Abstract
Objectives
To assess long-term efficacy, safety and tolerability of secukinumab up to 104 weeks in patients with active PsA.
Methods
Patients with PsA (n = 397) were randomized to s.c. secukinumab 300, 150 or 75 mg or placebo at baseline, weeks 1, 2, 3 and 4 and every 4 weeks thereafter. Placebo-treated patients were re-randomized to receive secukinumab 300 or 150 mg s.c. from week 16 (placebo non-responders) or week 24 (placebo responders). Exploratory endpoints at week 104 included 20, 50 and 70% improvement in ACR criteria (ACR20, 50, 70); 75 and 90% improvement in the Psoriasis Area Severity Index, 28-joint DAS with CRP, presence of dactylitis and enthesitis and other patient-reported outcomes. For binary variables, missing values were imputed; continuous variables were analysed by a mixed-effects model for repeated measures.
Results
A total of 86/100 (86%), 76/100 (76%) and 65/99 (66%) patients in the secukinumab 300, 150 and 75 mg groups, respectively, completed 104 weeks. At week 104, ACR20 response rates after multiple imputation in the 300, 150 and 75 mg groups were 69.4, 64.4 and 50.3%, respectively. Sustained clinical improvements were observed through week 104 with secukinumab across other clinically important domains of PsA. Responses were sustained through week 104 regardless of prior anti-TNF-α use. Over the entire treatment period the incidence, type and severity of adverse events were consistent with those reported previously.
Conclusion
Secukinumab provided sustained improvements in signs and symptoms and multiple clinical domains in patients of active PsA through 2 years of therapy. Secukinumab was well tolerated, with a safety profile consistent with that reported previously.
Trial registration
ClinicalTrials.gov (https://clinicaltrials.gov), NCT01752634
Keywords: spondyloarthritis, psoriatic arthritis, biological therapies, anti-TNF therapy, interleukin, long term, efficacy, safety, joints, swelling
Rheumatology key messages
Secukinumab provided rapid improvements sustained through 2 years in multiple clinical domains of PsA.
Clinical improvements with secukinumab were observed in PsA patients regardless of prior anti-TNF exposure.
Secukinumab was well tolerated in PsA, with a safety profile consistent with that reported previously.
Introduction
PsA is a chronic, immune-mediated, inflammatory disease characterized by peripheral and axial arthritis, dactylitis and enthesitis that affects ∼30% of psoriasis patients and comprises up to 1% of the general population [1–3].
Biologic therapies such as anti-TNF-α and anti-IL-17A antibodies are recommended for the treatment of PsA if an inadequate response is noted to first-line treatment with NSAIDs and/or DMARDs [4, 5]. TNF-α inhibitors improve the signs and symptoms of PsA, reduce structural damage and enhance patient-reported measures of quality of life (QoL) [6, 7]. However, not all patients respond to or tolerate anti-TNF-α therapy; in some patients, response is lost over time. There remains significant unmet clinical need in PsA [8, 9].
Secukinumab, a high-affinity, fully human IgG1 mAb that selectively binds to and neutralizes IL-17A, has shown efficacy in treating immune-mediated inflammatory diseases such as psoriasis [4, 10–15], AS and PsA [16–18].
In the FUTURE 1 study (NCT01392326), secukinumab, administered with i.v. loading and s.c. maintenance dosing, demonstrated a rapid onset of efficacy and was shown to be superior to placebo in the treatment of patients with PsA through multiple components of the disease, including the arthritis and skin signs and symptom measures, structural damage and physical function in a population comprised of 70% patients naive to anti-TNF-α and 30% patients who were inadequate responders or intolerant to anti-TNF-α (anti-TNF-α-IR) therapy. Long-term efficacy with secukinumab was also demonstrated in the study through the maintenance of 20, 50 and 70% improvement in ACR criteria (ACR20, 50, 70) up to week 104 accompanied by significant and sustained inhibition of joint structural damage based on radiographic data [19].
Secukinumab, given via s.c. self-administration loading and maintenance dosing, significantly and rapidly improved the signs and symptoms of PsA at week 24 sustained through week 52 in the primary analysis of the phase 3 FUTURE 2 study (NCT01752634). FUTURE 2 is the first large study to report that inhibiting IL-17A with s.c. secukinumab provides significant and sustained improvements in important clinical domains of PsA, including joint and skin symptoms, physical function and QoL. Importantly, clinical benefits were demonstrated in both anti-TNF-α-naive and anti-TNF-α-IR patients. The results highlighted the key role of IL-17A in the pathogenesis of PsA and confirmed the potential of secukinumab as a novel therapy for patients with this disease [18].
Here we provide data describing the efficacy and safety of secukinumab through 104 weeks in the FUTURE 2 study that demonstrate the long-term (2 year) efficacy and safety results of secukinumab in patients with active PsA despite current or previous NSAIDs, DMARDs and/or anti-TNF-α therapy.
Methods
Study design
FUTURE 2 is an ongoing, multicentre, randomized, double-blind, parallel-group, placebo-controlled study, the design of which has been published in detail [18]. In brief, patients with active PsA were randomized (1:1:1:1) to receive s.c. secukinumab (300, 150 or 75 mg) or placebo at baseline, weeks 1, 2, 3 and 4 and every 4 weeks thereafter. Placebo patients were re-randomized to receive secukinumab 300 or 150 mg s.c. every 4 weeks depending upon ACR20 response at week 16; patients were classified as responders (⩾20% improvement from baseline in tender and swollen joint counts) or non-responders, with non-responders switching at week 16 and responders at week 24. Randomization was stratified, as pre-specified, by anti-TNF-α status (anti-TNF-α-naive and anti-TNF-α-IR) with ∼40% of randomized patients planned to be anti-TNF-α-IR.
The study was conducted in accordance with the principles of the Declaration of Helsinki [20], International Conference of Harmonization Good Clinical Practice and all applicable laws and regulations. All centres received approval from an independent ethics committee or institutional review board. Patients provided written informed consent before the study-related procedures were undertaken.
Patients
Key inclusion criteria
Adult patients with active disease, who met the ClASsification criteria for Psoriatic ARthritis, despite previous treatment with NSAIDs, DMARDs and/or anti-TNF-α agents were included. Patients who had previously used up to three anti-TNF-α agents could enrol if they had an inadequate response or had stopped treatment because of safety or tolerability reasons.
Key exclusion criteria
Previous use of any biologic agent other than anti-TNF-α agents; active inflammatory diseases other than PsA; active infection 2 weeks before randomization or a history of ongoing, chronic or recurrent infections; history of malignant disease within the past 5 years (excluding basal cell carcinoma or actinic keratosis, in situ cervical cancer or non-invasive malignant colon polyps) and pregnancy were considered as exclusion criteria.
A detailed description of the patient inclusion and exclusion criteria has been reported previously [18].
Outcomes
The primary efficacy endpoint was the proportion of patients achieving ACR20 response at week 24. Secondary objectives included the efficacy of secukinumab versus placebo at week 24 in ACR50 response; 75 and 90% improvement in the Psoriasis Area Severity Index (PASI 75 and 90) in patients with psoriasis affecting ⩾3% of the body surface area (BSA); change from baseline in the 28-joint DAS including levels of CRP (DAS28-CRP) and the incidence of dactylitis and enthesitis. QoL was measured as the change from baseline in the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) physical component summary (PCS) score. Physical function was assessed as the change from baseline in the HAQ Disability Index (HAQ-DI) score. ACR70 response at week 24 was an exploratory endpoint [18]. For the 2 year analysis, exploratory analysis of all primary and secondary endpoints continued to week 104. Exploratory endpoints assessed at week 104 are based on patients originally randomized to secukinumab at the beginning of the trial and included ACR20/50/70, PASI 75/90, DAS28-CRP, SF-36 PCS, HAQ-DI and resolution of dactylitis and enthesitis. The other patient-reported outcomes (PROs) and QoL measures assessed at week 104 included patient global assessment of disease activity, patient assessment of PsA pain by visual analogue scale (VAS), SF-36 mental component summary (MCS), Work Productivity And Activity Impairment–General Health, Dermatology Life Quality Index, Functional Assessment of Chronic Illness Therapy–Fatigue and the European Quality of Life 5-Dimensions (EQ-5D) health status questionnaire. All PROs were analysed in the full analysis set that comprised all randomized patients to whom study treatment had been assigned and entered the long-term extension.
The overall safety and tolerability was assessed by monitoring the frequency of adverse events (AEs), abnormalities in laboratory findings, ECG findings and vital signs. Biochemical investigations were classified according to the Common Terminology Criteria for Adverse Events (version 4) [21]. Blood samples were collected at baseline, immediately before dosing at week 24 and at week 104 for the assessment of secukinumab immunogenicity using a Meso Scale Discovery bridging immunoassay (Meso Scale Diagnostics, Rockville, MD, USA) [22].
Statistical analysis
The details of sample size calculation and analysis of primary and other efficacy endpoints have been reported previously [18]. Briefly, a sample size of 100 patients per group was estimated to provide about 92% power to detect a treatment difference of 26% for the primary endpoint of ACR20 response at week 24 with Fisher’s exact test and about 80% power for secondary endpoints. In the current analysis, missing binary variables up to week 104 were imputed using multiple imputation. Summary statistics are based on observed and imputed data. Continuous variables were analysed using a mixed-effects model for repeated measures, with treatment group, assessment visit and anti-TNF-α response status as factors, weight and baseline score as continuous covariates and treatment by analysis visit as well as baseline score by analysis visit as interaction terms. Results are given in mean (s.d.) or least squares mean (s.e.), as appropriate. Evaluations of efficacy were performed on the full analysis set. Dactylitis and enthesitis, when multiple imputation was considered, were evaluated in the subgroup of patients with these symptoms at baseline. PASI assessment was evaluated in the subgroup with at least 3% of the BSA affected by psoriatic skin involvement. Predefined subgroup analyses were carried out on the basis of previous anti-TNF-α therapy and concomitant MTX treatment for key efficacy endpoints. The safety analysis included all patients who received one or more doses of secukinumab and data presented as exposure-adjusted incidence rates (EAIRs) per 100 patient-years.
Results
Patients
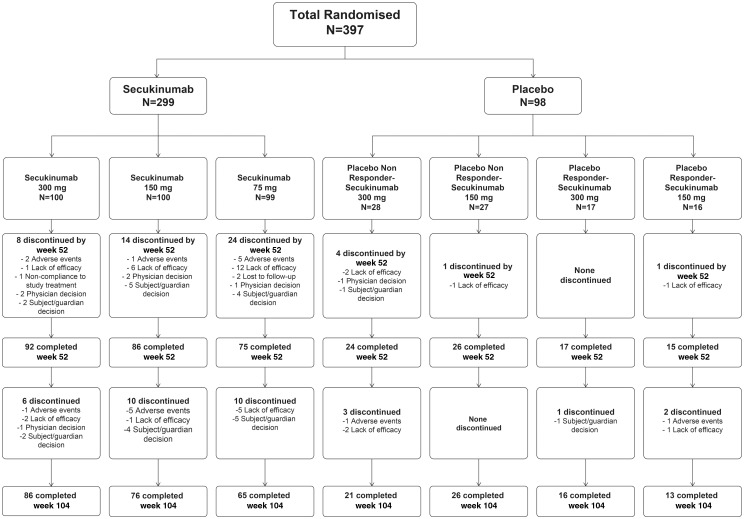
Of the 397 patients randomized overall, 335 (84%) completed 52 weeks of study. Eighty-six (86%), 76 (76%) and 65 (66%) patients in the secukinumab 300, 150 and 75 mg groups, respectively, completed 104 weeks of treatment (Fig. 1).
Fig. 1.
Patient flow through the study from randomization to week 104
All patients who were randomly allocated to treatment were included in the analysis of efficacy parameters at weeks 24, 52 and 104.
At baseline, demographic and disease characteristics were similar across groups: 184 (46%) of 397 randomized patients had psoriasis on at least 3% of their BSA, 258 (65%) were anti-TNF-α-naive and 185 (47%) and 81 (20%) received concomitant MTX and systemic glucocorticoid, respectively [18].
Discontinuation of study treatment
Of 299 patients originally randomized to secukinumab, 14/100 (14%), 24/100 (24%) and 34/99 (34%) patients discontinued the study treatment by week 104 in the secukinumab 300, 150 and 75 mg groups, respectively, of which 8 (8%), 14 (14%) and 24 (24%) patients, respectively, discontinued treatment by week 52. At week 104, 3/100 (3%), 7/100 (7%) and 17/99 (17%) patients had discontinued the treatment citing lack of efficacy, which was the most common cause of discontinuation in the secukinumab 300, 150 and 75 mg groups, respectively. In these three groups, 3/100 (3%), 6/100 (6%) and 5/99 (5%) patients discontinued due to an AE in the secukinumab 300, 150 and 75 mg groups, respectively.
Efficacy
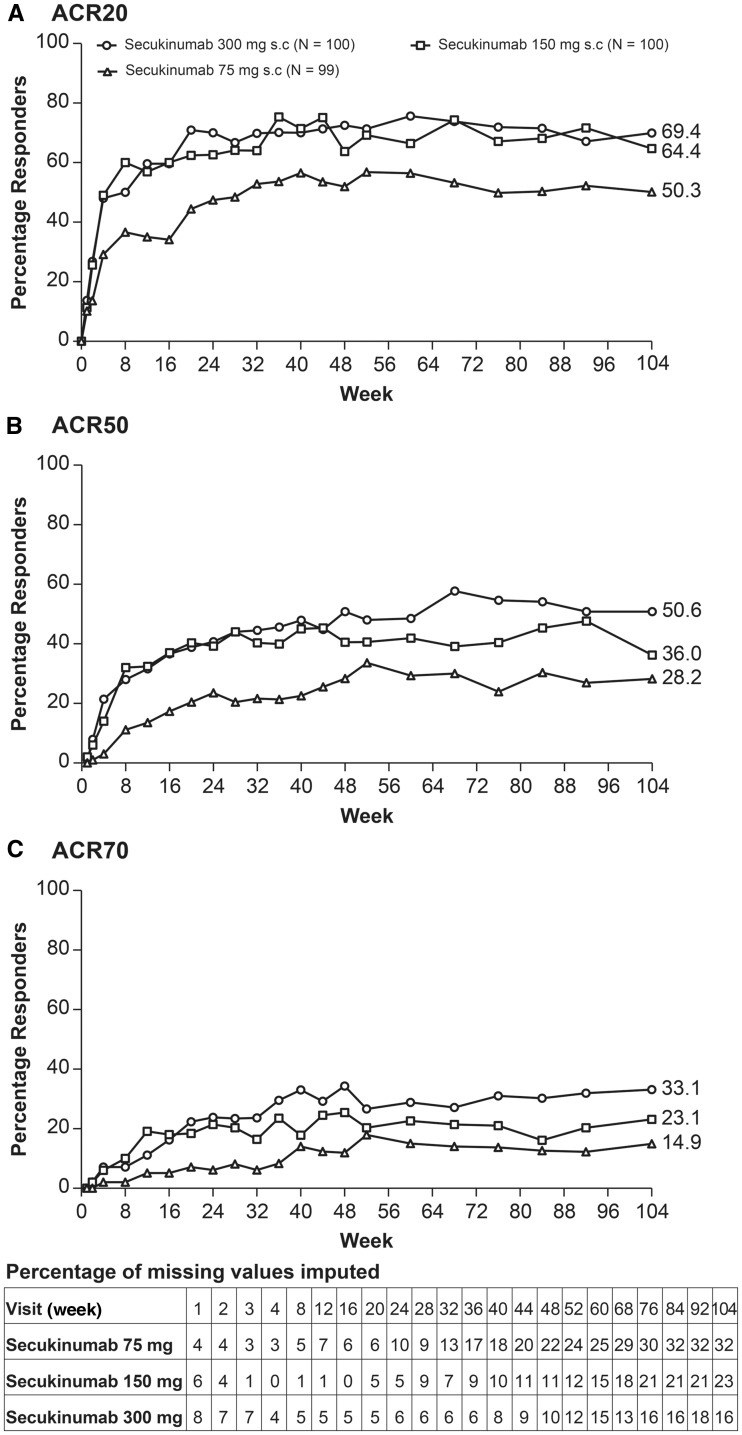
At week 104, ACR20 response rates in the secukinumab 300, 150 and 75 mg groups were 69.4, 64.4 and 50.3%, respectively (Fig. 2A). ACR50/70 response rates at week 104 in the secukinumab 300, 150 and 75 mg groups were 50.6%/33.1%, 36%/23.1% and 28.2%/14.9%, respectively (Fig. 2B and C). At week 104, PASI 75/90 response rates in the secukinumab 300, 150 and 75 mg groups were 79.5%/69.6%, 73.3%/52.5% and 58.4%/33.7%, respectively (Table 1). Secukinumab demonstrated efficacy across multiple clinically relevant domains of PsA outcomes, including DAS28-CRP, resolution of dactylitis and enthesitis and improvement in SF-36 PCS and HAQ-DI scores sustained through week 104 (Table 1).
Fig. 2.
ACR20, ACR50 and ACR70 response rates up to week 104
Data summaries until week 104 are based on multiple imputation as applied to missing variables. ACR20: at least 20% improvement in the ACR response criteria; ACR50: at least 50% improvement in the ACR response criteria; ACR70: at least 70% improvement in the ACR response criteria.
Table 1.
Summary of efficacy results at week 104
| Efficacy endpoint | Missing values considered | Observed data | ||||
|---|---|---|---|---|---|---|
| Secukinumab 300 mg | Secukinumab 150 mg | Secukinumab 75 mg | Secukinumab 300 mg | Secukinumab 150 mg | Secukinumab 75 mg | |
| Patients randomized to secukinumab, n | 100 | 100 | 99 | 100 | 100 | 99 |
| ACR response | ||||||
| Overall population | ||||||
| Patients, n | 100 | 100 | 99 | 84 | 77 | 67 |
| ACR20 response, % | 69.4 | 64.4 | 50.3 | 73.8 | 72.7 | 62.7 |
| ACR50 response, % | 50.6 | 36.0 | 28.2 | 56.0 | 42.9 | 37.3 |
| ACR70 response, % | 33.1 | 23.1 | 14.9 | 38.1 | 28.6 | 20.9 |
| Anti-TNF-α-naive | ||||||
| Patients, n | 67 | 63 | 65 | 56 | 53 | 51 |
| ACR20 response, % | 74.8 | 79.3 | 62.7 | 80.4 | 86.8 | 68.6 |
| ACR50 response, % | 58.5 | 46.1 | 38.0 | 66.1 | 50.9 | 43.1 |
| ACR70 response, % | 39.5 | 30.1 | 21.2 | 46.4 | 34.0 | 25.5 |
| Anti-TNF-α-IR | ||||||
| Patients, n | 33 | 37 | 34 | 28 | 24 | 16 |
| ACR20 response, % | 58.4 | 38.9 | 26.3 | 60.7 | 41.7 | 43.8 |
| ACR50 response, % | 34.1 | 18.9 | 9.4 | 35.7 | 25.0 | 18.8 |
| ACR70 response, % | 19.7 | 11.3 | 3.0 | 21.4 | 16.7 | 6.3 |
| PASI responsea | ||||||
| Patients, n | 41 | 58 | 50 | 36 | 47 | 38 |
| PASI 75 response, % | 79.5 | 73.3 | 58.4 | 80.6 | 78.7 | 63.2 |
| PASI 90 response, % | 69.6 | 52.5 | 33.7 | 72.2 | 59.6 | 34.2 |
| DAS28-CRP | ||||||
| Patients, n | 100 | 100 | 99 | 83 | 77 | 66 |
| Change from baselineb | –1.9 (0.1) | –1.7 (0.1) | –1.5 (0.1) | –2.0 (1.2) | –1.8 (1.1) | –1.7 (1.4) |
| Dactylitis resolutionc | 46 | 32 | 33 | 87 | 77 | 68 |
| Resolution rate, % | 79.9 | 78.0 | 88.6 | 88.5 | 92.2 | 95.6 |
| Enthesitis resolutionc | 56 | 64 | 68 | 87 | 77 | 68 |
| Resolution rate, % | 71.5 | 61.8 | 68.4 | 77.0 | 70.1 | 69.1 |
| SF-36 PCS | ||||||
| Patients, n | 100 | 100 | 99 | 85 | 79 | 66 |
| Change from baselineb | 6.8 (0.9) | 5.0 (0.9) | 4.1 (0.9) | 7.4 (9.4) | 5.6 (8.0) | 5.6 (8.4) |
| HAQ-DI | ||||||
| Patients, n | 100 | 100 | 99 | 86 | 77 | 67 |
| Change from baselineb | –0.6 (0.1) | –0.5 (0.1) | –0.3 (0.1) | –0.6 (0.6) | –0.6 (0.5) | –0.3 (0.6) |
Evaluated in subgroup with >3% of BSA affected by psoriatic skin involvement.
Mean (s.d.) (observed data) or least squares mean (s.e.) (mixed-effects model for repeated measures estimates).
Evaluated in the subgroup with these symptoms at baseline.
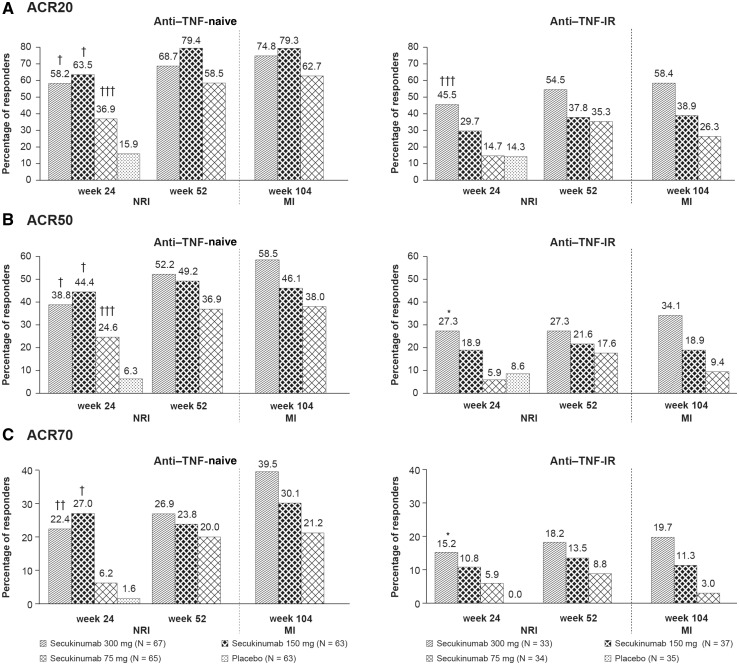
Clinical responses with secukinumab were sustained through week 104 in anti-TNF-α-naive patients and anti-TNF-α-IR. Greater ACR20/50/70 response rates were seen in TNF-α-IR patients receiving higher secukinumab doses. ACR20 response rates at week 104 in anti-TNF-α-naive patients were 74.8, 79.3 and 62.7% with secukinumab 300, 150 and 75 mg, respectively; corresponding rates in anti-TNF-α-IR patients were 58.4, 38.9 and 26.3%, respectively (Fig. 3). ACR20 response rates at week 104 in concomitant MTX patients were 70.6, 69.6 and 54.1% with 300, 150 and 75 mg, respectively; corresponding rates in patients not taking MTX were 68.5, 59.8 and 45.8%, respectively (Fig. 4).
Fig. 3.
ACR20, 50 and 70 response rates by baseline TNF status at weeks 24, 52 and 104
† P < 0.0001; ††P < 0.001; †††P < 0.01; *P < 0.05. P-values at week 24 derived from a logistic regression model with treatment as the factor and baseline weight as a covariate. Missing data were imputed as non-response until week 52. Data until week 52 were reported previously [18]. Data for week 104 are after multiple imputation applied to missing variables. ACR20: at least 20% improvement in the ACR response criteria; ACR50: at least 50% improvement in the ACR response criteria; ACR70: at least 70% improvement in the ACR response criteria; MI: multiple imputation; NRI: non-responder imputation.
Fig. 4.
ACR20, ACR50 and ACR70 response rates by baseline MTX use at weeks 24, 52 and 104
†P < 0.0001; ††P < 0.001; †††P < 0.01; *P < 0.05. P-values at week 24 derived from a logistic regression model with treatment as the factor and baseline weight as a covariate. Missing data were imputed as non-response until week 52. Data until week 52 are reported previously [18]. Data for week 104 is after multiple imputation applied to missing variable. ACR20: at least 20% improvement in the ACR response criteria; ACR50: at least 50% improvement in the ACR response criteria; ACR70: at least 70% improvement in the ACR response criteria; MI: multiple imputation; NRI: non-responder imputation.
PROs and QoL
The HAQ-DI scores and SF-36 PCS scores improved significantly in the secukinumab dose groups vs placebo at week 24 [18]; improvements were sustained in the secukinumab dose groups up to week 104 (Table 1). For the SF-36 MCS scores, improvements from baseline at week 24 were better in the secukinumab dose groups vs placebo; further improvements were observed at week 104 with secukinumab (supplementary Table S1, available at Rheumatology Online).
Least squares mean changes from baseline in patient global assessment of disease activity and patient assessment of PsA pain by VAS were higher in the secukinumab dose groups compared with placebo at week 24 and improved further at week 104 with secukinumab doses. Secukinumab resulted in better improvements from baseline in PsA QoL, Work Productivity And Activity Impairment–General Health, Dermatology Life Quality Index, Functional Assessment of Chronic Illness Therapy–Fatigue and EQ-5D scores at week 24 compared with placebo. Sustained or further improvements in these scores were observed with secukinumab at week 104. In patients who were employed, the decrease from baseline at week 24 in the percentage work time missed and work impairment due to health was higher with secukinumab doses vs placebo (supplementary Table S1, available at Rheumatology Online).
Safety
Over the treatment period [mean (s.d.) exposure to secukinumab of 709 (211.0) days] the incidence, type and severity of AEs were consistent with those reported previously by week 52 [19]. Over the entire treatment period, the EAIRs of AEs were 163.3, 181.2 and 159.2 per 100 patient-years for patients who received at least 1 dose of secukinumab 300, 150 and 75 mg, respectively. Discontinuations due to AEs in these three secukinumab dose groups occurred at rates of 3.4, 5.6 and 5.1%, respectively. No deaths were reported in the study. In particular, no case of suicide or suicidal ideation was reported in secukinumab-treated patients.
Infections and infestations were the most commonly reported AE with secukinumab, with an EAIR of 65.0/100 patient-years across the three dosage groups. The most common AEs by preferred term were upper respiratory tract infection (13.6/100 patient-years) and nasopharyngitis (12.6/100 patient-years). AEs were mostly mild or moderate in severity. The EAIRs of serious AEs were 7.0, 5.6 and 7.7 per 100 patient-years for patients who received at least one dose of secukinumab 300, 150 and 75 mg, respectively (Table 2).
Table 2.
Summary of safety data at week 104
| Variable | Secukinumab 300 mg (n = 145) | Secukinumab 150 mg (n = 143) | Secukinumab 75 mg (n = 99) | Any secukinumab (n = 387) |
|---|---|---|---|---|
| Duration of exposure, days, mean (s.d.) | 726.0 (190.1) | 715.5 (196.0) | 674.8 (254.8) | 709.0 (211.0) |
| Exposure, patient-years | 288.2 | 280.1 | 182.9 | 751.3 |
| EAIR/100 patient-years, n | ||||
| Any AE | 127 (163.3) | 126 (181.2) | 84 (159.2) | 337 (168.5) |
| Any SAEs | 19 (7.0) | 15 (5.6) | 13 (7.7) | 47 (6.6) |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Discontinuation due to AEsa | 5 (3.4) | 8 (5.6) | 5 (5.1) | 18 (4.7) |
| Common AEs, n (EAIR/100 patient-years)b | ||||
| Upper respiratory tract infection | 33 (13.4) | 30 (12.7) | 23 (15.6) | 86 (13.6) |
| Nasopharyngitis | 28 (11.3) | 33 (13.7) | 21 (13.1) | 82 (12.6) |
| Diarrhoea | 12 (4.4) | 13 (4.9) | 11 (6.3) | 36 (5.0) |
| Headache | 10 (3.7) | 15 (5.7) | 5 (2.9) | 30 (4.2) |
| Nausea | 9 (3.2) | 12 (4.5) | 7 (4.0) | 28 (3.9) |
| Urinary tract infection | 10 (3.6) | 12 (4.5) | 6 (3.4) | 28 (3.9) |
| Vomiting | 7 (2.5) | 7 (2.6) | 4 (2.3) | 18 (2.5) |
| AEs of special interest, n (EAIR/100 patient-years) | ||||
| Serious infections | 6 (2.1) | 5 (1.8) | 1 (0.6) | 12 (1.6) |
| Candida infections | 8 (2.9) | 8 (2.9) | 1 (0.5) | 17 (2.3) |
| Ulcerative colitis | 1 (0.3) | 1 (0.4) | 0 (0.0) | 2 (0.3) |
| Neutropenia | 1 (0.3) | 0 | 0 | 1 (0.1) |
| Malignancy/unspecified tumour | 1 (0.3) | 6 (2.2) | 3 (1.7) | 10 (1.3) |
| MACE | 1 (0.3) | 0 (0.0) | 1 (0.6) | 2 (0.3) |
EAIR was not calculated for study drug discontinuations due to AEs; percentages are shown instead.
AEs with incidence of at least 2.0 cases per 100 patient-years in the combined secukinumab group during the entire treatment period.
In the study cohort of secukinumab-treated patients, the EAIRs for serious infections, Candida infections, IBD, malignant/unspecified tumours and major adverse cardiac events (MACEs) were 1.6, 2.3, 0.5, 1.3 and 0.3, respectively. No new cases of tuberculosis were reported and no reactivation of latent tuberculosis occurred. Non-serious Candida infections over the entire treatment period were reported for 17 (4.4%) patients across the three secukinumab dose groups. These included 10 patients with oral candidiasis, 5 patients with vulvovaginal candidiasis and 1 patient each with oesophageal Candida and intertrigo Candida. All cases of Candida were mild or moderate in severity and resolved with antifungal treatment and all patients continued in the study.
Crohn’s disease was not reported as an AE in any patient during the entire treatment period. Two cases of ulcerative colitis (one each in the 300 and 150 mg groups) and one case each of haemorrhagic diarrhoea (300 mg group) and anal fistula (300 mg group) were reported with secukinumab. Of the two cases of ulcerative colitis reported with secukinumab, one was a flare in a patient with prior history and the other was a new onset. In the secukinumab groups over the entire treatment period, four cases of basal cell carcinoma, three cases of squamous cell carcinoma and one case each of Bowen’s disease, squamous cell carcinoma of the tongue and throat cancer were reported. No other malignancies were reported in the entire treatment period. One adjudicated MACE was reported during the 24 week analysis (myocardial infarction) in a patient on secukinumab 75 mg with a history of sinus tachycardia, ongoing hypertension and hyperlipidaemia. A case of haemorrhagic stroke was reported after the 24 week analysis in a patient on secukinumab 300 mg and was adjudicated as a MACE. One case of uveitis was reported in a patient randomized to secukinumab 300 mg, which was moderate in severity and the patient recovered without any intervention. No changes were necessitated to the study treatment regimen in this patient.
Transient Common Terminology Criteria for AEs grade 3 neutropenia occurred in two patients (300 mg group) over the entire treatment period. No patient withdrew from the study because of neutropenia. Treatment-emergent anti-secukinumab antibodies were detected in three patients, two patients originally allocated to secukinumab 150 mg and one originally allocated to placebo and switched to secukinumab 150 mg at week 16. Immunogenicity-related AEs or loss of efficacy were not reported in these patients.
Discussion
In phase 3 trials, secukinumab showed rapid-onset efficacy in the treatment of psoriasis and PsA [4, 18]. The two pivotal phase 3 studies investigating secukinumab in PsA, namely, FUTUREs 1 and 2, suggested that secukinumab is associated with a wide range of clinical benefits across outcome domains.
Whereas the primary results of the FUTURE 2 study showed that secukinumab was efficacious in patients with PsA, the current dataset demonstrates that the reported improvements in clinical outcomes with secukinumab in PsA were either sustained or further improved through 104 weeks of therapy in those patients remaining in the study. Secukinumab showed efficacy across multiple clinically relevant domains of PsA, including PASI 75/90 response rates, DAS28-CRP, resolution of dactylitis and enthesitis, SF-36 PCS and HAQ-DI score through week 104, consistent with the clinical benefits reported previously at weeks 24 and 52 [18]. The notable responses in PASI score and enthesitis are consistent with the proposed relationship of the IL-23/17 pathway to the skin–enthesial axis [23].
Discontinuations were low with secukinumab and sustainability on the study drug over the long term was notable in the present study, particularly in the 300 and 150 mg groups (86 and 76%, respectively), which is likely a reflection of the efficacy and tolerability of secukinumab.
The clinical responses with secukinumab were noted in both anti-TNF-α-naive and anti-TNF-α-IR patients, with responses generally greater in the anti-TNF-α-naive population for all doses of secukinumab. The response rates were markedly higher in the secukinumab 300 mg group compared with the 150 and 75 mg groups in the anti-TNF-α-IR patients, suggesting a dose response in these patients. However, the study was not powered to detect statistically significant differences between the doses. Improvements with secukinumab through 104 weeks were similar in both concomitant MTX and without MTX subgroups.
PsA places a substantial burden on patients, diminishing their capacity to carry out daily activities and reducing their health-related QoL. Therapies that improve these aspects of disease have the potential to reduce the broad burden of PsA and also reduce its impact on work productivity. Consistent with observations in other similar studies [24–26], patients in the FUTURE 2 study had impaired physical function, work productivity and health-related QoL at baseline. In this study, at week 24, secukinumab demonstrated clinically meaningful improvements in PROs, including global disease activity, pain, physical function, fatigue and generic and disease-specific measures of health-related QoL in patients with active PsA. The improvements in PRO scores with secukinumab were maintained or improved further at week 104.
Sustained improvements in joint and skin symptoms, physical function and QoL of patients with PsA observed after treatment with secukinumab 300 and 150 mg through 2 years suggest the potential for selection of IL-17 blockade in discrete phenotypes of PsA, such as patients with dominant enthesitis or significant concomitant psoriasis. Such possibilities should now be subject to investigation. These longer-term efficacy data are important, as they suggest the potential for expansion of the treatment armament for PsA.
The safety profile of secukinumab was consistent with that in previous reports on PsA [18] and psoriasis [4]. The types, severity, nature and incidence of AEs with secukinumab at week 104 were similar to those reported for the placebo-controlled period and at week 52 [19], with no apparent relation to the dose. The overall incidence of SAEs was low and did not show dose dependence across secukinumab dose groups. The rate of discontinuation because of AEs with secukinumab was low and no deaths occurred during the study. Immunogenicity with secukinumab was low and was not associated with a loss of efficacy or immunogenicity-related AEs.
The lack of a long-term comparator arm was a limitation of the study, although continuation of a placebo arm in the long term would be unethical. Statistical analysis of the data obtained at week 24 differed from that at weeks 52 and 104 since all patients originally randomized to placebo had switched to secukinumab by week 24.
In summary, secukinumab provided sustained improvements at 2 years across multiple clinical domains, including joint and skin, dactylitis and enthesitis, improved physical functioning and QoL in patients with active PsA. The safety profile of secukinumab showed no new or unexpected safety signals through 2 years of treatment.
Supplementary Material
Acknowledgements
The authors thank the patients who participated in the study, the study investigators and John Gallagher, a medical consultant for Novartis Pharma. The first draft of this manuscript was written by M.K. Vivek Sanker (Novartis, India) based on input from all the authors. The work on this manuscript was completed on behalf of the FUTURE 2 study group.
Funding: This work was supported by Novartis Pharma, Basel, Switzerland.
Disclosure statement: C.T.R. has received research grants, consultation fees or speaker honoraria from Amgen, UCB, AbbVie, Novartis and Janssen. I.B.M. has received research grants, consultation fees or speaker honoraria from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer and UCB. A.B.G. currently has consulting/advisory board agreements with Amgen, Astellas, Akros, Centocor (Janssen), Celgene, Bristol-Myers Squibb, Beiersdorf, Abbott Labs (AbbVie), TEVA, Actelion, UCB, Novo Nordisk, Novartis, Dermipsor, Incyte, Pfizer, Canfite, Lilly, Coronado, Vertex, Karyopharm, CSL Behring Biotherapies for Life, GlaxoSmithKline, Xenoport, Catabasis, Meiji Seika Pharma, Takeda, Mitsubishi, Tanabe Pharma Development America, Genentech, Baxalta, Kineta One, KPI Therapeutics, Crescendo Bioscience, Aclaris, Amicus, Reddy Labs, Valeant, Dermira and Allergan and has received research/educational grants from Centocor (Janssen), Amgen, Abbott (AbbVie), Novartis, Celgene, Pfizer, Lilly, Coronado, Levia, Merck, Dermira, Baxalta and Xenoport paid to Tufts Medical Center up to 5 November 2016, none thereafter. E.-M.D. is an employee of Novartis. L.P., S.M. and R.K. are employees of Novartis with Novartis stock. P.J.M. has received grant/research support from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma and UCB; consulted for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Crescendo Bioscience, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma and UCB and been on the speakers bureau for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer and UCB. B.K. has received research grants, consultation fees or speaker honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Lilly, MSD, Novartis, Roche and UCB. P.R. has received consulting fees from Abbott, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Janssen, Lilly, Novartis, Pfizer and Roche.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Zachariae H. Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 2003;4:441–7. [DOI] [PubMed] [Google Scholar]
- 2. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P.. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005;64(Suppl 2):ii14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehncke WH, Menter A.. Burden of disease: psoriasis and psoriatic arthritis. Am J Clin Dermatol 2013;14:377–88. [DOI] [PubMed] [Google Scholar]
- 4. Langley RG, Elewski BE, Lebwohl M. et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med 2014;371:326–38. [DOI] [PubMed] [Google Scholar]
- 5. Ash Z, Gaujoux-Viala C, Gossec L. et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. [DOI] [PubMed] [Google Scholar]
- 6. Saad AA, Symmons DP, Noyce PR, Ashcroft DM.. Risks and benefits of tumor necrosis factor-alpha inhibitors in the management of psoriatic arthritis: systematic review and metaanalysis of randomized controlled trials. J Rheumatol 2008;35:883–90. [PubMed] [Google Scholar]
- 7. Carneiro S, Azevedo VF, Bonfiglioli R. et al. Recommendations for the management and treatment of psoriatic arthritis. Rev Bras Reumatol 2013;53:227–41. [PubMed] [Google Scholar]
- 8. Golmia RP, Martins AH, Scheinberg M.. When anti-TNF fails, anti-IL12-23 is an alternate option in psoriasis and psoriatic arthritis. Rev Bras Reumatol 2014;54:247–9. [PubMed] [Google Scholar]
- 9. Fagerli KM, Lie E, van der Heijde D. et al. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis 2013;72:1840–4. [DOI] [PubMed] [Google Scholar]
- 10. Thaci D, Blauvelt A, Reich K. et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015;73:400–9. [DOI] [PubMed] [Google Scholar]
- 11. Gottlieb AB, Langley RG, Philipp S. et al. Secukinumab improves physical function in subjects with plaque psoriasis and psoriatic arthritis: results from two randomized, phase 3 trials. J Drugs Dermatol 2015;14:821–33. [PubMed] [Google Scholar]
- 12. Baeten D, Sieper J, Braun J. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015;373:2534–48. [DOI] [PubMed] [Google Scholar]
- 13. Kavanaugh A, McInnes IB, Mease PJ. et al. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. J Rheumatol 2016;43:1713–7. [DOI] [PubMed] [Google Scholar]
- 14. Strand V, Mease P, Gossec L. et al. Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (FUTURE 1). Ann Rheum Dis 2017;76:203–7. [DOI] [PubMed] [Google Scholar]
- 15. van der Heijde D, Landewe RB, Mease PJ. et al. Brief report: secukinumab provides significant and sustained inhibition of joint structural damage in a phase III study of active psoriatic arthritis. Arthritis Rheumatol 2016;68:1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mease PJ, McInnes IB, Kirkham B. et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med 2015;373:1329–39. [DOI] [PubMed] [Google Scholar]
- 17. McInnes IB, Sieper J, Braun J. et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 2014;73:349–56. [DOI] [PubMed] [Google Scholar]
- 18. McInnes IB, Mease PJ, Kirkham B. et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015;386:1137–46. [DOI] [PubMed] [Google Scholar]
- 19. Kavanaugh A, Mease PJ, Reimold AM. et al. Secukinumab for long-term treatment of psoriatic arthritis: 2-year follow-up from a phase 3, randomized, double-blind, placebo-controlled study. Arthritis Care Res 2017;69:347–55. [DOI] [PubMed] [Google Scholar]
- 20. General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 21.National Cancer Institute. Enterprise Vocabulary Services. https://evs.nci.nih.gov/ (8 February 2017, date last accessed).
- 22. Klein U, Liang E, Vogel B. et al. SAT0142 immunogenicity of the novel anti-Il-17A antibody, secukinumab, with intravenous and subcutaneous dosing regimens in healthy subjects and patients. Ann Rheum Dis 2013;72(Suppl 3):A630. [Google Scholar]
- 23. Sakkas LI, Bogdanos DP.. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 2017;16:10–15. [DOI] [PubMed] [Google Scholar]
- 24. Strand V, Schett G, Hu C, Stevens RM.. Patient-reported health-related quality of life with apremilast for psoriatic arthritis: a phase II, randomized, controlled study. J Rheumatol 2013;40:1158–65. [DOI] [PubMed] [Google Scholar]
- 25. Wallenius M, Skomsvoll JF, Koldingsnes W. et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis 2009;68:685–9. [DOI] [PubMed] [Google Scholar]
- 26. Zachariae H, Zachariae R, Blomqvist K. et al. Quality of life and prevalence of arthritis reported by 5,795 members of the Nordic Psoriasis Associations. Data from the Nordic Quality of Life Study. Acta Derm Venereol 2002;82:108–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.