Abstract
In cells with fluctuating energy demand (e.g., skeletal muscle), a transfer system of proteins across the inner and outer mitochondrial membranes links mitochondrial oxidative phosphorylation to cytosolic phosphorylated creatine (PCr) that serves as a phosphate reservoir for rapid repletion of cytosolic adenosine triphosphate (ATP). Crucial proteins of this energy transfer system include several creatine kinase (CK) isoforms found in the cytosol and mitochondria. In a recent proteomic study (Kong et al., 2016), several components of this system were up-regulated in high feed efficiency (FE) compared to low FE breast muscle; notably adenine nucleotide translocase (ANT), voltage dependent activated channel (VDAC), the brain isoform of creatine kinase (CK-B), and several proteins of the electron transport chain. Reexamination of the original proteomic dataset revealed that the expression of two mitochondrial CK isoforms (CKMT1A and CKMT2) had been detected but were not recognized by the bioinformatics program used by Kong et al. (2016a). The CKMT1A isoform was up-regulated (7.8-fold, P = 0.05) in the high FE phenotype but there was no difference in CKMT2 expression (1.1-fold, P = 0.59). From these findings, we hypothesize that enhanced expression of the energy production and transfer system in breast muscle of the high FE pedigree broiler male could be fundamentally important in the phenotypic expression of feed efficiency.
Keywords: feed efficiency, breast muscle, mitochondria, creatine kinase
INTRODUCTION
Creatine kinase (CK) is crucial for energy metabolism in tissues with high and/or fluctuating energy demand (e.g., skeletal, heart, smooth muscle, and brain). Jacobus and Lehninger (1973) credited Burger et al. (1964) with the first report of three izoenzymes of CK that included two cytosolic forms in skeletal muscle and brain, and a third found in mitochondria. Jacobus and Lehninger (1973) proposed a mechanism in which the mitochondrial CK would transfer phosphate groups from adenosine triphosphate (ATP) generated by mitochondrial oxidative phosphorylation to creatine (Cr) to form phosphocreatine (PCr) that would serve as a high energy phosphate reservoir used for rapid regeneration of cytosolic ATP during periods of high energy demand. It is now recognized that the brain (B) and muscle (M) isoforms of CK exist as dimers in the cytosol (either BB, MM, or MB) whereas mitochondrial CK is found as an octamer of two different isoforms present between the inner and outer mitochondrial membranes (see reviews by Brdiczka et al. (2006) and Schlattner et al. (2006)). The transfer of phosphate groups from mitochondrially-generated ATP to Cr to form PCr in the cytosol represents a coordination between; a) adenosine diphosphate (ADP)-ATP flux by adenine nucleotide translocase (ANT) located on the inner mitochondrial membrane to mtCK, and b) subsequent mitochondrial CK catalyzed transfer of the phosphate group to Cr to form PCr that enter and exit the outer mitochondrial membrane, respectively, through a voltage dependent activated channel (VDAC) protein. This coordinated energy transfer system (VDAC-CKMT-ANT) links mitochondrial ATP generation to a reservoir of high energy phosphate group (PCr) that can be drawn upon when cytosolic ATP levels fall in the cell in response to energy demand (cellular work).
A link between mitochondrial function and feed efficiency (FE, Gain: Feed) in a single pedigree male broiler line has been reported (Bottje et al., 2002; Bottje and Carstens, 2009). Pedigree male broilers (PedM) exhibiting a high FE phenotype had higher electron transport chain coupling, higher electron transport chain complex activities, lower amounts of mitochondrial reactive oxygen species (ROS) generation, and lower oxidative stress compared to the low FE phenotype across several tissues (Bottje and Carstens, 2009). It was hypothesized that increased expression of desmin in muscle was indicative of improved mitochondrial function in swine (Grubbs et al., 2014) as desmin is involved in mitochondrial distribution in the cell and more efficient mitochondrial respiration (Milner et al., 2000). More recently, global gene and global protein expression studies have been conducted on breast muscle tissue obtained from the same PedM line (Kong et al., 2011; 2016). In the proteomic study, the importance of mitochondria in this FE model was reemphasized in that; 1) there was a highly significant binomial skew of up-regulated to down-regulated mitochondrial proteins in high FE breast muscle that included several electron transport chain proteins as well as ANT and VDAC, and 2) the prediction of activation of the electron transport chain complexes 1, 3, 4, and 5 in the high FE phenotype that concurred with our previous findings. In addition, the brain isoform of CK (CK-B) was up-regulated (8.7-fold) whereas the muscle isoform of CK (CK-M) was down-regulated (−1.4-fold) in the high FE compared to the low FE breast muscle tissue (see Table 1, Kong et al., 2016). With regard to the energy production-transfer system described above, the only component missing was mitochondrial CK (CKMT) in the proteomic dataset. Since the binomial analysis of mitochondrial proteins revealed that more mitochondrial proteins were expressed at higher levels in the high FE compared to the low FE phenotype (Kong et al., 2016), we anticipated that the expression of CKMT might be found in greater amounts in high FE, but not necessarily attaining a statistical significance. Therefore, the purpose of this study was to search our original dataset for expression of CKMT and then to place this protein in the context of the energy production-transfer system in our PedM broiler FE model.
Table 1.
Protein expression associated with energy production and conveyance in breast muscle of pedigree broiler males. Probability (P value), fold differences (Fold Diff), and molecular weights (Mwt) are presented for up-regulated (positive number) and down-regulated (negative number) proteins in the high compared to low FE pedigree male broiler phenotype (n = 4 per group). Mitochondrial creatine kinase (CKMT1A and CKMT2) expression data were obtained from the original dataset but not recognized by bioinformatics analysis (Kong et al., 2016).
| Symbol | Accession number | Protein name (significance) | P value | Fold diff | Mwt |
|---|---|---|---|---|---|
| CKMT1A | F1NXR0_CHICK | Mitochondrial creatine kinase | P = 0.05 | 7.1 | 42 kDa |
| CKMT2 | F1NAD3_CHICK [4] | Mitochondrial creatine kinase 2 | P = 0.59 | 1.1 | 47 kDa |
| IDE | E1BTQ0_CHICK | Insulin Degrading Enzyme | P = 0.004 | 11.7 | 118 kDa |
| SL25A4 (ANT) | Q5ZMJ6_CHICK [2] | Solute carrier family 25 | P = 0.002 | 10.3 | 33 kDa |
| (mitochondrial carrier; adenine | |||||
| nucleotide translocator, ANT) | |||||
| CAV1 | A0M8T8_CHICK | Caveolin 1, caveolae protein 22kDa | P = 0.009 | 9.4 | 20 kDa |
| CK-B | sp|P05122-5|KCRB_CHICK | Creatine kinase, brain | P = 0.01 | 8.9 | 35 kDa |
| VDAC2 | Q9I9D1_CHICK | Voltage-dependent anion channel 2 (ATP transport) | P = 0.03 | 2.8 | 30 kDa |
| VDAC1 | E1BYN7_CHICK | Voltage-dependent anion channel 1 (Ca++ transport) | P = 0.003 | 2.4 | 31 kDa |
| CK-M | KCRM_CHICK | Creatine Kinase (muscle) | P = 0.04 | −1.4 | 35 kDa |
MATERIALS AND METHODS
All procedures for animal care complied with the University of Arkansas Institutional Animal Care and Use Committee (IACUC): Protocol #14012. A complete description of the procedures for this study is provided in Kong et al. (2016). Briefly, following protein extraction from breast muscle tissue (∼100 mg) obtained from PedM broilers exhibiting high and low FE phenotypes (n = 4 per group), proteins were subjected to shotgun proteomic analysis by in-gel trypsin digestion and tandem mass spectrometry (MS/MS) at the University of Arkansas for Medical Sciences (UAMS) Proteomics Core Lab (Little Rock, AR). Raw spectrometric data was analyzed by the Mascot (Matrix Science, Boston, MA) database search engine along with the UniProtKB (http://www.uniprot.org/help/uniprotkb) database, and compiled using the Scaffold program (Proteome Software, Portland OR). Normalization of data based on total spectral counts for each individual sample revealed no differences in actin, myosin, or tubulin expression that were used as house-keeping proteins in this study. A search for “CKMT” in the excel file of the normalized proteomic dataset revealed two CKMT protein isoforms (CKMT1A and CKMT2) that had been identified and given separate accession numbers. However, both isoforms but had not been assigned a gene name and therefore were not previously reported by Kong et al. (2016).
RESULTS AND DISCUSSION
A list of proteins, accession numbers, size, and fold difference in expression between high and low FE breast muscle tissue in the mitochondrial energy production and transfer system is provided in Table 1. The CKMT1A isoform was up-regulated (P = 0.05) in the high FE phenotype (7.1-fold) whereas the second isoform (CKMT2) was not differentially expressed (P = 0.59) but numerically higher (1.1-fold) in the high FE phenotype. The CKMT exists as an octamer within the inner and outer mitochondrial membrane and is comprised of both CKMT1A and CKMT2 isoforms (Fritz-Wolf et al., 1996; Khuchua et al., 1998). If protein expression in these broiler breast muscle correlates with enzyme activity as it does in human muscle (Barrerio et al., 2005), it is reasonable to hypothesize that CKMT enzyme activity would be higher in the high FE phenotype.
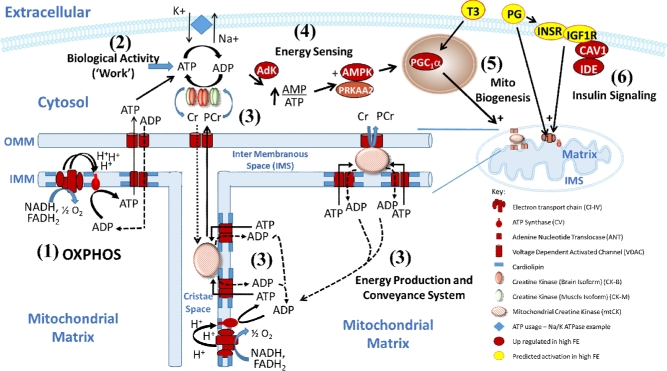
From the CKMT expression data shown in Table 1, a missing piece of the energy production and transfer system summarized by Brdiczka et al. (2006) and Schlattner et al. (2006), is now put in place in the context of protein expression in breast muscle of the high FE phenotype (see Figure 1). Molecules shown in red and green were up-regulated and down-regulated, respectively, in the high FE phenotype. The octameric CKMT is tethered to the inner mitochondrial membrane by cardiolipin along with ANT (Schlattner and Walliman, 2000; Schlattner et al., 2001). Cardiolipin, shown as blue patches on the inner mitochondrial membrane in Figure 1, is a unique “double” phosphoglyceride; i.e., it has 4 long-chain fatty acids compared to 2 side chains in other phospholipids (Hoch, 1992). Cardiolipin is found in high amounts in mitochondria and full activity of respiratory chain complexes (I to V) requires interaction with cardiolipin. Oxidative phosphorylation (OXPHOS, point 1, Figure 1) uses reducing equivalents from nicotinadenine nucleotide- and flavin adenine nucleotide-linked energy substrates to develop a proton motive force that is used to generate ATP from ADP as H+ ions flow back through the ATP synthase. We have reported a general increase in respiratory chain complex activities in high FE tissues (Bottje and Carstens, 2009) that has also been observed in skeletal muscle of more efficient lambs (Sharifabadi et al., 2012). The mRNA expression of ANT was also higher in breast muscle of the high FE pedigree male broiler (Bottje and Carstens, 2009). Four of the 5 complexes that make up the respiratory chain were also predicted to be activated in the proteomics dataset; the exception being complex II (succinate dehydrogenase) (Kong et al., 2016). The shuttling of ADP and ATP across the inner mitochondrial membrane (IMM) and outer mitochondrial membrane (OMM) would be enhanced in the high FE breast muscle due to increased expression of ANT and VDAC2 that were up-regulated in the high FE phenotype muscle by 10.3- and 2.4-fold, respectively (Table 1). This would facilitate ATP export from the mitochondria to support biological work activities (point 2 on Figure 1) using Na+/K+ ATPase in the cell membrane as an example. The ATP needed to accomplish biological work would be supported by cytosolic CK activity. Cytosolic CK, that consists of protein dimers of CK-M or CK-B proteins (combinations of MM, BB, or MB dimers), would enhance the transfer of phosphate from PCr to generate ATP from ADP in the energy production and conveyance system (point 3, Figure 1). Whereas CK-M was down-regulated (−1.4-fold) in the high FE phenotype, the CK-B isoform was up-regulated (8.9-fold) indicating that overall cytosolic CK expression would be higher in the high FE breast muscle tissue. Point 3 on Figure 1 also shows the catalytic activity of CK to transfer phosphate groups moving into the mitochondrial cristae or inter membranous space from ATP to Cr to form PCr that in turn are transferred across the OMM through the VDAC porin protein. As protein expression of CK and enzymatic activity of CK in human muscle are closely correlated (e.g., Barrerio et al., 2005), we would hypothesize that differences in protein expression in the high and low FE groups would also be linked to CK enzymatic activity.
Figure 1.
Depiction of energy production and conveying system enhanced in breast muscle of Pedigree Male Broilers exhibiting a high feed efficiency phenotype. The figure is modified from ones presented in Brdiczka et al. (2006) and Schlattner et al. (2006) using data from proteogenomic data reported previously (Kong et al., 2011; 2016, Bottje et al., 2012;2014). Molecules in red and green indicate proteins or genes up-regulated or down-regulated, respectively, in the high FE Pedigree Male Phenotype. Molecules in yellow were predicted to be activated in the high FE Pedigree Male phenotype based on expression of downstream molecules (Kong et al., 2016). Processes that are shown are described in the text in detail. Abbreviations: OMM (Outer mitochondrial membrane), IMM (Inner mitochondrial membrane), PG (progesterone), T3 (tri-iodothyronine).
Additional components of an energy production – transfer system includes the ability to sense energy status in the cytosol (point 4, Figure 1). During high energy demand, accumulation of ADP with increased formation of adenosine monophosphate (AMP) through adenosine kinase (AdK) activity can occur that in turn can stimulate mitochondrial biogenesis through peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) (number 5, Figure 1). We previously reported that gene expression of AdK, AMP kinase (AMP), and PGC1α were up-regulated in the high FE phenotype (Bottje et al., 2012; 2014; Lassiter et al., 2015). Expression of AMPK mRNA was also higher in commercial broilers in comparison to a slower growing, less efficient legacy broiler (Davis et al., 2015). However, there was no difference in expression of the phosphorylated (activated) AMPK between the high and low FE groups (Lassiter et al., 2015). The expression/activity of PGC1α could be enhanced by thyroxine (T3) that was predicted to be activated in our proteomics study (Kong et al., 2016). We have not conducted western analysis of PGC1α levels in these muscle samples, however. Further enhancement of mitochondrial function would occur through the insulin signaling pathway (point 6, Figure 1) and by the predicted activation (light orange color) of progesterone (PG), insulin receptor (INSR), and insulin like growth factor 1 receptor (IGF1R) shown in Figure 1 in the high FE muscle (Kong et al., 2016).
The results of this study provides a framework to hypothesize that enhanced energy production and transfer from the mitochondria to the cytosol could play a role in the phenotypic expression of feed efficiency in this pedigree broiler male model. This hypothesis is supported by a recent study in beef heifers in which circulating levels of creatine kinase were associated with feed efficiency that depended on whether the animal was pregnant and the stage of gestation (Gonano et al., 2014). Higher plasma levels of creatinine (a break down product of creatine) were observed in feed efficient heifers which could be associated with greater muscle mass (Fitzsimmons et al., 2013); there were no differences in creatinine levels between low-, medium, and high feed efficient bulls, however (Fitzsimmons et al., 2014). Since skeletal muscle comprises roughly 40% of total body mass in animals and accounts for ∼30% of basal metabolic rate in humans (Zurlo et al., 1990), this system could potentially contribute to an overall energetic efficiency in this high FE model. Further investigation is warranted in which activities of mitochondrial and tissue creatine kinase activity are measured to assess whether the differences in mitochondrial expression of proteins or enzymatic activity of creatine kinase in this study could be used as an indicator feed efficiency in poultry and livestock. Possibly, components of the mitochondrial-cytosolic energy conveyance system could be fertile areas to investigate for possible biomarkers for feed efficiency in poultry and livestock. Although speculative at this stage, detailed studies documenting FE-CK activity relationships pre- and post-slaughter might lead to the ability to conduct progeny testing on large groups of offspring without the necessity of measuring feed intake and weight gain on individual animals.
Acknowledgments
Funding for research was provided by USDA-NIFA (#2013-01953) and the Agricultural Experiment Station (Univ. of Arkansas, Fayetteville). I would like to dedicate this manuscript to my father, Will Gay Bottje, who influenced me to be creative and ‘push the envelop’ by his example.
REFERENCES
- Barreiro E., Gea J., Matar G., Hussain S. N. A.. 2005. Expression and carbonylation of creatine kinase in the quadriceps femoris muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 33:632–642. [DOI] [PubMed] [Google Scholar]
- Bottje W., Tang Z., Iqbal M., Cawthon D., Okimoto R., Wing T., Cooper M.. 2002. Association of mitochondrial function with feed efficiency within a single genetic line of male broilers. Poul. Sci. 81:546–555. [DOI] [PubMed] [Google Scholar]
- Bottje W. G., Carstens G., 2009. Association of mitochondria with feed efficiency in livestock and poultry. J. Anim. Sci. 87:E48–E63. [DOI] [PubMed] [Google Scholar]
- Bottje W. G., Kong B-W, Song J. J., Lee J. Y., Hargis B. M., Lassiter K., Wing T., Hardiman J.. 2012. Gene expression in breast muscle associated feed efficiency in a single male broiler line using a chicken 44k microarray II. Differentially expressed focus genes. Poult. Sci. 91:2576–2587. [DOI] [PubMed] [Google Scholar]
- Bottje W. G., Kong B-W., Lee J.Y., Washington T., Baum J., Dridi S., Wing T., Hardiman J.. 2014. Potential roles of mTOR and protein degradation pathways in the phenotypic expression of feed efficiency in broilers. Biochem. Physiol. 3:1. [Google Scholar]
- Brdiczka D. G., Zorov D. B., Sheu S-S.. 2006. Mitochondrial contact sites: Their role in energy metabolism and apoptosis. Biochem. Biophys. Acta. 1762:148–163. [DOI] [PubMed] [Google Scholar]
- Burger A., Richterich R., Aebi H.. 1964. Die heterogenitat der kreatin-kinase. Biochem. 339:305–314. [PubMed] [Google Scholar]
- Davis R. V. N., Lamont S. J., Rothschild M. F., Persia M. E., Ashwell C. M., Schmidt C. J.. 2015. PLOS ONE | DPO:10 /1371/journal.pone.0122525 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons C., Kenny D. A., Deighton M. H., Fahey A. G., McGee M.. 2013. Methane emissions, body composition, and rumen fermentation traits of beef heifers. J. Anim. Sci. 91:5789–5800. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons C., Kenny D. A., McGee M.. 2014. Visceral organ weights, digestion and carcass characteristics of beef bulls differing in residual feed intake offered a high concentrate diet. Animal. 8:949–959. [DOI] [PubMed] [Google Scholar]
- Fritz-Wolf K., Schnyder T., Wallimann T., Kabsch W.. 1996. Structure of mitochondrial creatine kinase. Nature. 381:341–345. [DOI] [PubMed] [Google Scholar]
- Gonano C. V., Montanholi Y. R., Schenkel F. S., Smith B. A., Cant J. P., Miller S. P.. 2014. The relationship between feed efficiency and the circadian profile of blood plasma analytes measured in beef heifers at different physiological stages. Animal 8:1684–1698. [DOI] [PubMed] [Google Scholar]
- Grubbs J. K., Huff-Lonergan E., Gabler N. K., Dekkers J. C. M., Lonergan S. M.. 2014. Liver and skeletal mitochondria proteomes are altered in pigs divergently selected for residual feed intake. J. Anim. Sci. 92:1995–2007. [DOI] [PubMed] [Google Scholar]
- Hoch F., 1992. Cardiolipins and biomembrane function. Biochim. Biophys. Acta. 1113:71–133. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L.. 1973. Creatine kinase of rat heart mitochondria: Coupling of creatine phosphorylation to electron transport. J. Biol. Chem. 248:4803–4810. [PubMed] [Google Scholar]
- Kong B-W., Song J. J., Lee J. Y., Hargis B. M., Wing T., Lassiter K., Bottje W.. 2011. Gene expression in breast muscle associated feed efficiency in a single male broiler line using a chicken 44k microarray. I. Top differentially expressed genes. Poult. Sci. 90:2535–2547; doi:10.3382/ps.2011-01435. [DOI] [PubMed] [Google Scholar]
- Kong B-W., Lassiter K, Piekarski A., Dridi S., Reverter-Gomez A., Hudson N., W Bottje. 2016. Proteomics of breast muscle tissue associated with the phenotypic expression of feed efficiency within a pedigree male broiler line. I. Highlight on mitochondria. PLOS One 11 |DOI:10.1371/journal.pone.0155679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuchua Z. A., Qin W., Bocro J., Cheng J., Payne R. M., Saks V. A., Strauss A. W.. 1998. Octomer formation and coupling of cardiac sarcomeric mitochondrial creatine kinase are mediated by charged N-terminal residues. J. Biol. Chem. 273:22990–22996. [DOI] [PubMed] [Google Scholar]
- Lassiter K., Kong B. W., Dridi S., Bottje W.. 2015. Investigating differential gene and protein expression to understand the cellular basis of feed efficiency in commercial broilers. Poult. Sci. 94:15. [Google Scholar]
- Milner D. J. M., Mavroids N., Weisleder N., Capetanaki Y.. 2000. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 150:1283–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlattner U., Walliman T.. 2000. Octamers of mitochondrial creatine kinase isozymes differ in stability and membrane binding. J. Biol. Chem. 275:17314–17320. [DOI] [PubMed] [Google Scholar]
- Schlattner U., Dolder M, Walliman T., Tokarska-Schlattner M.. 2001. Mitochondrial creatine kinase and mitochondrial outer membrane porin show a direct interaction that is modulated by calcium. J. Biol. Chem. 276:48027–48030. [DOI] [PubMed] [Google Scholar]
- Schlattner U., Tokarska-Schlattner M., Wallimann T.. 2006. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta. 1762:164–180. [DOI] [PubMed] [Google Scholar]
- Sharifabadi H. R., Zamir M. J., Rowghani E., Bottje W. G.. 2012. Relationship between the activity of mitochondrial respiratory chain complexes and feed efficiency in fat-tailed ghezel lambs J. Anim. Sci. 90:1807–1815. [DOI] [PubMed] [Google Scholar]
- Zurlo F., Larson K., Bogardus C., Ravussin E.. 1990. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Invest. 86:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]