFR. The result in the present study is consistent with a recent study by Hussain et al, which demonstrated some discrepancy between high‐resolution perfusion CMR and FFR in patients with multivessel disease.11 However, in their study, perfusion CMR images alone were visually assessed, without combination of global CFR assessment. A recent study by Shomanova et al found additive value of CFR assessment to perfusion CMR for detecting angiographically obstructive CAD.18 The present study demonstrated that multiparametric CMR combining stress‐rest perfusion CMR and global CFR determined by blood flow measurements in the CS substantially improved the diagnostic concordance of CMR in predicting reduced FFR in patients with multivessel disease. This is the first study to our knowledge to comprehensively assess both stress‐rest CS blood flow and myocardial perfusion in the prediction of flow‐limiting stenosis. Importantly, CS blood flow can be derived with only addition of 2 breath‐hold phase‐contrast cine CMR scans.
In accordance with previous studies,1, 2, 3, 4, 5, 6, 7 the results in our current study demonstrated that stress‐rest perfusion CMR allows for noninvasive prediction of flow‐limiting stenosis in patients with suspected or known CAD. However, we found that the assessment of stress‐rest perfusion CMR alone showed limited diagnostic concordance in predicting reduced FFR in multivessel disease. This is supported by the results by Melikian et al, who reported that the sensitivity and specificity of myocardial perfusion single‐photon emission computed tomography for detecting significant stenosis was 76% and 38%, respectively, in patients with multivessel disease.20 In contrast to single‐photon emission computed tomography, first‐pass stress‐rest perfusion CMR can theoretically reveal abnormal transmural blood flow patterns, thus avoiding false‐negative results that can occur in balanced myocardial ischemia in multivessel disease. However, in the setting of low CFR associated with severe diffuse and/or multivessel disease, the concordance of stress perfusion CMR may be limited as well. In addition, visual assessment relies on identifying relative differences in perfusion between adjacent myocardial territories or between subendocardial and subepicardial myocardium. Greenwood et al showed similar and high diagnostic performance of perfusion CMR between single and multivessel disease for detecting obstructive CAD.10 However, we focused mainly on the assessment of perfusion CMR against flow‐limiting stenosis determined by FFR; thereby there are significant differences in these 2 studies. The presence of coronary steal or a positive collateral flow reserve in multivessel disease can lead to some differences in diagnostic concordance between the physiological and anatomical severity of coronary stenosis as reference.21
Several investigators attempted to improve the diagnostic concordance of a stress CMR study. In a recent study by Bettencourt et al, coronary MR angiography and stress perfusion CMR were combined. However, additional coronary MR angiography failed to improve the diagnostic concordance at a cost of longer scanning time, leading to the conclusion that coronary MR angiography integration might not be routinely justified in the clinical setting.22 Given that the benefits from revascularization are most apparent in physiological stenosis rather than anatomical stenosis, the integration of functional blood flow assessment might be more appropriate. Our present study demonstrated that the combined assessment of CS blood flow and myocardial perfusion during stress and at rest provided the better diagnostic concordance on patient‐based as well as vessel‐based analyses in patients with multivessel disease. The CFR assessment using CS blood flow indicates not only the severity of a coronary stenosis measured by FFR but also microvascular information with endothelial dysfunction, and does not necessarily have an excellent concordance with FFR assessment. However, we found that patients with multivessel disease appeared more likely to have lower CFR. Considering that there is a considerable overlap between multivessel disease and lower CFR, the combined CMR approach has the potential to improve the diagnostic concordance in detecting reduced FFR beyond stress CMR alone approach. A positron emission tomography study by Taqueti et al demonstrated that global CFR was independently associated with increased risk of major adverse cardiac events regardless of angiographic CAD.23 In a recent CMR study by Kato et al, CFR using CS blood flow provided better risk stratification compared with perfusion CMR for patients with suspected CAD.19 Therefore, it might be plausible and reasonable that the integration of CS flow measurement can detect high‐risk populations with low CFR but not functionally angiographic CAD. It should be also noted that combination of perfusion CMR and CS flow measurement can be used in patients with prior coronary stents, while coronary MR angiography cannot assess stenosis and patency of coronary stents.
Quantitative assessment of perfusion CMR may also be useful for detecting reduced coronary blood flow during stress in patients with multivessel disease and/or diffuse endothelial dysfunction population. In previous reports, however, quantitative analysis could not necessarily improve the diagnostic concordance to detect flow‐limiting stenosis as compared with visual analysis of CMR.2, 24 In addition, quantitative assessment is time consuming and might be dependent on image quality and the presence of prior infarction. Currently, Kellman et al demonstrated in‐line automated and reliable perfusion mapping, which may increase the applicability of the technology in clinical and research settings.25 On the other hand, an advantage of blood flow assessment in the CS is that hemodynamic data can be derived from phase‐contrast images alone with additional imaging time of 2 breath‐holds and postprocessing time of a few minutes.
Limitations
Our study has several limitations. First, the present study is a single‐center, observational study with a relatively small sample size. Dose of adenosine 140 μg/kg per minute may not be sufficient in some patients to detect perfusion deficit on stress CMR. However, given that we used the same adenosine stress protocol for CMR and FFR measurements, adenosine under stress could not contribute to false‐negative findings on perfusion CMR. We studied patients undergoing stress CMR at 2 different MR scanners and different sequence parameter settings that might have an influence on the results. Our results are not applicable to the patients with coronary artery bypass grafts or a persistent left superior vena cava.
In conclusion, visual assessment of stress perfusion CMR provided high diagnostic concordance in predicting reduced FFR in single‐vessel disease. However, in multivessel disease, its concordance was limited. The integration of MR blood flow measurements in the CS in stress‐rest CMR examination substantially improved the diagnostic concordance in predicting reduced FFR in patients with multivessel. The blood flow assessment in the CS is a clinically feasible approach because it only requires additional imaging time of 2 breath‐holds. Larger multicenter studies are warranted to examine the value of the integrated stress CMR approach presented in this study in the selection of patients with multivessel disease indicated for revascularization therapy.
Disclosures
Sakuma, MD receives departmental research grant support from Siemens AG, Bayer AG, Guerbet SA, DAIICHI SANKYO Group, FUJIFILM Holdings Corporation and Nihon Medi‐Physics Co, Ltd. Masaaki Ito, MD receives departmental research grant support from Genzyme Japan K.K., Shionogi & Co, Ltd, Sumitomo Dainippon Pharma Co, Ltd, Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co, Ltd, Bayer AG, Pfizer Japan Inc, AstraZeneca K.K., MSD K.K., Bristol‐Myers Squibb, Astellas Pharma Inc, BIOTRONIK Japan Inc, Eisai Co, Ltd, Boston Scientific Japan K. K., Torii Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, and Nippon Shinyaku Co, Ltd.
Acknowledgments
We thank Susan M. Miller, MD, MPH at Houston Methodist Hospital for reviewing the article and for editorial assistance.
(J Am Heart Assoc. 2018;7:e007736 DOI: 10.1161/JAHA.117.007736.)29432130
References
- 1. Watkins S, McGeoch R, Lyne J, Steedman T, Good R, McLaughlin MJ, Cunningham T, Bezlyak V, Ford I, Dargie HJ, Oldroyd KG. Validation of magnetic resonance myocardial perfusion imaging with fractional flow reserve for the detection of significant coronary heart disease. Circulation. 2009;120:2207–2213. [DOI] [PubMed] [Google Scholar]
- 2. Lockie T, Ishida M, Perera D, Chiribiri A, De Silva K, Kozerke S, Marber M, Nagel E, Rezavi R, Redwood S, Plein S. High‐resolution magnetic resonance myocardial perfusion imaging at 3.0‐Tesla to detect hemodynamically significant coronary stenoses as determined by fractional flow reserve. J Am Coll Cardiol. 2011;57:70–75. [DOI] [PubMed] [Google Scholar]
- 3. Jogiya R, Kozerke S, Morton G, De Silva K, Redwood S, Perera D, Nagel E, Plein S. Validation of dynamic 3‐dimensional whole heart magnetic resonance myocardial perfusion imaging against fractional flow reserve for the detection of significant coronary artery disease. J Am Coll Cardiol. 2012;60:756–765. [DOI] [PubMed] [Google Scholar]
- 4. Manka R, Paetsch I, Kozerke S, Moccetti M, Hoffmann R, Schroeder J, Reith S, Schnackenburg B, Gaemperli O, Wissmann L, Wyss CA, Kaufmann PA, Corti R, Boesiger P, Marx N, Lüscher TF, Jahnke C. Whole‐heart dynamic three‐dimensional magnetic resonance perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve: determination of volumetric myocardial ischaemic burden and coronary lesion location. Eur Heart J. 2012;33:2016–2024. [DOI] [PubMed] [Google Scholar]
- 5. Chiribiri A, Hautvast GL, Lockie T, Schuster A, Bigalke B, Olivotti L, Redwood SR, Breeuwer M, Plein S, Nagel E. Assessment of coronary artery stenosis severity and location: quantitative analysis of transmural perfusion gradients by high‐resolution MRI versus FFR. JACC Cardiovasc Imaging. 2013;6:600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bettencourt N, Chiribiri A, Schuster A, Ferreira N, Sampaio F, Duarte R, Santos L, Melica B, Rodrigues A, Braga P, Teixeira M, Simões L, Leite‐Moreira A, Silva‐Cardoso J, Nagel E, Portugal P, Gama V. Cardiac magnetic resonance myocardial perfusion imaging for detection of functionally significant obstructive coronary artery disease: a prospective study. Int J Cardiol. 2013;168:765–773. [DOI] [PubMed] [Google Scholar]
- 7. Manka R, Wissmann L, Gebker R, Jogiya R, Motwani M, Frick M, Reinartz S, Schnackenburg B, Niemann M, Gotschy A, Kuhl C, Nagel E, Fleck E, Marx N, Luescher TF, Plein S, Kozerke S. Multicenter evaluation of dynamic three‐dimensional magnetic resonance myocardial perfusion imaging for the detection of coronary artery disease defined by fractional flow reserve. Circ Cardiovasc Imaging. 2015;8:e003061. [DOI] [PubMed] [Google Scholar]
- 8. Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al‐Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M, Johansson L. MR‐IMPACT: comparison of perfusion‐cardiac magnetic resonance with single‐photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–489. [DOI] [PubMed] [Google Scholar]
- 9. Schwitter J, Wacker CM, Wilke N, Al‐Saadi N, Sauer E, Huettle K, Schönberg SO, Luchner A, Strohm O, Ahlstrom H, Dill T, Hoebel N, Simor T. MR‐IMPACT II: magnetic resonance imaging for myocardial perfusion assessment in coronary artery disease trial: perfusion‐cardiac magnetic resonance vs. single‐photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775–781. [DOI] [PubMed] [Google Scholar]
- 10. Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, Bijsterveld P, Ridgway JP, Radjenovic A, Dickinson CJ, Ball SG, Plein S. Cardiovascular magnetic resonance and single‐photon emission computed tomography for diagnosis of coronary heart disease (CE‐MARC): a prospective trial. Lancet. 2012;379:453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hussain ST, Chiribiri A, Morton G, Bettencourt N, Schuster A, Paul M, Perera D, Nagel E. Perfusion cardiovascular magnetic resonance and fractional flow reserve in patients with angiographic multi‐vessel coronary artery disease. J Cardiovasc Magn Reson. 2016;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Rossum AC, Visser FC, Hofman MB, Galjee MA, Westerhof N, Valk J. Global left ventricular perfusion: noninvasive measurement with cine MR imaging and phase velocity mapping of coronary venous outflow. Radiology. 1992;182:685–691. [DOI] [PubMed] [Google Scholar]
- 13. Kawada N, Sakuma H, Yamakado T, Takeda K, Isaka N, Nakano T, Higgins CB. Hypertrophic cardiomyopathy: MR measurement of coronary blood flow and vasodilator flow reserve in patients and healthy subjects. Radiology. 1999;211:129–135. [DOI] [PubMed] [Google Scholar]
- 14. Sakuma H, Kawada N, Takeda K, Higgins CB. MR measurement of coronary blood flow. J Magn Reson Imaging. 1999;10:728–733. [DOI] [PubMed] [Google Scholar]
- 15. Hundley WG, Hillis LD, Hamilton CA, Applegate RJ, Herrington DM, Clarke GD, Braden GA, Thomas MS, Lange RA, Peshock RM, Link KM. Assessment of coronary arterial restenosis with phase‐contrast magnetic resonance imaging measurements of coronary flow reserve. Circulation. 2000;101:2375–2381. [DOI] [PubMed] [Google Scholar]
- 16. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS; American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging . Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 17. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision‐making. J Am Coll Cardiol. 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 18. Shomanova Z, Florian A, Bietenbeck M, Waltenberger J, Sechtem U, Yilmaz A. Diagnostic value of global myocardial perfusion reserve assessment based on coronary sinus flow measurements using cardiovascular magnetic resonance in addition to myocardial stress perfusion imaging. Eur Heart J Cardiovasc Imaging. 2017;18:851–859. [DOI] [PubMed] [Google Scholar]
- 19. Kato S, Saito N, Nakachi T, Fukui K, Iwasawa T, Taguri M, Kosuge M, Kimura K. Stress perfusion coronary flow reserve versus cardiac magnetic resonance for known or suspected CAD. J Am Coll Cardiol. 2017;70:869–879. [DOI] [PubMed] [Google Scholar]
- 20. Melikian N, De Bondt P, Tonino P, De Winter O, Wyffels E, Bartunek J, Heyndrickx GR, Fearon WF, Pijls NH, Wijns W, De Bruyne B. Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv. 2010;3:307–314. [DOI] [PubMed] [Google Scholar]
- 21. Werner GS, Figulla HR. Direct assessment of coronary steal and associated changes of collateral hemodynamics in chronic total coronary occlusions. Circulation. 2002;106:435–440. [DOI] [PubMed] [Google Scholar]
- 22. Bettencourt N, Ferreira N, Chiribiri A, Schuster A, Sampaio F, Santos L, Melica B, Rodrigues A, Braga P, Teixeira M, Leite‐Moreira A, Silva‐Cardoso J, Portugal P, Gama V, Nagel E. Additive value of magnetic resonance coronary angiography in a comprehensive cardiac magnetic resonance stress‐rest protocol for detection of functionally significant coronary artery disease: a pilot study. Circ Cardiovasc Imaging. 2013;6:730–738. [DOI] [PubMed] [Google Scholar]
- 23. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hamon M, Fau G, Née G, Ehtisham J, Morello R, Hamon M. Meta‐analysis of the diagnostic performance of stress perfusion cardiovascular magnetic resonance for detection of coronary artery disease. J Cardiovasc Magn Reson. 2010;12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kellman P, Hansen MS, Nielles‐Vallespin S, Nickander J, Themudo R, Ugander M, Xue H. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]


