Abstract
Paired oxygen-isotopic analyses of abiotic carbonate and benthic-ostracode shells from lake sediments provide a continuous quantitative record of growing-season temperature for the past 2000 years in the northwestern foothills of the Alaska Range. This record reveals three time intervals of comparable warmth: anno Domini (A.D.) 0–300, 850-1200, and post-1800, the latter two of which correspond to the Medieval Climatic Anomaly and climatic amelioration after the end of the Little Ice Age. The Little Ice Age culminated at A.D. 1700, when the climate was ≈1.7°C colder than at present. A marked climatic cooling also occurred around A.D. 600, coinciding with extensive glacial advances in Alaska. Comparisons of this temperature record with ostracode trace-element ratios (Mg/Ca, Sr/Ca) further suggest that colder periods were wetter and vice versa during the past 2000 years.
Knowledge of natural climatic variability is essential for evaluating possible human impacts on recent and future climate changes (1–4). Because of the paucity of lengthy instrumental records, such knowledge must be acquired through geological and biological archives of climatic change. This approach is particularly important for northern high-latitude regions where instrumental climate records are short (typically <75 years in the North American sub-Arctic) and where climate is thought to be most susceptible to anthropogenic alterations (5, 6). Despite the increasing appreciation for long-term high-resolution proxy data from these regions, few reliable quantitative records exist, and temperature variations at decadal to century scales remain poorly understood (6–8).
We conducted multiproxy geochemical analyses of a sediment core from Farewell Lake (62° 33′ N, 153° 38′ W, 320 m altitude) in the northwestern foothills of the Alaska Range (Fig. 1A). These analyses provide the first high-resolution (multidecadal) quantitative record of Alaskan climate variations that spans the last two millennia. Specifically, the oxygen-isotopic composition of abiotic carbonate in conjunction with that of benthic-ostracode shells are used to estimate changes in growing-season temperature, and the trace-element ratios (Mg/Ca, Sr/Ca) of ostracode shells are used to infer changes in effective moisture. These geochemical results provide information on climatic variations over the past two millennia, including those related to major hypothesized climatic events, such as the Little Ice Age and the Medieval Climatic Anomaly (9).
Figure 1.
(A) Map showing location of Farewell Lake (FL) in Alaska. (B) Bathymetry of Farewell Lake and coring location.
Study Site
Farewell Lake is within the ice limit of Late Wisconsin glaciation in the Alaska Range and lies on a large, gently north-sloping piedmont covered with moraines and glacial outwash. Bedrock in the area is primarily Paleozoic limestone with slate, phyllite, and chert (10). The lake is located in today's Interior Climate Zone of Alaska, characterized by large seasonal variations in temperature (11). The mean annual temperature of the region is −3.5°C, mean January temperature is −16.7°C, mean July temperature is 12.7°C, and mean annual precipitation is ≈400 mm. Modern vegetation is typical of closed boreal forests in Alaska (12). Poorly drained lowlands are characterized by Picea mariana muskegs, whereas well drained upland sites are dominated by mixed conifer–deciduous forests of Picea glauca, Betula papyrifera, and Populus tremuloides.
Farewell Lake is composed of three subbasins separated by prominent ridges (ref. 13; Fig. 1B). The sediment core for this study was obtained from the middle basin, which had a maximum depth of 39 m in April 1995. At present, the lake lacks distinct influent or effluent streams and has a surface area of about 4 km (2). The water of Farewell Lake is supersaturated with respect to calcite in summer, and Holocene sediments from the lake contain abundant calcareous material suitable for geochemical analysis (13).
Materials and Methods
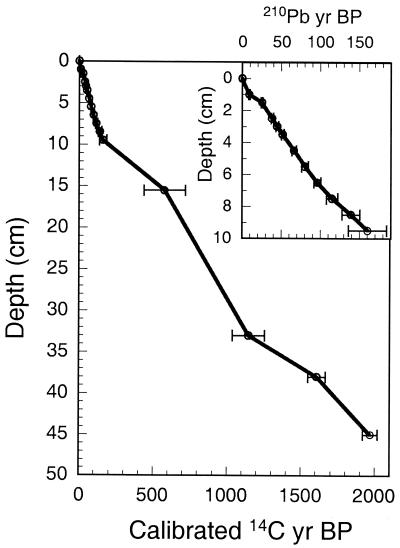
In 1995 we obtained from the lake a 45-cm-long sediment core by using a modified wedge-box freeze-corer (14, 15) with a mixture of dry ice and alcohol. The core was sectioned into continuous 1-cm intervals, each of which integrates on average ≈45 years of sedimentation. These samples were analyzed for organic and inorganic carbon content and for the oxygen and carbon isotopic composition of abiotic carbonate (bulk carbonate excluding ostracode and mollusk shells). We also analyzed the calcitic valves of the benthic-ostracode species Candona ikpikpukensis for oxygen and carbon isotopes as well as trace elements. The chronology of the record is based on 210Pb for the last 150 years and on linear interpolation between the oldest 210Pb date and 4 calibrated (http://depts.washington.edu/qil/) accelerator-MS 14C dates on plant macrofossils older than 150 years (Fig. 2). The 210Pb data indicate that, with our 1-cm-interval sampling of the core, the sedimentation rate at Farewell Lake is too low to permit calibration of our δ18O-derived temperature data against instrumental weather data. However, the broad consistency of our reconstructed temperature profile with tree-ring and geomorphic data at selected intervals (e.g., the Little Ice Age and a cooling at A.D. 600), as discussed below, suggests that our reconstruction is reasonable.
Figure 2.
Age-depth plot of the Farewell Lake core. Dates <150 calibrated year B.P. are based on 210Pb analysis of bulk sediment, and those >150 calibrated year B.P. are based on 14C analysis of Picea needles, Betula seeds, and unidentified twigs. Error bars represent ± 1σ SE.
To determine total carbon, we combusted freeze-dried samples in an oxygen atmosphere to convert organic and inorganic carbon to CO2. CO2 was then swept to a UIC (Joliet, IL) Carbon/Sulfur Analysis Coulometer where it was detected by automatic coulometric titration. To determine inorganic carbon, we treated samples with perchloric acid to dissolve carbonates and release CO2, the amount of which was measured by automatic titration in the coulometer. Organic-carbon content was determined by subtracting the amount of inorganic carbon from the amount of total carbon. We assumed that all inorganic carbon was derived from CaCO3, and we calculated the content of CaCO3 accordingly.
For the analyses of oxygen and carbon isotopes, abiotic carbonate samples were reacted at 25°C with 104% (vol/vol) H3PO4 made from ultra-pure P2O5 and triple-distilled H2O for 1 h to extract CO2. This reaction should not result in the substantial dissolution of dolomite, which would complicate the interpretations of δ18O data. The isotopic composition of the evolved CO2 was analyzed with a Finnigan-MAT (San Jose, CA) DELTA-E triple-collector mass spectrometer. Ostracodes were sieved and cleaned by following the methods of Xia et al. (16). Only well preserved valves of adult C. ikpikpukensis were used for the geochemical analysis. Ostracode samples were reacted with ultra-pure H3PO4 in an automated extraction device (Kiel II) connected to a Finnigan-MAT 252 IRMS. The acid solution was diluted 40 times with 0.5 M ultra-pure HCl and analyzed with a Perkin–Elmer/Sciex (Thornhill, ON, Canada) Elan 5000 inductively coupled plasma mass spectrometer (ICP-MS) for Mg, Sr, and Ca. Standard errors for the δ18O analysis of abiotic carbonate and ostracode valves are 0.09‰ and 0.06‰, respectively. Standard errors for the δ13C analysis of abiotic carbonate and ostracode valves are 0.04‰ and 0.02‰, respectively. Standard errors for Mg, Sr, and Ca concentrations are about 2%.
Rationale for δ18O-Based Temperature Reconstruction
Microscopic examination and x-ray diffraction analysis of selected samples from the Farewell Lake core indicate that abiotic carbonate in the core is composed primarily of fine-grain calcite. These calcite grains were most likely precipitated from surface water through algal photosynthetic uptake of CO2 during the growing season, which reduced the partial pressure of CO2 and caused CaCO3 supersaturation in the surface water. We assume that the calcification of C. ikpikpukensis shells also occurred during the relatively short growing season, when maximum aquatic primary productivity led to abundant food resources. The isotopic differences between abiotic carbonate and ostracode valves reflect (i) environmental differences between surface water, where abiotic-carbonate precipitation occurred, and bottom water, where benthic ostracodes formed their shells, and (ii) isotopic fractionation related to physiological processes of ostracode shell formation—the so-called “vital effects” (17). We use oxygen-isotopic fractionation of carbonates resulting from the temperature difference between surface and bottom waters to derive past temperature anomalies (departures from the present; ref. 18), as discussed below.
The δ18O values of abiotic carbonate (δ18Oac) and benthic ostracodes (δ18Obo) are both functions of lake-water δ18O and temperature. Lake-water δ18O in turn reflects a number of factors, including the source and history of precipitation, within-basin evaporation, and watershed hydrologic processes, the effects of which should be similar for both δ18Oac and δ18Obo at the same lake. Although evaporation could potentially cause more 18O enrichment in surface water than in bottom water of a lake, this factor is likely negligible at Farewell Lake, because the lake has a large volume (maximum depth of the south basin: 55 m; surface area: ≈4 km2) and because no major moisture deficit exists in the region. This argument is supported by the fact that the δ18O values (12.2–12.5‰) of water samples taken weekly from the lake surface during July 19–September 25, 2000, show no time-dependent trend. Furthermore, there exists no systematic δ18O variation among the growth rings of the gastropod Valvata sincera helicoidea from the sediments of Farewell Lake (B. F. Clegg and F.S.H., unpublished data). These growth rings probably formed during different seasons, as evidenced by the large seasonal changes in δ13C related to aquatic primary productivity and organic-matter decomposition. The lack of corresponding changes in δ18O suggests that seasonal changes in lake-water δ18O were not substantial.
δ18Oac reflects changes in atmospheric temperature, as abiotic carbonate precipitation occurs in surface water, where the temperature fluctuates in response to atmospheric temperature changes (19). In contrast, because Farewell Lake is deep and has a large water volume, the bottom-water temperature likely remains constant at 4°C, as it was in June 1990 and July 2000, based on field measurements. Therefore, the deviation of δ18Oac from contemporaneous δ18Obo results from oxygen-isotope fractionation induced by the temperature difference between surface and bottom waters after accounting for any vital effects. This temperature difference can be calculated from the temperature dependence of oxygen-isotopic fractionation between inorganic calcite and lake water (−0.25‰/oC; refs. 20 and 21). We do not know the magnitude of the vital effect of C. ikpikpukensis on the oxygen-isotopic composition of its shells. However, this factor should remain constant through time for the same species and can be removed in estimating past surface water-temperature (SWT) anomalies.
The rationale described above can be summarized by
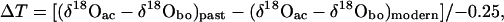 |
1 |
where ΔT denotes the temperature anomaly of the past from the modern.
Results and Discussion
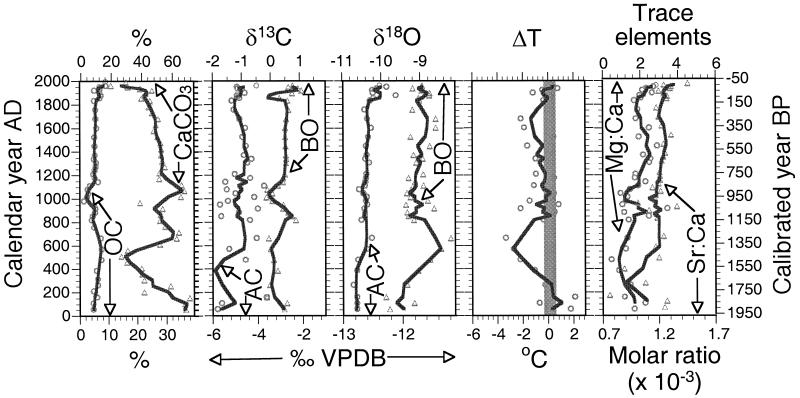
The Farewell Lake sediments of the last 2000 years contain relatively low organic carbon (1.37–9.01%) and abundant CaCO3 (19.50–71.00%; Fig. 3). δ18Oac and δ13C (δ13Cac) of abiotic carbonate range from −12.83‰ to −12.11‰ and from −6.70‰ to −3.62‰, respectively, whereas δ18Obo and δ13C (δ13Cbo) of C. ikpikpukensis valves range from −9.93‰ to −8.22‰ and from −1.15‰ to 0.95‰, respectively (Fig. 3). Overall, the amplitude of stratigraphic variations is greater for δ18Oac than for δ18Obo, suggesting that the isotopic-fractionation effects of SWT changes must have dampened the effects of lake-water δ18O changes on δ18Oac. We applied Eq. 1 to our oxygen-isotopic data to estimate temperature changes. The results suggest that at Farewell Lake SWT was as warm as the present at A.D. 0–300, after which it decreased steadily by ≈3.5°C to reach a minimum at A.D. 600 (Fig. 3). SWT increased by ≈3°C during the period A.D. 600–850 and then exhibited fluctuations of 0.5–1.0°C until A.D. 1200. Between A.D. 1200–1700, SWT decreased gradually by 1.25°C, and from A.D. 1700 to the present, SWT increased by 1.75°C, with a small reversal in the early 1900s.
Figure 3.
Climate records for the past 2000 years. These records include gross sediment composition, carbon and oxygen isotopic values, growing-season temperature anomalies, and trace-element ratios. OC, organic carbon; CaCO3, carbonate as calculated from inorganic carbon content; AC, abiotic carbonate (bulk carbonate excluding shells of ostracodes, bivalves, and gastropods); BO, valves of benthic ostracode C. ikpikpukensis; ΔT, growing-season temperature anomaly; VPDB, Vienna Peedee belemnite. The vertical bar in the ΔT plot marks the temperature range of the 20th century, as represented by the uppermost three samples. Open symbols are original data, and lines are three-point moving averages to emphasize overall trends. Calibrated year B.P. is referenced to the year 1950.
In addition to pronounced changes in SWT, effective moisture probably varied substantially in the Farewell Lake region over the last 2000 years, as suggested by stratigraphic changes in the molar ratios of Sr/Ca and Mg/Ca in C. ikpikpukensis valves. Farewell Lake has no major inlet and outlet streams, and its water is highly supersaturated with calcium carbonate. In such a basin the Sr/Ca ratio of ostracodes is a negative function of effective moisture and is independent of water temperature (22), whereas the Mg/Ca ratio is a negative function of effective moisture and a positive function of water temperature (23). Over the last 2000 years, the molar ratios of Sr/Ca at Farewell Lake ranged from 0.84 × 10−3 to 1.14 × 10−3, and those of Mg/Ca ranged from 1.24 × 10−3 to 3.80 × 10−3 (Fig. 3). The stratigraphic trends are generally similar for Sr/Ca and Mg/Ca despite some differences in the exact timing and magnitude of changes. This similarity suggests that effective moisture was the dominant control and that temperature was probably unimportant in determining these ratios. A comparison of the Sr/Ca and Mg/Ca profiles with that of ΔT suggests that colder periods were wetter and vice versa. Such climatic conditions in Alaska are typically driven by enhanced westerly flow through an eastward shift in the east-Asian trough and positive pressure anomalies in the North Pacific area (24).
Our SWT reconstruction at Farewell Lake provides, to our knowledge, the first quantitative temperature record that continuously spans the last 2000 years from Alaska. It is unlikely that this record reflects only local environmental conditions, because several major features of our reconstructed SWT profile are strikingly similar to those based on existing tree-ring and glacial geomorphic data from elsewhere in Alaska and adjacent Canada (25–28). In particular, both the pronounced temperature minimum centered at A.D. 600 and the culmination of the Little Ice Age cooling at A.D. 1700 in the Farewell Lake region coincide with extensive glacial advances in the southern coasts and the Brooks Range of Alaska (25–28). The cooling event around A.D. 600 might have also caused the demise of the Kachemak culture in the northwestern Gulf of Alaska at this time (29). In addition, concurrent climatic changes exist between Alaska and other regions, although the specific characteristics of these climatic changes differ among various regions. For example, the warmth before A.D. 300 at Farewell Lake coincides with a warm episode extensively documented in northern Europe (30) and an overall wet period inferred from tree-ring analysis in the American Southwest (31), whereas the A.D. 600 cooling is coeval with the European “Dark Ages” (30) and a prolonged dry period in the American Southwest (31). The relatively warm climate A.D. 850-1200 at Farewell Lake corresponds to the Medieval Climatic Anomaly, a time of marked climatic departure over much of the planet (9, 32). These concurrent changes suggest large-scale teleconnnections in natural climatic variability during the last two millennia, likely driven by atmospheric controls. However, caution must be exerted in comparing climatic fluctuations in these widely spaced areas, because recent studies (4, 8) have provided compelling evidence against the over-simplified concept of a globally synchronous climatic history.
Our SWT reconstruction at Farewell Lake indicates that although the 20th century, represented by the uppermost three samples, was among the warmest periods of the past two millennia, two earlier intervals may have been comparably warm (A.D. 0–300 and A.D. 850-1200). These data agree with tree-ring evidence from Fennoscandia, indicating that the recent warmth is not atypical of the past 1000 years (33, 34). In contrast, diatom and tree-ring records from the Canadian and Russian arctics suggest that the warmth of the 20th century is unprecedented in the late Holocene (35, 36). Such geographic disparities emphasize the complexity of the climate system and the need for a greater number of detailed records from a wider network of sites. The SWT record from Farewell Lake, however, is inadequate for understanding the context of the 20th century climate. Specifically, the low sedimentation rates at this site do not allow us to discern detailed patterns of SWT variation during the 20th century. In addition, various other aspects of climate, such as seasonal variability and winter temperature, may be more sensitive than the growing-season temperature to increasing CO2 concentrations and cannot be assessed with our reconstructed SWT record. Furthermore, our climatic reconstructions at Farewell Lake must be verified by similar studies at other sites in the region.
The 20th century climate is a major societal concern in the context of greenhouse warming (1, 2, 6). The scarcity of quantitative climatic information over longer periods, however, severely hampers our understanding of the nature and causes of the recent warming, especially for high-latitude regions (6). This research demonstrates that paired stable-isotope analyses of abiotic calcite and benthic ostracodes can be used as an effective tool to estimate past temperature changes. Such paleolimnological data, in conjunction with other long-term quantitative paleoclimate proxies (e.g., tree rings, ice-core geochemistry), promise to advance our knowledge of continental climatic variability, thereby enhancing our ability to understand mechanisms of environmental change.
Acknowledgments
Special thanks to Jeff Le Page (Farewell Lake Wilderness Lodge caretaker) who meticulously took weekly lake-water samples for this project from July to September in 2000. We thank S. Ambrose, C. Augspurger, L. Brubaker, R. Forester, C. Mock, G. Wiles, and H. E. Wright for comments. We are grateful to P. Calkin, S. Stine, and J. Teranes for constructive reviews. The University of Arizona Isotope Laboratory conducted isotopic analyses of water samples. This work was supported by National Science Foundation Grants OPP-9616288 and ATM-9619583. National Science Foundation Paleoenvironmental Arctic Sciences (PARCS) contribution 165 and Limnological Research Center-University of Minnesota contribution 583.
Abbreviations
- A.D.
anno Domini
- SWT
surface water-temperature
References
- 1.Crowley T J. Science. 2000;289:270–277. doi: 10.1126/science.289.5477.270. [DOI] [PubMed] [Google Scholar]
- 2.Mann M E, Bradley R S, Hughes M K. Nature (London) 1998;397:779–787. [Google Scholar]
- 3.Jones P D, Bradley R S, Jouzel J. Climate Variations and Forcing Mechanisms of the Last 2000 Years. New York: Springer; 1996. [Google Scholar]
- 4.Wright H E J, Kutzbach J E, Webb T I, Ruddiman W F, Street-Perrott F A, Bartlein P J. Global Climate Since the Last Glacial Maximum. Minneapolis: University of Minnesota Press; 1993. [Google Scholar]
- 5.PARCS. The Arctic Paleosciences in the Context of Global Change Research. Washington, DC: Am. Geophys. Union; 1999. [Google Scholar]
- 6.Overpeck J, Hughen K, Hardy D, Bradley R, Case R, Douglas M, Finney B, Gajewski K, Jacoby G, Jennings A, et al. Science. 1997;278:1251–1256. [Google Scholar]
- 7.Barnett T P, Santer B D, Jones P D, Bradley R S, Briffa K R. Holocene. 1996;6:255–263. [Google Scholar]
- 8.Hughes M K, Diaz H F. Clim Change. 1994;26:109–142. [Google Scholar]
- 9.Stine S. Nature (London) 1994;369:546–549. [Google Scholar]
- 10.Fernald A T. US Geol Surv Bull. 1960;1071-G:191–275. [Google Scholar]
- 11.National Oceanic and Atmospheric Administration. Climatography of the United States. Monthly Normals of Temperature, Precipitation, and Heating and Cooling Degree Days by State. Washington, DC: Natl. Oceanic Atmos. Admin.; 1951–1980. [Google Scholar]
- 12.Viereck L A, Dyrness C T, Batten A R, Wenzlick K J. The Alaska Vegetation Classification. Portland, OR: U.S. Dept. Agric. For. Serv. Pac. Northwest Res. Station; 1992. [Google Scholar]
- 13.Hu F S, Ito E, Brubaker L B, Anderson P M. Quat Res. 1998;49:86–95. [Google Scholar]
- 14.Renberg I. Boreas. 1981;10:255–258. [Google Scholar]
- 15.Wright H E., Jr J Paleolimnol. 1991;6:37–49. [Google Scholar]
- 16.Xia J J, Haskell B J, Ito E, Engstrom D R. J Paleolimnol. 1997;17:85–100. [Google Scholar]
- 17.Xia J J, Ito E, Engstrom D R. Geochim Cosmochim Acta. 1997;61:377–382. [Google Scholar]
- 18.Lister G. Ecol Geol Helv. 1989;82:219–234. [Google Scholar]
- 19.Livingstone D M, Lotter A F. J Paleolimnol. 1998;19:181–198. [Google Scholar]
- 20.Epstein S, Buchsbaum H A, Lowenstam H A, Urey H C. Bull Geol Soc Am. 1953;64:1315–1326. [Google Scholar]
- 21.Craig H, Gordon L. In: Stable Isotopes in Oceanographic Studies and Paleotemperatures, Spoleto 1965. Tongiorgi E, editor. Pisa, Italy: Conoglio Nazionale delle Ricerche; 1965. pp. 9–130. [Google Scholar]
- 22.Chivas A R, Deckker P D, Shelley J M G. Nature (London) 1985;316:251–253. [Google Scholar]
- 23.Chivas A R, Deckker P D, Shelley J M G. Palaeogeogr Palaeoclimatol Palaeoecol. 1986;54:43–61. [Google Scholar]
- 24.Mock C J, Bartlein P J, Anderson P M. Int J Clim. 1998;18:1085–1104. [Google Scholar]
- 25.Calkin P E, Wiles G C, Barclay D J. Quat Sci Rev. 2001;20:449–461. [Google Scholar]
- 26.Calkin P E. Quat Sci Rev. 1988;7:159–184. [Google Scholar]
- 27.Jacoby G C, D'Arrigo R D. Clim Change. 1989;14:39–49. [Google Scholar]
- 28.Wiles G C, Barclay D J, Calkin P E. Holocene. 1999;9:163–173. [Google Scholar]
- 29.Workman W. Arctic Anthropol. 1999;35:146–159. [Google Scholar]
- 30.Lamb H H. Climate History and Modern World. Chapman & Hall, New York: Routledge; 1995. [Google Scholar]
- 31.Hughes M K, Graumlich L J. In: Climate Variations and Forcing Mechanisms of the Last 2000 Years, NATO ASI. Jones P D, Bradley R S, Jouzel J, editors. Berlin: Springer; 1996. , Series 1 and 41, pp. 109–124. [Google Scholar]
- 32.Broecker W S. Science. 2001;291:1497–1499. doi: 10.1126/science.291.5508.1497. [DOI] [PubMed] [Google Scholar]
- 33.Briffa K R, Bartholin T S, Eckstein D, Jones P D, Karlen W, Schweingruber F H, Zetterberg P. Nature (London) 1990;346:434–439. [Google Scholar]
- 34.Briffa K R, Jones P D, Bartholin T S, Eckstein D, Schweingruber F H, Karlen W, Zetterberg P, Eronen M. Climate Dyn. 1992;7:111–119. [Google Scholar]
- 35.Douglas M S V, Smol J P, Blake W., Jr Science. 1994;226:416–419. doi: 10.1126/science.266.5184.416. [DOI] [PubMed] [Google Scholar]
- 36.Briffa K R, Jones P D, Schweigruber F H, Shiyatov S G, Cook E R. Nature (London) 1995;376:156–159. [Google Scholar]