Summary
A systematic review and meta-analysis of trials comparing artemether-lumefantrine (AL) to non-artemisinin-based combination therapies for gametocyte clearance and transmission interruption demonstrated superiority of AL. The transmission-limiting potential of AL is relevant to drug deployment strategies in malaria treatment and chemoprevention.
Keywords: malaria, gametocytes, artemisinin-based combination therapy, artemether-lumefantrine, meta-analysis.
Abstract
Background.
Artemisinin-based combination therapies (ACTs) have been widely adopted as first-line agents to treat uncomplicated falciparum malaria due to their activity against multidrug resistant parasites. ACTs may also disrupt transmission through a direct antigametocyte effect, but the extent of this effect is uncertain. We assessed the evidence for and estimated the effects of the most widely-deployed ACT, artemether-lumefantrine (AL), relative to non-ACTs on gametocyte clearance and transmission interruption.
Methods.
We searched electronic databases for randomized controlled trials comparing AL to non-ACTs that reported gametocyte counts or results of mosquito-feeding assays. Two authors working independently assessed eligibility, extracted data, and evaluated the risk of bias. We conducted meta-analyses using a random-effects model.
Results.
We identified 22 eligible trials. The pooled odds of gametocytemia at 1 week were lower in AL- compared to non-ACT-treated participants (odds ratio [OR] 0.09; 95% confidence interval [CI], 0.06–0.15; I2 = 0.60, P < .01; 15 trials). The odds of transmission to mosquitoes were also lower in AL treatment groups (OR 0.06; 95% CI, 0.00–0.47, P < .01 at 7 days post-treatment; 1 trial; OR 0.56; 95% CI, 0.36–0.88, P = .01 at 14 days post-treatment; 1 trial).
Conclusion.
AL is superior to non-ACTs in reducing gametocytemia, and, based on limited evidence, abating transmission to mosquitoes. The transmission-limiting benefit of AL has relevance for policymakers planning optimal utilization of control strategies, including use of ACTs for malaria treatment and chemoprevention.
Malaria caused an estimated 212 million infections and 429000 deaths in 2015, predominantly in children in sub-Saharan Africa [1]. Recently, there has been a renewed call for malaria eradication, building on substantial gains made in the last 15 years [2]. Control and elimination currently center on case management with artemisinin-based combination therapy (ACT) and vector control with insecticide-treated bed nets. Less widely deployed, due to cost and technical constraints, are indoor spraying of insecticides, mass drug administration, and chemopreventive use of drugs. As long as a highly effective malaria vaccine remains unavailable, drugs that reduce human-to-mosquito transmission offer a vital contribution to control and elimination programs.
Malaria is transmitted by gametocytes in host blood. Following ingestion by mosquito vectors, malaria parasites migrate to the insect midgut and proceed through sporogony, developing from ookinetes to oocysts that contain infectious sporozoites. Few antimalarials possess antigametocyte activity, and individuals may remain infectious for several weeks after treatment [3, 4]. ACTs are more potent than non-ACTs against asexual parasites and have activity against immature gametocytes [5–11]. Some artemisinin derivatives may also possess sporonticidal activity, thereby limiting transmission from mosquitoes to humans [12]. The resultant transmission-limiting benefit of ACTs has relevance for policymakers planning optimal use of control and elimination approaches as stepping stones toward eradication.
Among ACTs, artemether-lumefantrine (AL) is the most widely adopted. Moreover, AL appears to be superior to most other widely-used ACTs for reducing gametocyte carriage and disrupting transmission [13–16]. The objective of this review is to systematically identify and synthesize the evidence for and quantify the effects of AL relative to non-ACTs on gametocyte carriage and transmission interruption in individuals with uncomplicated falciparum malaria.
METHODS
Eligibility Criteria
We included randomized controlled trials (RCTs) that compared AL (6-dose regimen) to one or more non-ACT regimens for gametocyte clearance or mosquito infectivity. We included studies in endemic regions of participants of any age with a diagnosis of uncomplicated falciparum malaria. We excluded studies of severe malaria and non-falciparum malaria, and trials in migrants, displaced peoples, travelers, and military personnel.
Outcomes
The primary outcomes were the proportion of study participants with circulating gametocytes 7 days after initiation of treatment, measured by microscopy, and the proportion of mosquitoes that developed gut oocysts in feeding studies at any day. Secondary outcomes included the proportion of study participants with circulating gametocytes at post-treatment day 14, mean duration of gametocyte carriage, mean gametocyte density, area under the curve (AUC) of gametocyte density, and proportion of participants in feeding studies who were infectious to mosquitoes.
Search Strategy
We designed an electronic search strategy using terms related to “malaria” and “antimalarial drugs” (Supplementary Table 1). We applied a filter for RCTs to electronic databases for which that feature was available. We searched PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov on February 5, 2016, and manually searched references of relevant papers. The search was not limited by year or language.
Data Extraction
We extracted data pertaining to study design, eligibility criteria, baseline characteristics of the study groups, co-administered interventions, features of the study setting including transmission intensity and locale (rural, urban, peri-urban), duration of follow-up, and attrition. We extracted gametocyte and mosquito-feeding outcomes from text or tables, or estimated them from figures when necessary. At time points for which a denominator was not reported, we estimated it as the sample size at the previous time point if available, otherwise as the initial sample size. Where continuous variables were presented as medians, we assumed a normal distribution and converted medians to means. One trial included a 4-dose AL group, but only the standard 6-dose AL group was considered in analyses [17]. For the single study that performed polymerase chain reaction (PCR) for gametocytes [18], we only extracted results of microscopy in order to maintain comparability across trials.
Assessment of Risk of Bias
We used the Cochrane Collaboration tool for risk of bias assessments of individual studies [19]. We considered selection, performance, detection, attrition, and reporting biases, graded independently by two authors as low risk, high risk, or unclear risk.
Analysis
We qualitatively assessed the comparability of characteristics and designs of included trials. We assessed statistical heterogeneity among the included trials through visual inspection of forest plots and computation of the I2 statistic. By convention, I2 values of 0.3–0.6 were interpreted as evidence of moderate heterogeneity, and values >0.6 as considerable heterogeneity [20]. When no substantial clinical, methodological, or statistical heterogeneity was present, we conducted meta-analyses using a random-effects model in RevMan 5.0 (The Cochrane Collaboration).
We conducted prespecified subgroup analyses by age (≤5 and >5 years of age), comparator drug (amodiaquine plus sulfadoxine/sulfalene-pyrimethamine, AQ-SP; and chloroquine plus sulfadoxine-pyrimethamine, CQ-SP), and geographic region (Africa and South/Southeast Asia). We assessed small-study effects by visual inspection of funnel plots for asymmetry. We performed a sensitivity analysis excluding studies with high or unclear risk of detection bias, based on whether study microscopists were masked.
RESULTS
Study Selection
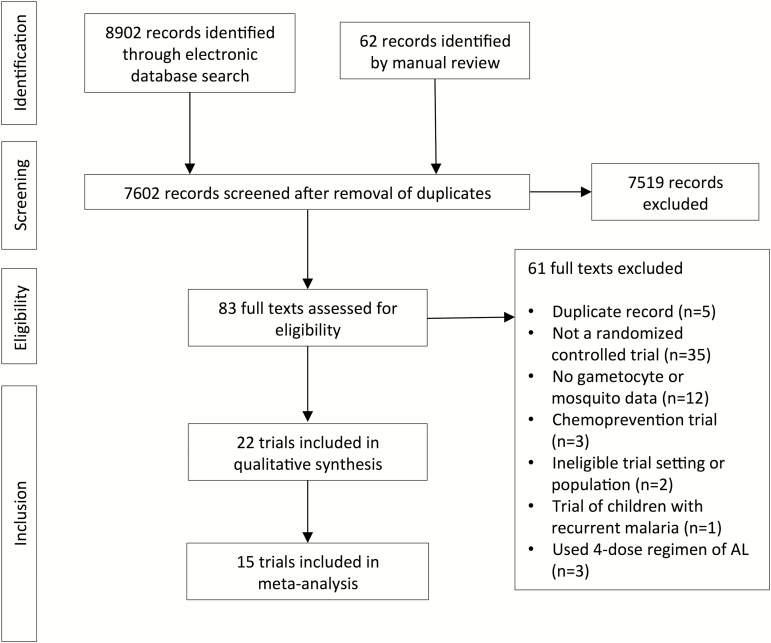
We identified 7602 unique records from our searches and included 22 RCTs in the systematic review (Figure 1). Fifteen studies reported sufficient data for inclusion in meta-analyses.
Figure 1.
Flowchart of inclusions and exclusions from the systematic review. Abbreviation: AL, artemether-lumefantrine.
Study Characteristics
Of the 22 included RCTs, only 2 were designed explicitly to evaluate malaria transmission [18, 21]. All others reported gametocyte and mosquito infectivity results as secondary outcomes. The trials were predominantly done in children; only 3 included adults [22–24]. Eight recruited children <5 years of age [25–32]. Most trials were conducted in sub-Saharan Africa (17 out of 22), the remainder in South and Southeast Asia.
The trials examined a total of 60 treatment groups, of which 23 groups received AL, 10 groups ACTs other than AL, and 27 groups non-ACTs (Table 1). Non-ACT regimens mainly comprised mono- or combination therapies of AQ (10 trials), CQ (7 trials), and SP (18 trials). Dosing schedules for the non-ACT groups were standard. Two trials reported co-interventions: 1 administered iron supplementation [33], and 1 administered the gametocytocide primaquine to study participants with gametocytemia at any follow-up visit, which resulted in a greater number of participants in the non-ACT group receiving the drug [22].
Table 1.
Characteristics of Included Trials
| Study Author and Publication Year | Country (No. sites) | Transmission Intensity | Study Period | Participant Ages | Day(s) of Assessment for Gametocytes (Mosquitoes) | Treatment Groupsa (No.) | Baseline Parasite Density (Count/μL) | Day 7 Gametocyte Carriage (%) | Day 14 Gametocyte Carriage (%) | Proportion of Infected Mosquitoes (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| von Seidlein 1998 [32] | Gambia (2) | … | 1996–1997 | 1–5 y | 2, 3, 4, 15 | AL (144) | 141000 | … | 0/125 (0.0) | … |
| SP (143) | 168000 | 37/128 (28.9) | ||||||||
| Hatz 1998 [25] | Tanzania (1) | … | … | 1–5 y | 1, 2, 3, 7 | AL (130) | 55017 | 2/130 (1.6) | … | … |
| CQ (130) | 53575 | 8/130 (6.2) | ||||||||
| Mayxay 2004 [35] | Laos (1) | … | 2002–2003 | ≥ 1 y | Not specified | AL (110) | 29363 | … | … | … |
| CQ-SP (110) | 26122 | |||||||||
| Sutherland 2005 [21] | Gambia (1) | … | 2002 | 1–10 y | 7, 14, 28 (7) | AL (406) | 56689 | 16/336 (4.8) | 11/356 (3.1) | 0/195 (0.0) |
| CQ-SP (91) | 55203 | 27/74 (36.5) | 27/79 (34.2) | 14/345 (4.1) | ||||||
| Mutabingwa 2005 [26] | Tanzania (1) | … | 2002–2004 | 4–59 mo | 14 | AL (519) | 24100 | … | 20/333 (6.0) | … |
| AQ (270) | 24350 | 24/128 (18.8) | ||||||||
| AQ-SP (507) | 24400 | 73/284 (25.7) | ||||||||
| Koram 2005 [27] | Ghana (2) | … | 2003 | 6–59 mo | 1, 2, 3, 5, 7, 14 | AL (51) | 28175 | 1/51 (2.0) | 1/47 (2.1) | … |
| SP (27) | 25064 | 10/27 (37.0) | 6/27 (22.2) | |||||||
| CQ (36) | 28134 | 1/36 (2.8) | 0/36 (0.0) | |||||||
| van den Broek 2005 [37] | Bangladesh (2) | High | 2003 | ≥ 1 y | 2, 3, 7, 14, 21, 28, 35, 42 | AL (121) | 11814 | 3/121 (2.5) | 1/121 (0.8) | … |
| CQ-SP (122) | 12016 | 22/51 (43.1) | 44/122 (36.1) | |||||||
| Bousema 2006 [18] | Kenya (1) | High | 2003–2004 | 6–120 mo | 3, 7, 14, 28 (14) | AL (75) | 11887 | 12/75 (16.0) | 5/75 (6.7) | 27/750 (3.6) |
| SP (152) | 10122 | 83/135 (61.5) | 58/152 (38.2) | 52/750 (6.9) | ||||||
| AQ-SP (127) | 11452 | 55/119 (46.2) | 38/127 (29.9) | 41/750 (5.5) | ||||||
| Mulenga 2006 [23] | Zambia (4) | Moderate | 2003–2005 | 15–50 y | 3, 7, 14, 28 | AL (485) | 8405 | 2/436 (0.5) | … | … |
| SP (486) | 8787 | 88/405 (21.7) | ||||||||
| Faye 2007 [17] | Senegal (5) | Moderate | 2002–2003 | … | 3, 7, 14, 21, 28 | AL (149) | 15398 | 9/149 (6.0) | 5/149 (3.4) | … |
| AQ-SP (161) | 27000 | 19/161 (11.8) | 0/161 (0.0) | |||||||
| Fanello 2007 [28] | Rwanda (2) | … | 2004–2005 | 12–59 mo | 2, 3, 7, 14, 21, 28 | AL (251) | 23323 | 2/246 (0.8) | 2/246 (0.8) | … |
| AQ-SP (249) | 22070 | 19/247 (7.7) | 9/247 (3.6) | |||||||
| Dorsey 2007 [56] | Uganda (1) | … | 2004–2006 | 1–10 y | 2, 3, 4–14 | AL (202) | 10939 | … | … | … |
| AQ-SP (253) | 13170 | |||||||||
| Zongo 2007a [33] | Burkina Faso (3) | High | … | ≥ 6 mo | Not specified | AL (188) | 27110 | … | … | … |
| AQ-SP (184) | 22884 | |||||||||
| Zongo 2007b [38] | Burkina Faso (3) | High | 2005 | ≥ 6 mo | 2, 3, 7, 14, 21, 28 | AL (275) | 27689 | 0/261 (0.0) | 0/275 (0.0) | … |
| AQ-SP (273) | 27137 | 3/260 (1.2) | 0/273 (0.0) | |||||||
| Thapa 2007 [22] | Nepal (1) | Low | 2005 | > 5 y | 3, 7, 14, 21, 28 | AL (66) | 6492 | 9/66 (13.6) | 1/66 (1.5) | … |
| SP (33) | 2997 | 27/33 (81.8) | 14/33 (42.4) | |||||||
| Sowunmi 2008 [34] | Nigeria (1) | High | 2005–2006 | ≤ 10 y | 1, 7, 14, 21, 28, 35 | AL (90) | 46541 | 1/90 (1.1) | 3/90 (3.3) | … |
| AQ-SP (91) | 50784 | 3/91 (3.3) | 2/91 (2.2) | |||||||
| Karunajeewa 2008 [29] | Papua New Guinea (2) | High | 2005–2007 | 6–59 mo | 1, 2, 3, 7, 14, 28, 42 | AL (127) | 48507 | 24/127 (18.9) | 6/127 (4.7) | … |
| CQ-SP (110) | 43869 | 91/110 (82.7) | 78/110 (70.9) | |||||||
| Gürkov 2008 [39] | Ethiopia (1) | … | 2006 | > 5 y | 0, 7, 28 | AL (30) | 21189 | 2/30 (6.7) | … | … |
| QN (35) | 15469 | 10/34 (29.4) | ||||||||
| AP (32) | 13028 | 16/32 (50.0) | ||||||||
| Achan 2009 [30] | Uganda (1) | High | 2007–2008 | 6–59 mo | 7, 14, 28 | AL (89) | 16124 | 1/89 (1.1) | 1/89 (1.1) | … |
| QN (86) | 14107 | 10/59 (1.7) | 5/62 (8.1) | |||||||
| Okafor 2010 [36] | Nigeria (1) | … | 2005 | 6–120 mo | 3, 7, 14, 28, 42 | AL (70) | 11320 | 2/69 (2.9) | 1/69 (1.4) | … |
| AQ-SP (70) | 9717 | 15/69 (21.7) | 8/69 (11.6) | |||||||
| Valecha 2012 [24] | India (3), Thailand (1) | … | … | 12–65 y | 28 | AL (80), OZ-PIP (160) | 22221, 21497 | … | … | … |
| Chandra 2015 [31] | Africa, multiple (6) | … | 2008–2010 | 6–59 mo | Not specified | AL (126) | … | … | … | … |
| CQ-AZ (120) |
Abbreviations: AL, artemether-lumefantrine; AP, Atovaquone-proguanil; AQ, amodiaquine; AZ, azithromycin; CQ, chloroquine; OZ, arterolane; PIP, piperaquine; QN, quinine; SP, sulfadoxine/sulfalene-pyrimethamine; …, not done or not reported.
aOmits artemisinin-containing study arms except AL.
Seventeen trials reported the proportion of participants with gametocytemia at post-treatment day 7 and/or 14, and 2 reported results of mosquito feeding assays. Additional gametocyte-related measures included mean or median gametocyte density [21–23, 34–36], cumulative prevalence of gametocytemia [22, 37], mean duration of gametocytemia [21], probability of remaining gametocyte-free [34], gametocyte clearance rate [31], AUC of gametocyte density [34], ratio of means of gametocyte density [21], total person-time with gametocytes [21, 30], and mean gametocyte sex ratio [34]. One trial restricted its follow-up gametocyte measurements only to participants who were gametocytemic at baseline [34]. Every trial included genotyping (e.g., pfmsp1 and pfmsp2 alleles) to distinguish reinfection from recrudescence in participants with recurrent parasitemia, but none provided information relating new or recrudescent asexual parasitemia to the presence of gametocytes.
Risk of Bias of Included Trials
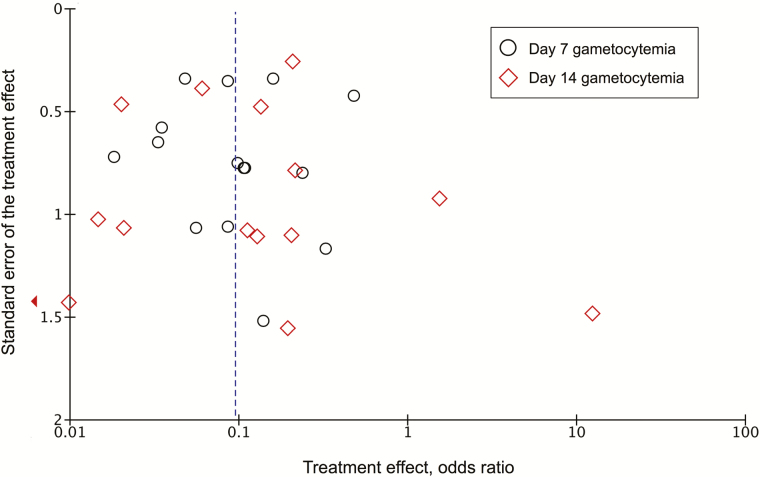
There was an overall low risk of bias within trials (Table 2, Supplementary Table 2). Outcome assessments were unmasked in 2 trials [25, 38] and not described in 8 others [17, 21, 24, 27, 33, 35, 37, 39]. Attrition bias was low, with retention rates >90% in nearly all trials; 3 trials reported losses to follow-up >20% in one or more treatment groups [21, 26, 33]. A funnel plot of effect estimates from studies that reported 7- and 14-day gametocyte prevalence displayed a near-symmetric distribution about the pooled estimate, indicating low evidence of small-study effects (Figure 2).
Table 2.
Summary of Risk of Bias of Included Trials
| Type of Bias | Risk of Bias, No. of Studies | ||
|---|---|---|---|
| Low | High | Unclear | |
| Random sequence generation (selection bias) | 16 | 3 | 3 |
| Allocation concealment (selection bias) | 10 | 4 | 8 |
| Blinding of participants and personnel (performance bias) | 2 | 15 | 5 |
| Blinding of outcome assessment (detection bias) | 12 | 2 | 8 |
| Incomplete outcome data (attrition bias) | 15 | 3 | 4 |
| Selective reporting (reporting bias) | 21 | 0 | 1 |
| Intention-to-treat analysis (bias due to incomplete reporting) | 19 | 0 | 3 |
| Group similarity at baseline (selection bias) | 17 | 4 | 1 |
| Co-interventions (performance bias) | 19 | 1 | 2 |
| Compliance (performance bias) | 16 | 2 | 4 |
| Timing of outcome assessment (detection bias) | 20 | 0 | 2 |
Figure 2.
Funnel plot of gametocyte clearance effect measures.
Relative Effects of Artemether-lumefantrine
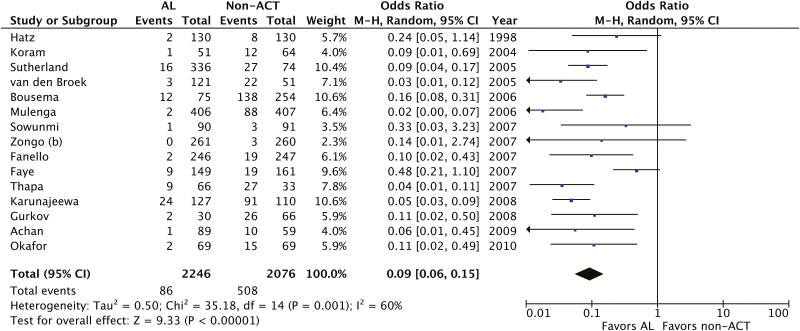
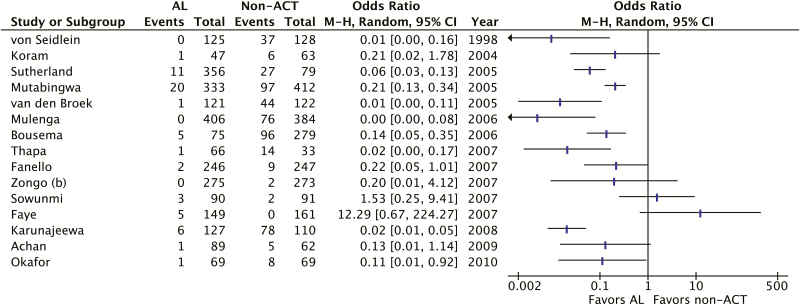
We found that AL significantly reduced the odds of gametocyte carriage one week after treatment compared to non-ACTs (OR 0.09; 95% confidence interval [CI], 0.06–0.15, I2 = 0.60, P < .01; 15 trials) (Figure 3). The proportion of participants with gametocytemia at one week ranged from 0–19% in the AL groups to 1–83% in the non-ACT groups. High statistical heterogeneity precluded pooled analysis of results at two weeks (I2 = 0.75; 15 trials), but 9 of the 15 trials showed AL to be significantly better than non-ACTs and, among the remainder, all except 2 trended toward AL (Figure 4). Trials that reported other gametocyte outcomes all showed fewer gametocytes or faster clearance of gametocytes among AL treatment groups than non-ACT groups, though not all results attained statistical significance (data not shown). The only trial that examined gametocyte sex ratios was limited by small sample size, and did not detect a statistically significant difference between AL and the non-ACT comparator [33]. We performed a sensitivity analysis including only trials that reported a procedure for masking study microscopists during gametocyte assessments. The sensitivity analysis showed similar results to the primary meta-analysis (OR 0.07; 95% CI, 0.04–0.13; I2 = 0.52, P < .01; 8 trials).
Figure 3.
Meta-analysis of trials comparing AL to non-ACTs for gametocyte clearance at post-treatment day 7. Abbreviations: ACT, artemisinin-based combination therapy; AL, artemether-lumefantrine; CI, confidence interval; M-H, Mantel-Haenszel.
Figure 4.
Summary of results of trials comparing AL to non-ACTs for gametocyte clearance at post-treatment day 14. High statistical heterogeneity (I2 = 0.75) precluded meta-analysis of these trials. Abbreviations: ACT, artemisinin-based combination therapy; AL, artemether-lumefantrine; CI, confidence interval; M-H, Mantel-Haenszel.
We identified 2 trials that carried out mosquito feeding assays [18, 21]. One trial performed assessments on post-treatment day 7 in participants treated with either AL or CQ-SP [21], the other on day 14 in participants receiving AL, SP, or AQ-SP [18]. Both demonstrated significantly fewer infected mosquitoes among those fed on blood from AL-treated participants compared to non-ACT-treated participants (day 7: OR 0.06; 95% CI, 0.00–0.47; P < .01; day 14: OR 0.56; 95% CI, 0.36–0.88; P = .01).
Subgroup Analyses
We performed subgroup analyses by participant age, comparator drug, and geographic region (Table 3). We found no detectable difference in gametocyte clearance between children ≤5 years (OR 0.07; 95% CI, 0.04–0.11; I2 = 0, P < .01; 5 trials) and children >5 years (OR 0.04; 95% CI, 0.01–0.10; I2 = 0.36, P < .01; 3 trials). We found a smaller effect size in trials comparing AL to AQ-SP (OR 0.22; 95% CI, 0.12–0.40; I2 = 0.17, P < .01; 6 studies) than those comparing it to CQ-SP (OR 0.06; 95% CI, 0.04–0.10; I2 = 0.15, P < .01; 3 trials) with non-overlapping CIs. AL appeared to exhibit greater effect in trials done in South and Southeast Asia (OR 0.04; 95% CI, 0.03–0.07; I2 = 0, P < .01; 3 trials) compared to sub-Saharan Africa (OR 0.12; 95% CI, 0.07–0.21; I2 = 0.50, P < .01; 12 trials), but the association with geographic region is likely confounded by the exclusive use CQ-SP comparators in trials in Asia, whereas the African trials included a mix of comparators.
Table 3.
Results of Subgroup Meta-analyses of AL vs. Non-ACTs for Gametocyte Clearance at Post-treatment Day 7
| Subgroup | Description | No. Studies | OR (95% CI) | I2-Statistic | P Value |
|---|---|---|---|---|---|
| All studies | 15 | 0.09 (0.06–0.15) | 0.60 | <.01 | |
| Age | Children ≤ 5 years | 5 | 0.07 (0.04–0.11) | 0.00 | <.01 |
| Children > 5 years | 3 | 0.04 (0.01–0.10) | 0.36 | <.01 | |
| Comparator drug | AQ-SP | 6 | 0.22 (0.12–0.40) | 0.17 | <.01 |
| CQ-SP | 3 | 0.06 (0.04–0.10) | 0.15 | <.01 | |
| Geographic region | Sub-Saharan Africa | 12 | 0.12 (0.07–0.21) | 0.50 | <.01 |
| South and Southeast Asia | 3 | 0.04 (0.03–0.07) | 0.00 | <.01 |
Abbreviations: AQ, amodiaquine; CI, confidence interval; CQ, chloroquine; OR, odds ratio; SP, sulfadoxine/sulfalene-pyrimethamine.
DISCUSSION
We assessed the body of evidence comparing the effects of AL and non-ACTs on P. falciparum gametocyte clearance and transmission interruption. We found a consistent, large effect favoring AL. At 1 week following treatment, AL reduced the odds of gametocyte carriage by 91% relative to non-ACTs and disrupted transmission to mosquitoes. The quality of the evidence was judged to be good overall, with low risk of bias. Thus, an important advantage of AL over non-ACT regimens, in addition to better efficacy against clinical illness, is better ability to prevent transmission to mosquitoes.
The combination of artemether’s direct antigametocyte action and faster killing of P. falciparum asexual stages likely explains the decreased gametocyte carriage and mosquito infectivity of AL-treated participants compared to non-ACT-treated participants [9–11]. Direct activity against early-stage gametocytes precludes maturation to transmissible late-stage gametocytes, and clearance of asexual stages is hypothesized to deplete the pool from which gametocytes arise [40]. Artemisinins also may interrupt gametocyte progression through sporogony once they are taken up by vector mosquitoes [12]. The non-artemisinin component of AL, lumefantrine, also may have antigametocyte and sporonticidal action [41–43]. Subgroup analyses illustrated a hierarchy of antigametocyte efficacy among specific comparators (AL > AQ-SP > CQ-SP), consistent with results previously reported in the literature [44]. The results of the trial that administered the gametocytocide primaquine to participants with gametocytemia at any follow-up visit were consistent with those observed in other trials despite more in the non-ACT group receiving primaquine, offering a conservative estimate of the relative effect of AL [22].
Gametocytemia is an imperfect marker of infectivity; infected individuals can remain infective after gametocytes fall below microscopically detectable concentrations, and factors in addition to absolute counts, such as gametocyte sex ratios, may modulate transmission [45–47]. Mosquito feeding assays remain the most direct means of assessment of transmissibility, but these are challenging to do. Only 2 trials included feeding assays [18, 21]. They showed that blood from AL-treated participants reduced infectivity to mosquitoes on post-treatment days 7 and 14 compared to blood from those treated with non-ACT regimens. The single trial that correlated treatment regimens to gametocyte sex ratios found no difference between AL and the non-ACT comparator, AQ-SP, but its small number of gametocytemic individuals may have led to insufficient statistical power to detect a difference [34].
Age-related immunity is believed to contribute to gametocyte clearance [3], and we hypothesized it would influence the observed effect sizes in children of different age groups. However, in subgroup meta-analyses comparing trials of children above and below 5 years of age, we found no significant difference in gametocyte clearance. This finding was unexpected, as older children have been shown to display increased immune responses directed against gametocytes compared to younger children [3, 48]. Surprisingly, field trials suggest gametocyte carriage may persist longer in older than younger children [49]. Immune-mediated clearance of gametocytes remains incompletely characterized, and age-related immunity appears to differ in regard to clearance of gametocytes and that of asexual parasites [49–51].
Impressive gains in malaria control and elimination made over the last decade are threatened by political instability, repercussions of climate change, stagnating funding, rising insecticide resistance, and the prospect of artemisinin-resistant parasites spreading to sub-Saharan Africa [2, 52]. These threats can be mitigated by optimizing our use of available tools. To this end, future trials of the relative effects of different ACTs on transmission of parasites to mosquitoes will be helpful. A network meta-analysis that extends the scope of the present study to compare the relative effects of different ACTs to each other and to non-ACTs would be informative.
Beyond malaria case management, the relative transmission impacts of antimalarials may have relevance for other drug deployment strategies. Treated symptomatic cases continue to contribute to the infectious reservoir due to persistent circulation of gametocytes for a duration that varies by therapy. But asymptomatic cases, a heterogeneous group, also contribute to the infectious reservoir [53–55]. Asymptomatic cases are targeted by intermittent preventive therapy, mass drug administration, and screen-and-treat programs. Studies and mathematical models of the comparative effects of these interventions using different antimalarial drugs could further aid in optimizing malaria control strategies.
There were limitations to this review. We only included trials with an AL treatment group, limiting the generalizability of our results to other ACTs. We compared AL to a number of different regimens, limiting our ability to tease out effects of individual drugs. Only 2 trials were designed to assess treatment impact on transmission. Misclassification bias was potentially high, due to reliance mainly on microscopy rather than more sensitive PCR-based methods of gametocyte detection. Although all included trials used genotyping to distinguish treatment failure from reinfection, none reported the association between gametocyte outcomes and reinfection or recrudescence; it was therefore not possible to distinguish among persistent gametocytemia following initial treatment, recrudescent gametocytemia, or new gametocytemia due to reinfection. Despite these limitations, the large effect size for AL strongly suggests that it offers benefit over non-ACT regimens in abating malaria transmission.
CONCLUSIONS
AL is superior to non-ACT regimens in reducing gametocyte carriage and interrupting transmission in individuals with uncomplicated falciparum malaria. In a meta-analysis of 15 trials, treatment with AL led to a 91% reduction in the odds of gametocytemia at one week compared to non-ACTs. Two trials showed reduction in transmission to mosquitoes at 1 and 2 weeks post-treatment. The benefit of transmission interruption conferred by ACTs has relevance for policy makers planning optimal utilization of currently available control and elimination tools, including the use of ACTs for malaria treatment and chemoprevention.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgements. This review was initiated as coursework at the Johns Hopkins Bloomberg School of Public Health. We acknowledge the course director, Dr Kay Dickersin, and teaching assistants Nicole Fusco and Jimmy Le. We also thank Donna Hesson from the Johns Hopkins William H. Welch Medical Library for her assistance in developing the electronic search strategy.
Financial support. M. M. I. and E. W. were supported by the National Institute of General Medical sciences (T32GM066691, T32GM007546), J. J. was supported by the National Heart, Lung, and Blood Institute (T32HL1252391), and N. M. was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (T32AR048522) of the National Institutes of Health.
Potential conflicts of interest. T. L. was the course instructor and received salary support from the Johns Hopkins Bloomberg School of Public Health. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. World Malaria Report. Geneva, Switzerland: WHO Global Malaria Programme, 2016. [Google Scholar]
- 2. Bhatt S, Weiss DJ, Cameron E et al. . The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526:207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 2011; 24:377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vinetz JM, Clain J, Bounkeua V, Eastman RT, Fidock D. Chemotherapy of Malaria. In: Hilal-Dandan R, Laurence B, eds. Goodman and Gilman’s Manual of Pharmacology and Therapeutics. New York: McGraw-Hill, 2015. [Google Scholar]
- 5. Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database Syst Rev 2009:CD007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smithuis F, Kyaw MK, Phe O et al. . Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis 2010; 10:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Price RN, Nosten F, Luxemburger C et al. . Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996; 347:1654–8. [DOI] [PubMed] [Google Scholar]
- 8. Bousema T, Okell L, Shekalaghe S et al. . Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J 2010; 9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen PQ, Li GQ, Guo XB et al. . The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J 1994; 107:709–11. [PubMed] [Google Scholar]
- 10. Kumar N, Zheng H. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol Res 1990; 76:214–8. [DOI] [PubMed] [Google Scholar]
- 11. Mehra N, Bhasin VK. In vitro gametocytocidal activity of artemisinin and its derivatives on Plasmodium falciparum. Jpn J Med Sci Biol 1993; 46:37–43. [DOI] [PubMed] [Google Scholar]
- 12. Chotivanich K, Sattabongkot J, Udomsangpetch R et al. . Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother 2006; 50:1927–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WorldWide Antimalarial Resistance Network Lumefantrine PK/PD Study Group. Artemether-lumefantrine treatment of uncomplicated Plasmodium falciparum malaria: a systematic review and meta-analysis of day 7 lumefantrine concentrations and therapeutic response using individual patient data. BMC Med 2015; 13:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abay SM. Blocking malaria transmission to Anopheles mosquitoes using artemisinin derivatives and primaquine: a systematic review and meta-analysis. Parasit Vectors 2013; 6:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makanga M. A review of the effects of artemether-lumefantrine on gametocyte carriage and disease transmission. Malar J 2014; 13:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WorldWide Antimalarial Resistance Network Gametocyte Study Group. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med 2016; 14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faye B, Ndiaye JL, Ndiaye D, Dieng Y, Faye O, Gaye O. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malar J 2007; 6:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bousema JT, Schneider P, Gouagna LC et al. . Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis 2006; 193:1151–9. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gøtzsche PC et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chapter 9.5.d Identifying and measuring heterogeneity. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011]: The Cochrane Collaboration, 2011. [Google Scholar]
- 21. Sutherland CJ, Ord R, Dunyo S et al. . Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med 2005; 2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thapa S, Hollander J, Linehan M et al. . Comparison of artemether-lumefantrine with sulfadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria in eastern Nepal. Am J Trop Med Hyg 2007; 77:423–30. [PubMed] [Google Scholar]
- 23. Mulenga M, Van Geertruyden JP, Mwananyanda L et al. . Safety and efficacy of lumefantrine-artemether (Coartem) for the treatment of uncomplicated Plasmodium falciparum malaria in Zambian adults. Malar J 2006; 5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valecha N, Krudsood S, Tangpukdee N et al. . Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated Plasmodium falciparum malaria: a comparative, multicenter, randomized clinical trial. Clin Infect Dis 2012; 55:663–71. [DOI] [PubMed] [Google Scholar]
- 25. Hatz C, Abdulla S, Mull R et al. . Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1–5 years. Trop Med Int Health 1998; 3:498–504. [DOI] [PubMed] [Google Scholar]
- 26. Mutabingwa TK, Anthony D, Heller A et al. . Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 2005; 365:1474–80. [DOI] [PubMed] [Google Scholar]
- 27. Koram KA, Abuaku B, Duah N, Quashie N. Comparative efficacy of antimalarial drugs including ACTs in the treatment of uncomplicated malaria among children under 5 years in Ghana. Acta Trop 2005; 95:194–203. [DOI] [PubMed] [Google Scholar]
- 28. Fanello CI, Karema C, van Doren W, Van Overmeir C, Ngamije D, D’Alessandro U. A randomised trial to assess the safety and efficacy of artemether-lumefantrine (Coartem) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwanda. Trans R Soc Trop Med Hyg 2007; 101:344–50. [DOI] [PubMed] [Google Scholar]
- 29. Karunajeewa HA, Mueller I, Senn M et al. . A trial of combination antimalarial therapies in children from Papua New Guinea. N Engl J Med 2008; 359:2545–57. [DOI] [PubMed] [Google Scholar]
- 30. Achan J, Tibenderana JK, Kyabayinze D et al. . Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial. BMJ (Clinical research ed) 2009; 339:b2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chandra R, Ansah P, Sagara I et al. . Comparison of azithromycin plus chloroquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in children in Africa: A randomized, open-label study. Malar J 2015; 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Seidlein L, Bojang K, Jones P et al. . A randomized controlled trial of artemether/benflumetol, a new antimalarial and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. Am J Trop Med Hyg 1998; 58:638–44. [DOI] [PubMed] [Google Scholar]
- 33. Zongo I, Dorsey G, Rouamba N et al. . Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis 2007; 45:1453–61. [DOI] [PubMed] [Google Scholar]
- 34. Sowunmi A, Balogun T, Gbotosho GO et al. . Activities of artemether-lumefantrine and amodiaquine-sulfalene-pyrimethamine against sexual-stage parasites in falciparum malaria in children. Chemotherapy 2008; 54:201–8. [DOI] [PubMed] [Google Scholar]
- 35. Mayxay M, Khanthavong M, Lindegårdh N et al. . Randomized comparison of chloroquine plus sulfadoxine-pyrimethamine versus artesunate plus mefloquine versus artemether-lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People’s Democratic Republic. Clin Infect Dis 2004; 39:1139–47. [DOI] [PubMed] [Google Scholar]
- 36. Okafor HU, Shu EN, Oguonu T. Therapeutic efficacy and effect on gametocyte carriage of an artemisinin and a non-based combination treatment in children with uncomplicated P. falciparum malaria, living in an area with high-level chloroquine resistance. J Trop Pediatr 2010; 56:398–406. [DOI] [PubMed] [Google Scholar]
- 37. van den Broek IV, Maung UA, Peters A et al. . Efficacy of chloroquine + sulfadoxine–pyrimethamine, mefloquine + artesunate and artemether + lumefantrine combination therapies to treat Plasmodium falciparum malaria in the Chittagong Hill Tracts, Bangladesh. Trans R Soc Trop Med Hyg 2005; 99:727–35. [DOI] [PubMed] [Google Scholar]
- 38. Zongo I, Dorsey G, Rouamba N et al. . Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet 2007; 369:491–8. [DOI] [PubMed] [Google Scholar]
- 39. Gürkov R, Eshetu T, Miranda IB et al. . Ototoxicity of artemether/lumefantrine in the treatment of falciparum malaria: a randomized trial. Malar J 2008; 7:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baker DA. Malaria gametocytogenesis. Mol Biochem Parasitol 2010; 172:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Delves M, Plouffe D, Scheurer C et al. . The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLoS Med 2012; 9:e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bolscher JM, Koolen KM, van Gemert GJ et al. . A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J Antimicrob Chemother 2015; 70:1357–66. [DOI] [PubMed] [Google Scholar]
- 43. Lucantoni L, Duffy S, Adjalley SH, Fidock DA, Avery VM. Identification of MMV malaria box inhibitors of plasmodium falciparum early-stage gametocytes using a luciferase-based high-throughput assay. Antimicrob Agents Chemother 2013; 57:6050–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zwang J, Olliaro P, Barennes H et al. . Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: a multi-centre analysis. Malar J 2009; 8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. White NJ, Ashley EA, Recht J et al. . Assessment of therapeutic responses to gametocytocidal drugs in Plasmodium falciparum malaria. Malar J 2014; 13:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dicko A, Brown JM, Diawara H et al. . Primaquine to reduce transmission of Plasmodium falciparum malaria in Mali: a single-blind, dose-ranging, adaptive randomised phase 2 trial. Lancet Infect Dis 2016; 16:674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Robert V, Read AF, Essong J et al. . Effect of gametocyte sex ratio on infectivity of Plasmodium falciparum to Anopheles gambiae. Trans R Soc Trop Med Hyg 1996; 90:621–4. [DOI] [PubMed] [Google Scholar]
- 48. Saeed M, Roeffen W, Alexander N, Drakeley CJ, Targett GA, Sutherland CJ. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS One 2008; 3:e2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ayanful-Torgby R, Oppong A, Abankwa J, Acquah F, Williamson KC, Amoah LE. Plasmodium falciparum genotype and gametocyte prevalence in children with uncomplicated malaria in coastal Ghana. Malar J 2016; 15:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paul NH, Vengesai A, Mduluza T et al. . Prevalence of Plasmodium falciparum transmission reducing immunity among primary school children in a malaria moderate transmission region in Zimbabwe. Acta Trop 2016; 163:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stone WJ, Dantzler KW, Nilsson SK et al. . Naturally acquired immunity to sexual stage P. falciparum parasites. Parasitology 2016; 143:187–98. [DOI] [PubMed] [Google Scholar]
- 52. WHO. World Malaria Report. Geneva, Switzerland: WHO Global Malaria Programme, 2016. [Google Scholar]
- 53. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 2014; 30:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 2013; 11:623–39. [DOI] [PubMed] [Google Scholar]
- 55. Ogutu B, Tiono AB, Makanga M et al. . Treatment of asymptomatic carriers with artemether-lumefantrine: an opportunity to reduce the burden of malaria? Malar J 2010; 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dorsey G, Staedke S, Clark TD et al. . Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA 2007; 297:2210–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.