Abstract
Objective
Patients with SLE have increased morbidity and premature mortality. Whether this mortality gap has improved in recent years, as in RA, is unknown.
Methods
We conducted a population-based cohort study using a medical records database representative of the general population of the UK. We identified incident SLE cases and matched non-SLE controls between 1999 and 2014, divided into two subgroups based on year of SLE diagnosis, forming the early cohort (1999–2006) and late cohort (2007–14). We compared the mortality rates and hazard ratios, adjusting for potential confounders.
Results
We identified 1470 and 1666 incident SLE cases in the early and late cohorts, respectively. In both cohorts, SLE patients had similar levels of excess mortality compared with their matched comparators [15.9 vs 7.9 deaths/1000 person-years (PY) in the early cohort and 13.8 vs 7.0 deaths/1000 PY in the late cohort]. The corresponding mortality hazard ratios were 2.15 (95% CI 1.63, 2.83) and 2.12 (95% CI 1.61, 2.80) in the early and late cohorts, respectively (P-value for interaction = 0.95). The absolute mortality differences were 8.0 (95% CI 4.3, 11.8) and 6.8 (95% CI 3.5, 10.0) deaths/1000 PY, respectively (P-value for interaction = 0.61).
Conclusion
This general population–based cohort study suggests that excess mortality has not improved among SLE patients in recent years, remaining greater than double that of comparators, unlike RA during the same period. This highlights a critical unmet need for the development of new therapeutic agents and improved management strategies for SLE and its comorbidities.
Keywords: lupus, mortality, trend analysis, quality of care, treatment
Rheumatology key messages
The premature mortality gap in SLE patients has not closed over a recent 16-year period.
SLE patients continue to have nearly double the premature mortality risk of their peers.
This highlights a critical unmet need for improved management of SLE, including new treatments.
Introduction
Patients with SLE have a wide range of morbidities and premature mortality [1–7]. With treatment advances largely occurring before the new millennium, a large international multicentre SLE cohort study found a standardized mortality ratio decline of 60% from the 1970s to 1990s [7]. However, it remains unknown whether this trend in mortality improvement has continued into the new millennium.
Recently it was found that the mortality gap in RA, another protean systemic autoimmune disease associated with premature mortality, has closed substantially in the post-biologic era [8]. For example, RA cases diagnosed between 1999 and 2006 had a 56% increased risk of death compared with those without RA, whereas those diagnosed between 2007 and 2014 had a 29% increased risk of death [9]. This mortality reduction is likely due to significant improvements in RA care (e.g. the availability of highly effective biologic therapies and improved management strategies) and perhaps improved comorbidity management [10–12]. The past decade has seen a multitude of drug therapies in development for lupus. However, only one new medication has been recently approved by the US Food and Drug Administration (FDA) for SLE. Given the lack of major breakthroughs in SLE care to date, unlike in RA, the mortality gap in SLE may not have closed further in the new millennium.
To examine this key issue we conducted a population-based cohort study to investigate the secular trend of all-cause mortality among patients with SLE over the same period as the recent study in RA (1999–2014) in the same general population database [9]. As overall mortality in the UK has improved substantially over the past decades, it was important to incorporate this background mortality improvement to assess excess mortality in patients with SLE [13].
Methods
Source population
The base population of our study was The Health Improvement Network (THIN), an anonymized electronic medical records database from general practitioners (GPs) in the UK. The THIN database is representative of the general UK population in terms of demographics and the prevalence of common medical diagnoses [14]. It includes >11 million patients, comprising 6.2% of the UK population. Health data available in THIN include demographic information, details from GP visits, lifestyle factors, diagnoses from hospital admissions and specialists (e.g. rheumatologist visits), medications and laboratory results. The specific diagnoses are recorded by the Read code classification system, which is a standard thesaurus of clinical terms used by the National Health Service [15]. The Multifunctional Standardized Lexicon for European Community Language classification system is used to code medications [16].
All data in this study were anonymous to the investigators. This study was approved by the Partners Human Research Committee and the Health Improvement Network’s Scientific Review Committee.
Study design and cohort definition
We conducted a population-based matched cohort study. We identified all individuals between 18 and 89 years of age who had a new diagnosis of SLE, defined by at least one Read code for SLE between 1 January 1999 and 31 December 2014, following a recent study that determined the incidence and prevalence of SLE in a UK GP database between 1999 and 2012 using this case definition [17]. We considered incident cases as those who had an index date occurring at least 1 year after the date of entry into the study cohort. Our alternative definition of SLE additionally required a positive immunologic blood test result (positive ANA or ENAs), a prescription for a medication specific to lupus (i.e. HCQ, MTX, AZA, mycophenolate, CYC, rituximab or glucocorticoids) or an evaluation by a rheumatologist, as was also adopted in the same UK study [17]. Validation of other autoimmune diseases in the UK GP database has shown positive predictive values of >90% and UK GPs are unlikely to use a Read code for SLE unless it has been confirmed by a hospital specialist [17, 18].
To evaluate changes in mortality, we then divided the SLE cohort into two calendar time-based cohorts according to each patient’s year of diagnosis, forming the early (1999–2006) and late cohorts (2007–14). For the comparison cohorts corresponding to each time-based SLE cohort, we selected up to five individuals without SLE matched on age, sex and entry time. All non-SLE individuals also had to be continuously enrolled in the THIN database for at least 1 year with at least one GP visit prior to inclusion in the cohort.
Assessment of outcome
The primary outcome of this study was all-cause mortality. This was determined by the death date recorded in the THIN database, which is automatically updated when death is registered in the Personal Demographics Service database. The Personal Demographics Service is the national database that contains demographic data for patients registered with the National Health Service in the UK. The automatic update of deceased status does not require input by practice staff in the THIN database and it has been shown to be an accurate reflection of national death rates in the UK [14].
Assessment of covariates
We determined information from the most recent available data in THIN prior to the index date on sociodemographic and anthropometric characteristics (age, sex and BMI) as well as lifestyle factors (alcohol consumption and tobacco use). We obtained information on medication use within 1 year prior to cohort entry, including aspirin, beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, diuretics and low-dose aspirin. We identified prior comorbid medical conditions using Read codes, including diabetes, hypertension, congestive heart failure, myocardial infarction, stroke, peripheral vascular disease, dementia, chronic obstructive pulmonary disease, chronic kidney disease, liver disease, cancer and rheumatologic disease. We calculated modified Charlson comorbidity index (CCI) scores, excluding CTD, based on comorbidities recorded by GPs [19]. We assessed health care utilization by determining the number of GP office visits in the year prior to cohort entry.
Statistical analysis
We compared the baseline characteristics of individuals in the SLE cohorts and corresponding non-SLE cohorts. Person-years (PY) of follow-up for each subject were calculated as the amount of time from the index date until either death, the end of the follow-up period for their cohort (either 31 December 2006 for the early cohort or 31 December 2014 for the late cohort, ensuring equal follow-up time between the two cohorts to allow for a fair comparison) or disenrollment from the THIN database. We then calculated all-cause mortality rates per 1000 PY and plotted cumulative mortality incidence curves for each cohort.
We examined the relationship of SLE and all-cause mortality for each time-specific SLE cohort using a Cox proportional hazards model, adjusting for age, sex and entry time into the study cohort. We calculated multivariable-adjusted hazard ratios (HRs), additionally adjusting for BMI, alcohol consumption, tobacco use, health care utilization (i.e. the number of GP visits in the prior year), medication usage and the modified CCI score. We also examined the difference in all-cause mortality between the SLE cohorts and comparison cohorts using an additive hazard model in which the hazard was modelled as a linear function of SLE status. The effect estimate generated from the model can be interpreted as the number of excess deaths attributable to SLE per 100 PY [20].
To evaluate whether the relationship between SLE and all-cause mortality varied according to time, we combined the two SLE cohorts and tested an interaction term (i.e. SLE status × calendar time-based cohort) in the multivariable regression model. Given the predilection of SLE among women, we also analysed the mortality trends restricted to women. For all analyses, missing values for covariates (i.e. BMI, alcohol consumption and smoking status) were imputed by a sequential regression method based on a set of covariates as predictors [IVEware for SAS, version 9.2; SAS Institute, Cary, NC, USA].
Results
The early cohort consisted of 1470 patients with incident SLE and 7348 matched non-SLE individuals. During the follow-up period, 76 and 189 individuals died in the SLE and non-SLE cohorts, respectively. In the late cohort of 1666 patients with incident SLE and 8318 matched non-SLE individuals, 76 and 196 died during the follow-up period. The mean follow-up time for each cohort was similar, ranging between 3.2 and 3.3 years.
The subjects were well balanced in both the early and late cohorts among the SLE and non-SLE participants in terms of age, sex, BMI and alcohol use, as shown in Table 1. Notably, the mean age is similar to that found in other population-based studies of incident SLE [21]. However, the patients with SLE were more likely to use tobacco, more often used cardiovascular medications, had greater health care utilization and had higher CCI scores than non-SLE individuals in both time-based cohorts (Table 1).
Table 1.
Baseline characteristics of cohort subsets by time period and disease status
| Characteristics | 1996–2006 | 2007–2014 | ||
|---|---|---|---|---|
| SLE (n = 1470) | Non-SLE comparators (n = 7348) | SLE (n = 1666) | Non-SLE Comparators (n = 8318) | |
| Sex, male, n (%) | 268 (18.2) | 1339 (18.2) | 275 (16.5) | 1371 (16.5) |
| Age, mean (s.d.), years | 50.3 (15.3) | 50.3 (15.3) | 51.2 (15.7) | 51.2 (15.7) |
| BMI, mean (s.d.), kg/m2 | 25.8 (5.2) | 25.9 (5.2) | 27.0 (5.8) | 26.9 (5.9) |
| Smoking status, n (%) | ||||
| Current smoker | 446 (30.3) | 1624 (22.1) | 470 (28.2) | 1579 (19.0) |
| Alcohol use, n (%) | ||||
| Current drinker | 931 (63.3) | 4, 514 (61.4) | 1, 076 (64.6) | 5, 544 (66.7) |
| GP visit, mean (s.d.) | 5.1 (4.4) | 2.6 (3.1) | 6.3 (4.4) | 3.1 (3.3) |
| Medication use, n (%) | ||||
| Diuretics | 209 (14.2) | 790 (10.8) | 255 (15.3) | 874 (10.5) |
| ARBs | 49 (3.3) | 129 (1.8) | 102 (6.1) | 363 (4.4) |
| Beta-blockers | 156 (10.6) | 617 (8.4) | 193 (11.6) | 729 (8.8) |
| Calcium channel blockers | 138 (9.4) | 398 (5.4) | 238 (14.3) | 698 (8.4) |
| Low-dose aspirin | 166 (11.3) | 476 (6.5) | 198 (11.9) | 680 (8.2) |
| ACE inhibitors | 108 (7.3) | 417 (5.7) | 198 (11.9) | 838 (10.1) |
| Charlson comorbidity index, mean (s.d.) | 0.27 (0.76) | 0.18 (0.66) | 0.41 (0.96) | 0.28 (0.79) |
ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers.
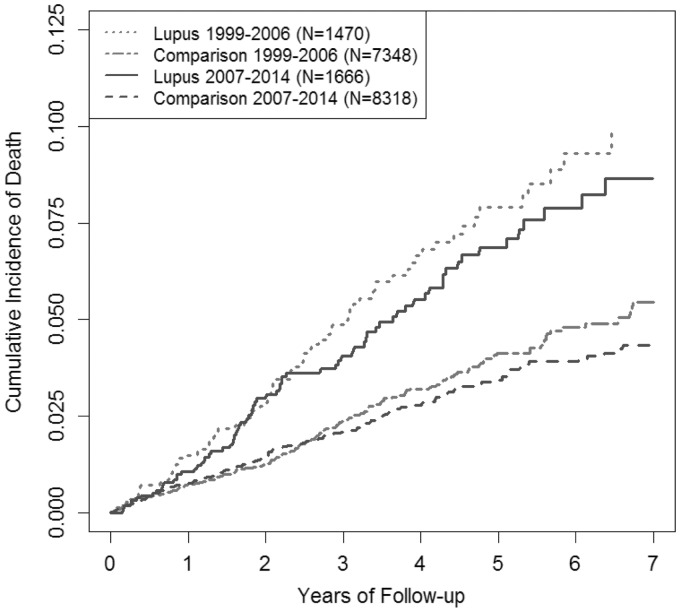
Figure 1 depicts the cumulative mortality of participants with SLE and non-SLE individuals for both the early and late cohorts during the follow-up. Participants with SLE had higher cumulative mortality than non-SLE comparators in both the early and late cohorts and the magnitude of excess mortality was similar between the two cohorts. Overall mortality rates declined between time period–defined cohorts for both SLE participants and matched comparators, but the excess mortality gap for SLE patients did not decline (Table 2). The age-, sex- and entry time–matched HR for mortality was 2.15 (95% CI 1.63, 2.83) in the early cohort compared with 2.12 (95% CI 1.61, 2.80) in the late cohort (Table 2), indicating that there was no improvement in the mortality gap between patients with SLE and the general population over time (P-value for interaction = 0.95). The multivariable-adjusted HRs for all-cause mortality also did not differ between time periods [1.94 (95% CI 1.44, 2.61) for the early cohort and 1.80 (95% CI 1.32, 2.44) for the late cohort; P-value for interaction =0.76]. The age-, sex- and entry time–adjusted mortality rate difference was similar (8.0 vs 6.8 excess deaths in SLE participants per 1000 PY; P-value for interaction = 0.61) and corresponding multivariable-adjusted mortality rate differences were also similar (7.4 vs 5.7; P-value for interaction = 0.48).
Fig. 1.
Cumulative mortality over time in early and late cohorts
Table 2.
Association between SLE and all-cause mortality according to time period
| Outcomes | 1996–2006 | 2007–14 | |||
|---|---|---|---|---|---|
| SLE (n = 1470) | Non-SLE comparators (n = 7348) | SLE (n = 1666) | Non-SLE comparators (n = 8318) | P-value for interaction | |
| Follow-up, mean (s.d.), PY | 3.2 (2.1) | 3.3 (2.1) | 3.3 (2.2) | 3.3 (2.3) | |
| Number of deaths | 76 | 189 | 76 | 195 | |
| Death rate/1000 PY (95% CI) | 15.9 (12.7, 20.0) | 7.9 (6.8, 9.1) | 13.8 (10.9, 17.3) | 7.0 (6.1, 8.1) | |
| Age-, sex- and entry year–matched HR (95% CI) | 2.15 (1.63, 2.83) | 1.00 (Ref) | 2.12 (1.61, 2.80) | 1.00 (Ref) | 0.95 |
| Multivariable-adjusteda HR (95% CI) | 1.93 (1.42, 2.62) | 1.00 (Ref) | 1.78 (1.30, 2.44) | 1.00 (Ref) | 0.52 |
| Age-, sex- and entry year–matched rate difference/1000 PY (95% CI) | 8.0 (4.3, 11.8) | 0.0 (Ref) | 6.8 (3.5, 10.0) | 0.0 (Ref) | 0.61 |
| Multivariable-adjusteda rate difference/ 1000 PY (95% CI) | 7.4 (3.7, 11.0) | 0.0 (Ref) | 5.7 (2.5, 8.9) | 0.0 (Ref) | 0.48 |
In addition to the matching variables, multivariable models were adjusted for the number of GP visits, BMI, smoking status (i.e. non-smokers, ex-smokers and current smokers), alcohol consumption (i.e. non-drinkers, ex-drinkers and current drinkers), CCI score and medication use prior to the index date.
When we repeated the analyses using our alternative definition of SLE (i.e. SLE diagnosis plus immunologic evidence of lupus, evaluation by a rheumatologist or a prescription for medication specific to lupus), the results remained similar (Fig. 2 and Table 3). After adjusting for covariates, the multivariable-adjusted HR for mortality did not differ between the early and late cohorts [2.13 (95% CI 1.56, 2.91) vs 1.97 (95% CI 1.50, 2.58); P-value for interaction = 0.45]. Similarly, the multivariable-adjusted mortality rate difference also did not differ between the two cohorts (7.9 vs 6.5 deaths/1000 PY; P-value for interaction = 0.35) (Fig. 2 and Table 3).
Fig. 2.
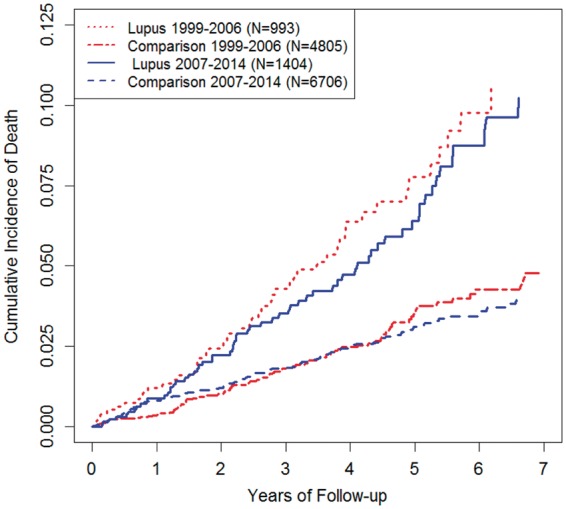
Cumulative mortality of patients with SLE with immunologic evidence, lupus-specific medication and/or rheumatologist referral
Table 3.
Second definition of SLE and all-cause mortality according to time period
| Outcomes | 1996–2006 | 2007–14 | |||
|---|---|---|---|---|---|
| SLE (n = 993) | Non-SLE comparators (n = 4805) | SLE (n = 1404) | Non-SLE comparators (n = 6706) | P-value for interaction | |
| Follow-up, mean (s.d.), PY | 3.2 (2.1) | 3.2 (2.1) | 3.3 (2.2) | 3.3 (2.3) | |
| Number of deaths | 49 | 99 | 63 | 139 | |
| Death rate/1000 PY (95% CI) | 15.4 (11.4, 20.3) | 6.4 (5.2, 7.8) | 13.5 (10.4, 17.3) | 6.2 (5.2, 7.3) | |
| Age-, sex- and entry year–matched HR (95% CI) | 2.62 (1.83, 3.74) | 1.00 (Ref) | 2.24 (1.64, 3.05) | 1.00 (Ref) | 0.52 |
| Multivariable-adjusteda HR (95% CI) | 2.13 (1.56, 2.91) | 1.00 (Ref) | 1.97 (1.50, 2.58) | 1.00 (Ref) | 0.43 |
| Age-, sex- and entry year–matched rate difference/1000 PY (95% CI) | 9.0 (4.5, 13.4) | 0.0 (Ref) | 7.3 (3.8, 10.8) | 0.0 (Ref) | 0.57 |
| Multivariable-adjusteda rate difference/1000 PY (95% CI) | 7.9 (3.6, 12.1) | 0.0 (Ref) | 6.5 (3.2, 9.8) | 0.0 (Ref) | 0.35 |
In addition to the matching variables, multivariable models were adjusted for the number of GP visits, BMI, smoking status (i.e. non-smokers, ex-smokers and current smokers), alcohol consumption (i.e. non-drinkers, ex-drinkers and current drinkers), CCI score and medication use prior to the index date.
In a sensitivity analysis using a case definition of SLE requiring two or more Read codes for SLE, our results remained similar. After adjusting for covariates, the multivariable-adjusted HR for mortality did not differ between the early and late cohorts [2.60 (95% CI 1.70, 4.00) vs 2.00 (95% CI 1.30, 3.10); P-value for interaction = 0.11]. In a secondary analysis limited to females, the findings again remained similar. After adjusting for covariates, the multivariable HR did not differ between the early and late cohorts [1.87 (95% CI 1.34, 2.61) vs 1.80 (95% CI 1.27, 2.54); P-value for interaction = 0.96]. Similarly, the multivariable-adjusted mortality rate difference also did not differ between the two cohorts (5.8 vs 4.5 deaths per 1000 PY; P-value for interaction = 0.65).
Discussion
In this general population–based cohort study, we found that excess all-cause mortality did not improve among SLE patients in recent years. Overall mortality rates were lower in the late SLE cohort than the early cohort over this 16-year period; however, the improving trend paralleled that of the general population, leaving the premature mortality gap unclosed. This is in clear contrast to the aforementioned substantial reduction in excess mortality observed among patients with RA during the same period in the same general population database [9]. To our knowledge, this study is the first to investigate premature mortality trends in patients with SLE in recent years, and our findings urgently call for renewed attention and continued efforts to close the persisting mortality gap, as was achieved in RA [8, 9].
Our study findings extend the previous mortality gap trend data in SLE [22] to a contemporary era (1999–2014). A prior large multinational cohort study comprising SLICC and Canadian Network for Improved Outcomes in Systemic Lupus consortiums found that the standardized mortality ratio has declined from 4.9 in the 1970s to 2 in the 1990s. However, our findings indicate that the mortality gap in SLE did not improve further in the 2000s up until 2014, remaining approximately double that of non-SLE comparators [7]. While the prior study used external reference population mortality rates, our study used individually matched internal non-SLE comparison cohorts, allowing for multivariable adjustment of potential confounders such as smoking, alcohol use, cardiovascular medication use and major comorbidities.
Compared with crude HRs, the multivariable-adjusted HRs for all-cause mortality were slightly attenuated for each time period, which likely reflect the baseline differences in tobacco use and comorbidities. Notably, we found a higher frequency of baseline tobacco use among SLE patients than in age- and sex-matched comparators, which has also been reported in prior studies [23–25]. Furthermore, BMIs and CCI scores were higher for both SLE patients and controls in the late cohort than those in the early cohort, likely reflecting the secular trend of increased obesity in the general population and more rigorous recordkeeping of comorbidities in recent years. Regardless, even after these adjustments, the independent mortality impact of SLE remained nearly double that of non-SLE comparators.
The persistent mortality gap in SLE may be related to the lack of major breakthroughs in SLE care, unlike those seen in RA. The treatment options for RA have improved dramatically, particularly with regard to the advance of effective biologic therapies and increased recognition of cardiovascular comorbidities over recent decades, which has presumably contributed to the reduction in excess mortality in RA [12, 23]. However, despite progress in our understanding of the pathogenesis of SLE and the ongoing endeavour to develop and appropriately evaluate new therapies for SLE, there have been few major breakthroughs to date [24, 25]. For example, only one new medication, belimumab, has been approved for treating SLE since HCQ was approved by the FDA in 1955 [26–28], while trials of other candidate therapies have failed to show significant effects [29–31]. In the past decade, mycophenolate has emerged as an improved standard-of-care treatment for LN [32, 33]. However, as shown in a large recent international cohort study, LN continues to be associated with increased mortality and end-stage renal disease, suggesting the need for further advancements in LN treatment [34]. Furthermore, SLE confers an increased risk of a wide range of complications and comorbidities, including cardiovascular disease, malignancy, infections, pregnancy complications and venous thromboembolism [5, 6, 35–41]. These would add to the mortality risk of SLE patients beyond that directly related to the disease itself [3]. As such, our findings are likely to be multifactorial, calling for the need for improved SLE care as well as better prevention and management of comorbidities.
Our study has several strengths. First, this study is based on a general population cohort, thus our findings are likely to be generalizable. Another important strength of our study is the large number of subjects with incident SLE we identified through the population-based database. Unlike previous studies that adjusted for age, sex and region to obtain mortality ratios [21, 22], our internal cohort comparison group allowed for adjustment of other key confounders prior to SLE diagnosis (i.e. smoking, obesity, comorbidities and medication use) to help isolate the mortality impact of SLE. Furthermore, using incident SLE cases minimizes the selection bias [42] that could underestimate the risk of death if prevalent surviving SLE cases were included in the analysis. Since our study examined the mortality gap for up to 8 years after SLE onset in each cohort, this gap likely reflects the level of initial lupus morbidity and the current level of lupus care. As in all studies with mortality endpoints, with a longer follow-up, a smaller effect size is generally expected, with more individuals in both SLE and non-SLE groups dying progressively until all have eventually died. Nevertheless, there was a clear improvement in the premature mortality gap in RA over the same period and with the same duration of follow-up in the same general population database [9].
Although we followed a recent study that determined the incidence and prevalence of SLE in a UK GP database for our case definition of lupus, uncertainty surrounding its diagnostic accuracy is a potential concern. However, the incidence rate of SLE (4.9/100 000 PY) from the UK GP database was virtually the same as the recent incidence estimate from the Rochester Epidemiology Project, defined by SLICC criteria (4.9/100 000 PY) [43]. The racial breakdown in the Rochester Epidemiology Project is similar to that of the UK general population per recent UK census data (i.e. 2.7% black/African American [44] vs 3.4% black/African British or black Caribbean [45]). Furthermore, mortality rates in SLE are also compatible with previous estimates given the general trends of improvement [22]. For example, the mortality rate in the SLICC cohort (until 2001), which was composed of both incident and prevalent (survived) SLE cases, was 16.3/1000 PY, whereas the all-cause mortality rates of our early and late incident SLE cohorts were 15.9 and 13.8/1000 PY, respectively. Moreover, several validation studies have demonstrated significant diagnostic accuracy of electronic medical records–based databases, including this UK GP database, to assess important medical outcomes for autoimmune diseases, with positive predictive values of >90% [18, 46]. Finally, our findings were similar after conducting a secondary analysis that used a stricter definition of SLE with further evidence of SLE in the medical record.
Race/ethnicity data were not available for individual patients in the THIN database and are poorly recorded in UK death records in general [47]; however, recent UK studies suggest that the life expectancy of Asian and Black women in the UK tends to be longer than that of Caucasians, unlike in the USA [47–49]. As such, the increased risk of mortality in SLE observed in our study reflective of the UK general population is unlikely to be explained by an overrepresentation of these ethnic/racial minorities in SLE [50]. Nevertheless, it would be valuable for future studies to investigate mortality trends stratified by racial and ethnic groups. While our study suggests that there has not been a significant survival impact from more recently available lupus treatments (e.g. mycophenolate or rituximab), future studies could specifically examine such potential benefits. As belimumab was not approved for use in the UK until 2016, our findings do not reflect mortality trends related to belimumab use [35]. Our study focused on all-cause mortality, as cause-specific mortality data tend to be incomplete in the THIN database. Nevertheless, the overall mortality trends are critically important in their own right, as all-cause mortality represents the overall net health outcome of various benefits and risks associated with disease management.
In conclusion, this general population–based cohort study demonstrates that over a recent 16 year period, the mortality gap persisted among SLE patients, with double the risk of overall mortality compared with their non-SLE peers. While mortality is decreasing for the population at large, the excess mortality attributable to SLE has not declined significantly, in contrast to the closing mortality gap seen in RA [9]. This indicates a clear unmet need for both further research into the causes of persistently high mortality rates in SLE patients as well as the development of new therapeutic agents and improved management strategies for SLE and its associated comorbidities.
Funding: This project was supported in part by the T32 Ruth L. Kirschstein Institutional National Research Service Award, National Institutes of Health (NIH) grant P60-AR-047785 and NIH grant R01-AR-065944.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Abu-Shakra M, Gladman DD, Urowitz MB.. Mortality studies in SLE: how far can we improve survival of patients with SLE. Autoimmun Rev 2004;3:418–20. [DOI] [PubMed] [Google Scholar]
- 2. Borchers AT, Keen CL, Shoenfeld Y, Gershwin ME.. Surviving the butterfly and the wolf: mortality trends in systemic lupus erythematosus. Autoimmun Rev 2004;3:423–53. [DOI] [PubMed] [Google Scholar]
- 3. Lee Y, Choi S, Ji J, Song G.. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016;25:727–34. [DOI] [PubMed] [Google Scholar]
- 4. Urowitz M, Bookman A, Koehler B. et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 1976;60:221–5. [DOI] [PubMed] [Google Scholar]
- 5. Aviña-Zubieta JA. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res 2017;69:849–56. [WorldCat] [DOI] [PubMed] [Google Scholar]
- 6. Navarra SV, Leynes MS.. Infections in systemic lupus erythematosus. Lupus 2010;19:1419–24. [DOI] [PubMed] [Google Scholar]
- 7. Bernatsky S, Boivin J-F, Joseph L. et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 8. Humphreys JH, Warner A, Chipping J.. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis Register. Arthritis Care Res 2014;66:1296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Lu N, Peloquin C.. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis 2017;76:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters MJ, Symmons DP, McCarey D. et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010;69:325–31. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Landewé R, Breedveld FC. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haraoui B. The anti-tumor necrosis factor agents are a major advance in the treatment of rheumatoid arthritis. J Rheumatol 2005;72:46–7. [PubMed] [Google Scholar]
- 13. Nash A. Past trends in life expectancy. In: Mortality, 2014-based national population projections reference volume. Office for National Statistics, 2016. March. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/compendium/nationalpopulationprojections/2014basedreferencevolumeseriespp2/chapter4mortality2014basednationalpopulationprojectionsreferencevolume (April 2017, date last accessed).
- 14. Blak BT, Thompson M, Dattani H, Bourke A.. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care 2011;19:251–5. [DOI] [PubMed] [Google Scholar]
- 15. NHS Digital: Read Codes. https://digital.nhs.uk/article/1104/Read-Codes (April 2017, date last accessed).
- 16. FDB Solutions: Multilex. www.fdbhealth.co.uk/solutions/multilex (April 2017, date last accessed).
- 17. Rees F, Doherty M, Grainge M. et al. The incidence and prevalence of systemic lupus erythematosus in the UK, 1999–2012. Ann Rheum Dis 2016;75:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrett E, Thomas SL, Schoonen WM. et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan NF, Perera R, Harper S. et al. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rod NH, Lange T, Andersen I. et al. Additive interaction in survival analysis: use of the additive hazards model. Epidemiology 2012;23:733–7. [DOI] [PubMed] [Google Scholar]
- 21. Rees F, Doherty M, Grainge MJ. et al. Mortality in systemic lupus erythematosus in the United Kingdom 1999–2012. Rheumatology 2016;55:854–60. [DOI] [PubMed] [Google Scholar]
- 22. Bartels CM, Buhr KA, Goldberg JW. et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol 2014;41:680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Her M, Kavanaugh A.. Advances in use of immunomodulatory agents—a rheumatology perspective. Nat Rev Gastroenterol Hepatol 2015;12:363–8. [DOI] [PubMed] [Google Scholar]
- 24. Jordan N, D’Cruz D.. Current and emerging treatment options in the management of lupus. Immunotargets Ther 2016;2:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rai SK, Yazdany J, Fortin PR, Avina-Zubieta JA.. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res Ther 2015;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gatto M, Saccon F, Zen M. et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun 2016;74:94–105. [DOI] [PubMed] [Google Scholar]
- 27. Mosak J, Furie R.. Breaking the ice in systemic lupus erythematosus: belimumab, a promising new therapy. Lupus 2013;22:361–71. [DOI] [PubMed] [Google Scholar]
- 28. Ruiz-Irastorza G, Khamashta MA.. Hydroxychloroquine: the cornerstone of lupus therapy. Lupus 2008;17:271–3. [DOI] [PubMed] [Google Scholar]
- 29. Rovin BH, Furie R, Latinis K. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- 30. Merrill JT, Neuwelt CM, Wallace DJ. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010;62:222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Isenberg DA, Petri M, Kalunian K. et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016;75:323–31. [DOI] [PubMed] [Google Scholar]
- 32. Chan TM. Treatment of severe lupus nephritis: the new horizon. Nat Rev Nephrol 2014; 11:46–61. [DOI] [PubMed] [Google Scholar]
- 33. Dall’Era M. Treatment of lupus nephritis: current paradigms and emerging strategies. Curr Opin Rheumatol 2017;29:241–7. [DOI] [PubMed] [Google Scholar]
- 34. Hanly JG, O’Keeffe AG, Su L. et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hak AE, Karlson EW, Feskanich D. et al. Systemic lupus erythematosus and the risk of cardiovascular disease: results from the nurses’ health study. Arthritis Rheum 2009;61:1396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schoenfeld S, Kasturi S, Costenbader K.. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 2013;43:77–95. [DOI] [PubMed] [Google Scholar]
- 37. Aviña-Zubieta JA, Vostretsova K, De Vera MA, Sayre EC, Choi HK.. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: a general population-based study. Semin Arthritis Rheum 2015;45:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goobie GC, Bernatsky S, Ramsey-Goldman R, Clarke AE.. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol 2015;27:454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lateef A, Petri M.. Management of pregnancy in systemic lupus erythematosus. Nat Rev Rheumatol 2012;8:710–8. [DOI] [PubMed] [Google Scholar]
- 40. Danza A, Ruiz-Irastorza G.. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 2013;22:1286–94. [DOI] [PubMed] [Google Scholar]
- 41. Bundhun PK, Soogund MZ, Huang F.. Impact of systemic lupus erythematosus on maternal and fetal outcomes following pregnancy: A meta-analysis of studies published between years 2001–2016. J Autoimmun 2017;79:17–27. [DOI] [PubMed] [Google Scholar]
- 42. Choi HK, Nguyen U-S, Niu J. et al. Selection bias in rheumatic disease research. Nat Rev Rheumatol 2014;10:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ungprasert P, Sagar V, Crowson CS. et al. Incidence of systemic lupus erythematosus in a population-based cohort using revised 1997 American College of Rheumatology and the 2012 Systemic Lupus International Collaborating Clinics classification criteria. Lupus 2017;26:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jarukitsopa S, Hoganson DD, Crowson CS. et al. Epidemiology of systemic lupus erythematosus and cutaneous lupus erythematosus in a predominantly white population in the United States. Arthritis Care Res 2015;67:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.2011 Census: Key Statistics for England and Wales, March 2011. Office for National Statistics; London: UK Statistics Authority, 2012.
- 46. Coleman N, Halas G, Peeler W. et al. From patient care to research: a validation study examining the factors contributing to data quality in a primary care electronic medical record database. BMC Fam Pract 2015;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jarman B, Aylin P.. Death rates in England and Wales and the United States: variation with age, sex, and race. BMJ 2004;329:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wohland P, Rees P, Nazroo J, Jagger C.. Inequalities in healthy life expectancy between ethnic groups in England and Wales in 2001. Ethn Health 2015;20:341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gruer L, Cezard G, Clark E. et al. Life expectancy of different ethnic groups using death records linked to population census data for 4.62 million people in Scotland. J Epidemiol Commun Health 2016;70:1251–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moss KE, Ioannou Y, Sultan SM, Haq I, Isenberg DA.. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis 2002;61:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]