Abstract
Dynamic simulation models provide vector abundance estimates using only meteorological data. However, model outcomes may heavily depend on the assumptions used to parameterize them. We conducted a sensitivity analysis for a model of Aedes aegypti (L.) abundance using weather data from two locations where this vector is established, La Margarita, Puerto Rico and Tucson, Arizona. We tested the effect of simplifying temperature-dependent development and mortality rates and of changing development and mortality thresholds as compared with baselines estimated using biophysical models. The simplified development and mortality rates had limited effect on abundance estimates in either location. However, in Tucson, where the vector is established but has not transmitted viruses, a difference of 5 °C resulted in populations either surviving or collapsing in the hot Arizona mid-summer, depending on the temperature thresholds. We find three important implications of the observed sensitivity to temperature thresholds. First, this analysis indicates the need for better estimates of the temperature tolerance thresholds to refine entomologic risk mapping for disease vectors. Second, our results highlight the importance of extreme temperatures on vector survival at the marginal areas of this vector’s distribution. Finally, the model suggests that adaptation to warmer temperatures may shift regions of pathogen transmission.
Keywords: Aedes aegypti, climate, temperature threshold, population dynamics
Aedes aegypti (L.) is one of the primary vectors for (re)emerging arboviruses causing diseases like dengue, chikungunya, and Zika. To estimate the risk of disease transmission occurring in new areas, presence of a disease vector is necessary. Moreover, the vector must be present in sufficient abundance, survive long enough to become infected, and and have enough human contact to successfully transmit virus. When coupled with human travel information, entomologic risk is helpful in the early stages of an outbreak to quickly delineate the regions where disease transmission may occur. Such approaches may rely on the presence of the vector (Khan et al. 2014) or on more sophisticated models of vector abundance (Bogoch et al. 2016, Monaghan et al. 2016). However, incomplete understanding of the interactions and susceptibilities of vectors, hosts, and pathogen continues to hamper the development of early warning systems (Louis et al. 2014).
Entomologic risk, with respect to modeling disease transmission, involves understanding both the seasonal population dynamics (because vectors must be sufficiently abundant for hosts to incur an infectious bite) and adult female longevity (because they must live long enough to become infectious and successfully find another host). Previous sensitivity analyses have shown that survival thresholds associated with temperature extremes were most influential in patterns of Ae. aegypti population dynamics and dengue transmission (Xu et al. 2010, Ellis et al. 2011). Specifically, temperature may influence the survival of adult females beyond the extrinsic incubation period (Brady et al. 2013). However, the implication of these temperature thresholds has not been evaluated in regions along the periphery of the vector’s established range.
With summer high temperatures exceeding 38°C and winter low temperatures <4 °C, Tucson, Arizona provides a testing ground for the influence of extreme temperatures on a model of Ae. aegypti abundance. The vector is established but the viruses transmitted by this vector are not (Fink et al. 1998). This situation differs from La Margarita, Puerto Rico, where the vector is present year-round due to temperature and precipitation patterns (Barrera et al. 2011), and dengue is endemic (Barrera 2010), with recent outbreaks of chikungunya and Zika (Sharp et al. 2014, Adams et al. 2016). Herein, we conduct a sensitivity analysis to evaluate the effect of temperature on the predictions of an Ae. aegypti abundance model in these two climates. We assess how the estimated vector abundance changes when simplifying modeling assumptions and which factors, e.g., temperature thresholds, development and mortality rates, are most influential.
Materials and Methods
Study Area
We compare modeled vector abundance between two locations where Ae. aegypti are established. Weather data at a single point for each location for the study period (3 October 2011–18 November 2014) were derived from a HOBO meteorological station (HOBO Data Loggers, Onset Computer Corporation, Boume, MA) in La Margarita, a tropical environment in southern Puerto Rico (17.9716667°, −66.3027778°), and from the National Climatic Data Center for Tucson, an arid city in southern Arizona (32.221667°, −110.9263389°). Over the 2 yr of the study period, for which we have a complete year’s weather data (2012 and 2013), the median monthly total precipitation in La Margarita was 41.52 cm (range 2.27–199.4 cm) and an average mean temperature of 27.2°C (maximum high temperature recorded was 35.5 °C and minimum low temperature was 18.6 °C), whereas Tucson experienced a median monthly total of 0.86 cm (range 0–10.5 cm) of precipitation and an average mean temperature of 21.8 °C (maximum high temperature recorded was 44.4 °C and minimum low temperature was −8.3 °C).
Model Description
We used DyMSiM, an established mosquito abundance model that uses daily temperature and precipitation data to simulate the development of individual mosquitoes from egg to adult, yielding a daily estimated adult mosquito population for a given location (Morin and Comrie 2010, Brown et al. 2015, Morin et al. 2015). Our version of DyMSiM is implemented in MATLAB R2013b (MathWorks, Natick, MA) and was parameterized for Ae. aegypti using specific temperature-dependent development and mortality rates, as well as minimum and maximum temperature thresholds for each immature and adult stage, obtained from published empirical studies (Otero et al. 2006, Eisen et al. 2014).
Because the model has a stochastic component (random events include, e.g., whether a specific mosquito survives until the next day or whether a specific gravid female lays eggs), different simulations with identical initial conditions and climate input lead to different daily estimates but to consistent seasonal trends. The standard deviation of daily predictions from individual runs changes as a function of time and location. On average, it is about 13% of the mean in PR and 26% of the mean in Tucson (this latter number increases to 49% when values for which the mean is near zero are included). To obtain a low-variability estimation of the expected abundance on any given day, we therefore average the output of the code over a fixed number of simulations (set to N = 30; see Model Optimization in the Results section for details).
Analysis
For each life stage, the development and mortality rates, and the temperature thresholds for development and survival were changed serially (one value changed per life stage and rate per threshold combination), and then concurrently across all stages. For the development and mortality rates, we tested whether a simplified model (a uniform or a linear dependence on temperature, Table 1) would significantly alter the estimates from current biophysical models (Otero et al. 2006). For the development and survival thresholds, increments of 5 °C above and below the maximum and minimum thresholds were individually tested by life stage and then tested concurrently across all life stages. The value of the increment (5 °C) is about 10% of the annual temperature range in Tucson and about a third of the annual temperature range in Puerto Rico, and is therefore, significant in both areas. Temperature tolerance thresholds were derived from a recent review of Ae. aegypti bionomics (Eisen et al. 2014).
Table 1.
The linear development rates including the temperature range for which the formula was applied for each life stage
| Range (°C) | Formula | R2 | |
|---|---|---|---|
| Egg | 5–49 | 0.0171*T − 0.102 | 0.90 |
| IS1 | 4.7–30 | 0.0379*T − 0.1908 | 0.89 |
| 30–38 | −0.0784*T + 3.3396 | 0.98 | |
| IS2 | 4.7–28 | 0.0575*T − 0.5439 | 0.82 |
| 28–39 | −0.1166*T + 4.6635 | 0.99 | |
| IS3 | 4.7–30 | 0.0497*T − 0.5012 | 0.85 |
| 30–39 | −0.0544*T + 2.8747 | 0.96 | |
| IS4 | 4.7–33 | 0.0258*T − 0.2472 | 0.92 |
| 33–39 | −0.0118*T + 1.0303 | 0.94 | |
| Pupal | 17–39 | 0.0345*T − 0.3252 | 0.88 |
The coefficient of determination (R2) is reported for the linear fit to the empirical data.
Daily estimated abundance for baseline (using original parameters) and adjusted models were compared visually, and root mean square error (RMSE) was calculated by comparing the daily difference between baseline and adjusted model. Root mean square error should be low when the estimated daily mosquito abundance between simulations are close. Calculation of RMSE and graphing was performed in Excel 2010 (Microsoft, Redmond, WA).
Results
Model Optimization
Averaging over 30 independent runs resulted in stable sample mean estimates of the expected value (mean) of predicted daily mosquito abundance. Specifically, the variability of these estimates, measured as the time average of the daily standard deviation scaled to the daily mean, was 2.3% in PR and 4.7% in Tucson. The Tucson average is only over values for which the mean is significant; including winter estimates, when the mean abundance is near zero, increases the average variability to 8.5% (see panel B of each figure below). Changing the coefficient for the environmental suitability of immature habitat had little effect on the dynamics of the abundance but, as expected given the nature of this parameter, scaled the number of mosquitoes predicted. Thus, we set the environmental suitability at 1 for all analyses.
Mean Development Rates
First, we evaluated the effect of using the average development rate from egg to adult rather than stage-specific development rates (Otero et al. 2006). Then, stage-specific development rates were replaced with their average calculated between 5°C and 38 °C. These temperature values were selected to capture the widest range of temperature-dependent development data. This was performed for each individual life stage separately, and then concurrently for all life stages together.
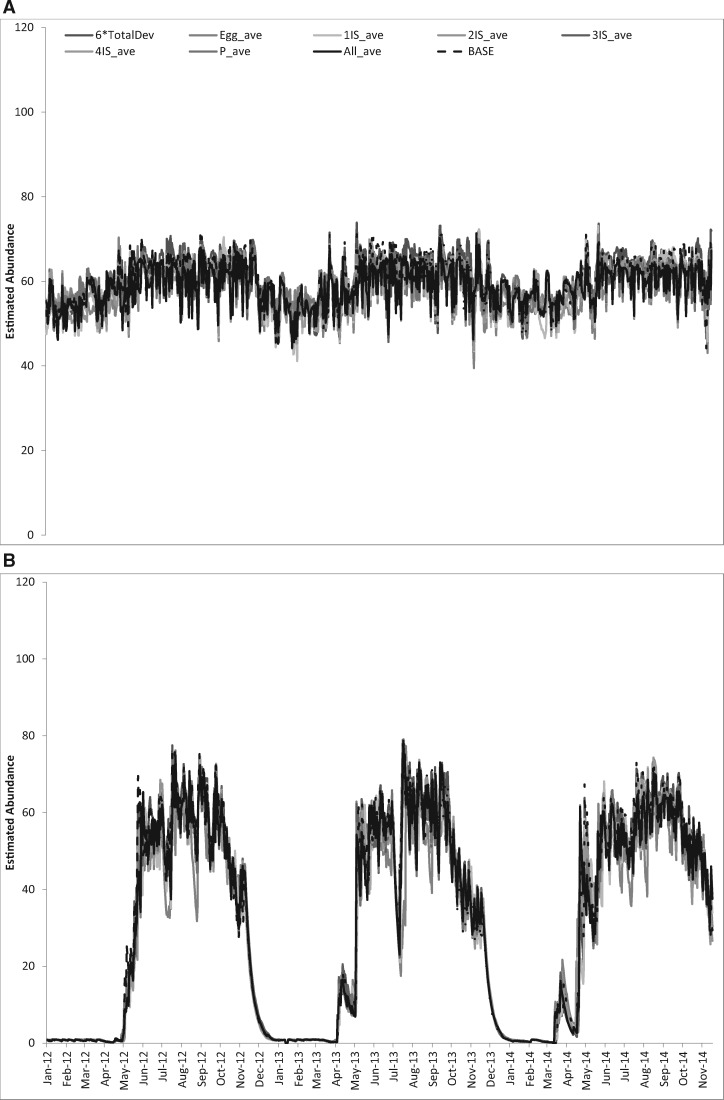
La Margarita. All of the above simplifications had a negligible impact on the estimated daily abundance, with RMSEs of about 10% of the total daily abundance (Fig. 1A).
Fig. 1.
Comparison of abundance using average development rates. (A) La Margarita. (B) Tucson. Baseline (BASE), egg (EggDev_ave), 1–4 larval instars (#IS_ave) and pupal (P_ave) and egg to adults (6*TotalDev).
Tucson. Likewise, in Tucson using average development rates led to fairly similar estimates, with RMSEs of about 10% (Fig. 1B).
Linear Development Rates
Next, the stage-specific development rates were replaced with a linear fit of temperature-dependent development rates. For the larval stages, the temperature-dependent development rates increase and then decrease as the temperature increases. Thus, the data were divided and a linear fit applied to pre- and postpeak data independently. For eggs and pupae, a single linear fit was used. For each, only one stage was changed at a time, and the model was reset to the original values between tests. The linear fit of each life stage was then used for all stages.
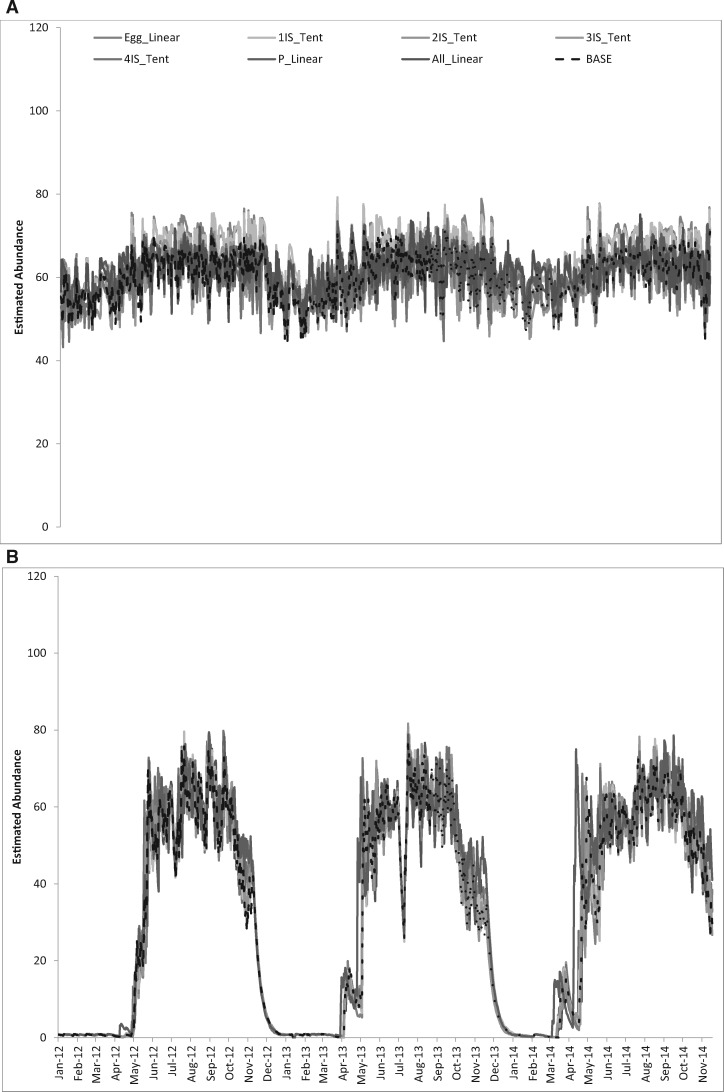
La Margarita. Applying a linear fit provides an estimate within the same range as the originally parameterized model (Fig. 2A), still capturing the seasonality, and is a reasonably close approximation with low RMSE (<6.6 mosquitoes per day, where the daily average is around 60; i.e., ∼10%). Changing the egg and third-instar development rates had the greatest effect (RMSE = 6.41 and 6.0, respectively), one overestimating (mean number of females = 65, SD = 7) and the other underestimating (mean number of females = 60, SD = 6) the average number of mosquitoes (baseline model = 62 females, SD = 7). When replacing all fits with a linear approximation, the estimated total was still around 60 adult females (62, SD = 5) compared with the baseline model, with an average of 60 adult females (SD = 6).
Fig. 2.
Comparison of abundance using linear development rates. (A) La Margarita. (B) Tucson. Baseline (BASE), egg (Egg_Linear), 1–4 larval instar development rates (#IS_Tent), pupal (P_Linear), all stages (All_Linear).
Tucson. Applying a linear fit provides an estimate within the same range as the originally parameterized model, still capturing the seasonality, and is a reasonably close approximation (Fig. 2B). Changing all development rates to their linear approximation had the greatest effect (RMSE = 10.13), though still providing similar estimates of average number of female mosquitoes (N = 38, SD = 26) as the baseline (N = 36, SD = 26).
Thresholds Associated With Development
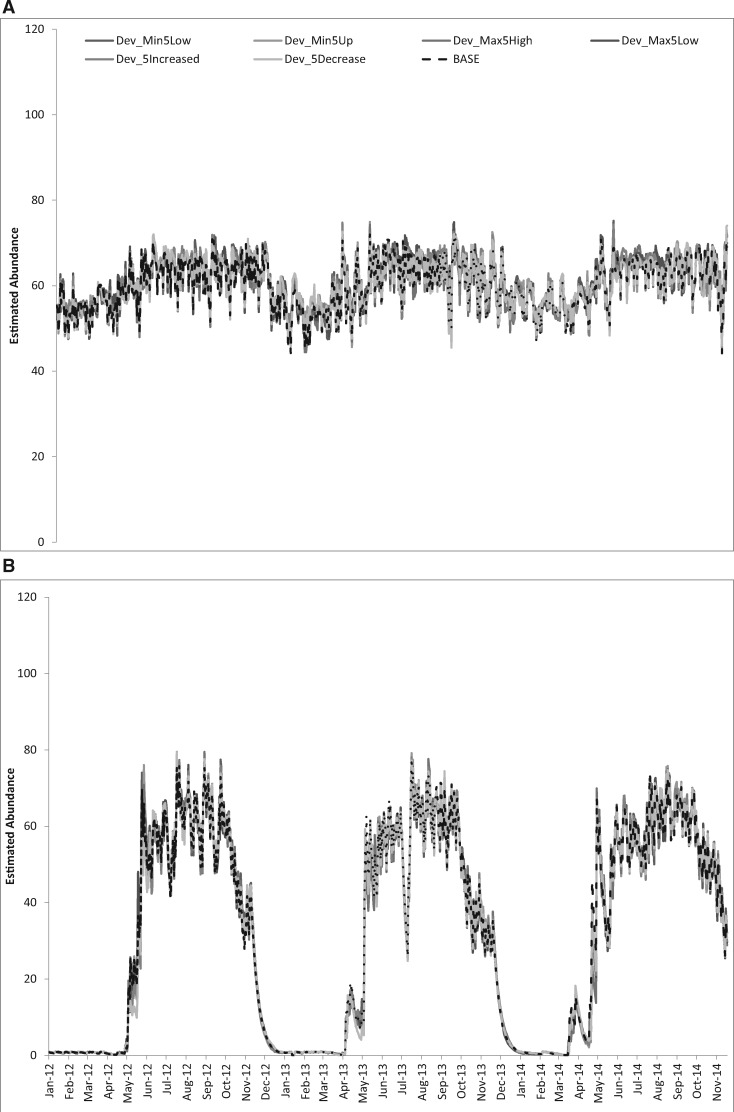
Minimum and maximum temperature development thresholds were lowered and raised by 5 °C yielding increased and decreased suitable temperature ranges. First, all stages were evaluated individually, and then all stages jointly. Only the estimates obtained when all stage-specific values were changed concurrently are presented in Fig. 3.
Fig. 3.
Comparison of abundance when minimum and maximum development thresholds were changed by 5 °C across all life stages. (A) La Margarita. (B) Tucson. Baseline (BASE), thresholds reduced (Dev_Min5Low; Dev_Max5Low), thresholds increased (Dev_Min5High; Dev_Max5High), range for suitable development increased (Dev_5Increase: minimum threshold lowered and maximum threshold raised), suitable development range decreased (Dev_5Decrease: minimum threshold raised and maximum threshold lowered).
La Margarita. In La Margarita, the mean temperature (27.2 °C) is well within the development temperature thresholds and the observed minimum and maximum (18.6°C, 35.5 °C) temperatures also remain within baseline thresholds. As a consequence, the sensitivity analysis showed negligible effect when compared with the variance across baselines yielding a RMSE of <2 (Fig. 3A and Table 2).
Table 2.
Error and estimates (mean daily abundance and standard deviation) associated with 5 °C changes in temperature tolerance thresholds
| Baseline | Low widened | Upper widened | Both widened | Low narrow | Upper narrow | Both narrow | ||
|---|---|---|---|---|---|---|---|---|
| Development | ||||||||
| La Margarita | RMSE | – | 1.70 | 1.69 | 1.86 | 1.75 | 1.90 | 1.82 |
| Mean | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| SD | 7 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Tucson | RMSE | – | 2.05 | 2.63 | 2.04 | 2.15 | 2.00 | 2.26 |
| Mean | 36 | 36 | 36 | 36 | 36 | 36 | 36 | |
| SD | 26 | 26 | 26 | 26 | 26 | 26 | 26 | |
| Survival | ||||||||
| La Margarita | RMSE | – | 1.88 | 1.84 | 1.87 | 1.81 | 1.75 | 1.78 |
| Mean | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| SD | 7 | 6 | 6 | 6 | 6 | 7 | 6 | |
| Tucson | RMSE | – | 1.88 | 2.65 | 2.09 | 11.99 | 31.03 | 29.80 |
| Mean | 36 | 36 | 36 | 36 | 31 | 18 | 17 | |
| SD | 26 | 26 | 26 | 26 | 28 | 21 | 21 | |
Tucson. For the time period of this study, the lowest minimum temperature reported for Tucson was −8.3 °C and the maximum was 44.4 °C. As with La Margarita, differences between baselines for stage-specific changes, as well as when all values were changed, were negligible with RMSE < 3 (Fig. 3B and Table 2).
Changing Death Rates
We changed the immature death rate for temperatures between 4.85°C and 29.85 °C from the formula presented in Otero et al. (2006), given by ), where T is temperature in °C, to a constant (1 − 0.970969). This value was calculated as the average of the temperature-dependent death rate in degree increments using the Otero et al. (2006) model. Using La Margarita and Tucson data had a negligible effect when compared with the unchanged model, with a RMSE for La Margarita of 2.08 and a mean of 60 females (SD = 6), and a RMSE for Tucson of 2.04 with a mean of 35 females (SD = 26). The other mortality rates were already constant between temperature thresholds (Otero et al. 2006).
Changing Thresholds Associated With Survival
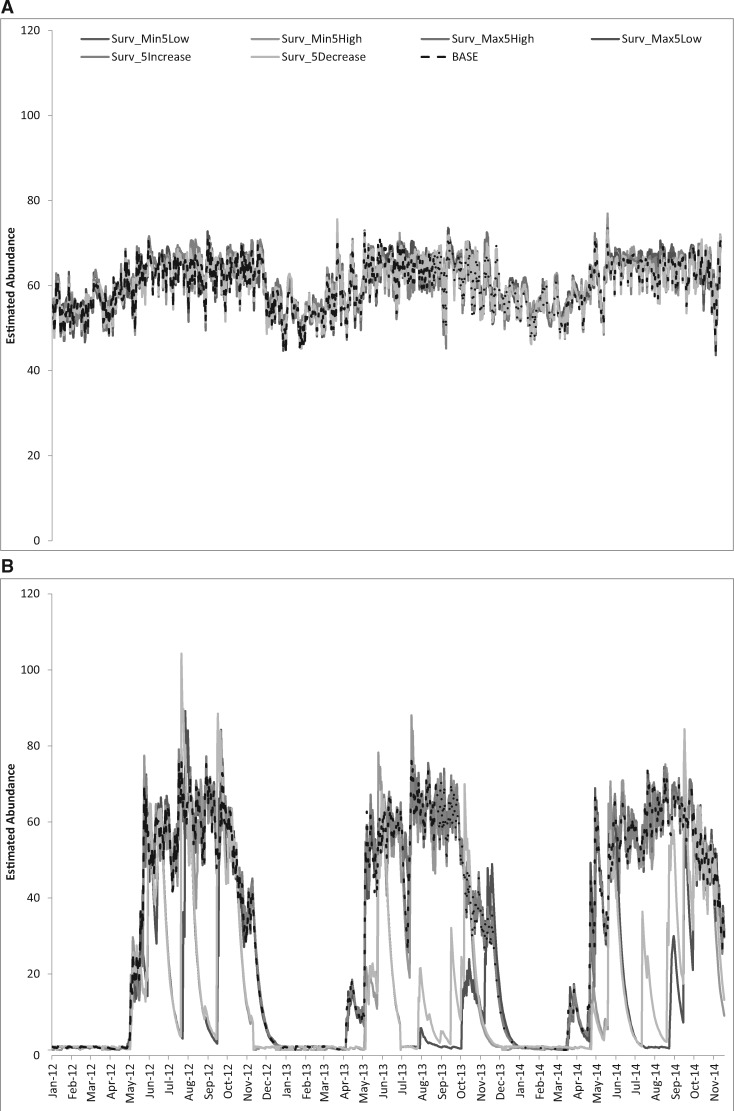
Fig. 4 presents the estimated abundance when all stage-specific values were changed simultaneously.
Fig. 4.
Comparison of abundance when survival thresholds were changed by 5 °C across all life stages. (A) La Margarita. (B) Tucson. Baseline (BASE), minimum and maximum thresholds reduced (Surv_Min5Low; Surv_Max5Low), minimum and maximum increased (Surv_Min5High; Surv_Max5High), suitable survival range increased (Surv_5Increase: minimum threshold lowered and maximum threshold raised), suitable survival range decreased (Surv_5Decrease: minimum threshold raised and maximum threshold lowered).
La Margarita. Changing the minimum temperature threshold by 5°C above or below the minimum temperature thresholds estimated from the graphs in Eisen et al. (2014) for this species remained well within the temperatures experienced in La Margarita (Table 3). Thus, we saw little effect of changing the minimum temperature thresholds, with an average number of females similar to the baselines and a RMSE <2.2 mosquitoes per day (Fig. 4A and Table 2). While the highest maximum temperature reported during this time period (35.5 °C) was closer to the stage-specific thresholds, these high temperatures were infrequent and had negligible effect, with a RMSE <2 mosquitoes per day (Fig. 4A and Table 2).
Table 3.
Development and survival thresholds (°C) estimated from the graphs in Eisen et al. (2014)
| Article I. | Egg | Hatch | Larvae | Pupa | Adult |
|---|---|---|---|---|---|
| Development | |||||
| Min | 10 | NA | 11.8 | 10.3 | NA |
| Max | 36 | 39 | (39) | ||
| Survival | |||||
| Min | 10 | 13 | 13 | 12 | 4 |
| Max | 36 | 38 | 36 | 38 | 41 |
Tucson. Unlike La Margarita, the temperatures in Tucson reach the minimum and maximum temperature thresholds during this time series—lowest minimum −8.3 °C and highest maximum 44.4 °C. Reducing the range of temperatures in which this vector survives by lowering the maximum temperature thresholds caused populations to collapse in the mid-summer with RMSE = 31.03 and mean number of females = 18 (Fig. 4B). This collapse is primarily driven by the effect of lowering the maximum survival threshold for larvae from 36°C to 31 °C (outside of which the mortality is set to 0.05). We also observed an effect when the minimum threshold for the daily survival rate for larvae and pupae was increased from 13°C to 18 °C and when the adult survival threshold was changed from 4 °C estimated from Eisen et al. (2014) to 9 °C. This caused the population to collapse about 20 d earlier in the fall.
Discussion
We conducted a sensitivity analysis to assess the effect of simplified development and mortality rates and to establish which parameters are most critical in understanding vector dynamics. We compared two very different regions (La Margarita and Tucson), which have very different climates. While both have established Ae. aegypti populations, their disease transmission profiles differ. Counter to our expectations, replacing the development rates with their averages or linear approximations had a negligible (∼10% error) effect on the estimated daily abundance in either location. This sensitivity analysis also shows the key role of temperature thresholds in marginal vector or extreme climate areas, and suggests these thresholds may be important for understanding regional risks of disease transmission.
The observation that simplified development rates have a negligible effect (∼10% error) on the predicted daily abundance suggests that empirical studies aiming for better estimates of development rates at constant temperatures may not be as necessary as addressing other aspects of vector growth dynamics. However, we saw an effect in Tucson when using the linear approximation concurrently for all development rates, which was not observed with any stage-specific change alone—a widening of the mosquito season with an earlier start to the population growth. This widening was most noticeable in the second yr of the study period, where the population began to build from mid-March to early April 2013 and again early March through early April 2014 (Fig. 2B). During these two periods, the average maximum and mean temperatures were about 2 °C warmer than the comparable period in 2012 (maximum temperature: 25.6°C, 27.7°C, and 27.0°C; mean temperature: 17.0°C, 19.4°C, 18.8°C in 2012, 2013, 2014, respectively) and the average minimum temperature 2–3 °C warmer (8.4°C, 11.2°C, 10.6°C, in 2012, 2013, 2014, respectively). Understanding this effect has implications for modeling disease transmission, as an extension of the activity season may translate to increased probability of a mosquito acquiring an infectious bloodmeal. Ginsberg et al. (2010) found earlier positive pools were positively associated with later West Nile virus activity in Culex species.
Although changing the thresholds associated with development had little effect, changing the survival thresholds influenced vector dynamics in Tucson. Cool temperatures are implicated in limiting the distribution of Ae. aegypti (Eisen and Moore 2013). Similar abundance modeling in cooler locations of Australia found the effect of cool temperatures on adult activity may have explained Ae. aegypti disappearance in marginal areas (Williams et al. 2010). Changes to development or mortality rates or temperature tolerance thresholds did not result in simulated populations surviving through the winter collapses in Tucson. This finding is likely because of the development at lower temperature ranges is near zero for immature stages (Otero et al. 2006, Eisen et al. 2014). Thus, even with shifting the cold temperature tolerance, unless the low-temperature development rates are also changed, the populations are not sustained over the winter.
This analysis suggests that the mid-summer heat is having an impact on the seasonality of Ae. aegypti in Tucson at the periphery of its geographic range. Our model does not include humidity related mortality, which has been suggested by some as a factor in Ae. aegypti survival in the arid Southwest (Hayden et al. 2010, Walker et al. 2011). Nonetheless, this analysis indicates heat alone is negatively impacting the populations. The modeled mosquito population dynamics was most sensitive in Tucson, where the survival thresholds reported in the literature for all immature stages were close to the observed summer temperatures. When using thresholds from the comprehensive summary provided by Eisen et al. (2014) as done here, the simulated populations survived. However, when using the thresholds estimated by Christophers (1960) and Otero et al. (2006), the mid-summer population collapsed as observed in the analysis reported here, with the reduction of the maximum temperature threshold. Simulated populations surviving or collapsing depending on the empirically derived threshold used, together with existing differences in reported development and survival thresholds, highlights a need to better understand temperature thresholds for Ae. aegypti. However, for estimating abundance in tropical regions where the temperatures often do not reach the thresholds, a simplified fit of the development rates within the reported thresholds may be sufficient to estimate vector abundance.
The development rates estimated for our model are based on constant temperatures and do not include diurnal fluctuations which have been shown to lengthen development time and reduce larval survival (Carrington et al. 2013). The results of this manuscript suggest that diurnal fluctuations may be simply accounted for by estimating general trends and revising the average development rates used in the model accordingly.
We observed an effect of maximum temperature thresholds limiting populations in the periphery of the geographic limits of Ae. aegypti. This study reinforces the need for better data to study how this vector behaves at the extreme temperatures experienced at the periphery of the vector’s range (Brady et al. 2013, Eisen et al. 2014).
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K01AI101224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References Cited
- Adams L., Bello-Pagan M., Lozier M., Ryff K. R., Espinet C., Torres J., Perez-Padilla J., Febo M. F., Dirlikov E., Martinez A., et al. 2016. Update: Ongoing Zika virus transmission-Puerto Rico, November 1, 2015–July 7, 2016. MMWR Morb. Mortal. Wkly. Rep. 65: 774–779. [DOI] [PubMed] [Google Scholar]
- Barrera R. 2010. Dengue and Aedes aegypti dynamics in Puerto Rico. Rev. Biomed. (Mexico) 21: 179–195. [Google Scholar]
- Barrera R., Amador M., MacKay A. J.. 2011. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl. Trop. Dis. 5: e1378.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch I. I., Brady O. J., Kraemer M. U., German M., Creatore M. I., Kulkarni M. A., Brownstein J. S., Mekaru S. R., Hay S. I., Groot E., et al. 2016. Anticipating the international spread of Zika virus from Brazil. Lancet 387: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady O. J., Johansson M. A., Guerra C. A., Bhatt S., Golding N., Pigott D. M., Delatte H., Grech M. G., Leisnham P. T., Maciel-de-Freitas R., et al. 2013. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit. Vectors 6: 351–3305. 6–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. E., Young A., Lega J., Andreadis T. G., Schurich J., Comrie A.. 2015. Projection of climate change influences on U.S. West Nile virus vectors. Earth Interact. 19: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington L. B., Seifert S. N., Willits N. H., Lambrechts L., Scott T. W.. 2013. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life-history traits. J. Med. Entomol. 50: 43–51. [DOI] [PubMed] [Google Scholar]
- Christophers S. R. 1960. Aedes aegypti (L.): The yellow fever mosquito. Cambridge University Press, London, United Kingdom. [Google Scholar]
- Eisen L., Moore C. G.. 2013. Aedes (Stegomyia) aegypti in the continental United States: A vector at the cool margin of its geographic range. J. Med. Entomol. 50: 467–478. [DOI] [PubMed] [Google Scholar]
- Eisen L., Monaghan A. J., Lozano-Fuentes S., Steinhoff D. F., Hayden M. H., Bieringer P. E.. 2014. The impact of temperature on the bionomics of Aedes (Stegomyia) aegypti, with special reference to the cool geographic range margins. J. Med. Entomol. 51: 496–516. [DOI] [PubMed] [Google Scholar]
- Ellis A. M., Garcia A. J., Focks D. A., Morrison A. C., Scott T. W.. 2011. Parameterization and sensitivity analysis of a complex simulation model for mosquito population dynamics, dengue transmission, and their control. Am. J. Trop. Med. Hyg. 85: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M. T., Hau B., Baird B. L., Palmer S., Kaplan S., Ramberg F. B., Mead D. G., Hagedorn H.. 1998. Aedes aegypti in Tucson, Arizona. Emerg. Infect. Dis. 4: 703.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Rochlin I., Campbell S. R.. 2010. The use of early summer mosquito surveillance to predict late summer West Nile virus activity. J. Vector Ecol. 35: 35–42. [DOI] [PubMed] [Google Scholar]
- Hayden M. H., Uejio C. K., Walker K., Ramberg F., Moreno R., Rosales C., Gameros M., Mearns L. O., Zielinski-Gutierrez E., Janes C. R.. 2010. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, U.S./Sonora, MX border. EcoHealth 7: 64–77. [DOI] [PubMed] [Google Scholar]
- Khan K., Bogoch I., Brownstein J. S., Miniota J., Nicolucci A., Hu W., Nsoesie E. O., Cetron M., Creatore M. I., German M., et al. 2014. Assessing the origin of and potential for international spread of chikungunya virus from the Caribbean. PLoS Curr. Outbreaks 6: 10.1371/currents.outbreaks.2134a0a7bf37fd8d388181539fea2da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis V. R., Phalkey R., Horstick O., Ratanawong P., Wilder-Smith A., Tozan Y., Dambach P.. 2014. Modeling tools for dengue risk mapping-A systematic review. Int. J. Health Geogr. 13: 50–072X. 13–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan A. J., Morin C. W., Steinhoff D. F., Wilhelmi O., Hayden M., Quattrochi D. A., Reiskind M., Lloyd A. L., Smith K., Schmidt C. A., et al. 2016. On the seasonal occurrence and abundance of the Zika virus vector mosquito Aedes aegypti in the contiguous United States. PLoS Curr. Outbreaks 8: 10.1371/currents.outbreaks.50dfc7f46798675fc63e7d7da563da76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin C. W., Comrie A. C.. 2010. Modeled response of the West Nile virus vector Culex quinquefasciatus to changing climate using the dynamic mosquito simulation model. Int. J. Biometeorol 54: 517–529. [DOI] [PubMed] [Google Scholar]
- Morin C. W., Monaghan A. J., Hayden M. H., Barrera R., Ernst K.. 2015. Meteorologically driven simulations of dengue epidemics in San Juan, PR. PLoS Negl. Trop. Dis. 9: e0004002.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero M., Solari H. G., Schweigmann N.. 2006. A stochastic population dynamics model for Aedes aegypti: Formulation and application to a city with temperate climate. Bull. Math. Biol. 68: 1945–1974. [DOI] [PubMed] [Google Scholar]
- Sharp T. M., Roth N. M., Torres J., Ryff K. R., Perez Rodriguez N. M., Mercado C., Pilar Diaz Padro M. D., Ramos M., Phillips R., Lozier M., et al. 2014. Chikungunya cases identified through passive surveillance and household investigations–Puerto Rico, May 5–August 12, 2014. MMWR Morb. Mortal. Wkly. Rep. 63: 1121–1128. [PMC free article] [PubMed] [Google Scholar]
- Walker K. R., Joy T. K., Ellers-Kirk C., Ramberg F. B.. 2011. Human and environmental factors affecting Aedes aegypti distribution in an arid urban environment. J. Am. Mosq. Control Assoc. 27: 135–141. [DOI] [PubMed] [Google Scholar]
- Williams C. R., Bader C. A., Kearney M. R., Ritchie S. A., Russell R. C.. 2010. The extinction of dengue through natural vulnerability of its vectors. PLoS Negl. Trop. Dis. 4: e922.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Legros M., Gould F., Lloyd A. L.. 2010. Understanding uncertainties in model-based predictions of Aedes aegypti population dynamics. PLoS Negl. Trop. Dis. 4: e830.. [DOI] [PMC free article] [PubMed] [Google Scholar]