Abstract
Background
The classical renin‐angiotensin system is known as the angiotensin (Ang)‐converting enzyme/Ang II/Ang type 1 receptor axis, which induces various organ damage including cognitive decline. The angiotensin‐converting enzyme 2/Ang‐(1‐7)/Mas axis is known to exert antagonistic actions against the classical renin‐angiotensin system axis in the cardiovascular system. However, its roles in the brain remain unclear. We examined possible roles of the angiotensin‐converting enzyme 2/Ang‐(1‐7)/Mas axis in cognitive function, employing vascular cognitive impairment model mice.
Methods and Results
Male 10‐week‐old C57BL6 (wild‐type mice, Mas1 knockout mice, Ang II type 2 receptor knockout mice, and Ang II type 2 receptor/Mas1 double knockout mice were subjected to bilateral carotid artery stenosis (BCAS) surgery. Six weeks after treatment, they were subjected to cognitive tasks. Brain samples were used for histopathological analysis. Cognitive function was significantly impaired in wild‐type and double knockout mice after BCAS. On the other hand, the cognitive function of Mas1 knockout mice was maintained in spite of the reduction of cerebral blood flow with BCAS. Total cell number in the dentate gyrus region was significantly reduced after BCAS in wild‐type but not in Mas1 knockout mice. The number of doublecortin‐positive cells in the subgranular zone was not significantly different between wild‐type and Mas1 knockout mice. Ang‐(1‐7) administration did not improve cognitive function in all mice after BCAS surgery.
Conclusions
Lack of the Mas receptor may have a protective effect against chronic brain ischemia when the Ang II type 2 receptor exists.
Keywords: angiotensin II, angiotensin II type 2 receptor, angiotensin receptor, angiotensin‐(1‐7), Mas receptor, neurogenesis, neuroprotection, vascular cognitive impairment, vascular dementia
Subject Categories: ACE/Angiotension Receptors/Renin Angiotensin System, Cognitive Impairment, Neurogenesis
Clinical Perspective
What Is New?
This is the first report to assess the effect of Mas receptor deficiency in chronic cerebral hypoperfusion other than a cerebral infarction model and it is also notable that we employed Mas/angiotensin II type 2 receptor double knockout mice to examine the correlation of the 2 possible protective molecular axes.
What Are the Clinical Implications?
Since the angiotensin‐converting enzyme 2/angiotensin‐(1‐7)/Mas axis has been highlighted as a protective arm of the renin‐angiotensin system, its contribution to cognitive function is intriguing.
Introduction
Dementia is becoming a serious health problem worldwide, yet the efficacy of current treatment is limited. One of the most important risk factors for dementia is hypertension, and some antihypertensive drugs are expected to reduce the prevalence of dementia.1, 2 The classical renin‐angiotensin system (RAS) is closely associated with the pathogenesis of hypertension and other cardiovascular disease, and we have reported that activation of the classical RAS causes deterioration of cognitive function.3
In contrast to the classical RAS, there are some organ‐protective components, one of which is the angiotensin (Ang) type 2 (AT2) receptor. We previously reported that activation of the AT2 receptor improved learning and memory in a vascular dementia model.4 Recently, the angiotensin‐converting enzyme 2 (ACE2)/Ang‐(1‐7)/Mas receptor axis has also aroused interest as another protective arm of RAS.5 It is known that the Mas receptor is highly expressed in the hippocampus and blood vessels in the brain.6 Since the hippocampus plays an important role in the formation of new memories, a relationship between cognitive function and this axis is suggested. Consistent with this concept, Hellner reported that Ang‐(1–7) could increase long‐term potentiation (LTP) in the hippocampus through its interaction with the Mas receptor.7
Ang‐(1‐7) is a main product of Ang II degradation by ACE2 and is known to be an endogenous ligand of the Mas receptor. Ang‐(1‐7) is reported to increase cerebral blood flow (CBF) and reduce vascular resistance.8 We recently reported that ACE2 deficiency caused cognitive decline and Ang‐(1‐7) treatment prevented the worsening of cognitive function.9 However, the effect of Mas receptor deficiency on cognitive function is still unclear. In previous reports, Mas1 knockout (MasKO) mice showed impaired novel object cognition but no worsening of spatial memory. Interestingly, MasKO mice are reported to show a greater increase of LTP compared with wild‐type (WT) mice.10, 11
To highlight the role of this axis in cognitive function, we employed a disease model. Because of the vasoactive properties of Ang‐(1‐7), a vascular dementia model seems to be the most suitable way to investigate its role in cognitive function. Therefore, we decided to use a vascular dementia mouse model. Considering the possible interaction between RAS components, we conducted 2‐step experiments. First, we evaluated the degree of cognitive decline after bilateral carotid artery stenosis (BCAS) in WT, MasKO, and Mas/AT2 receptor double knockout (DKO) mice to investigate the additional impact of the AT2 receptor on MasKO mice. After examining the results, we evaluated the effect of Ang‐(1‐7) administration on WT, MasKO, AT2 receptor knockout mice (AT2KO), and DKO mice.
Methods
This study was performed in accordance with the National Institutes of Health guidelines for the use of experimental animals. All animal studies were reviewed and approved by the Animal Studies Committee of Ehime University. The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure. They are available from the corresponding author upon reasonable request. Additional methods are described in Data S1.
Animals
Ten‐week‐old male C57B1/6 (WT), MasKO, AT2KO, and Mas/AT2 receptor DKO mice were enrolled in these experiments. The animals were housed in a room with a 12‐hour light/dark cycle with a temperature of 25±1°C. They were given standard laboratory chow (MF, Oriental Yeast Co, Ltd) and water ad libitum.
Osmotic pumps (Micro‐Osmotic Pump Model 1004, Alzet) were used for intraperitoneal drug administration. A month before the Morris water maze (MWM) test, an osmotic pump with Ang‐(1‐7) (0.5 mg/kg per day) or vehicle (saline) was implanted into the abdominal cavity of WT, MasKO, AT2KO, and DKO mice. Ang‐(1‐7) dissolved in saline was applied to the osmotic pumps and incubated overnight at 37°C before implantation. Through a peritoneal incision made under anesthesia, the osmotic pump was gently placed in the space between the intestines. The incision was closed with sutures.
Bilateral Common Carotid Artery Stenosis
Micro‐coils with an inner diameter of 0.18 mm, pitch 0.5 mm, and total length 2.5 mm were used to create artificial stenosis in the bilateral common carotid arteries (CCAs). Before the procedure, mice were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneal). Through a midline cervical incision, both CCAs were exposed and freed from their sheaths. The artery was gently lifted with a silk suture and then placed between the loops of a micro‐coil. The micro‐coil was twined around by rotating it around the CCA. Then another micro‐coil was applied to the other CCA. After placing the coils, the incision was closed with sutures. Samples with cerebral infarction were identified at the dissection stage and excluded from analysis. Cerebral infarction was detected by an apparent unilateral decrease of CBF and/or apparent color change of the brain.
Evaluation of Cognitive Function
The apparatus for the Y‐maze test has 3 arms, 400 mm in length and 150 mm in height, each separated by an angle of 120° (Brain Science Idea Co, Ltd). Each mouse was placed in the center of the apparatus and allowed to explore the maze for 8 minutes. We manually recorded their arm choices. Any 3 consecutive choices of 3 different arms were counted as an alternation. Working memory was evaluated as the percentage of alternation in all arm choices. The total number of arm entries is considered an indicator of spontaneous locomotive activity. Animals whose spontaneous locomotive activity was less than 10 were removed from analysis because working memory could not be evaluated properly.
The MWM test was performed 6 weeks after BCAS surgery, as previously described.12 Mice were trained 5 times a day at 20‐minute intervals for 5 consecutive days. In each trial, mice were given 120 seconds to find the platform. Swimming was video‐tracked (AnyMaze, Stoelting Co), and latency, path length, swim speed, and cumulative distance from the platform were recorded. Mean swim latency for each day was evaluated and compared between groups.
Measurement of CBF
After the cognitive tasks, CBF was measured by laser speckle flowmetry (Omegazone laser speckle blood flow imager; Omegawave), which obtains high‐resolution 2‐dimensional images in seconds, as previously described.13 Mice were anesthetized with pentobarbital, and a midline incision was made in the scalp. Blood pressure (BP) was not affected by anesthesia. The skull was exposed and wet with saline. A 780‐nm semiconductor laser illuminated the whole skull surface. Mean CBF on the skull surface was measured. Light intensity was accumulated in a charge‐coupled device camera and transferred to a computer for analysis. Image pixels were analyzed to produce average perfusion values.
Real‐Time Reverse Transcription–Polymerase Chain Reaction Method
Samples of hippocampus were frozen in liquid nitrogen and stored at −80°C until analysis. Total mRNA was extracted from brain samples after homogenization in Sepazol (Nacalai Tesque Inc). Real‐time quantitative reverse‐transcription polymerase chain reaction was performed with an SYBR green I kit (MJ Research, Inc). The polymerase chain reaction (primers were as follows: 5′‐ATGTAGGCCATGAGGTCCAC‐3′ (forward) and 5′‐TGCGACTTCAACAGCAACTC‐3′ (reverse) for GAPDH, 5′‐AGTCGCACTCAAGCCTGTCT‐3′ (forward) and 5′‐ACTGGTCCTTTGGTCGGAG‐3′ (reverse) for angiotensin II type 1 receptor (AT1 receptor), 5′‐CCTGCATGAGTGTCGATAGGT‐3′ (forward) and 5′‐CCAGCAGACCACTGAGCATA‐3′ (reverse) for AT2 receptor, 5′‐AGGACAGCAAAGCCACAATGT‐3′ (forward) and 5′‐AGGTTCCCACCGCTGTGTTC‐3′ (forward) and 5′‐TCTTGCCCTGGGTCACTTCA‐3′ (reverse) for Mas1, 5′‐CCACTTCACAAGTCGGAGGCTTA‐3′ (forward) and 5′‐GCAAGTGCATCATCGTTGTTCATAC‐3′ (reverse) for interleukin 6, 5′‐GTGTCCCAAAGAAGCTGTAGTTTT‐3′ (forward) and 5′‐TCATTTGGTTCCGATCCGATCCAGGTTT‐3′ (reverse) for monocyte chemotactic protein‐1, and 5′‐CCTTCATGCAACCGAAGTATG‐3′ (reverse) for brain‐derived neurotrophic factor.
Histological Analysis
After measuring CBF, mice were perfused transcardially with saline and euthanized under deep anesthesia. The brains were collected and weighed immediately then stored in a −80°C freezer. For histopathological analysis, certain numbers of mice were selected from each group and perfused with PBS transcardially. Brain samples were fixed with 4% paraformaldehyde and stored as paraffin‐embedded samples. To assess the histopathological changes after BCAS surgery, we prepared 5‐μm‐thick paraffin sections and stained them with hematoxylin–eosin after deparaffinization. Coronal slices were selected between 1.82 to 2.31 mm posterior to the bregma to observe the hippocampal area. Samples were examined with an upright microscope (Axioskop 2, Carl Zeiss) at ×400 magnification. The center of the cornu ammonis area 1 and the innermost portion of the granular cell layer in the dentate gyrus were set as the observation range. The average cell number per field from 2 slices with a 200‐μm interval was counted with computer‐imaging software (Densitograph, ATTO).
To assess the degree of neurogenesis in the hippocampus, we conducted immunofluorescent staining to detect doublecortin (DCX)‐positive cells. Paraffin‐embedded brain samples were sliced into 5‐μm‐thick sections. After this, brain sections were incubated for 1 hour in blocking solution. Goat anti‐DCX antibody (DCX, sc‐8066, Santa Cruz Biotechnology Inc) was used as the primary antibody. Anti‐DCX antibodies were used at a 1:100 dilution with diluent (Dako Antibody Diluent, Agilent Technologies). Samples were incubated overnight at 4°C. As secondary antibodies, Cy3‐conjugated donkey anti‐goat IgG (Jackson ImmunoResearch Laboratories Inc) was applied to the samples at a 1:500 dilution. Slices were then washed with PBS and mounted with 4′,6‐diamidino‐2‐phenylindole (1:10 000) to detect cell nuclei. These samples were examined with an All‐in‐One Fluorescence Microscope (BZ‐9000, Keyence) equipped with a computer‐based imaging system at ×80 magnification. The total number of DCX‐positive cells per 4′,6‐diamidino‐2‐phenylindole–positive dentate gyrus area was counted with computer‐imaging software (Densitograph, ATTO).
Statistical Analysis
All values are expressed as mean±SEM. Comparisons between 2 groups were analyzed using Levene test for equal variances then Student (equal variances) or Welch (unequal variances) t tests as appropriate. Comparisons between ≥3 groups were analyzed using ANOVA, then, when significant differences were indicated, were followed by post hoc pairwise comparisons using Tukey‐Kramer method to adjust for multiple hypothesis testing. For each experiment, the overall significance threshold was set to 0.05. Statcel 3 (OMS Inc) add‐in software for Microsoft Excel was used for statistical analysis.
Results
Body Weight, Brain Weight, and BP
Table S1 shows the characteristics of the mice. Sham‐operated MasKO mice showed significantly heavier body weight compared with WT‐sham, but there was no significant difference in brain/body weight ratio. Systolic BP in AT2KO‐BCAS and DKO‐BCAS mice were significantly higher than those in sham mice. Multiple comparison analysis showed no significant difference among all BCAS groups.
Change in CBF After BCAS
Figure 1 shows mean CBF measured by laser speckle flowmetry. CBF significantly decreased after BCAS compared with that in sham mice in each group, although no significant intergroup difference was seen among the BCAS‐treated groups. Time‐course analysis of CBF also showed no significant difference in each group (Figure S1). This means that DKO mice did not show a further decrease in CBF compared with MasKO mice.
Figure 1.
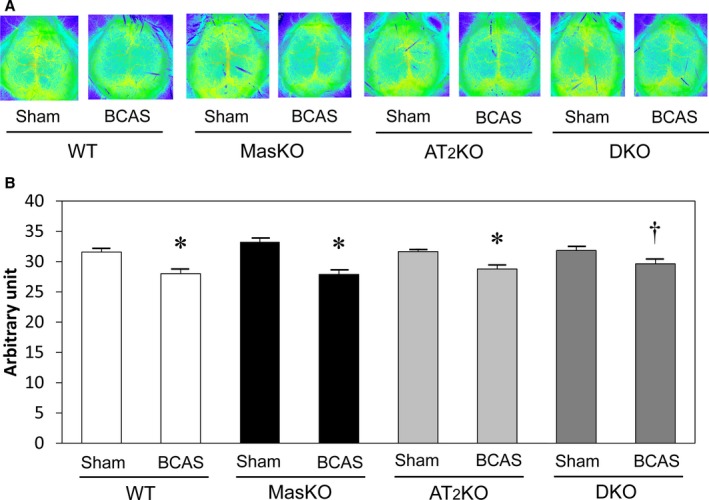
Cerebral blood flow (CBF) changes after bilateral common carotid artery stenosis (BCAS) surgery. A, Representative CBF images of groups assessed by laser speckle flowmetry 6 weeks after surgery. B, Quantitative analysis of CBF indicated as arbitrary unit determined by Doppler method. CBF decreased significantly after BCAS in each group (n=7–22 for each group). *P<0.01 vs sham. † P<0.05 vs sham. AT2KO indicates angiotensin II type 2 receptor knockout mice; DKO, Mas/angiotensin II type 2 receptor double knockout mice; MasKO, Mas receptor knockout mice; WT, wild‐type mice.
Cognitive Function
We evaluated working memory and spontaneous locomotive activity with the Y‐maze test (Figure 2A and 2B). The total number of arm entries was significantly lower in MasKO‐sham mice than WT‐sham and DKO‐sham mice. WT‐BCAS mice showed significantly lower working memory than WT‐sham mice. On the other hand, MasKO mice did not show significant deterioration after BCAS surgery. A slight reduction in alternation was also observed in the MasKO mice, but the effect did not reach statistical significance (P=0.79). AT2KO mice also exhibited lower spontaneous locomotive activity than DKO‐sham mice. However, there was no significant difference among BCAS‐treated mouse groups. Working memory in DKO mice tended to decrease after BCAS (P=0.07). Multiple comparison analysis did not show a significant difference among all groups with BCAS. To avoid visual problems after BCAS operation,14 we performed visible platform task. No significant difference was observed in the BCAS‐induced impairment of cognition in each mouse.
Figure 2.
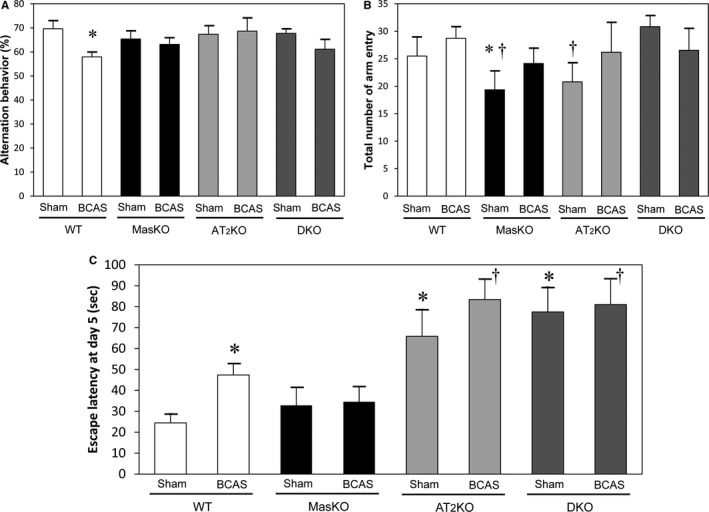
Cognitive function. Working memory and spontaneous locomotive activity in mouse groups determined with Y‐maze test. A, The percentage of alternation behavior is used to estimate working memory. Working memory was significantly reduced in wild‐type mice (WT) after bilateral common carotid artery stenosis (BCAS) but not in the other groups (n=8–14 for each group). B, Spontaneous locomotive activity is defined as the total number of arm entries. Mas receptor knockout mice (MasKO)–sham and angiotensin II type 2 receptor knockout mice (AT2KO) showed significantly lower locomotive activity compared with WT and Mas/AT2KO (DKO) (n=7–15 for each group). *P<0.05 vs WT‐sham. † P<0.05 vs DKO‐sham. C, Escape latency of the Morris water maze test on the last day was significantly longer in WT‐BCAS than in WT‐sham. AT2KO‐sham and DKO‐sham mice showed markedly longer latency compared with WT and MasKO (n=8–22 for each group). *P<0.05 vs WT‐sham. † P<0.05 vs WT‐BCAS.
We also evaluated spatial memory with the MWM test (Figure 2C). WT mice showed significant deterioration of cognitive function after BCAS. However, MasKO mice did not show a significant decline after BCAS. Compared with other groups, AT2KO and DKO mice showed significantly lower performance in the MWM test even without BCAS treatment, and still showed worse cognitive function than WT‐BCAS mice. There was no significant deference between AT2KO and DKO mice with BCAS treatment.
mRNA Expression
We measured mRNA expression of AT1R, AT2R, Mas1R, and inflammatory cytokines in the hippocampus with real‐time quantitative reverse‐transcription polymerase chain reaction. As shown in Figure 3, there was no significant difference in AT1 receptor and AT2 receptor expression among the groups. The expression of Mas1 receptor was significantly higher in AT2KO‐BCAS than WT‐BCAS mice. There was also no significant difference in the expression of brain‐derived neurotrophic factor and inflammatory cytokines (interleukin 6 and monocyte chemotactic protein‐1).
Figure 3.
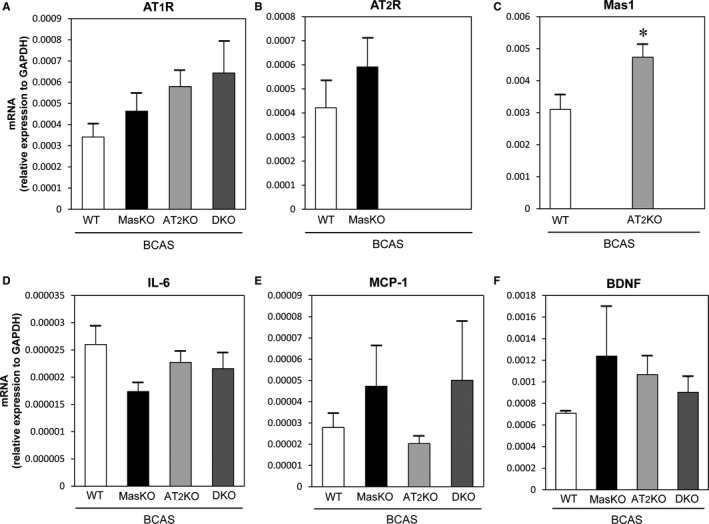
Relative expression of mRNA in the hippocampus 6 weeks after bilateral common carotid artery stenosis (BCAS) surgery. No significant difference was detected in the expression of angiotensin II type 1 receptor (AT1R) and angiotensin II type 2 receptor (AT2R) knockout mice (A and B), inflammatory cytokines (D and E), and brain‐derived neurotrophic factor (BDNF; F). As shown in Panel C, Mas1 expression was significantly higher in AT2 knockout mice (AT2KO)–bilateral common carotid artery stenosis mice (BCAS) than wild‐type mice (WT)–BCAS (n=5–8 for each group). *P<0.05. DKO indicates Mas/AT2KO; IL‐6, interleukin 6; MasKO, Mas receptor knockout mice; MCP‐1, monocyte chemotactic protein‐1.
Cell Number and Proliferation in Dentate Gyrus
We assessed morphological changes with hematoxylin‐eosin staining. The area ratio of the hippocampus did not significantly differ from that in samples with BCAS (data not shown). There was no significant difference in the cell number in the cornu ammonis area 1 among all groups. However, the cell number in DKO mice only showed the tendency to decrease after BCAS treatment in the area. On the other hand, WT‐BCAS mice showed significantly reduced cell number in dentate gyrus area compared with sham mice. MasKO mice and DKO mice did not show significant changes after BCAS treatment in the dentate gyrus area (Figure 4A and 4B). There was no significant difference in the number of pyknotic cells between WT and MasKO mice (data not shown).
Figure 4.
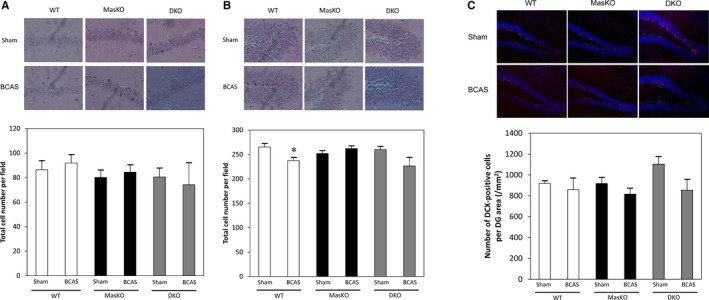
Effect of bilateral common carotid artery stenosis (BCAS) on cell number and proliferation in dentate gyrus. The hippocampus was histologically examined with hematoxylin‐eosin staining. A, Representative images and total cell number of cornu ammonis area 1 of hippocampus in wild‐type mice (WT) and Mas receptor knockout mice (MasKO). B, Representative images and total cell numbers of dentate gyrus (DG) in the hippocampus in WT and MasKO. *P<0.05 vs WT‐sham (n=4–5 for each group). C, Representative images of immunohistochemistry in the subgranular zone (SGZ) in WT and MasKO mice. There was no significant difference in number of doublecortin (DCX)‐positive cells in the SGZ (n=4–6 for each group). DKO indicates Mas/angiotensin receptor type 2 double knockout mice.
To estimate the amount of neurogenesis, we counted the number of DCX‐positive cells in the subgranular zone. The number of DCX‐positive cells per area did not significantly change after BCAS treatment in all mouse groups, but the cell number in DKO mice tended to decrease in the BCAS‐treated group (P=0.09). Although the number of DCX‐positive cells per area was the highest in DKO‐sham mice, this did not reach statistical significance with 1‐way ANOVA (P=0.07) (Figure 4C).
Effect of Ang‐(1‐7) on Cognitive Function
We administered Ang‐(1‐7) intraperitoneally using an osmotic minipump at a dose of 0.5 mg/kg per day to assess its effect on the vascular cognitive impairment model. Ang‐(1‐7) administration did not significantly affect body weight, brain weight, or brain/body ratio. The BP in AT2KO was significantly lower with Ang‐(1‐7)–treated mice than with vehicle mice. However, there was no significant intergroup difference (Table S2).
CBF did not significantly change after Ang‐(1‐7) administration in all groups, and multiple comparison analysis showed no significant intergroup difference (data not shown). We assessed the effect of Ang‐(1‐7) administration on cognitive function with BCAS‐treated mice. There was no significant difference between Ang‐(1‐7)–administered mice and vehicle‐treated mice in all groups with MWM test. The reason we did not observe any preventive effects of Ang‐(1‐7) on cognitive function is the duration of Ang‐(1‐7) treatment. We administered Ang‐(1‐7) from 2 weeks after BCAS operation to avoid inflammatory effects of pump replacement because we used a minipump for 4 weeks of treatment. Further analysis is necessary to confirm of the effect of Ang‐(1‐7) treatment (Figure 5).
Figure 5.
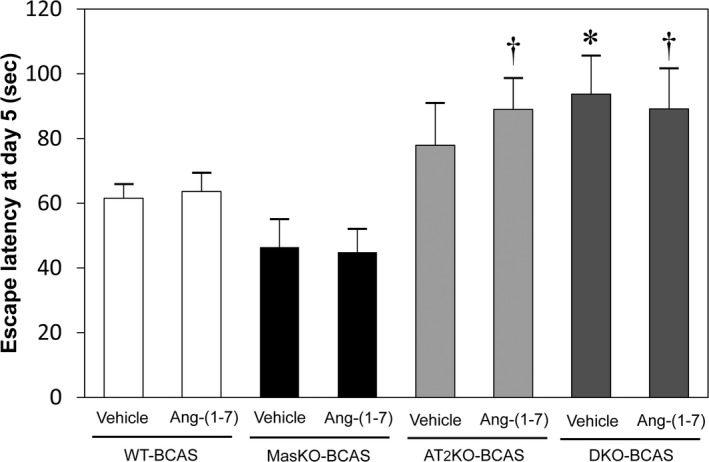
Effect of Ang‐(1‐7) on cerebral blood flow and cognitive function. Effect of Ang‐(1‐7) was estimated with the Morris water maze test. All mice underwent bilateral common carotid artery stenosis (BCAS) surgery before drug administration. The mean escape latency did not significantly change between sham mice and BCAS‐treated mice among all groups. (n=8–14 for each group). *P<0.05 vs Mas receptor knockout mice (MasKO)–BCAS with vehicle. † P<0.05 vs MasKO‐BCAS with Ang‐(1‐7). AT2KO indicates angiotensin II type 2 receptor knockout mice; DKO, Mas/angiotensin II type 2 receptor double knockout mice; WT, wild‐type mice.
Discussion
This study showed that deficiency of the Mas receptor did not worsen cognitive function in vascular dementia model mice. Moreover, the degree of deterioration in cognitive function was significantly smaller than that in WT mice.
In general, the ACE2/Ang‐(1‐7)/Mas axis is believed to be an organ‐protective axis and is expected to provide a beneficial effect on cognitive function in the brain. However, the role of the Mas receptor in the central nervous system is not clearly understood and there remains controversy concerning its effect on memory and other cognitive function. The total number of arm entries was significantly lower in MasKO‐sham mice than WT‐sham and DKO‐sham mice. AT2KO mice also exhibited lower spontaneous locomotive activity than DKO‐sham mice. However, there was no significant difference among BCAS‐treated mouse groups. Spontaneous locomotive activity is associated with the function of amygdala and could be influenced by emotional conditions such as fear and anxiety. MasKO mice were reported to present increased anxiety and contextual fear memory.11, 15 Similarly, anxiety‐like behavior in AT2KO mice has also been reported.16 We believe these behavioral phenotypes could affect the spontaneous activity, but do not yet have enough evidence to understand the mechanism. As the baseline performance is different in AT2KO and DKO mice, we should take this into account when interpreting the results of behavioral tests. In 1998, Walther et al11 reported that MasKO mice show increased LTP, and reasonably asserted that the Mas receptor is a memory suppressor. Yet, they failed to show a difference in cognitive function between WT and MasKO mice with the MWM test.11 Later, their team found that Ang‐(1‐7) enhanced LTP in the hippocampus in WT mice but not in MasKO mice.7 We assumed that the function of the ACE2/Ang‐(1‐7)/Mas axis becomes more obvious in pathological conditions such as a dementia model. Because Ang‐(1‐7) is reported to be formed in vascular endothelial cells and has the potential to increase CBF,17 we decided to use a vascular dementia mouse model to assess the effects.
In this study, mean CBF was significantly reduced in MasKO mice with BCAS, and there was no significant difference to that in WT‐BCAS mice. We assessed the CBF changes at 4 different timepoints after BCAS treatment (just after or 1, 2, or 4 weeks after the surgery). Overall, there was no significant difference in the percentile changes in CBF among all groups (data not shown). Long‐term reduction in CBF is reported to impair neuronal function,18 but MasKO mice did not show inferior performance in both the Y‐maze test and the MWM test, in contrast to WT mice. In spite of the cerebral hypoperfusion, we could not detect significant changes in histological analysis in MasKO mice. We also did not observe a significant difference in mRNA expression of inflammatory cytokines, which are believed to be related to cognitive function. Moreover, we did not detect changes of gliosis evaluated by glial fibrillary acidic protein immunohistochemistry among WT, MasKO, and DKO‐BCAS mice in the brain after 6 weeks of BCAS operation (data not shown). Kluver‐Barrera staining also did not show a significant difference in the severity of white matter lesions as evaluated in a previous report among BCAS‐treated groups (Figure S2).14 To investigate the reason for this resilience to ischemia, we focused on the amount of neurogenesis in the hippocampus. Freund et al19 reported that Mas deficiency resulted in an increase in the number of DCX‐positive cells in the dentate gyrus, and discussed the possibility that increased neurogenesis leads to enhanced LTP in MasKO mice. However, no significant difference was seen in the number of DCX‐positive cells between WT and MasKO mice. Moreover, DCX‐positive cells tended to decrease in MasKO after BCAS. The discordance with the previous report might be attributable to the different scanned area (whole brain or single slice) and relatively smaller sample size of MasKO‐sham. In any case, neurogenesis does not seem to fully explain the maintenance of cognitive function in MasKO.
Another conceivable mechanism is that deletion of the Mas receptor altered the balance of other RAS components and provided a beneficial effect indirectly. In this study, mRNA expression of the AT1 and AT2 receptors was not significantly affected by deficiency of the Mas receptor. On the other hand, the expression of Mas receptor was significantly higher in AT2KO mice than in WT mice after BCAS treatment. So, we assumed that unbound Ang‐(1‐7), which was caused by Mas deletion, could act as a ligand for other receptors. Generally, Ang‐(1‐7) has been believed to be a specific ligand for the Mas receptor. However, there is a report that shows it has relatively higher binding affinity to the AT2 receptor rather than the AT1 receptor.20 Leonhardt et al21 reported the functional interaction between AT2 and Mas receptor showing the evidence of heterodimerization with fluorescence resonance energy transfer technology. They also reported their functional dependence in mouse astrocytes. According to the report, the lack of one receptor impairs the other receptor's response to its ligand. We previously reported that Ang‐(1‐7) administration reduced neointimal formation in injured arteries, through both Mas and AT2 receptor stimulation.22 In addition, we previously reported that direct AT2 receptor stimulation prevents cognitive decline after BCAS.4 Accordingly, we assumed that unbound Ang‐(1‐7) could activate the AT2 receptor and maintain cognitive function in MasKO mice. To test this hypothesis, we administered Ang‐(1‐7) to WT, MasKO, AT2KO, and DKO mice. As a result, Ang‐(1‐7) did not affect BP or CBF (please see Supplemental Table S2). A statistically significant difference between MasKO and DKO mice with Ang‐(1‐7) administration was only seen in the MWM test. This means that the combination of activated AT2 receptor and Mas receptor deficiency could be an effective way to preserve spatial learning.
Angiotensin‐converting enzyme inhibition works not only on the reduced conversion of Ang I to Ang II, which blocks AT1 receptor signaling, but also on the increased conversion of Ang‐(1‐9) to Ang‐(1‐7), enhancing organ‐protective effects. Therefore, it has been suggested that the vasoconstrictive/proliferative actions by Ang II of Ang‐(1‐7) depends on the angiotensin‐converting enzyme/ACE2 ratio balance.5 WT‐BCAS mice administered perindopril at the dose of 1 mg/kg per day in drinking water after 2 weeks from BCAS treatment showed better cognitive function than those administered normal drinking water (data not shown). Therefore, angiotensin‐converting enzyme inhibition may have potent or diverse strengths related to enzyme inhibition or AT1 receptor blockade.
Taking all of these results into account, we conclude that Mas receptor deficiency has a beneficial effect on vascular cognitive impairment, and this protective effect is only brought about under the existence of the AT2 receptor. Since the mechanistic insights remain limited, focusing more on dose‐response relationships, sex differences, pretreatment versus posttreatment, and use of aged mice could help us understand the detailed mechanisms in future studies. However, we believe this study creates a stir in the comprehension of the ACE2/Ang‐(1‐7)/Mas axis in the central nervous system.
Conclusions
We demonstrated that Mas receptor deficiency brought about a beneficial effect on cognitive function in a vascular cognitive impairment mouse model in which the AT2 receptor was preserved. This result could enable us to think about brain protection from another viewpoint.
Sources of Funding
This study was supported by JSPS KAKENHI (grant number 25293310 to Horiuchi, 25462220 to Mogi, 15K19974 to Iwanami, and 26860567 to Min) and research grants from pharmaceutical companies Astellas Pharma Inc, Bayer Yakuhin, Ltd, Daiichi‐Sankyo Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Novartis Pharma K. K., Shionogi & Co Ltd, and Takeda Pharmaceutical Co, Ltd. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Body Weight, Brain Weight, and Systolic Blood Pressure in Each Group
Table S2. Body weight, brain weight and systolic blood pressure according to Ang‐(1‐7) treatment
Figure S1. Time‐course analysis of cerebral blood flow (CBF) changes after bilateral common carotid artery stenosis (BCAS) surgery.
Figure S2. Severity of white matter lesion.
Acknowledgments
We thank Takeshi Kiyoi, Hirotomo Nakaoka, and Yuta Yanagihara for their devoted technical support of this work.
(J Am Heart Assoc. 2018;7:e008121 DOI: 10.1161/JAHA.117.008121.)29431106
References
- 1. Czuriga‐Kovacs KR, Czuriga D, Csiba L. Influence of hypertension, alone and in combination with other vascular risk factors on cognition. CNS Neurol Disord Drug Targets. 2016;15:690–698. [DOI] [PubMed] [Google Scholar]
- 2. Zhuang S, Wang HF, Li J, Wang HY, Wang X, Xing CM. Renin‐angiotensin system blockade use and risks of cognitive decline and dementia: a meta‐analysis. Neurosci Lett. 2016;624:53–61. [DOI] [PubMed] [Google Scholar]
- 3. Inaba S, Iwai M, Furuno M, Tomono Y, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M. Continuous activation of renin‐angiotensin system impairs cognitive function in renin/angiotensinogen transgenic mice. Hypertension. 2009;53:356–362. [DOI] [PubMed] [Google Scholar]
- 4. Iwanami J, Mogi M, Tsukuda K, Wang XL, Nakaoka H, Kan‐no H, Chisaka T, Bai HY, Shan BS, Kukida M, Horiuchi M. Direct angiotensin II type 2 receptor stimulation by compound 21 prevents vascular dementia. J Am Soc Hypertens. 2015;9:250–256. [DOI] [PubMed] [Google Scholar]
- 5. Santos RA, Ferreira AJ, Simoes ESAC. Recent advances in the angiotensin‐converting enzyme 2‐angiotensin(1‐7)‐Mas axis. Exp Physiol. 2008;93:519–527. [DOI] [PubMed] [Google Scholar]
- 6. Freund M, Walther T, von Bohlen und Halbach O. Immunohistochemical localization of the angiotensin‐(1‐7) receptor Mas in the murine forebrain. Cell Tissue Res. 2012;348:29–35. [DOI] [PubMed] [Google Scholar]
- 7. Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin‐(1‐7) enhances LTP in the hippocampus through the G‐protein‐coupled receptor Mas. Mol Cell Neurosci. 2005;29:427–435. [DOI] [PubMed] [Google Scholar]
- 8. Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin‐(1‐7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985–H1994. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Iwanami J, Min L, Tsukuda K, Nakaoka H, Bai H, Shan B, Kan‐no H, Kukida M, Chisaka T, Yamauchi T, Higaki A, Mogi M, Horiuchi M. Deficiency of angiotensin‐converting enzyme 2 causes deterioration of cognitive function. NPJ Aging Mech Dis. 2016;2:16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lazaroni TL, Raslan AC, Fontes WR, de Oliveira ML, Bader M, Alenina N, Moraes MF, Dos Santos RA, Pereira GS. Angiotensin‐(1‐7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem. 2012;97:113–123. [DOI] [PubMed] [Google Scholar]
- 11. Walther T, Balschun D, Voigt JP, Fink H, Zuschratter W, Birchmeier C, Ganten D, Bader M. Sustained long term potentiation and anxiety in mice lacking the Mas protooncogene. J Biol Chem. 1998;273:11867–11873. [DOI] [PubMed] [Google Scholar]
- 12. Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Fujita T, Iwai M, Ito M, Horiuchi M. Sex‐different effect of angiotensin II type 2 receptor on ischemic brain injury and cognitive function. Brain Res. 2009;1300:14–23. [DOI] [PubMed] [Google Scholar]
- 13. Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin‐(1‐7)‐stimulated nitric oxide and superoxide release from endothelial cells. Hypertension. 2001;37:72–76. [DOI] [PubMed] [Google Scholar]
- 14. Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. [DOI] [PubMed] [Google Scholar]
- 15. Lazaroni TL, Bastos CP, Moraes MF, Santos RS, Pereira GS. Angiotensin‐(1‐7)/Mas axis modulates fear memory and extinction in mice. Neurobiol Learn Mem. 2016;127:27–33. [DOI] [PubMed] [Google Scholar]
- 16. Okuyama S, Sakagawa T, Chaki S, Imagawa Y, Ichiki T, Inagami T. Anxiety‐like behavior in mice lacking the angiotensin II type‐2 receptor. Brain Res. 1999;821:150–159. [DOI] [PubMed] [Google Scholar]
- 17. Pena Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke. 2012;43:3358–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sekhon LH, Morgan MK, Spence I, Weber NC. Chronic cerebral hypoperfusion and impaired neuronal function in rats. Stroke. 1994;25:1022–1027. [DOI] [PubMed] [Google Scholar]
- 19. Freund M, Walther T, von Bohlen Und Halbach O. Effects of the angiotensin‐(1‐7) receptor Mas on cell proliferation and on the population of doublecortin positive cells within the dentate gyrus and the piriform cortex. Eur Neuropsychopharmacol. 2014;24:302–308. [DOI] [PubMed] [Google Scholar]
- 20. Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond). 2011;121:297–303. [DOI] [PubMed] [Google Scholar]
- 21. Leonhardt J, Villela DC, Teichmann A, Munter LM, Mayer MC, Mardahl M, Kirsch S, Namsolleck P, Lucht K, Benz V, Alenina N, Daniell N, Horiuchi M, Iwai M, Multhaup G, Schulein R, Bader M, Santos RA, Unger T, Steckelings UM. Evidence for heterodimerization and functional interaction of the angiotensin type 2 receptor and the receptor Mas. Hypertension. 2017;69:1128–1135. [DOI] [PubMed] [Google Scholar]
- 22. Ohshima K, Mogi M, Nakaoka H, Iwanami J, Min LJ, Kanno H, Tsukuda K, Chisaka T, Bai HY, Wang XL, Ogimoto A, Higaki J, Horiuchi M. Possible role of angiotensin‐converting enzyme 2 and activation of angiotensin II type 2 receptor by angiotensin‐(1‐7) in improvement of vascular remodeling by angiotensin II type 1 receptor blockade. Hypertension. 2014;63:e53–e59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Body Weight, Brain Weight, and Systolic Blood Pressure in Each Group
Table S2. Body weight, brain weight and systolic blood pressure according to Ang‐(1‐7) treatment
Figure S1. Time‐course analysis of cerebral blood flow (CBF) changes after bilateral common carotid artery stenosis (BCAS) surgery.
Figure S2. Severity of white matter lesion.


