Abstract
Objective. The importance of hypomethylation in SLE is well recognized; however, the significance of hypermethylation has not been well characterized. We screened hypermethylated marks in SLE and investigated their possible implications.
Methods. DNA methylation marks were screened in SLE whole-blood DNA by microarray, and two marks (CD3Z and VHL hypermethylations) were confirmed by a methylation single-base extension method in two independent ethnic cohorts consisting of 207 SLE patients and 151 controls. The correlation with clinical manifestations and the genetic influence on those epigenetic marks were analysed.
Results. Two epigenetic marks, CD3Z and VHL hypermethylation, were significantly correlated with SLE: CD3Z hypermethylation (odds ratio = 7.76; P = 1.71 × 10−13) and VHL hypermethylation (odds ratio = 3.77; P = 3.20 × 10−8), and the increased CD3Z methylation was correlated with downregulation of the CD3ζ-chain in SLE T cells. In addition, less genetic influence on CD3Z methylation relative to VHL methylation was found in analyses of longitudinal and twin samples. Furthermore, a higher CD3Z methylation level was significantly correlated with a higher SLE disease activity index and more severe clinical manifestations, such as proteinuria, haemolytic anaemia and thrombocytopenia, whereas VHL hypermethylation was not.
Conclusion. CD3Z hypermethylation is an SLE risk factor that can be modified by environmental factors and is associated with more severe SLE clinical manifestations, which are related to deranged T cell function by downregulating the CD3ζ-chain.
Keywords: CD3Z hypermethylation, systemic lupus erythematosus, environmental factor
Rheumatology key messages
CD3Z hypermethylation in SLE T cells is correlated with SLE disease severity.
CD3Z methylation can be related to the reduced CD3ζ-chain expression in SLE T cells.
Controlling CD3Z methylation in SLE patients can provide a new therapeutic approach.
Introduction
SLE is a prototypic autoimmune disease that is related to abundant production of autoantibodies and formation of immune complexes. Epigenetic factors are important in the pathogenesis of SLE [1] because demethylating agents cause lupus-like symptoms [2, 3]. In accordance with this, significant epigenetic differences have been revealed in SLE-discordant twins [4], and several hypomethylated epigenetic marks in such genes, including ITGAL (CD11a), PRF1 (perforin), CD70, GADD45A and IFNs [5–8], were identified, which strongly implicates the aetiological role of epigenetic factors in SLE. Most studies, however, have focused on hypomethylated marks, and hypermethylated marks have not been well characterized thus far. Hypermethylated epigenetic marks can potentially provide another mechanism for SLE pathogenesis. In addition, the associations between epigenetic marks and clinical manifestations are unclear.
In the present study, we used a microarray method to screen hypermethylated epigenetic marks in SLE patients and controls. The methylation single-base extension (MSBE) method was used to validate those marks in Korean and American cohorts comprising 207 patients with SLE and 151 controls. The correlation between the hypermethylated level of an epigenetic mark and the expression of its protein was evaluated by FACS analysis. Environmental and genetic influences on the hypermethylated marks were studied with serial samples collected at two time points and samples collected from monozygotic (MZ), dizygotic (DZ) and SLE-discordant MZ (DMZ) twins. Additionally, the associations between hypermethylation and clinical manifestations of SLE were evaluated.
Methods
Patients and controls
All patients with SLE met the ACR classification criteria [9]. Use of samples and clinical information for this study was approved by the Institutional Review Boards of Seoul National University Hospital, University of California Los Angeles Medical Center and the National Institutes of Health. The subject’s written consents were obtained in compliance with the Declaration of Helsinki, excepting those for the Korean samples obtained before 2005 for which consents were waived by the Insitutional Review Board of Seoul National University Hospital owing to lack of any relevant law in Korea at that time. The demographic or clinical characteristics of the American and Korean study subjects relevant to the microarray analysis and/or validation are summarized in supplementary Tables S1 and S2, available at Rheumatology online. Use of blood DNA samples from patients with breast cancer was approved by the Institutional Review Board of the National Cancer Center.
Genome-wide methylation microarray analysis
The Illumina HumanMethylation27 BeadChip (Illumina, San Diego, CA, USA) was used to screen candidate epigenetic marks in DNA samples purified from whole blood using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA). The EZ DNA methylation kit (Zymo Research, Irvine, CA, USA) was used for bisulfite conversion of blood DNA samples (500 ng). DNA amplification, hybridization and allele-specific extension preparatory to the Beadchip array procedure were performed according to the manufacturer’s protocol (Macrogen, Seoul, Korea). The array slides were scanned on a BeadArray Reader (Illumina).
The image intensities were extracted using Illumina’s BeadScan software, and the resulting intensity files were analysed with Illumina’s GenomeStudio software, which generates β-values (i.e. M/(M + UM), where M is methylated signal intensity and UM is unmethylated signal intensity). The microarray data for all samples are publicly available at Gene Expression Omnibus (GSE57869, http://www.ncbi.nlm.nih.gov/geo).
Significant epigenetic marks were selected based on the following criteria: presence of the probe in the promoter CpG islands, P < 0.05 by t-test and change in β-values (mean signal value difference) >0.08 or <−0.08 between the patients with SLE and the controls. Hierarchical clustering of selected marker genes was performed using Ward’s linkage with Euclidean distance for samples and Pearson’s correlation coefficients for the probes. Genes for which DNA methylation levels changed significantly were analysed for gene networks using the Ingenuity Pathway Analysis software (Ingenuity Systems; http://www.ingenuity.com).
Determining promoter methylation
The quantitative MSBE method was used to validate different methylation levels (supplementary Fig. S1A and B, available at Rheumatology Online). After bisulphite conversion on 500 ng of whole-blood DNA samples with the EZ DNA methylation kit (Zymo Research), gene-specific amplification was performed using the PCR primers shown in supplementary Table S3, available at Rheumatology Online, in the following conditions: initial incubation at 95 °C for 10 min, followed by 35 cycles of 30 s at 95 °C, 30 s at 56 °C and 1 min at 72 °C in a mixture containing 1× PCR buffer II (Roche, Mannheim, Germany) with 1.5 mM MgCl2, 0.2 mM dNTPs, 10 pmol of each primer and 50–100 ng bisulphite-treated genomic DNA. The amplified products were purified using the AxyPrep PCR Clean up kit (Axygen, Union City, CA, USA), and the single-base-extension reaction was performed using the SNaPshot kit (Applied Biosystems, Foster City, CA, USA) and the extension primers (supplementary Table S3, available at Rheumatology Online) in the following conditions: 15 cycles of 10 s at 96 °C, 5 s at 50 °C and 30 s at 60 °C. The single-base-extended product was analysed on an ABI sequencer (Applied Biosystems), and the M/UM ratio was calculated, where M is the peak height of methylated cytosine and UM is that of unmethylated cytosine.
Bisulphite sequencing from cell-line DNA
Bisulphite modification and PCR amplification were performed as described above. Then, the PCR-amplified products were subcloned into a T-vector (pGEM-T Easy; Promega, Madison, WI, USA) and sequenced using the T7 primer. The ratio of methylated-to-unmethylated cytosine residues within the amplified sequence was used to compare the MSBE results.
T cell sub-fractionation
T cells were isolated by magnetic cell sorting using the Pan T cell isolation kit II (MACS; Miltenyi Biotech, Bergisch Gladbach, Germany) in 22 peripheral blood samples from the patients with SLE. Briefly, a freshly prepared peripheral blood lymphocyte suspension in MACS buffer (0.5% BSA and 2 mM EDTA in PBS) and antibody in the kit were incubated for 10 min at 4 °C. After adding microbeads, the mixture was incubated for 15 min at 4 °C and applied to the column. The eluate was used as an enriched T cell fraction.
CD3ζ-chain-expressing T cell fraction by FACS analysis
Peripheral blood lymphocytes were separated from heparinized peripheral blood of 22 SLE patients by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density-gradient centrifugation and immediately subjected to FACS, using a FACScan flow cytometer (BD Biosciences, San Diego, CA, USA), CellQuest software (BD Biosciences), anti-CD3ε-FITC for the T cell surface glycoprotein CD3ε-chain (BD Biosciences) and anti-CD3ζ-chain phycoerythrin for the T cell surface glycoprotein CD3ζ-chain (Beckman Coulter, Marseille Cedex 9, France). CD3ζ-chain expression was determined by the bright/dim ratio of the cells expressing high (CD3ζ bright) or low (CD3ζ dim) levels of the T cell surface glycoprotein CD3ζ-chain, as described previously [10].
Clinical analysis
The patients’ clinical information was obtained by reviewing patient records within 1 month of blood sampling. Based on the daily CS dosage and use of immunosuppressants throughout the 3 months before the sampling date, the patients were classified as follows: low-dose CS (lowS; ⩽10 mg/day prednisolone); high-dose CS (highS; >10 mg/day prednisolone); and immunosuppressant subgroups (Imm; use of CYC, AZA, MTX, tacrolimus or MMF) regardless of CS use.
Statistical analyses
Student's unpaired t-test was used to select the candidate epigenetic marks from the BeadChip microarray data, and the two-sided Mann–Whitney U-test was used to validate the candidate epigenetic marks. The bisulfite sequencing and MSBE results were analysed using the two-sided Pearson’s correlation analysis after logarithmic transformation. Logistic regression was used to estimate the odds ratios (ORs) and 95% CIs for the SLE risk of DNA methylation. The intraclass correlation (ICC) test was used to analyse the correlation in methylation levels between the MZ, DZ or SLE DMZ twin pairs. The two-sided Pearson’s correlation test was used after logarithmic transformation to correlate CD3Z methylation from whole-blood or T cells with the CD3ζ-chain bright/dim ratio. Correlations between CD3Z methylation level in the whole-blood and T cell DNA were tested using the two-sided Pearson’s correlation test after logarithmic transformation. Correlations between CD3Z methylation and clinical parameters were analysed using the two-sided Mann–Whitney U-test. SAS (SAS Institute, Cary, NC, USA) and GraphPad Prism version 5 software (GraphPad Software, San Diego, CA, USA) were used for all statistical analyses.
Results
Screening of SLE epigenetic marks by microarray
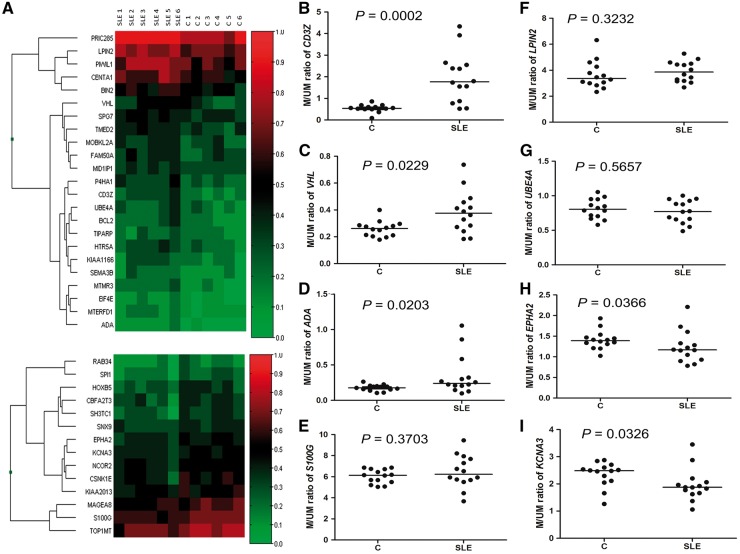
Epigenetic marks with differential methylation levels in the patients with SLE were screened by microarray using six SLE and six control blood DNA samples. Thirty-seven candidate epigenetic marks (Fig. 1A) were selected after screening across ∼27 000 probes, based on the criteria specified in the Methods section; 14 were hypomethylated in the patients with SLE and 23 hypermethylated (Fig. 1A).
Fig. 1.
Microarray analysis for epigenetic marks and validation using the methylation single-base extension method
(A) Heatmap of genes showing differential DNA methylation levels in their CpG islands between patients with SLE (SLE1–SLE6) and controls (C1–C6). Thirty-seven candidate epigenetic marks were selected and hierarchical clustering was performed. Hypermethylated marks are shown in the upper panel and hypomethylated marks in the lower panel. Higher methylation levels are shown in red and lower methylation levels in green. Colour intensity reflects the magnitude of the methylation level. Among the 37 marks, eight epigenetic marks in the CD3Z (B), VHL (C), ADA (D), S100G (E), LPIN2 (F), UBE4A (G), EPHA2 (H) and KCNA3 (I) genes, which have known immunological functions, were further validated using the methylation single-base extension method. Statistical differences were determined by the Mann–Whitney U-test for each mark. Horizontal lines are the median M/UM values, where M is height of the methylated cytosine peak, and UM is that of the unmethylated cytosine peak, in the methylation single-base extension results.
Validation of epigenetic marks
Out of 37 candidate marks, eight candidate ones (CD3Z, VHL, ADA, S100G, LPIN2, UBE4A, EPHA2 and KCNA3), for which immunological functions have been reported (supplementary Table S4, available at Rheumatology Online), were validated with the MSBE method for their differential methylation. An analysis using the Ingenuity Pathway Analysis program revealed a gene list with differential promoter CpG island methylation (supplementary Table S5, available at Rheumatology Online), on which the two marks, namely those in CD3Z and VHL, were most significant.
MSBE assays were established for eight candidate epigenetic marks, and two of these are shown in supplementary Fig. S1A and B, available at Rheumatology Online. Significant correlations were observed between the MSBE and bisulfite sequencing results for two epigenetic marks (R = 0.9429, P = 0.0167 for CD3Z; and R = 0.9367, P = 0.0059 for VHL; supplementary Fig. S1C and D, available at Rheumatology Online), indicating the reliability of the MSBE method to quantify the methylation level. With the MSBE method, the whole-blood DNA samples of 14 patients and 14 controls were tested. Significantly higher methylation levels of three marks, namely those in CD3Z (P = 0.0002), VHL (P = 0.0229) and ADA (P = 0.0203), and significantly lower methylation levels of two marks, namely those in EPHA2 (P = 0.0366) and KCNA3 (P = 0.0326), were reproduced (Fig. 1B–I).
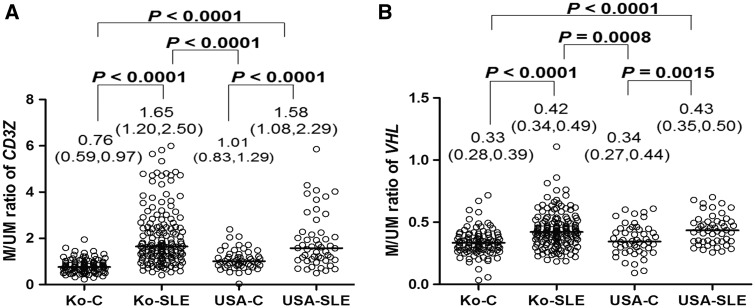
Given that general T cell hypomethylation [11] and several hypomethylated marks [5–7] have already been reported, only two marks with higher methylation levels (i.e. CD3Z [CD247, OMIM 186780] and VHL [OMIM 608537]) were further validated in the present study using 157 Korean patients with SLE and 101 Korean controls. A significant increase in DNA methylation was also observed in the Korean patients with SLE (Fig. 2A and B; P < 0.0001 for the two marks). The age-adjusted ORs for SLE risk, when the cut-off value was set at the median for the methylated vs the unmethylated peak ratio in the controls, were 15.49 for CD3Z and 3.85 for VHL (Table 1).
Fig. 2.
CD3Z and VHL methylation levels in Korean and American patients with SLE and controls
The Korean patients with SLE (Ko-SLE, n = 157) showed significantly higher CpG islands methylation levels for the CD3Z (A) and VHL (B) marks than those observed in controls (Ko-C, n = 101). Significantly higher methylation for the two marks was also observed in the American cohorts (USA-SLE, American patients with SLE, n = 50; and USA-C, American controls, n = 50). P-values were determined with the Mann–Whitney U-test. The Y-axis is the M/UM ratio, where M is height of the methylated cytosine peak, and UM is that of the unmethylated cytosine peak, in the methylation single-base extension results. Horizontal lines are the median M/UM values, and the first and third quartile values for each group are shown.
Table 1.
Higher CD3Z or VHL methylation levels in patients with SLE
| Gene | Country | M/UM ratioa | Control | Case | Odds ratiob (95% CI) | P-value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| CD3Z | Korea | High (≥0.755) | 48 (50.0) | 147 (74.9) | 15.49 (7.10, 33.78) | 5.58×10−12 |
| Low (<0.755) | 48 (50.0) | 10 (25.1) | ||||
| USA | High (≥1.010) | 25 (50.0) | 41 (82.0) | 4.53 (1.78, 11.49) | 1.47×10−3 | |
| Low (<1.010) | 25 (50.0) | 9 (18.0) | ||||
| Total | High (≥0.833) | 73 (50.0) | 183 (88.4) | 7.76 (4.50, 13.38) | 1.71×10−13 | |
| Low (<0.833) | 73 (50.0) | 24 (11.6) | ||||
| VHL | Korea | High (≥0.333) | 51 (50.0) | 123 (78.3) | 3.85 (2.20, 6.73) | 2.25×10−6 |
| Low (<0.333) | 50 (50.0) | 34 (21.7) | ||||
| USA | High (≥0.341) | 25 (50.0) | 40 (80.0) | 4.18 (1.67, 10.44) | 2.21×10−3 | |
| Low (<0.341) | 25 (50.0) | 10 (20.0) | ||||
| Total | High (≥0.336) | 76 (50.3) | 162 (78.3) | 3.77 (2.35, 6.03) | 3.20×10−8 | |
| Low (<0.336) | 75 (49.5) | 45 (21.7) |
The median M/UM ratios in the controls were used as cut-off values.
Odds ratios are adjusted by age, sex and ethnicity. M: peak height of methylated cytosine; UM: peak height of unmethylated cytosine.
The significance of two marks found in the Korean patients with SLE was also observed in another validation set comprising 50 American patients with SLE (USA-SLE in Fig. 2A and B) and 50 American controls (USA-C in Fig. 2A and B); the age-adjusted ORs for SLE risk were 4.53 for CD3Z and 4.18 for VHL (Table 1). When the all cases and controls for both countries were analysed together, the age-adjusted ORs for SLE risk were 7.76 for CD3Z (P = 1.71 × 10−14) and 3.77 for VHL (P = 3.20 × 10−8).
Downregulation of the CD3ζ-chain in T cells has also been reported in patients with breast cancer [12]. The CD3Z methylation level was measured in whole-blood DNA from patients with breast cancer (n = 21), but no significant difference (Mann–Whitney U-test, P = 0.5911) was found from that of the controls (supplementary Fig. S2, available at Rheumatology Online). At the same time, the VHL methylation level was not significantly different in patients with breast cancer (P = 0.9471; supplementary Fig. S2, available at Rheumatology Online).
We compared our data with the previously reported results of Coit et al. [13], who identified 44 significant epigenetic marks (for 86 probe sites). When we applied our criteria on those marks, four marks (CD3Z, PLSCR1, PRIC285 and TRIM22) were significant, and the directions of the methylation level increase or decrease were consistent for all marks (supplementary Fig. S3, available at Rheumatology Online). Even after extending our criteria as shown in the legend of supplementary Fig. S3, available at Rheumatology Online, the directions were again consistent for all seven significant marks.
Correlation between CD3Z methylation level and the CD3ζ-chain-expressing T cell fraction
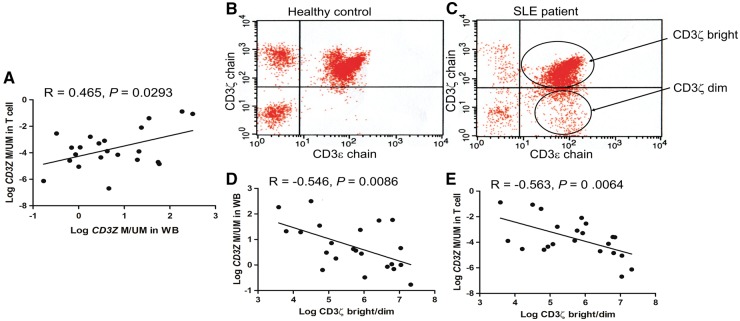
A significant positive correlation of CD3Z methylation levels was found between whole-blood cells and T cells in 22 SLE patients (P = 0.0293; Fig. 3A). Furthermore, the CD3ζ-chain-expressing T cell fraction was inversely correlated with the CD3Z methylation level in whole-blood DNA (P = 0.0086; Fig. 3D) and with that in T cell DNA (P = 0.0064; Fig. 3E), indicating that CD3Z methylation in whole-blood cells is related to both CD3ζ-chain-expressing T cells and the CD3Z methylation levels in T cells. For this analysis, CD3ζ-chain expression in the T cell fraction was evaluated by the CD3ζ-chain bright/dim ratio among the CD3ε-chain-positive cells (Fig. 3B and C).
Fig. 3.
Correlations between whole-blood or T cell CD3Z methylation level and CD3ζ-chain-expressing T cells
(A) CD3Z methylation levels between whole-blood and T cells of the 22 patients with SLE were significantly correlated. (B and C) A flow-cytometric analysis was performed to measure the CD3ζ-chain-expressing T cells, and the CD3ζ-chain bright/dim ratio was determined among the CD3ε-chain-positive cells in the controls (B) and the patients with SLE (C). (D and E) The number of CD3ζ-chain-expressing T cells was correlated with the CD3Z methylation levels in whole-blood cells (D) and T cells (E) of the patients with SLE. Pearson’s correlation analysis was performed after logarithmic transformation of the values. WB: whole blood.
DNA methylation levels for two marks during the SLE disease course
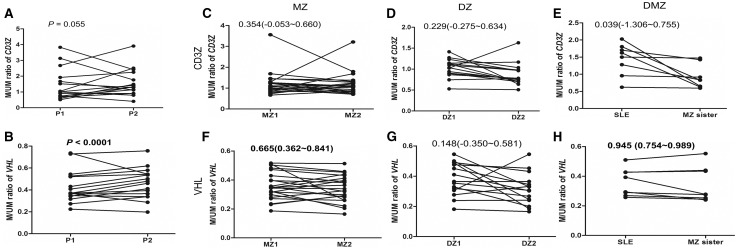
Serial samples from 16 SLE cases at two time points were analysed to evaluate changes in DNA methylation levels during the SLE disease course. The median time difference between the two blood samples was 24.2 months (interquartile range: 16.3–118.3 months). The correlation of the CD3Z methylation levels between the two time points from each patient was not significant (Mann–Whitney U-test, P = 0.055, R = 0.488; Fig. 4A), indicating that the CD3Z methylation level can change during the SLE disease course. In contrast, the DNA methylation levels between the two time points from each patient were closely correlated for VHL (P < 1.00 × 10−5, R = 0.942; Fig. 4B), suggesting that the VHL methylation level is relatively stable during the SLE disease course.
Fig. 4.
Longitudinal changes and environmental influences in methylation levels
(A and B) The longitudinal changes in CD3Z (A) and VHL (B) methylation levels from blood samples taken at two time points (P1 and P2, where P1 is the earlier point) in SLE patients (n = 16) are shown. (C–H) Difference in methylation level between pairs of monozygotic (MZ; C and F; n = 22), dizygotic (DZ; D and G; n = 15) and SLE-discordant MZ (DMZ; E and H; n = 8) twins were analysed. The MZ and DZ twins were controls without SLE. Twin sisters 1 and 2 (MZ1 and MZ2 or DZ1 and DZ2) were assigned randomly.
Correlation of CD3Z and VHL methylations between MZ, DZ or SLE DMZ twin pairs
Methylation levels in whole-blood DNA were compared between pairs of MZ or DZ healthy female twin controls to determine the genetic or environmental influences. CD3Z methylation levels were not correlated between the MZ (ICC coefficient, 0.354; 95% CI: −0.053, 0.660; Fig. 4C) or DZ twins (ICC = 0.229; 95% CI: −0.275, 0.634; Fig. 4D), indicating an effect of environmental factors on CD3Z methylation level. However, VHL levels were significantly correlated between the MZ pairs (ICC = 0.665; 95% CI: 0.362, 0.841; Fig. 4F) but not between the DZ pairs (ICC = 0.148; 95% CI: −0.350, 0.581; Fig. 4G), suggesting less influence of environmental factors on VHL methylation level. Likewise, CD3Z methylation level was not correlated between pairs of DMZ twins with SLE (ICC = 0.039; 95% CI: −1.306, 0.755; Fig. 4E). However, VHL methylation level was correlated (ICC = 0.945; 95% CI: 0.754, 0.989; Fig. 4H), demonstrating that the SLE disease process or therapy may have no effect or a minimal effect on VHL methylation level, which is consistent with the results for the healthy twin pairs.
Correlations between methylation level and various clinical parameters in patients with SLE or with various treatments
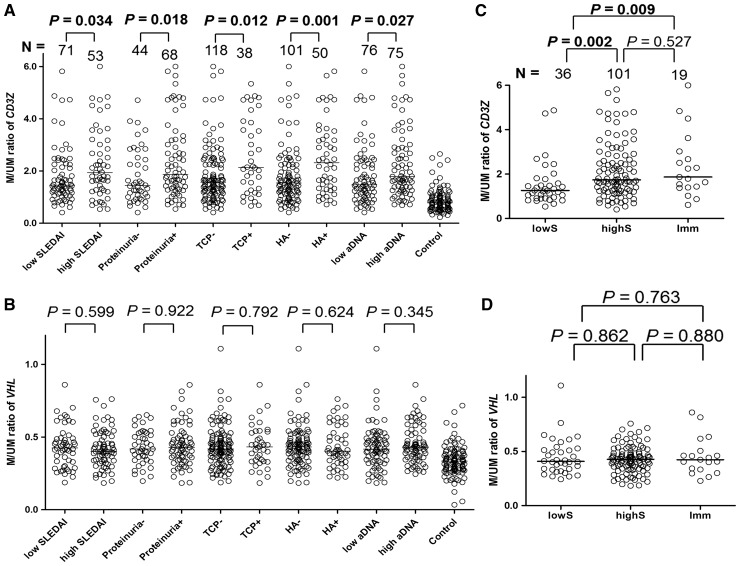
In the clinical correlation analysis of DNA methylation levels, higher CD3Z methylation was significantly correlated with the SLE disease activity index (P = 0.034), proteinuria (⩾500 mg/day, P = 0.018), thrombocytopenia (<100 000/mm3, P = 0.012), haemolytic anaemia (P = 0.001) and higher anti-dsDNA antibody titre (P = 0.027; Fig. 5A). However, higher VHL methylation was not significantly correlated with these clinical parameters (Fig. 5B). The correlation between methylation levels and the other clinical parameters is shown in supplementary Fig. S4, available at Rheumatology Online.
Fig. 5.
Correlations between DNA methylation level and clinical parameters
(A) Higher CD3Z methylation was related to higher SLEDAI, proteinuria, thrombocytopenia (TCP), haemolytic anaemia (HA) and a higher anti-dsDNA titer (α-DNA). Median values were used to divide the SLEDAI and anti-dsDNA titre subgroups. (B) VHL methylation was not correlated with any of the clinical manifestations. (C) The CD3Z methylation level was significantly higher in the high-dose CS-treated subgroup (highS; prednisolone >10 mg/day, P = 0.002) and immunosuppressant-treated subgroup (Imm; P = 0.009) than that in the low-dose CS-treated subgroup (lowS; prednisolone ≤10 mg/day). (D) VHL methylation levels were not different among the three treatment subgroups. Horizontal lines are median M/UM values, where M is height of the methylated cytosine peak, and UM is that of the unmethylated cytosine peak, in the methylation single-base extension results. Case number (N) is shown.
The three treatment subgroups (lowS, highS and Imm) were analysed to determine correlations between methylation level and SLE drug treatment. Use of HCQ was not considered, because no correlation with methylation level was found (data not shown). CD3Z methylation levels differed significantly among the subgroups (Fig. 5C); the highS (P = 0.002) and the Imm subgroups (P = 0.009) had significantly higher CD3Z methylation levels than those in the lowS subgroup. Considering that highS or immunosuppressant treatments are usually administered to patients resistant to lowS, CD3Z hypermethylation could be related to a more severe disease course. However, VHL (Fig. 5D) methylation levels were not significantly different among the three treatment subgroups.
Discussion
In the present study, we identified two hypermethylation marks in the CD3Z and VHL gene promoters in SLE patients, and these marks were confirmed in two independent cohorts, suggesting that they are epigenetic risk factors for SLE. A significant inverse correlation between CD3Z methylation level and CD3ζ-chain-expressing T cells in the present study suggests that CD3Z hypermethylation can be a mechanism for downregulation of the CD3ζ-chain in SLE T cells. In clinical correlation, CD3Z hypermethylation was related to more severe clinical manifestations of SLE and with more intensive drug treatment.
Abundant production of autoantibodies and the formation of immune complexes cause tissue damage; therefore, SLE is considered a B cell disease. However, the importance of helper T cells for triggering B cell immunity has also been recognized with the defective CD3ζ expression or function [14–17]. Reduced expression of the CD3ζ-chain has also been recognized in patients with cancer, infection and other autoimmune diseases, such as RA, but it is transient [18]. In contrast, reduced CD3ζ-chain expression is maintained throughout the SLE disease course in more than half of patients [19–21].
Several mechanisms for the CD3ζ-chain downregulation in patients with SLE have been suggested, including low transcriptional activity, increased ubiquitination and increased caspase 3-dependent proteolysis [18]. Mutations or deletions in CD3Z [22] and abnormal CD3ζ-chain transcripts [15, 22–24] have also been reported, but their roles in CD3ζ-chain downregulation in SLE T cells are not clear. In our present study, a significant increase in CD3Z hypermethylation in patients with SLE and its significant correlation with the reduced CD3ζ-expressing T cell fraction suggest that CD3Z hypermethylation may be a mechanism for CD3ζ-chain downregulation. Although downregulation of the CD3ζ-chain has also been reported in patients with breast cancer [12], our results show that the CD3Z methylation level was not significantly different in patients with breast cancer, which was related to previous reports suggesting that the mechanism of reduced CD3ζ-chain expression in cases other than SLE may be different [18–21]. Therefore, CD3Z hypermethylation in patients with SLE may be a mechanism for the sustained downregulation of the CD3ζ-chain in patients with SLE. To the best of our knowledge, this is the first report suggesting a relationship between CD3Z hypermethylation and CD3ζ-chain downregulation in patients with SLE.
CD3Z methylation levels were significantly higher in patients with higher SLEDAI and more severe clinical manifestations, such as proteinuria, thromobocytopenia, haemolytic anaemia and a higher anti-dsDNA titre. Furthermore, CD3Z methylation levels were significantly higher in patients with more intensive drug treatments, which are administered to patients with severe symptoms or to patients unresponsive to low-dose CS treatment, suggesting that a higher CD3Z methylation level is related to more severe disease. SLEDAI [25] and the anti-dsDNA titer are the most commonly used indicators for disease activity, but our results suggest that CD3Z methylation may be another excellent indicator.
We suggested that CD3Z methylation can be one of the environmentally modified epigenetic factors, which drew attention because they explain several characteristics that single nucleotide polymorphisms cannot, such as age dependency and the disease severity of multifactorial diseases [26]. Correspondingly, several twin studies have identified non-heritable epigenetic modulations occurring during ageing or disease processes [27–30]. In SLE, non-heritable epigenetic modulation has also been demonstrated in discordant twin samples [4]. In the present study, we showed that CD3Z methylation was not consistent in MZ SLE twins and not stable during the disease course, suggesting a greater environmental influence on CD3Z methylation. Notably, the OR for SLE risk of environmentally modifiable CD3Z methylation was higher (OR = 15.49 in Korean patients with SLE) than for VHL methylation, which may underscore the importance of non-heritable epigenetic factors in the pathogenesis of SLE.
Our analyses of twin and serial SLE samples indicated that VHL methylation can be a heritable risk factor for SLE. The VHL methylation level was maintained stably in the serial samples during the median 2-year SLE disease course. In addition, the VHL methylation level was significantly correlated between the MZ twin pairs, but not between the DZ pairs. Furthermore, the VHL methylation level was significantly correlated between the SLE DMZ twin pairs. These results point to a more heritable nature for VHL methylation. Although epigenetic inheritance in humans is not considered a major determinant of disease susceptibility because of an efficient eraser of epigenetic marks during the early embryonic period [31], recent reports suggest the existence of such inheritance in Lynch syndrome [32, 33] or chronic lymphocytic leukaemia [34]. The suggestion that VHL methylation is a heritable risk for SLE in the present study may be preliminary because of the limited twin samples, but this could be another demonstration of the heritable epigenetic risk for multifactorial diseases. The involvement of VHL product in immune regulation has been posited [35, 36]. However, no role for VHL has been described in SLE. Identifying VHL methylation as a risk factor for SLE in the present study could provide further insights into SLE pathogenesis.
In conclusion, we identified novel hypermethylation marks, such as CD3Z and VHL hypermethylation, as risks for SLE. In particular, CD3Z hypermethylation, which is an environmentally modified epigenetic mark, could provide an important mechanism for the CD3ζ-chain downregulation in SLE T cells. Furthermore, we found that DNA hypermethylation of CD3Z was associated with more severe clinical manifestations in patients with SLE, suggesting that CD3Z hypermethylation is a disease severity marker. Our results will contribute significantly to the current understanding of the epigenetic factors at play in the pathogenesis of SLE; thus, opening avenues for further research into epigenetic pathogenesis in human multifactorial complex diseases.
Supplementary Material
Acknowledgements
This study was partially supported by a grant from the National Research Foundation, Korea (no. NRF-2015R1A2A2A04007432 to K.-M.H.), by a grant from the National Cancer Center (1510121 to K.-M.H.), by the Intramural Research Program of the US National Institutes of Health, the National Institute of Environmental Health Sciences, USA, by a US National Institutes of Health grant (R01AR043814) and by a grant from the National Project for Personalized Genomic Medicine (no. A111218-12-GM10), Ministry for Health and Welfare, Republic of Korea. Y.-W.S. was supported by the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant no: HI13C1754). We are grateful for the use of the NCC FACS Core Laboratory. We thank Dr Terrance P. O’Hanlon for his technical help, and Drs Ejaz Shamim and Paul Wade for their useful comments on the manuscript. We also thank all other relevant individuals and family members for their participation.
Funding: This study is supported by the Korea Health Technology R&D project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant no: HI13C1754).
Disclosure statement: D.K.M. owns Amgen and Remeron stocks, but not a controlling interest. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Patel DR, Richardson BC. Epigenetic mechanisms in lupus. Curr Opin Rheumatol 2010;22:478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cornacchia E, Golbus J, Maybaum J. et al. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 1988;140:2197–200. [PubMed] [Google Scholar]
- 3. Quddus J, Johnson KJ, Gavalchin J. et al. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest 1993;92:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javierre BM, Fernandez AF, Richter J. et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res 2010;20:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu Q, Kaplan M, Ray D. et al. Demethylation of ITGAL (CD11a) regulatory sequences in systemic lupus erythematosus. Arthritis Rheum 2002;46:1282–91. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan MJ, Lu Q, Wu A, Attwood J, Richardson B. Demethylation of promoter regulatory elements contributes to perforin overexpression in CD4+ lupus T cells. J Immunol 2004;172:3652–61. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Zhao M, Yin H. et al. Overexpression of the growth arrest and DNA damage-induced 45α gene contributes to autoimmunity by promoting DNA demethylation in lupus T cells. Arthritis Rheum 2010;62:1438–47. [DOI] [PubMed] [Google Scholar]
- 8. Absher DM, Li X, Waite LL. et al. Genome-wide DNA methylation analysis of systemic lupus erythematosus reveals persistent hypomethylation of interferon genes and compositional changes to CD4+ T-cell populations. PLoS Genet 2013;9:e1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 10. Gorman CL, Russell AI, Zhang Z. et al. Polymorphisms in the CD3Z gene influence TCRζ expression in systemic lupus erythematosus patients and healthy controls. J Immunol 2008;180:1060–70. [DOI] [PubMed] [Google Scholar]
- 11. Balada E, Ordi-Ros J, Vilardell-Tarrés M. DNA methylation and systemic lupus erythematosus. Ann N Y Acad Sci 2007;1108:127–36. [DOI] [PubMed] [Google Scholar]
- 12. Dworacki G, Meidenbauer N, Kuss I. et al. Decreased zeta chain expression and apoptosis in CD3+ peripheral blood T lymphocytes of patients with melanoma. Clin Cancer Res 2001;7:947s–57s. [PubMed] [Google Scholar]
- 13. Coit P, Jeffries M, Altorok N. et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naïve CD4+ T cells from lupus patients. J Autoimmun 2013;43:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kammer GM, Perl A, Richardson BC, Tsokos GC. Abnormal T cell signal transduction in systemic lupus erythematosus. Arthritis Rheum 2002;46:1139–54. [DOI] [PubMed] [Google Scholar]
- 15. Takeuchi T, Tsuzaka K, Pang M. et al. TCR ζ chain lacking exon 7 in two patients with systemic lupus erythematosus. Int Immunol 1998;10:911–21. [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi T, Tsuzaka K, Abe T. Altered expression of the T cell receptor-CD3 complex in systemic lupus erythematosus. Int Rev Immunol 2004;23:273–91. [DOI] [PubMed] [Google Scholar]
- 17. Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest 1998;101:1448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baniyash M. TCR ζ-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 2004;4:675–87. [DOI] [PubMed] [Google Scholar]
- 19. Pang M, Setoyama Y, Tsuzaka K. et al. Defective expression and tyrosine phosphorylation of the T cell receptor zeta chain in peripheral blood T cells from systemic lupus erythematosus patients. Clin Exp Immunol 2002;129:160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brundula V, Rivas LJ, Blasini AM. et al. Diminished levels of T cell receptor ζ chains in peripheral blood T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum 1999;42:1908–16. [DOI] [PubMed] [Google Scholar]
- 21. Nambiar MP, Mitchell JP, Ceruti RP, Malloy MA, Tsokos GC. Prevalence of T cell receptor zeta chain deficiency in systemic lupus erythematosus. Lupus 2003;12:46–51. [DOI] [PubMed] [Google Scholar]
- 22. Nambiar MP, Enyedy EJ, Warke VG. et al. Polymorphisms/mutations of TCR-ζ-chain promoter and 3′ untranslated region and selective expression of TCR ζ-chain with an alternatively spliced 3′ untranslated region in patients with systemic lupus erythematosus. J Autoimmun 2001;16:133–42. [DOI] [PubMed] [Google Scholar]
- 23. Tsuzaka K, Setoyama Y, Yoshimoto K. et al. A splice variant of the TCR ζ mRNA lacking exon 7 leads to the down-regulation of TCR ζ, the TCR/CD3 complex, and IL-2 production in systemic lupus erythematosus T cells. J Immunol 2005;174:3518–25. [DOI] [PubMed] [Google Scholar]
- 24. Tsuzaka K, Fukuhara I, Setoyama Y. et al. TCRζ mRNA with an alternatively spliced 3′-untranslated region detected in systemic lupus erythematosus patients leads to the down-regulation of TCRζ and TCR/CD3 complex. J Immunol 2003;171:2496–503. [DOI] [PubMed] [Google Scholar]
- 25. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 26. Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet 2004;20:350–8. [DOI] [PubMed] [Google Scholar]
- 27. Weksberg R, Shuman C, Caluseriu O. et al. Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith–Wiedemann syndrome. Hum Mol Genet 2002;11:1317–25. [DOI] [PubMed] [Google Scholar]
- 28. Dempster EL, Pidsley R, Schalkwyk LC. et al. Disease-associated epigenetic changes in monozygotic twins discordant for schizophrenia and bipolar disorder. Hum Mol Genet 2011;20:4786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Talens RP, Christensen K, Putter H. et al. Epigenetic variation during the adult lifespan: cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell 2012;11:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bjornsson HT, Sigurdsson MI, Fallin MD. et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA 2008;299:2877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heard E, Martienssen RA. Transgenerational epigenetic inheritance: myths and mechanisms. Cell 2014;157:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ligtenberg MJL, Kuiper RP, Chan TL. et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet 2009;41:112–7. [DOI] [PubMed] [Google Scholar]
- 33. Hitchins MP, Rapkins RW, Kwok CT. et al. Dominantly inherited constitutional epigenetic silencing of MLH1 in a cancer-affected family is linked to a single nucleotide variant within the 5′UTR. Cancer Cell 2011;20:200–13. [DOI] [PubMed] [Google Scholar]
- 34. Raval A, Tanner SM, Byrd JC. et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell 2007;129:879–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cramer T, Yamanishi Y, Clausen BE. et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 2003;112:645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walmsley SR, McGovern NN, Whyte MK, Chilvers ER. The HIF/VHL pathway: from oxygen sensing to innate immunity. Am J Respir Cell Mol Biol 2008;38:251–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.