Abstract
Background
Animal models support dietary omega‐3 fatty acids protection against abdominal aortic aneurysm (AAA), but clinical data are scarce. The sum of red blood cell proportions of the omega‐3 eicosapentaenoic and docosahexaenoic acids, known as omega‐3 index, is a valid surrogate for long‐term omega‐3 intake. We investigated the association between the omega‐3 index and the prevalence and progression of AAA. We also investigated associations between AAA and arachidonic acid, an omega‐6 fatty acid that is a substrate for proinflammatory lipid mediators.
Methods and Results
We obtained blood samples from 498 AAA patients (maximal aortic diameter ≥30 mm) within a population‐based ultrasound‐screening trial in men and from 199 age‐matched controls who screened negative. We determined the fatty acids of red blood cells by gas chromatography. During a median follow‐up of 4.85 years, 141 AAA patients reached criteria for vascular surgical repair. Participants were high consumers of omega‐3 (average omega‐3 index: 7.6%). No significant associations were found for omega‐3 index. In contrast, arachidonic acid in AAA patients was higher than in controls (P<0.001), and individuals in the upper tertile of arachidonic acid at baseline had higher probability of having AAA (odds ratio: 1.309; 95% confidence interval, 1.021–1.678; P=0.033). AAA patients at the upper tertile of arachidonic acid at baseline had a 54% higher risk of needing surgical repair during follow‐up (hazard ratio: 1.544; 95% confidence interval, 1.127–2.114; P=0.007).
Conclusions
Omega‐3 index is unrelated to men with AAA from a country in which fish consumption is customarily high. Arachidonic acid is associated with AAA presence and progression.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00662480.
Keywords: abdominal aortic aneurysm, diet, inflammation
Subject Categories: Biomarkers, Vascular Biology
Clinical Perspective
What Is New?
Omega‐3 index is not associated with abdominal aortic aneurysm (AAA) presence and progression in Denmark, a country with a high omega‐3 index.
Red blood cell arachidonic acid is related to increased prevalence and progression of AAA.
What Are the Clinical Implications?
Omega‐3 index is not valuable as a biomarker of AAA, at least in this cohort of patients with high fatty fish consumption.
Arachidonic acid is associated with AAA progression independent of aortic size, suggesting its potential role as a prognostic biomarker.
Modulation of the arachidonic acid pathway could be a therapeutic strategy to prevent AAA growth.
Introduction
Abdominal aortic aneurysm (AAA) is characterized by a focal dilation of the aortic diameter ≥30 mm. AAA is asymptomatic until aortic rupture, which is fatal in ≈90% of cases. Main mechanisms of AAA are proteolysis and oxidative stress, which triggers the inflammatory response of the aortic wall, contributing to the AAA continuum. Oxidative stress and particularly inflammation are inhibited by the fish‐derived omega‐3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). This prompted the notion that dietary omega‐3 might protect against AAA.1 Such a hypothesis was confirmed in experimental models (angiotensin II–infused apolipoprotein E–knockout mice2, 3, 4). To date, however, clinical data are limited to a recent study conducted in a small Japanese population that reported a low proportion of EPA in serum related to AAA growth and size.5
In the frame of a population‐based ultrasound‐screening trial for AAA in Danish men aged 65 to 74 years (VIVA [Viborg Vascular] trial6), we conducted an observational study that aimed to test whether long‐term intake of omega‐3 related to decreased prevalence and progression of AAA. In observational studies, the optimal approach to address this issue is by using the fatty acid composition of body tissues, given the difficulties of accurately measuring fat intake from the diet records.7 Although the fatty acid profile of adipose tissue is the best surrogate of long‐term fat intake, circulating fatty acids are a convenient and accepted alternative.7 The turnover of red blood cells (RBCs; 120‐day life span) makes RBCs more suitable for objective assessment of omega‐3 fatty acid status than serum or plasma.8 We focused on the sum of proportions of EPA and DHA in RBCs, known as the omega‐3 index, because it is a valid surrogate for long‐term omega‐3 intake9 and has been proposed as a risk factor for cardiovascular diseases, particularly sudden cardiac death.10 We also determined the RBC proportion of arachidonic acid, an omega‐6 fatty acid that is a substrate for the synthesis of proinflammatory lipid mediators once released from cell membranes.
Materials and Methods
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Access to the data can be requested by applying for permission from the Danish Data Protection Agency and Statistics Denmark.
Setting
This observational study was conducted in the frame of a population‐based image‐screening trial for AAA in Danish men aged 65 to 74 years (VIVA, ClinicalTrials.gov identifier NCT00662480). The protocol design has been reported in detail elsewhere.6 In brief, between October 2008 and October 2010, ≈50 000 individuals were randomized to receive an invitation for vascular screening or to be a control. In total, 18 750 of the 25 000 individuals offered vascular screening agreed to participate. All participants gave informed consent, and the local ethics committee of Viborg Hospital approved the study, which was performed in accordance with the Helsinki Declaration. Trained project nurses performed ultrasound scans of the aorta; measured the ankle–brachial index; and collected anthropometric, clinical, and lifestyle data in 3 mobile units at local hospitals in the region of Jutland, Denmark.
To visualize the aorta, the ultrasound 4‐MHz transducer was placed longitudinally just above and a little to the left of the navel. In cases of dilation, the maximal perpendicular anteroposterior diameter was measured. If no dilation was found, the anteroposterior diameter was measured 2 cm above the bifurcation. In the case of infrarenal aortic dilatation, such findings were pre‐AAA (25–30 mm) or AAA (≥30 mm). The interobserver variation of aortic diameter measurements was 1.52 mm.11 Patients with AAA <50 mm diameter were offered annual control scans by the screening team, whereas patients with AAA ≥50 mm were referred for a computed tomography scan and vascular surgical evaluation. Elective surgery was conducted in patients with AAA ≥55 mm. If no repair was indicated, the vascular surgical department took over management because of a need for closer surveillance.
Ankle systolic blood pressure was also measured, and maximal anterior–posterior diameter of the infrarenal aorta was measured at the peak of systole from the inner edge to the outer edge of the aorta. The ankle–brachial index was calculated as the mean of the 2 recorded ankle arterial blood pressures divided by the brachial systolic blood pressure. Peripheral arterial disease was defined as an ankle–brachial index <0.90 or >1.4.
We obtained fasting blood samples at diagnosis from 498 AAA patients and 199 age‐matched controls free of AAA and peripheral arterial disease who were recruited from the original cohort at baseline after the screening tests. Growth rate was calculated based on the ultrasound scans from the baseline and annual follow‐up visits using individual linear regression between sizes and time. During a median follow‐up of 4.85 years, 141 AAA patients reached size criteria for recommending vascular repair. One participant in this cohort had emergency surgery and was excluded from further analyses.
RBC Membrane Fatty Acid Analysis
Overnight fasting‐period (>10 hours) blood samples were obtained by venipuncture and stored at −80°C until fatty acid analysis. The RBC fatty acid profile was determined, as described.9 In brief, cells contained in a 100‐μL aliquot of EDTA‐collected blood were hemolyzed and spun. The pellet (>99% RBC membranes) was dissolved in 1 mL BF3 methanol solution and heated to hydrolyze and methylate glycerophospholipid fatty acids. The fatty acid methyl esters were isolated by adding n‐hexane and were separated by gas chromatography using an Agilent HP 7890 gas chromatograph equipped with a 30 m×0.25 μm×0.25 mm SupraWAX‐280 capillary column (Teknokroma), an autosampler, and a flame ionization detector. The amount of each fatty acid was expressed as a percentage of the total identified fatty acids in the sample. The omega‐3 index was calculated as the sum of the percentages of EPA and DHA. As reported,9 the fatty analysis method has been cross‐validated against the method used in the original definition of the omega‐3 index, with a coefficient of variance of 3% for EPA and DHA.12
Statistical Analyses
When the sampling occurred, the hypothesis about an association between AAA and fatty acids had not yet been formulated. To our knowledge, there is a single report of data on RBC fatty acids in Denmark.13 Given that no data on the omega‐3 index are available, sample size was calculated according to arachidonic acid. We assumed that if the mean of arachidonic acid is 15.6 and standard deviation is ±1.51, the smallest detectable difference between AAA and controls is ±0.65, corresponding to <5% of the mean at a 1% significance level and 99% power, suggesting a very robust study concerning the comparison between controls and AAA patients. We could not find any reasonable assumptions to perform a power calculation concerning need for later repair.
Normal distribution of data was assessed using graphical methods and the Shapiro–Wilk test. Residuals were assessed in histograms and p‐p plots. Differences between AAA patients and controls regarding exposures of interest (omega‐3 index and arachidonic acid) and main clinical characteristics were assessed by Student t test. Independent associations between AAA prevalence and both exposures (arachidonic acid and omega‐3 index) of interest (divided into tertiles) were assessed by logistic regression analyses with adjustments for active smoking, hypertension, use of statins, use of low‐dose aspirin, body mass index, diastolic blood pressure, and peripheral arterial disease at screening. In addition, in the subset of AAA patients, we used the Pearson correlation coefficient to study the association between exposures of interest and maximal aortic diameter and growth rate. Because of a nontransformable nonnormal distribution of C‐reactive protein, Spearman correlation tests were used when exploring correlations with C‐reactive protein. We also explored whether patients at the upper tertile of each exposure at baseline had an increased risk of needing surgical repair. To address this issue, we obtained a Kaplan–Meier curve for cumulative freedom by constructing a multivariate Cox proportional hazards model, adjusted for active smoking, hypertension, use of low‐dose aspirin, use of statins, peripheral arterial disease at screening, body mass index, diabetes mellitus, use of beta blockers, C‐reactive protein, and baseline maximal aortic diameter.
Results
The Student t test revealed expected differences in clinical characteristics between AAA patients and controls (Table 1). No significant differences were found between AAA patients and controls for omega‐3 index. The average omega‐3 index was 7.6% and was <4% (the proposed cutoff for high cardiovascular risk10) in only 4.9% of the study population (Figure 1A). Arachidonic acid proportion in AAA patients was higher than in controls (mean±SD: 15.90%±2.58 versus 15.06%±2.33, respectively; P<0.001; Figure 1B). Participants in the upper tertile of arachidonic acid at baseline consistently had significantly higher prevalence of AAA, independent of potential confounding factors (adjusted odds ratio: 1.309; 95% confidence interval [CI], 1.021–1.678; P=0.033; Table 2). In AAA patients, the proportion of arachidonic acid in RBCs directly correlated with maximal aortic diameter (R=0.091, P=0.042; Figure 2A) but not with aneurysmal growth rate (R=−0.020, P=0.702). No correlation was noted concerning maximal aortic diameter and aneurysmal growth rate for omega‐3 index (R=−0.037, P=0.476, and R=0.001, P=0.979, respectively), EPA (R=−0.06, P=0.117, and R=−0.02, P=0.967, respectively), or DHA (R=−0.08, P=0.872, and R=0.01, P=0.816, respectively). Finally, after a median follow‐up of 4.85 years, AAA patients in the upper tertile of arachidonic acid at baseline showed a 54% higher risk of needing surgical repair (adjusted hazard ratio: 1.544; 95% CI, 1.127–2.114; P=0.007; Figure 2B), independent of potential confounding factors, including aortic diameter (Table 3). No significant associations were found in AAA patients in the upper tertile of EPA (hazard ratio: 0.957; 95% CI, 0.709–1.292; P=0.773), DHA (hazard ratio: 0.832; 95% CI, 0.613–1.130; P=0.239), or omega‐3 index (hazard ratio: 0.906; 95% CI, 0.669–1.227; P=0.520). Finally, C‐reactive protein levels inversely correlated with EPA (R=−0.112, P=0.015), DHA (R=−0.148, P=0.001), and the omega‐3 index (R=−0.147, P=0.001), but no significant correlation was found for arachidonic acid levels (R=0.052, P=0.261).
Table 1.
Clinical Baseline Characteristics of the Study Population
| AAA (n=498) | Age‐Matched Controls (n=199) | P Valuea | AAA, Vascular Repair (n=141) | AAA, No Repair (n=357) | P Valueb | |
|---|---|---|---|---|---|---|
| Baseline aortic size, mm | 40.8 (11.8) | 18.3 (3.0) | <0.001 | 47.7 (13.7) | 35.6 (6.4) | <0.001 |
| PAD, n (%) | 130 (26.1) | 0c | <0.001 | 27 (19.1) | 103 (28.9) | 0.013 |
| BMI, kg/m2 | 27.4 (3.6) | 26.2 (3.3) | <0.001 | 27.4 (3.5) | 27.3 (3.7) | 0.640 |
| Current smoking, n (%) | 203 (40.8) | 39 (19.6) | <0.001 | 57 (40.4) | 146 (40.9) | 0.878 |
| Diabetes mellitus, n (%) | 54 (10.8) | 29 (14.6) | 0.181 | 15 (10.6) | 39 (10.9) | 0.608 |
| Hypertension, n (%) | 266 (53.3) | 91 (45.7) | 0.030 | 80 (56.7) | 186 (52.1) | 0.305 |
| Diastolic blood pressure, mm Hg | 87.9 (12.1) | 81.1 (10.2) | <0.001 | 89.1 (12.5) | 87.0 (11.8) | 0.064 |
| Use of statins, n (%) | 260 (52.1) | 73 (36.7) | <0.001 | 74 (52.5) | 186 (52.1) | 0.972 |
| Use of low‐dose aspirin, n (%) | 247 (49.5) | 54 (27.1) | <0.001 | 64 (45.4) | 183 (51.3) | 0.199 |
| Use of bronchodilators, n (%) | 39 (7.8) | 11 (5.5) | 0.260 | 8 (5.7) | 31 (8.7) | 0.289 |
| Use of beta blockers, n (%) | 150 (30.1) | 46 (23.1) | 0.053 | 35 (24.8) | 115 (32.2) | 0.079 |
Data are expressed as mean (SD), except for quantitative variables, expressed as %. AAA indicates abdominal aortic aneurysm; BMI, body mass index; PAD, peripheral artery disease.
Comparison between AAA and controls. P obtained by Student t test.
Comparison between AAA needing vascular repair vs not. P obtained by Student t test.
Controls were free of PAD by definition.
Figure 1.
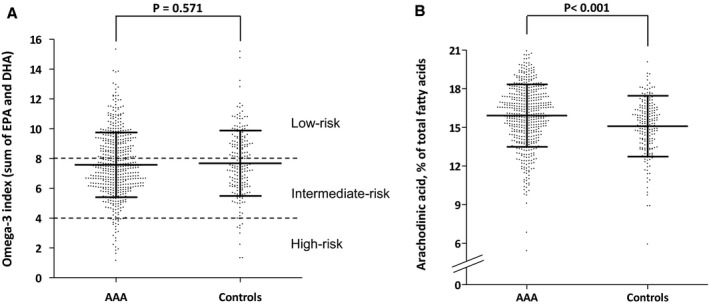
Red blood cell proportions (percentage of total fatty acids) of (A) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)(EPA+DHA=omega‐3 index) and (B) arachidonic acid in 498 patients with abdominal aortic aneurysm (AAA) and 199 age‐matched controls who screened negative. Dots are individual participant data, and bars represent mean±SD. P obtained by Student t test. In panel (A), discontinuous lines at 8% and 4% indicate proposed low‐ and high‐risk cutoffs for cardiovascular risk, respectively.10
Table 2.
Independent Determinants of AAA by Multivariate Logistic Regression
| Variable | B | SE | OR (95% CI) | P Value |
|---|---|---|---|---|
| Being at the upper tertile of arachidonic acid at baseline, yes | 0.269 | 0.127 | 1.309 (1.021–1.678) | 0.033 |
| Current smoking, yes | 1.413 | 0.248 | 4.110 (2.528–6.682) | <0.001 |
| Hypertension, yes | 0.062 | 0.211 | 1.064 (0.703–1.610) | 0.768 |
| Use of low‐dose aspirin, yes | 1.082 | 0.249 | 2.951 (1.809–4.812) | <0.001 |
| Use of statin, yes | 0.178 | 0.238 | 1.194 (0.750–1.903) | 0.454 |
| PAD, yes | 2.233 | 0.486 | 9.331 (3.598–24.197) | <0.001 |
| BMI, increase by 1 kg/m2 | 0.088 | 0.032 | 1.092 (1.026–1.162) | 0.005 |
| DBP, increase by 1 mm Hg | 0.074 | 0.010 | 1.077 (1.056–1.099) | <0.001 |
| Constant | −9.144 | 1.184 | 0.0002 | <0.001 |
AAA indicates abdominal aortic aneurysm; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; OR. odds ratio; PAD, peripheral artery disease.
Figure 2.
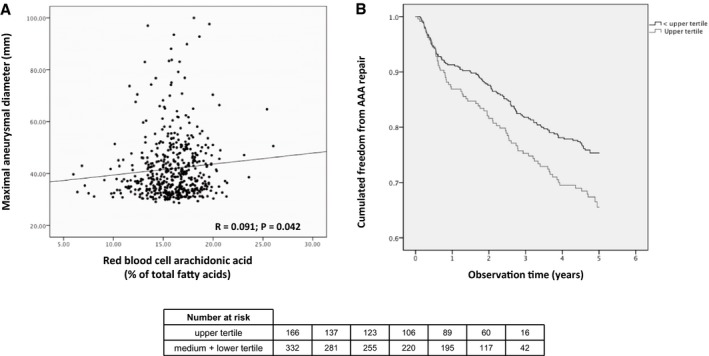
In 498 patients with abdominal aortic aneurysm (AAA), (A) a scatter plot shows the red blood cell proportion of arachidonic acid and the maximal aneurysm diameter, and (B) the Kaplan–Meier curve shows cumulative freedom from needing vascular repair, stratified by being in the upper tertile of red blood cell proportion of arachidonic acid at baseline vs not. Data were obtained using a multivariate Cox proportional hazards model adjusted for active smoking, hypertension, use of low‐dose aspirin, use of statins, peripheral arterial disease at screening, body mass index, diabetes mellitus, use of beta blockers, C‐reactive protein, and baseline maximal aortic diameter.
Table 3.
Independent Determinants of Needing Surgical Repair in 498 Patients With AAA by Cox Regression Analysis
| Variable | B | SE | HR (95% CI) | P Value |
|---|---|---|---|---|
| Being at the upper tertile of arachidonic acid at baseline, yes | 0.434 | 0.160 | 1.544 (1.127–2.114) | 0.007 |
| Current smoking, yes | 0.093 | 0.163 | 1.097 (0.797–1.510) | 0.570 |
| Hypertension, yes | 0.451 | 0.174 | 1.570 (1.117–2.207) | 0.009 |
| Use of low‐dose aspirin, yes | −0.225 | 0.190 | 0.799 (0.550–1.160) | 0.799 |
| Use of statin, yes | 0.153 | 0.189 | 1.166 (0.805–1.689) | 0.418 |
| PAD, yes | −0.197 | 0.207 | 0.821 (0.547–1.234) | 0.343 |
| BMI, increase by 1 kg/m2 | −0.028 | 0.023 | 0.973 (0.930–1.017) | 0.220 |
| Diabetes mellitus, yes | −0.042 | 0.280 | 0.959 (0.554–1.658) | 0.880 |
| Use of beta blockers, yes | −0.314 | 0.199 | 0.730 (0.494–1.079) | 0.730 |
| C‐reactive protein, increase by 1 mg/L | <0.001 | 0.007 | 1.000 (0.985–1.014) | 0.953 |
| Baseline aortic size, increase by 1 mm | 0.083 | 0.005 | 1.087 (1.076–1.097) | <0.001 |
AAA indicates abdominal aortic aneurysm; BMI, body mass index; CI, confidence interval; HR. hazard ratio; PAD, peripheral artery disease.
Discussion
The main conclusion of our study is that in a population with a high intake of omega‐3, the omega‐3 index was unrelated to AAA. In contrast, the RBC proportion of arachidonic acid, a substrate for generation of lipid mediators with proinflammatory properties, related to an increased prevalence of AAA and, in those diagnosed with AAA, an increased risk of needing surgical repair.
Although the role of EPA and DHA in primary prevention of coronary heart disease has long been explored, clinical research on omega‐3 and AAA is limited to a recent study conducted in 67 Japanese participants with AAA. Aikawa et al reported that the proportion of EPA in serum, a surrogate marker for EPA intake,7 inversely related to AAA size and growth after a mean follow‐up of 30.6 months.5 In conflict with this finding, we found that in our population, omega‐3 in RBC membranes was unrelated to both AAA prevalence and progression. This discrepancy could underlie several issues. First, the use of fatty acid composition of total serum, which does not reflect long‐term intake as accurately as adipose tissue or RBC do,7 precludes the adscription of observed effects to membrane changes with long‐term omega‐3 intake. In contrast, the omega‐3 fatty acid status in RBCs has stability documented over a 6‐week period8; the use of RBCs also allowed us to examine the omega‐3 index, defined as the sum of the percentages of EPA and DHA in RBC membranes, and its role as a risk marker for cardiovascular disease is emerging. Second, the number of AAA patients in our study was noticeably larger and had longer follow‐up. Third, white race is a well‐defined risk factor associated with the development of AAA, thus hampering direct comparisons regarding the influence of environmental risk factors for AAA between Asian and Western populations.
A plausible explanation for our neutral findings on omega‐3 and AAA could be the existence of a threshold of protection of EPA and DHA against AAA in non‐Asian individuals, largely exceeded by both controls and AAA patients in our study (given the average omega‐3 index of 7.6% in our population). This value resembles those described in other Scandinavian populations,14 resulting from the customarily high consumption of fatty fish in this area.15, 16 Future research should confirm or dispel whether the benefits of increasing dietary EPA and DHA on AAA would be observed only with a low background intake of omega‐3. This would be similar to prevention of coronary heart disease, for which few benefits are observed beyond intakes of 500 mg/d,17 an amount easily achievable through 2 weekly servings of fatty fish. Of particular interest are cohorts in the United States, a country with low omega‐3 index14 and high AAA prevalence.18
We observed increased levels of arachidonic acid in AAA patients. Although EPA and DHA act by generating anti‐inflammatory and vasoprotective compounds (eg, lipoxins, protectins, maresins), arachidonic acid mostly generates proinflammatory molecules on release of cell membranes. Arachidonic acid levels at baseline were associated with increased need of surgical repair independent of C‐reactive protein. Interestingly, C‐reactive protein showed no association with arachidonic acid, whereas significant inverse associations were observed for omega‐3 fatty acids. These findings support the view that the association of arachidonic acid with AAA presence and progression is not simply a reflection of increased systemic inflammation. However, the absence of correlation with C‐reactive protein does not preclude the potential contribution of arachidonic acid to vascular (local) inflammatory response. In this regard, increased levels of leukotriene B4 (an arachidonic acid‐derived compound) have been observed in tissues of AAA patients.19 Moreover, protection against experimental AAA was afforded by blockade of enzymes involved in arachidonic acid metabolism, such as lipooxygenase.20 Further research is needed to identify determinants of arachidonic acid in cell membranes and to elucidate the role of this fatty acid, particularly regarding its lipid‐derived mediators, on AAA progression.
The main limitation of our study is its observational design. A cause–effect relationship would be established only by a randomized controlled trial involving a nutritional intervention in a large population, ideally, with low background omega‐3 intake and high risk of AAA, followed for several years. In addition, cumulative average estimates of arachidonic acid would provide a more robust association with AAA progression than a single baseline measurement. The study also has strengths. Selection bias seems unlikely because the study group members were participants in a population‐based screening trial with an attendance rate of 74%.21 Information bias also seems unlikely because ultrasound‐based measurement of the aorta was performed by a validated method showing high position, and information on the need for later AAA repair was based on nationwide registry data in which all AAA repair procedures are recorded by law and for reimbursement.22 Consequently, all participants had follow‐up without missing data. Finally, the analyses were adjusted for known potential risk factors for AAA.
In conclusion, we found that omega‐3 index, an objective marker of omega‐3 intake, was unrelated to AAA in men from a country in which fish consumption is customarily high. In contrast, the content of arachidonic acid was related to prevalence and progression of AAA.
Sources of Funding
Martin‐Ventura funding includes the Spanish MINECO (SAF2016‐80843‐R), Fondo de Investigaciones Sanitarias ISCiii‐FEDER (Biobancos PT13/0010/0012). Sala‐Vila holds a Miguel Servet fellowship (CP12/032999) from the Ministry of Economy and Competitiveness through the Instituto de Salud Carlos III (Spain).
Disclosures
None.
Acknowledgments
CIBEROBN and CIBERCV are initiatives of the Instituto de Salud Carlos III, Spain.
(J Am Heart Assoc. 2018;7:e007790 DOI: 10.1161/JAHA.117.007790.)29374048
References
- 1. Meital LT, Sandow SL, Calder PC, Russell FD. Abdominal aortic anurysm and omega‐3 polyunsaturated fatty acids: mechanisms, animal models, and potential treatment. Prostaglandins Leukot Essent Fatty Acids. 2017;118:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Wang JH, Eguchi K, Matsumoto S, Fujiu K, Komuro I, Nagai R, Manabe I. The ω‐3 polyunsaturated fatty acid, eicosapentaenoic acid, attenuates abdominal aortic aneurysm development via suppression of tissue remodeling. PLoS One. 2014;9:e96286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshihara T, Shimada K, Fukao K, Sai E, Sato‐Okabayashi Y, Matsumori R, Shiozawa T, Alshahi H, Miyazaki T, Tada N, Daida H. Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage‐mediated inflammation. Circ J. 2015;79:1470–1478. [DOI] [PubMed] [Google Scholar]
- 4. Kavazos K, Nataatmadja M, Wales KM, Hartland E, Williams C, Russell FD. Dietary supplementation with omega‐3 polyunsaturated fatty acids modulate matrix metalloproteinase immunoreactivity in a mouse model of pre‐abdominal aortic aneurysm. Heart Lung Circ. 2015;24:377–385. [DOI] [PubMed] [Google Scholar]
- 5. Aikawa T, Miyazaki T, Shimada K, Sugita Y, Shimizu M, Ouchi S, Kadoguchi T, Yokoyama Y, Shiozawa T, Hiki M, Takahashi S, Al Shahi H, Dohi S, Amano A, Daida H. Low serum levels of EPA are associated with the size and growth rate of abdominal aortic aneurysm. J Atheroscler Thromb. 2017;24:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grøndal N, Søgaard R, Henneberg EW, Lindholt JS. The Viborg Vascular (VIVA) screening trial of 65‐74 year old men in the central region of Denmark: study protocol. Trials. 2010;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. [DOI] [PubMed] [Google Scholar]
- 8. Harris WS, Thomas RM. Biological variability of blood omega‐3 biomarkers. Clin Biochem. 2010;43:338–340. [DOI] [PubMed] [Google Scholar]
- 9. Sala‐Vila A, Harris WS, Cofán M, Pérez‐Heras AM, Pintó X, Lamuela‐Raventós RM, Covas MI, Estruch R, Ros E. Determinants of the omega‐3 index in a Mediterranean population at increased risk for CHD. Br J Nutr. 2011;106:425–431. [DOI] [PubMed] [Google Scholar]
- 10. Harris WS. The omega‐3 index: from biomarker to risk marker to risk factor. Curr Atheroscler Rep. 2009;11:411–417. [DOI] [PubMed] [Google Scholar]
- 11. Grøndal N, Bramsen MB, Thomsen MD, Rasmussen CB, Lindholt JS. The cardiac cycle is a major contributor to variability in size measurements of abdominal aortic aneurysms by ultrasound. Eur J Vasc Endovasc Surg. 2012;43:30–33. [DOI] [PubMed] [Google Scholar]
- 12. Harris WS, Pottala JV, Vasan RS, Larson MG, Robins SJ. Changes in erythrocyte membrane trans and marine fatty acids between 1999 and 2006 in older Americans. J Nutr. 2012;142:1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lauritzen L, Harsløf LB, Hellgren LI, Pedersen MH, Mølgaard C, Michaelsen KF. Fish intake, erythrocyte n‐3 fatty acid status and metabolic health in Danish adolescent girls and boys. Br J Nutr. 2012;107:697–704. [DOI] [PubMed] [Google Scholar]
- 14. Stark KD, Van Elswyk ME, Higgins MR, Weatherford CA, Salem N Jr. Global survey of the omega‐3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res. 2016;63:132–152. [DOI] [PubMed] [Google Scholar]
- 15. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group NutriCoDE . Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country‐specific nutrition surveys. BMJ. 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welch AA, Lund E, Amiano P, Dorronsoro M, Brustad M, Kumle M, Rodriguez M, Lasheras C, Janzon L, Jansson J, Luben R, Spencer EA, Overvad K, Tjønneland A, Clavel‐Chapelon F, Linseisen J, Klipstein‐Grobusch K, Benetou V, Zavitsanos X, Tumino R, Galasso R, Bueno‐De‐Mesquita HB, Ocké MC, Charrondière UR, Slimani N. Variability of fish consumption within the 10 European countries participating in the European Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2002;5:1273–1285. [DOI] [PubMed] [Google Scholar]
- 17. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. [DOI] [PubMed] [Google Scholar]
- 18. Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population‐a meta‐analysis. PLoS One. 2013;8:e81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Houard X, Ollivier V, Louedec L, Michel JB, Bäck M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil‐derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009;23:1376–1383. [DOI] [PubMed] [Google Scholar]
- 20. Bhamidipati CM, Whatling CA, Mehta GS, Meher AK, Hajzus VA, Su G, Salmon M, Upchurch GR Jr, Owens GK, Ailawadi G. 5‐Lipoxygenase pathway in experimental abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2014;34:2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grøndal N, Søgaard R, Lindholt JS. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65‐74 years from a population screening study (VIVA trial). Br J Surg. 2015;102:902–906. [DOI] [PubMed] [Google Scholar]
- 22. Laustsen J, Jensen LP, Hansen AK; Danish National Vascular Registry . Accuracy of clinical data in a population based vascular registry. Eur J Vasc Endovasc Surg. 2004;27:216–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Access to the data can be requested by applying for permission from the Danish Data Protection Agency and Statistics Denmark.


