Abstract
STUDY QUESTION
What is the live-birth rate (LBR) and cost-effectiveness of fertility preservation with oocyte cryopreservation (FP–OC) compared to expectant management in cancer patients age 25–40 based on estimated gonadotoxicity of treatments 5 years after cancer diagnosis?
SUMMARY ANSWER
Oocyte cryopreservation prior to cancer treatment is more costly, yet more effective (producing more live births), than not undergoing oocyte cryopreservation but it is most beneficial for patients undergoing high-risk chemotherapy (HRC).
WHAT IS KNOWN ALREADY
The decision to undergo FP prior to treatment is multifactorial and can be costly and delay treatment. Not all treatments carry the same gonadotoxicity and patients may choose to undergo FP–OC based on the probability of premature ovarian insufficiency, predicted outcomes and cost. A comprehensive model that incorporates age at diagnosis and toxicity of treatment to help guide patients in the decision to undergo FP–OC does not yet exist.
STUDY DESIGN, SIZE DURATION
This study used a Decision Analysis Model to estimate effectiveness and cost of FP for cancer patients.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Age-based estimates of LBR and cost per live birth were calculated for ages 25–40 years based on gonadotoxicity of treatment. A decision analysis model was constructed using Treeage Pro 2015 with case base probabilities derived from national registries, practice guidelines and medical records from a national network of infertility practices (IntegraMed).
MAIN RESULTS AND THE ROLE OF CHANCE
Compared to no FP–OC, FP–OC improved LBRs for women of all ages undergoing either low-risk chemotherapy (LRC) or HRC; however, it was most cost effective for women undergoing LRC at older ages or HRC at younger ages. Although FP–OC results in higher LBRs, it was always more costly. Using donor oocyte IVF can be a successful alternative to autologous FP–OC.
LIMITATIONS REASONS FOR CAUTION
Decision tree results reflect probabilities of certain events and are compiled from multiple reputable sources but are not directly derived from a recruited cohort of patients. Outcomes are based on United States estimates and should be interpreted in the broader context of individual patient diagnoses, treatment care plans and country of origin.
WIDER IMPLICATIONS OF THE FINDINGS
The development of this analytic model will help guide practitioners in their counseling of women from age 25 to 40 years, who are considering FP–OC at the time of cancer diagnosis. It provides a realistic pathway from diagnosis to LB and accounts for the majority of costs and outcome possibilities.
STUDY FUNDING/COMPETING INTEREST(s)
This study was partially funded by a grant from National Institute of Health (NIH)/National Institute of Child Health and Human Development (NICHD) (R01 HD67683) to A.Z.S. There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
N/A.
Keywords: fertility preservation, oocyte cryopreservation, cost-effectiveness, cancer, gonadotoxic, chemotherapy
Introduction
By 2030 the World Health Organization (WHO) estimates that 1.4 million reproductive aged women will be diagnosed with cancer each year. Advances in cancer treatment have dramatically improved survival rates and women under the age of 40 have a 5-year cancer survival rate of over 50% (UK, 2014). However, many cancer treatments are gonadotoxic, leaving women infertile following therapy. If patients have a loss of ovarian function before the age of 40 years, they meet criteria for a diagnosis of premature ovarian insufficiency (POI) (Anderson et al., 2016). Preserving the ability to have biological children is of great importance to many cancer survivors (Jeruss and Woodruff, 2009).
The 2013 American Society of Clinical Oncology (ASCO) guidelines state that oncologists have a responsibility to address fertility preservation (FP) options with all reproductive aged women (Loren et al., 2013). FP options include embryo or oocyte cryopreservation. Women in a stable relationship tend to favor banking embryos created with their partner. Reproductive aged women who do not have a partner either choose to use donor sperm to create embryos or frequently have a preference to undergo oocyte cryopreservation (FP–OC). Previously less successful than embryo cryopreservation, FP–OC has now reached IVF success rates comparable to those of fresh oocytes and frozen embryos (Cobo et al., 2010).
The decision to undergo FP prior to treatment is multifactorial (Lee et al., 2006). It may not be feasible or desirable to undergo FP at the time of cancer diagnosis as it can be costly and stressful and may delay the start of cancer treatment. Furthermore, not all treatments carry the same gonadotoxicity. Chemotherapy can be categorized as high risk (HRC) or low risk (LRC) depending on the gonadotoxicity (Lee et al., 2006). Treatments such as conditioning regimens for hematopoietic stem cell transplant for hematologic malignancies (leukemia, lymphoma, myeloma) used in Hodgkin lymphoma are HRC while those such as ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) used in Hodgkin lymphoma constitute LRC.
Patients may choose not to undergo FP in favor of attempting natural conception or achieving pregnancy with assisted reproductive technology in the future. Some patients will consider the back-up option of IVF with donor oocytes as a reasonable alternative to freezing their own oocytes and a preferable option to traditional adoption, while other reproductive age women may not desire children all.
Currently, there is not an established method of assessing the cost-effectiveness of FP–OC to help practitioners and patients weigh the risks and benefits. The nature of FP–OC makes it impossible to design a randomized controlled trial to address this question. However, decision modeling with sensitivity analysis utilizing multiple variables affecting the probability of live birth (LB) can be used to predict future fertility scenarios following cancer treatment. The objective of this study was to create a decision tree model to determine, at 5 years after cancer diagnosis, the live-birth rate (LBR) and cost-effectiveness of FP–OC versus no FP–OC in cancer patients age 25–40, based on estimated gonadotoxicity of treatments.
Materials and Methods
Decision analysis model
A Simple Node and Branch Decision Analysis model was constructed (Treeage Pro 2015) to determine LBR and cost-effectiveness for women with a new diagnosis of cancer choosing to undergo FP–OC prior to cancer treatment. Details of the basis for this decision analysis model have been described previously (Mesen et al., 2015). Briefly, the primary outcome for effectiveness of FP–OC versus no FP–OC was LB and primary outcome for cost-effectiveness was the incremental cost effectiveness ratio (ICER). The length of time patients with cancer are instructed to wait to conceive after cancer diagnosis is variable and can range anywhere from 2 to 5 years depending on the type of cancer, age at diagnosis, length of cancer treatment and risk of recurrence (Schapira, 2016). We used a conservative 5-year interval (‘Horizon’) for our model. Given the impact chemotherapy has on ovarian aging and the likelihood of decreased natural fecundability in couples who have not conceived within 6 months, our model used 6 months as the time to allow for natural conception before seeking fertility treatment (Fritz and Speroff, 2011; Schapira, 2016). The model was run for both LRC and HRC at each age of diagnosis from 25 to 40 years. Estimates of LBR and cost per LB were calculated for each age.
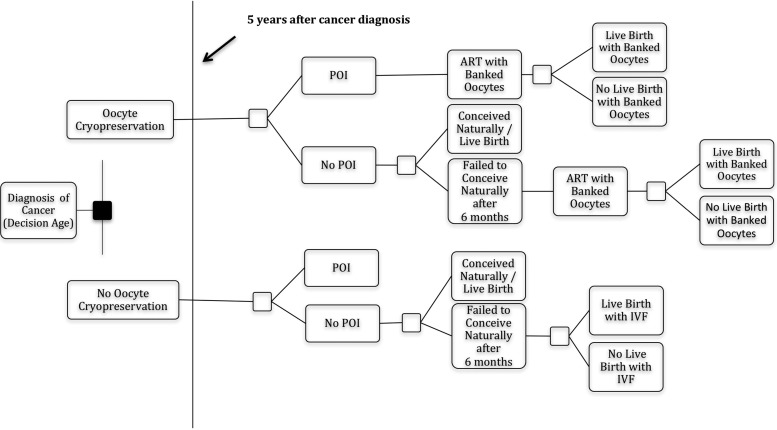
The model (Fig. 1) incorporates the following probabilities: (i) risk of POI after LRC and HRC; (ii) pregnancy rates for 6 months of attempted unassisted conception at the Horizon age (age at 5 years after cancer diagnosis); (iii) miscarriage (if pregnancy results in miscarriage, unassisted conception is attempted for an additional 6 months); (iv) LBR for patients who undergo IVF with cryopreserved oocytes (IVF–OC) or LBR after traditional IVF (IVF-T); and (v) probability of one frozen embryo transfer (FET) (based on number of embryos frozen from IVF). Base-case probability data was compiled from multiple sources including national registries and surveys, published research studies, practice guidelines and medical records from a large network of infertility practices (IntegraMed) (Table I) (Centers for Disease Control and Prevention, 2013; Loren et al., 2013; Mesen et al., 2015). The American Society of Clinical Oncology (ASCO) categorizes treatments based on the approximate gonadotoxic risk from published reports. Risk of POI was derived from ASCO guidelines based on estimated degree of gonadotoxicity for LRC and HRC (Lee et al., 2006).
Figure 1.
Simplified decision tree model without donor oocyte option. For a simplified representation of the decision tree, the chance nodes for the probability of a second cycle from frozen oocytes, a first and second frozen embryo transfer and miscarriage are not shown. Analyses were repeated for diagnosis at ages 25–40 years, assuming a 5-year time horizon before attempting pregnancy after cancer treatment. POI = premature ovarian insufficiency.
Table I.
Decision tree model and base-case estimates for cost and probability of events.
| Cost averages from seven United States fertility clinicsa | Costs (US $) |
|---|---|
| Fresh IVF cycle | 12 108 |
| Oocyte cryopreservation cycle/first year storage | 9253 |
| Frozen embryo transfer | 3355 |
| Oocyte warming, ICSI, embryo culture | 6373 |
| Donor oocyte cycle | 24 538 |
| Probability estimates | Reference | Scenario 1 (age 30, HRC) | Scenario 2 (age 40, LRC) |
| Risk of amenorrhea from cancer treatment | |||
| Low risk (LRC) | Lee et al. (2006) | 0.2 | 0.2 |
| High risk (HRC) | Lee et al. (2006) | 0.8 | 0.8 |
| Natural conception in 6 months | Mesen et al. (2015) | 0.7 | 0.3 |
| Miscarriage rate | Mesen et al. (2015) | 0.2 | 0.4 |
| Number of oocytes retrieved (by age) | IntegraMed database | 11.6 | 7.6 |
| Supernumerary embryos for FET | IntegraMed database | 0.4 | 0.1 |
| IVF-T live-birth rate | SART Data 2013 | 0.1 | 0.2 |
| IVF–OC live-birth rate | SART Data 2013 | 0.6 | 0.4 |
| IVF-D live-birth rate | SART Data 2013 | 0.6 | 0.7 |
aIndividual clinics listed in references.
LRC = low-risk chemotherapy; HRC = high-risk chemotherapy; IVF-T = traditional IVF; IVF–OC = IVF using own cryopreserved oocytes; IVF-D = donor oocyte IVF.
To illustrate this model, consider Scenario 1: a 30-year-old woman who is diagnosed with aggressive lymphoma planning HRC. Five years from diagnosis (Horizon age of 35 years), she will attempt natural conception for 6 months. If she does not become pregnant, she will then proceed with either IVF using her banked oocytes (IVF–OC) or traditional IVF (IVF-T) if she had not banked oocytes. If these efforts have still not resulted in a LB, she may undergo one FET, depending on the probability of having remaining embryos. If she has not had a LB after this, she has the option of IVF with fresh donor oocytes (IVF-D). She is allowed up to one FET if initial IVF-D does not result in LB. Scenario 2 is a 40-year-old woman, diagnosed with aggressive lymphoma, who is planning to receive LRC, and would have a similar path of decisions. See Table I for the specific probability input for Scenarios 1 and 2.
Determination of cost and ICER
Costs were averaged from actual charges from seven representative, diverse US fertility centers including both academic and private practice institutions (Supplementary Table S1). Direct charges, including laboratory costs, medical services and procedures were included in the model (Table I).
The ICER is the ratio of overall difference in cost between choosing FP–OC versus not choosing FP–OC divided by the incremental difference in LB ([ICER = costFP–CC – costNo FP–OC]/[probability LBFP–OC – probability LBNo FP–OC]). This is interpreted as the cost per additional LB when undergoing FP–OC. We calculated the ICER for horizon ages 25, 30, 35 and 40 years using TreeAge Pro 2015.
Sensitivity analysis
To assess the robustness of the model and account for error in probability and cost estimates, we performed a sensitivity analysis under the assumption that the cost of OC for cancer patients might be covered by health insurance in the future. We assumed that the initial cost of OC and the first 5 years of storage were free. The only cost for this analysis was for the subsequent procedures when patients came back to use the cryopreserved oocytes (cost of oocyte thaw, IVF, embryo transfer, etc.).
Ethical approval
This study was considered to be exempt by the University of North Carolina Institutional Review Board.
Results
Gonadotoxic effects of chemotherapy on LBR from natural conception
After a diagnosis of cancer, both LRC and HRC decreased the probability of LB at the horizon age (5 years after diagnosis) (Fig. 2). Maximum probability of LB without any ART intervention for LRC was 55% for LRC and <20% at any age for HRC (Fig. 2).
Figure 2.
The y axis represents the probability of live birth after 6 months of attempted conception beginning 5 years from the time of diagnosis. Probabilities of live birth are shown after treatment with low-risk chemotherapy (LRC) or high-risk chemotherapy (HRC). Probability of live birth with no cancer diagnosis and therefore no treatment is shown for comparison. Age at diagnosis is presented on the x axis.
Oocyte cryopreservation prior to HRC and LRC
When comparing FP–OC to no FP–OC, maximum improvement in LBR was achieved at age 37 for LRC and at age 27 for HRC (Fig. 3A and B). When patients who received LRC or HRC chose not to undergo FP–OC, their probability of LB after attempting natural conception and IVF-T was a maximum of 56 and 14%, respectively. In women over the age of 40, FP–OC increased LB by at least 20% for both treatment types (Table IIA and B).
Figure 3.
The y axis represents the probability of live birth 5 years after low-risk chemotherapy (LRC) (A) and high-risk chemotherapy (HRC) (B) with and without oocyte cryopreservation (FP–OC). Age at diagnosis is presented on the x axis.
Table II.
Live-birth rate (LBR) and incremental cost effectiveness ratio (ICER).
| Age at Diagnosis (years) | No FP–OC (LBR) | FP–OC (LBR) | ICER ($) |
|---|---|---|---|
| A. Low-risk chemotherapy (LRC) | |||
| 25 | 0.56 | 0.84 | 83 424 |
| 30 | 0.53 | 0.83 | 77 006 |
| 35 | 0.37 | 0.68 | 53 129 |
| 40 | 0.17 | 0.37 | 53 482 |
| B. High-risk chemotherapy (HRC) | |||
| 25 | 0.14 | 0.66 | 34 194 |
| 30 | 0.13 | 0.63 | 35 345 |
| 35 | 0.09 | 0.51 | 41 174 |
| 40 | 0.04 | 0.26 | 75 970 |
FP–OC = fertility preservation using oocyte cryopreservation.
To illustrate this idea, consider Scenario 1 (first addressed in Materials and Methods) where a 30-year-old woman undergoes HRC. When combining the probability of each of the above events taking place, the overall probability of LB for the now 35 years old who underwent FP–OC at the age 30 (age at diagnosis in Table IIB) is 63%. However, if she did not undergo FP–OC, her probability of LB at 35 years is 13% (Table IIB and Fig. 3B). In Scenario 2, a 40-year-old woman, who received LRC, could increase her probability of LB at the age of 45 from 17 to 37% by undergoing FP–OC prior to treatment (Table IIA and Fig. 3A).
Donor oocytes
If patients failed to achieve a LB with any of the above interventions, the option for IVF with a donor oocyte was added to the model (IVF-D). With this addition, there was at least a 58% chance of LB after HRC and a chance of LB as high as 87% after LRC (Supplementary Figure SI). Donor oocytes were more effective than banked autologous oocytes for all women diagnosed at 35 years of age or older.
Cost-effectiveness of oocyte cryopreservation prior to chemotherapy
For both LRC and HRC, patients had the greatest probability of achieving LB (increased ‘effectiveness’) when they underwent FP–OC, but it was always more costly than no FP–OC. When undergoing FP–OC prior to LRC, women between ages 35 and 40 years had the lowest ICER suggesting cost effectiveness is maximized in this age group (Table IIA and Fig. 4A). In contrast, patients undergoing HRC received the greatest benefit for the lowest cost at younger ages (25–30 years) (Table IIB and Fig. 4B). Cost per additional LB (ICER) for LRC ranged from $44 645 at older ages to $83 424 at younger ages with FP–OC being most cost effective at age 37 (Fig. 4A). For HRC, the ICER for FP–OC ranged from $34 194 to $75 970, with cost effectiveness maximized at the age of 25 (Fig. 4B).
Figure 4.
Cost per additional live birth after fertility preservation with oocytes (FP–OC) at 5 years from cancer diagnosis for both low-risk chemotherapy (LRC) (A) and high-risk chemotherapy (HRC) (B).
When the cost of initial oocyte cryopreservation and 5 years of storage was removed for our sensitivity analysis, the ICER for patients who underwent FP–OC prior to HRC ranged from $12 388 to $24 957 between the ages of 25 and 40 years resulting in a decrease in cost per LB (ICER) of up to $50 000. For LRC, the ICER for age 25 and 30 was $3528 and $3125, respectively, with a decrease in cost per LB of up to $80 000. For women who undergo LRC, it becomes more cost effective to undergo FP–OC than to undergo IVF after treatment after the age of 34.
Discussion
Our results showed that FP–OC (compared to no FP–OC) improved LB for women of all ages undergoing either LRC or HRC but was most cost-effective at older ages for LRC and at younger ages for HRC. Though FP–OC always has higher LBRs than attempting either natural conception or IVF after cancer treatment, it is also more costly. We also demonstrated that donor oocyte IVF can be a successful alternative to autologous FP–OC.
Even the lowest risk cancer treatment decreases the probability of LB by 10–15%, suggesting that any patient of reproductive age could benefit from fertility preservation (Fig. 2). In our model, LRC caused natural fertility at any given age to be equivalent to someone two years older while HRC reduced fertility for women at all ages to that comparable to 40 years old (Fig. 2). We used Scenarios 1 and 2 to illustrate the effects of gonadotoxic treatment in women diagnosed with cancer. Scenario 1 describes a 30-year-old woman who underwent FP–OC prior to HRC and subsequently increased her probability of LB by almost 5-fold. Similarly, in Scenario 2, a 40-year old woman who underwent LRC doubled her probability of LB with FP–OC (Fig. 3A and B). These scenarios demonstrate the decrease in fertility by both the gonadotoxic effects and the age-related decline in fertility resulting from the 5-year delay in attempted conception.
Despite the universal improvement in LBR when women with cancer in our study chose to undergo FP, cost is always a significant component of the decision to undertake any medical procedure. A cost effectiveness analysis (CEA) is a form of economic analysis that compares the costs and outcomes of different courses of action. It can help to inform patients and practitioners and guide them in their decision making process by incorporating measures such as financial feasibility which place a monetary value on the measure of effect (Bleichrodt and Quiggin, 1999). Our study illustrates that undergoing FP–OC will lead to improvement in LBR for women undergoing gonadotoxic cancer treatment; however, on average, it is more costly. For patients who want to maximize the cost-effectiveness of FP–OC, we identified differences when examining by age at diagnosis and treatment type and found the most cost-effective age for FP–OC in women undergoing LRC to be older ages (>35 years) and for women undergoing HRC, the younger ages are most cost-effective (<30 years).
While identifying maximum cost-effectiveness for FP–OC is an important feature of most CEAs, a measure of willingness to pay is often incorporated into the analysis (Bleichrodt and Quiggin, 1999). However, it can be challenging to place a monetary value on reproductive potential. Patients who strongly desire genetic offspring might interpret risk differently from those who do not plan for children or who prefer to pursue adoption. For example, Scenario 2 describes a 40-year-old woman, who undergoes FP–OC prior to LRC and increases her probability of LB from 17 to 37% (Fig. 3A). An overall probability of LB of <50% might be considered suboptimal for some women, while others might consider this a substantial increase and be willing to incur the financial burden. Future projects could incorporate feedback about family goals and financial considerations to help navigate and individualize recommendations regarding FP for women at the time of cancer diagnosis.
Some countries have mandated FP be covered by health insurance and others are beginning to explore its feasibility. To evaluate cost-effectiveness of even partial coverage of FP, we performed a sensitivity analysis that evaluated the cost effectiveness of strategies when the cost of the initial oocyte banking was dropped to zero. When this was done, the ICER was reduced by over 3-fold making FP equivalent in cost to other quality of life interventions, such as reconstructive breast surgery, that are currently part of comprehensive cancer health care coverage for women with breast cancer in the USA (Jeruss and Woodruff, 2009; Woodruff et al., 2017). As improvements in FP–OC continue to increase its efficiency, the cost-effectiveness of FP could lead to more widespread health coverage and make this a more viable option for many women with cancer.
Though undergoing FP–OC at time of cancer diagnosis has become a more streamlined process, FP is still not always feasible or desired and alternative options must be considered. A unique feature of our model was the incorporation of the option for donor oocyte IVF for patients who chose not to undergo FP–OC and suffered POI. With traditional adoption processes becoming increasingly difficult, many people are opting to using donor oocyte to complete their family (Bracewell-Milnes et al., 2016). In our model, the effectiveness of donor oocytes almost always surpassed that of the patient's own oocytes, from FP–OC, and always exceeded the effectiveness of natural fertility after gonadotoxic treatment (Supplementary Figure S1). Furthermore, depending on the age at which oocytes are frozen, it is possible that choosing not to undergo FP–OC and instead planning to use donor oocyte IVF after treatment, could save significant costs of failed autologous IVF and potentially make donor oocyte IVF more cost effective than FP–OC.
The strengths of our study include the development of a unique analytic model that provides a concrete tool and realistic pathway to help guide practitioners in their counseling at time of cancer diagnosis. The use of large databases combined with risk estimates allow for conservative estimates and the model accounts for the majority of costs and outcome possibilities. Unique to our model is the information on cost effectiveness of alternative options such as IVF post cancer treatment or use of donor oocytes by incorporating risk of POI for patients who choose not to undergo fertility preservation at the time of diagnosis.
Although the study is a useful starting point to counsel patients who are considering FP–OC, it does have limitations. It is possible that oocytes frozen at the time of cancer diagnosis may not be used if a patient conceives naturally or does not find a suitable partner. Limited data from large studies is available to estimate LBR in cancer patients who preserve oocytes and it is plausible that in addition to the gonadotoxicity of treatment, cancer itself has an effect on fertility that is not currently reflected in our model. We chose a time horizon of 5 years to attempt conception, though individual patients may choose shorter or longer time-frames. The model does not account for some of the costs including IVF medications as this varies significantly from patient to patient. In addition, this may not always be a cost incurred by patients as non-profit organizations often provide significant financial support for cancer patients to cover the cost of IVF medications for FP. Finally, the majority of costs are based on United States estimates and the costs presented in this paper are likely to be higher than in other parts of the world.
Advances in cryopreservation have resulted in successful outcomes and the probability that FP will result in LB is high, especially for younger women. Therefore, if patients have a strong desire for genetic offspring and the resources are available, our model suggests that FP–OC should be strongly considered for patients at all ages undergoing any cancer treatment. This model is a good starting point to help practitioners facilitate these difficult discussions by providing more information about cost and outcomes associated with FP–OC. However, we also hope it will serve to positively inform ongoing discussions about health care coverage for FP with the goal of improving life after cancer for patients.
Supplementary data
Supplementary data are available at Human Reproduction online.
Authors’ roles
B.L.S., N.G., T.B.M., A.Z.S. and J.E.M. contributed to the conception and design of the study. B.L.S. and N.G. collected data, developed the model and performed the data analysis. B.L.S., N.G. and J.E.M. drafted the article. T.B.M. and A.Z.S. revised and approved the final version.
Funding
Grant from National Institute of Health (NIH)/National Institute of Child Health and Human Development (NICHD) (R01 HD67683) to A.Z.S.
Conflict of interest
None.
Supplementary Material
References
- American Society for Reproductive Medicine; Society for Assisted Reproductive Technology . Criteria for number of embryos to transfer: a committee opinion. Fertil Steril 2013;99:44–46. [DOI] [PubMed] [Google Scholar]
- Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervost E, Janse F, Liao L, Vlaisavljevic V et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Human Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Bleichrodt H, Quiggin J. Life-cycle preferences over consumption and health: when is cost-effectiveness analysis equivalent to cost-benefit analysis. J Health Econ 1999;18:681–708. [DOI] [PubMed] [Google Scholar]
- Bracewell-Milnes T, Saso S, Bora S, Ismail AM, Al-Memar M, Hamed AH, Abdalla H, Thum M-Y. Investigating psychosocial attitudes, motivations and experiences of oocyte donors, recipients and egg sharers: a systematic review. Hum Reprod Update 2016;22:450–465. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention ASfRM; Society for Assisted Reproductive Technology . 2013Assisted reproductive technology national summary report. US Department of Health and Human Services, Atlanta (GA), 2013.
- Cobo A, Meseguer M, Remohi J, Pellicer A. Use of cryo-banked oocytes in an ovum donation programme: a prospective, randomized, controlled, clinical trial. Human Reprod 2010;9:2239–2246. [DOI] [PubMed] [Google Scholar]
- Fritz M, Speroff L. Assisted Reproductive Technologies in Clinical Gynecologic Endocrinology and Infertility. 8th edn, 2011.
- Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. New Engl J Med 2009;360:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace W, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology Recommendations on fertility preservation in cancer patients. J Clin Oncol 2006;24:2917–2931. [DOI] [PubMed] [Google Scholar]
- Loren AW, Mangu PB, Beck LN, Brennan LV, Magdalinski AJ, Partridge AH, Quinn G, Wallace W, Kutluk O. Fertility preservation for patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2013;31:2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesen T, Mersereau J, Kane J, Steiner A. Optimal timing for elective egg freezing. Fertil Steril 2015;103:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira L. Having a Baby After Cancer: Pregnancy In Cancer.Net (ed), 2016.
- UK CR One-, five- and ten-year survival for all cancers combined, 2014.
- Woodruff TK, Xu S, Walter JR. A call for fertility preservation coverage for breast cancer patients: the cost of consistency. J Natl Cancer Inst 2017;109:109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.