Summary
This study addresses the clinical relevance of antibiotic resistance in primary or ambulatory care. It shows that antibiotic resistance significantly impacts on patients’ illness burden in the community and may also impact on primary-care workload.
Keywords: antibiotic resistance, primary care, clinical significance
Abstract
Background.
Antibiotic use is the main driver for carriage of antibiotic-resistant bacteria. The perception exists that failure of antibiotic treatment due to antibiotic resistance has little clinical impact in the community.
Methods.
We searched MEDLINE, EMBASE, PubMed, Cochrane Central Register of Controlled Trials, and Web of Science from inception to 15 April 2016 without language restriction. We included studies conducted in community settings that reported patient-level data on laboratory-confirmed infections (respiratory tract, urinary tract, skin or soft tissue), antibiotic resistance, and clinical outcomes. Our primary outcome was clinical response failure. Secondary outcomes were reconsultation, further antibiotic prescriptions, symptom duration, and symptom severity. Where possible, we calculated odds ratios with 95% confidence intervals by performing meta-analysis using random effects models.
Results.
We included 26 studies (5659 participants). Clinical response failure was significantly more likely in participants with antibiotic-resistant Escherichia coli urinary tract infections (odds ratio [OR] = 4.19; 95% confidence interval [CI] = 3.27–5.37; n = 2432 participants), Streptococcus pneumoniae otitis media (OR = 2.51; 95% CI = 1.29–4.88; n = 921 participants), and S. pneumoniae community-acquired pneumonia (OR = 2.15; 95% CI = 1.32–3.51; n = 916 participants). Clinical heterogeneity precluded primary outcome meta-analysis for Staphylococcus aureus skin or soft-tissue infections.
Conclusions.
Antibiotic resistance significantly impacts on patients’ illness burden in the community. Patients with laboratory-confirmed antibiotic-resistant urinary and respiratory-tract infections are more likely to experience delays in clinical recovery after treatment with antibiotics. A better grasp of the risk of antibiotic resistance on outcomes that matter to patients should inform more meaningful discussions between healthcare professionals and patients about antibiotic treatment for common infections.
Antibiotic resistance is recognized as an important societal health issue. Yet members of the public consider the risk of antibiotic resistance to apply to society at large and in the distant future, rather than constituting a risk to their own health, and primary-care clinicians report that they rarely encounter treatment failure because of antibiotic resistance, leading to the perception that antibiotic resistance is remote from prescribing decisions [1–3]. This major evidence gap may influence expectations for antibiotics and antibiotic-prescribing decisions in the community [4, 5].
Although the consequences of antibiotic-resistant infections in hospitalized patients are known (increased mortality, longer hospital stays, and increased healthcare costs) [6, 7], antibiotic resistance may also have important consequences for patients with common infections managed in the community [8]. Approximately 300 million primary-care consultations in the United Kingdom and 490 million consultations in the United States each year [14, 17] are for respiratory-tract (10%–20%) [9–11], urinary-tract (1%–3%) [12, 13], and skin and soft-tissue infections (1%) [14–16]. Almost 75% of all antibiotics in the United Kingdom are prescribed in primary care [18] and at considerable cost [19–21].
Antibiotic use is also the most important risk factor for carriage of antibiotic-resistant bacteria [22, 23] and the development of subsequent antibiotic-resistant infections. However, the clinical relevance of antibiotic resistance for patients with common infections in the community is less well understood. This systematic review aims to compare clinical outcomes between antibiotic-resistant and antibiotic-sensitive infections in the community for patients with respiratory-tract, urinary-tract, and skin or soft-tissue infections.
METHODS
Search Strategy and Inclusion Criteria
We systematically searched electronic databases (MEDLINE, EMBASE, PubMed, Cochrane Central Register of Controlled Trials, and Web of Science) from inception to 15 April 2016 with no language restrictions. We used the Medical Subject Headings (MeSH) terms and validated search filters for “antibiotic resistance” [23] and “primary care/community setting” [24] and keywords “antibiotic resistance,” “skin or soft tissue infections,” “respiratory tract infections,” “otitis media,” and “urinary tract infections” (Supplementary Data Files 1 and 2).The review protocol was registered on the PROSPERO database (CRD42015032441).
Observational studies and randomized controlled trials (RCTs) were eligible for inclusion if the study was conducted in a community setting (general practice, hospital outpatient clinic, or emergency department) and reported patient-level data on laboratory-confirmed potentially pathogenic infections, antibiotic resistance, and clinical outcomes. Studies solely conducted in hospital inpatient settings, involving patients with hospital-acquired infections and highly specific patient groups in whom specialised antibiotic treatment strategies are recommended (eg, cystic fibrosis), were excluded.
We categorized respiratory-tract infections (RTIs) into community-acquired pneumonia (CAP), sore throat/pharyngitis, acute otitis media (AOM), and acute maxillary sinusitis (AMS).
Our primary outcome was clinical response failure, which we defined as the persistence of symptoms after completion of antibiotic treatment. Where studies reported outcomes at >1 time point, we selected the time point closest to 7–14 days from baseline to reflect the duration of typical antibiotic regimens. Secondary outcomes were reconsultation, further antibiotic prescriptions (both within 30 days from baseline), symptom duration, and symptom severity.
Data Extraction and Risk of Bias Assessment
Two reviewers (O. V. H., J. J. L.) independently extracted data on the characteristics of included studies (Table 1 and Supplementary Data File 2). For RCTs, outcome data for antibiotic-resistant and antibiotic-sensitive infections were extracted separately for each treatment arm because RCT studies only determined whether infections were antibiotic-resistant or antibiotic-sensitive after patients had already been randomized, hence randomization was not stratified according to antibiotic resistance.
Table 1.
Characteristics of 26 Included Studies (Primary and/or Secondary Outcomes) According to Infection
| Study | Country | Setting | Infection type | Study design | Participants | Potential pathogen being studied | Total no. recruited | Total no. with potential pathogen being studied | No. of potential pathogens being studied with evidence of antibiotic resistance | No. of patients where resistance and outcome data available | Primary outcome time pointa, b | Secondary outcomesc | Treatment antibiotic/ antibiotic classd |
Antibiotic to which resistance measured |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urinary-tract infection | ||||||||||||||
| Brown et al (2002) [35] | United States | OP/ PHC |
UTI | Obs (R) | Women | E | NR | 601 isolatese | 44 | 104 | … | Recc | TMP-SMX | TMP-SMX |
| Butler et al (2006) [8] | United Kingdom | PHC | UTI | CC Obs (P) | Aduluts | E | 932 | 922 | 94 | 797 [1] 862 (Rec) [2] 816 (Fab) [2] 420 (Sdur) [2] |
Within 30 days | Recc Fabc Sdurc |
Not specified | To prescribed antibiotic [1] To at least 1 antibiotic [2] |
| Gupta et al (2007) [37] | United States | PHC | UTI | RCT | Women | E | 338 | 276 | 34 | 308f | Day 3b | … | TMP-SMX Nitrofurantoin | TMP-SMX Nitrofurantoin |
| Little et al (2010) [50] | United Kingdom | PHC | UTI | Obs (P) | Women | NRg | 843 | NR | NR | 264 (Sdur) 264 (Ssev) |
- | Sdurc Ssevc |
Not specified | To >1 antibiotics |
| McNulty et al (2006) [32] | United Kingdom | PHC | UTI | Obs (P) | Women | E | 497 | 298h | 44 | 207 (Sdur)f 317 (Rec) |
Day 7b | Sdurc Recc |
Trimethoprim | Trimethoprim |
| Noskin et al (2001) [33] | United States | OP | UTI | Obs (P) | Women | E | 156 | 89 | 42 | 71 | NRb | … | Not specified | To >1 antibiotics |
| Raz et al (2002) [36] | Israel | OP | UTI | Obs (P) | Women | E | 618 | 425 | 30 | 484f | Days 5–9b | … | TMP-SMX | TMP-SMX |
| Soraas et al (2014) [34] | Norway | Other | UTI | Obs (P) | Adults | ESBL-E | 343 | 343i | 81 (ESBL-E) | 343 | Within 14 daysb | Fabc | Mecillinam Nonmecillinam |
Mecillinam and ESBL status |
| Vallano et al (2006) [39] | Spain | PHC | UTI | Obs (P) | Women | E | 220 | 88 | 15f | 108f | Within 14 daysb | … | Not specified | To >1 antibiotics |
| Van Merode et al (2005) [38] | Netherlands | PHC | UTI | RCT | Women | E | 324 | 80 | 17 | 114f | Days 6–8b | … | Trimethoprim | Trimethoprim |
| Community-acquired pneumonia | ||||||||||||||
| Cao et al (2010) [49] | China | OP | RTI (CAP) | Obs (P) | Adults; adolescents | MP | 356 | 67 | 46 | 59 | … | Fabc Sdurc |
Not specified | Erythromycin |
| Hagberg et al (2003) [29] | Multiple | IP/OP | RTI (CAP) | Pooled data from 6 phase III trials | Adults | SP | 1373 | 174 | 23j | 174 | Days 3–5b | … | Telithromycin | Penicillin or erythromycin |
| Kawai et al (2012) [51] | Japan | OP | RTI (CAP) | Obs (P) | Children; adolescents | MP | 476 | 50 | 21 | 30 | … | Fabc | Not specified | To >1 macrolide |
| O’Doherty et al (1997) [40] | United Kingdom; Ireland | OP | RTI (CAP) | RCT | Adults | SP | 264 | 30k | 6 | 30 | Days 3–5b | … | Grepafloxacin Amoxicillin |
Amoxicillin |
| Van Rensburg et al (2005) [30] | Multiple | OP | RTI (CAP) | Pooled RCT (8 phase III trials and 1 phase II study) |
Adults | SP | 2339 | 418 | 61 | 327 | Days 17–24b | … | Telithromycin | To erythromycin and penicillin |
| Yanagihara et al (2004) [41] | Japan | OP | RTI (CAP) | Obs (R) | Adults | SP | 306 | 306 | 129 | 306 | NRb | … | Not specified | Penicillin |
| Zhanel et al (2014) [31] | Multiple | Other | RTI (AMS) RTI (CAP) RTI (AOM) |
Pooled RCT (11 RCTs; 2 phase III trials) |
Adults; children | SP | 872l 309 CAP |
CAP 79 | CAP 27 | CAP 79 | NRb | … | Azithromycin | Azithromycin |
| Acute otitis media | ||||||||||||||
| Barry et al (1994) [27] | France | OP | RTI (AOM) | Pooled data from 3 RCTs | Children | SP | 1092 | 236 | 54m | 219 | Day 10 | … | B-lactams (combined) | Penicillin; B-lactams |
| Dagan et al (1996) [42] | Israel | ER | RTI (AOM) | RCT | Children | SP | 266 | 98 | 18 | 77 | Day 10 | … | Cefuroxime Cefaclor |
Cefuroxime Cefaclor |
| Hoberman et al (1996) [47] | Multiple | IP/OP | RTI (AOM) | Obs (P) | Children | SP | 917 | 298 | 82 | 260 | Days 12–14b | … | Co-amoxiclav | Penicillin |
| Hoberman et al (2005) [45] | Multiple | OP | RTI (AOM) | RCT | Children | SP | 730 | 229 | 158 | 188 | Days 12–14b | … | Co-amoxiclav Azithromycin | Penicillin Azithromycin |
| Zhanel et al (2014) [31] | Multiple | Other | RTI (AMS) RTI (CAP) RTI (AOM) |
Pooled RCT (11 RCTs; 2 phase III trials) |
Adults; children | SP | 872l AOM 402 |
AOM 177 | AOM 41 | AOM 177 | NR | … | Azithromycin | Azithromycin |
| Acute sore throat | ||||||||||||||
| Quinn et al (2003) [46] | United States; Canada | OP | RTI (sore throat) |
RCT | Adults; adolescents | Spy | 526 | 360n | 9 | 285 | Days 16–23b | … | Telithromycin Clarithromycin |
Erythromycin |
| Seppala et al (2002) [48] | Finland | OP | RTI (sore throat) |
Obs (R) | NR | Spy | NR | 529 | 76 | 273 | NR | … | Erythromycin Penicillin |
Erythromycin |
| Acute maxillary sinusitis | ||||||||||||||
| Buchanan et al (2005) [28] | Sweden | Other | RTI (AMS) | Pooled data from 3 RCTs | Adults; adolescents | SP | 1298 | 126 | 1 | 78 | Days 17–24b | … | Telithromycin | Telithromycin |
| Zhanel et al (2014) [31] | Multiple | Other | RTI (AMS) RTI (CAP) RTI (AOM) |
Pooled RCT (11 RCTs; 2 phase III trials) |
Adults; children | SP | 872l AMS 161 |
AMS 57 | AMS 19 | AMS 57 | NR | … | Azithromycin | Azithromycin |
| Skin and soft-tissue infection | ||||||||||||||
| Dagan et al (1992) [43] | Israel | OP | Skin (Imp) | RCT | Children | SA | 102 | 90 | 27 | 89 | Days 3–8 | … | Erythromycin Mupirocin |
Erythromycin, Mupirocin |
| Giordano et al (2006) [44] | United States | Other | Skin (USSSI) | RCT | Adults; adolescents | SA | 392 | 171 | 79 | 151 | Days 17–24b | … | Cefdinir Cephalexin |
Methicillin |
| Overall | 15580 | 5659 [3] | ||||||||||||
Abbreviations: AMS, acute maxillary sinusitis; AOM, acute otitis media; CAP, community-acquired pneumonia; CC, case control; E, Escherichia coli; ER, Emergency room; ESBL-E, extended spectrum β-lactamase Escherichia coli; Imp, impetigo; IP, hospital inpatients; MP, Mycoplasma pneumoniae; NR, not reported; Obs (P), prospective observational; Obs (R), retrospective observational; OP, hospital outpatients; Other, community setting (not specified); PHC, primary care clinic/general practice; RCT, randomized controlled trial; RTI, respiratory-tract infection; SA, Staphylococcus aureus; SP, Streptococcus pneumoniae; Spy, Streptococcus pyogenes; TMP-SMX, trimethoprim-sulfamethoxazole; USSSI, uncomplicated skin and skin structure infections (eg, cellulitis, erysipelas, impetigo, simple abscess, wound infection, furunculosis, folliculitis); UTI, urinary-tract infection.
aPrimary outcome: “response failure” defined as the persistence of symptoms after completion of antibiotic treatment. Where the outcome was reported as “clinical cure” in the study, we calculated the proportion of patients that had failed to respond to antibiotic treatment within the designated timescale (ie, 1 − proportion of patients with clinical cure).
bData on clinical cure, rather than clinical response failure, were reported by 10 randomized controlled trials [28–31, 37, 38, 40, 44–46] and 7 observational studies [32–34, 36, 39, 41, 47]. Overall, clinical response failure was assessed 3–5 days from baseline in 3 studies [29, 37, 40], 6–10 days from baseline in 6 studies [27, 32, 36, 38, 42, 43], 11–14 days from baseline in 4 studies [34, 39, 45, 47], 20–30 days from baseline in 5 studies [8, 28, 30, 44, 46], and not reported in 4 studies [31, 33, 41, 48].
cSecondary outcomes: reconsultation (Rec), further antibiotic prescriptions (Fab), symptom duration (Sdur), and symptom severity (Ssev).
dMultiple antibiotics prescribed in separate study arms.
eWe assumed 1 isolate per participant.
fAll combined pathogens.
gSpecific organism not reported.
hColiforms; 242 single isolates were sent to HPA Antibiotic Resistance Monitoring and Reference Laboratory (ARMRL) of which Escherichia coli accounted for 90% (n = 219/242).
iESBL E. coli and non-ESBL E. coli only.
jPenicillin- or erythromycin-resistant (all Streptococcus pneumoniae isolates were susceptible to telithromycin).
kOnly 81 were evaluated microbiologically.
lExcluded acute exacerbations of chronic bronchitis.
mOne child in the SpRP group did not complete the treatment course because of adverse events and was not evaluable for clinical response.
nPositive screening for group A ß-haemolytic streptococcus.
…not applicable [1].: Resistance measured to prescribed antibiotic [2];: Resistance measured to at least one antibiotic [3];: where more than one outcome data available, the lowest number was taken.
Data had to be reported in sufficient detail to assess relevant outcomes between patients with antibiotic-resistant and antibiotic-sensitive infections in order to construct a 2 × 2 contingency table. Where possible, we extracted outcomes for antibiotic-resistant and antibiotic-sensitive infections whereby resistance and sensitivity were defined in relation to the same antibiotic or class of antibiotic as the antibiotic being prescribed. If studies reported intermediate levels of antibiotic resistance for certain infections, these were classified as antibiotic-resistant infections in our analysis. If there was no agreement between susceptibility and treatment antibiotic, or the study did not report the type of antibiotic prescribed, studies were still included but specifically highlighted.
The quality of the included studies was assessed independently by 2 reviewers (O. V. H., J. L.) for RCTs and observational studies based on their respective risk-of-bias tool, namely the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials and critical appraisal skills programme (CASP) checklist for cohort studies (Supplementary Data File 3) [25, 26].
Statistical Analysis
To compare the odds of clinical response failure between antibiotic-resistant and antibiotic-sensitive infections, we calculated odds ratios (ORs) with 95% confidence intervals (Cis) for infections where data were available from ≥3 studies for the same bacterial pathogen using random effects meta-analysis. Heterogeneity was assessed using the χ2 test and I2 statistic. Odds ratios in relation to reconsultation and further antibiotic prescriptions were calculated using similar methods. For continuous data, we planned to plot survival curves where possible for duration and severity of symptoms in antibiotic-resistant versus antibiotic-sensitive infections.
Subgroup analyses were performed according to study design (observational studies vs RCTs) and type of healthcare setting (general practice, hospital outpatient clinic, or emergency department). Results were summarized narratively where data were not sufficient to perform meta-analysis or plot survival curves. Analysis was conducted using StataSE version 13.
RESULTS
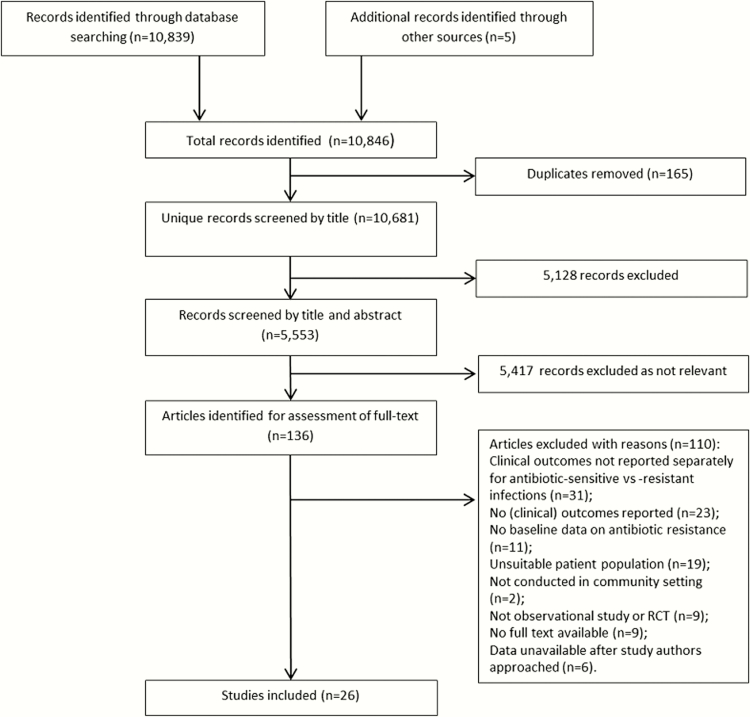
We identified 10681 records, of which 136 full-text articles were assessed. The most common reason for exclusion (n = 31/110) was that clinical outcomes were not reported separately for antibiotic-resistant versus antibiotic-sensitive infections.
Twenty-six studies were included (Figure 1), of which 13 were observational studies, 8 were RCTs, and 5 were secondary analyses of pooled RCT data [27–31]. Six studies were conducted in primary care/general practice, 12 in hospital outpatients, 1 in a mixed outpatient/primary care setting, 2 in a mixed outpatient/inpatient setting, 1 in an emergency department setting, and 4 in another community setting which was not clearly defined (Table 1). Our included RCTs and secondary analyses of pooled RCTs did not report any duplicate data.
Figure 1.
Study selection. Abbreviation: RCT, randomized controlled trial.
Data relating to ≥1 study outcomes were available for 15580 patients, of whom 6617 patients had a laboratory-confirmed potentially pathogenic bacterial infection. Data on whether the infection was antibiotic-resistant or antibiotic-sensitive were also available for 5659 of these patients (antibiotic-resistant: n = 1268; antibiotic-sensitive: n = 4391) (Table 2).
Table 2.
Data Related to 1 or More Study Outcomes According to Infection Type and Bacterial Pathogen
| Infection | Bacteria | No. of studies | No. of antibiotic-resistant infections | No. of antibiotic-sensitive infections |
| UTI [8, 32–39, 50] | Escherichia coli | 10 | 523 | 2277 |
| CAP [29–31, 40, 41] | Streptococcus pneumoniae | 5 | 246 | 670 |
| CAP [49, 51] | Mycoplasma pneumoniae | 2 | 63 | 24 |
| AOM [27, 31, 42, 45, 47] | Streptococcus pneumoniae | 5 | 225 | 696 |
| Sore throat [46, 48] | Group A ß-haemolytic Streptococcus | 2 | 85 | 473 |
| AMS [28, 31] | Streptococcus pneumoniae | 2 | 20 | 115 |
| Skin infection [43, 44] | Staphylococcus aureus | 2 | 106 | 134 |
Abbreviations: AMS, acute maxillary sinusitis; AOM, acute otitis media; CAP, community-acquired pneumonia; UTI, urinary tract infection.
Clinical criteria for obtaining urine samples and diagnosing urinary-tract infections (UTIs) varied between studies. Diagnostic thresholds used to define Escherichia coli UTIs were reported as being >104 colony-forming units (CFU) in 3 studies (Supplemetary Data File 4A) [32–34]. Four studies obtained urine samples from patients with urinary symptoms and positive urine dipstick test [32, 33, 35, 36], 2 studies obtained urine samples from patients with urinary symptoms only [37, 38], 1 study obtained urine samples from patients with “clinically suspected” UTI [8], and 2 studies did not report selection criteria for obtaining urine samples [34, 39]. Most UTI studies counted infections of mixed uropathogens as indicating an infection; however, the dominant bacterium (>65%) was E. coli in all UTI studies. Where calculations were possible, the proportion of clinically suspected UTIs that had a laboratory-confirmed infection was 57%–95%. Three studies based clinical diagnosis of S. pneumoniae CAP on symptoms, radiographic evidence, and blood tests [29, 40, 41], 1 study based diagnosis on symptoms and blood tests [30], and 1 study [31] did not report how a diagnosis was established (Supplementary Data File 4B). Diagnostic criteria for S. pneumoniae AOM (Supplementary Data File 4C) were more uniform (symptoms, examination, and tympanocentesis) except for 1 study for which this was not reported [31].
Data relating to our primary outcome (clinical response failure) were available from 13 RCTs [27–31, 37, 38, 40, 42–46] and 9 observational studies [8, 32–34, 36, 39, 41, 47, 48]. Three observational studies reported data on reconsultations [8, 32, 35]; 4 studies reported data on further antibiotic prescriptions [8, 34, 49, 50]; 4 studies reported data on symptom duration [8, 32, 49, 50]; and 1 study reported data on symptom severity [50]. Data on these outcomes were not reported by any RCTs or secondary analyses of pooled RCT data.
The appendix (Supplementary Data File 3) summarizes our risk of bias assessment of included studies. For 12 of 13 RCTs, there was low risk of reporting bias [27–29, 31, 37, 38, 40, 42–46]. Only 1 RCT reported assessing outcomes blinded from knowledge of whether the infection was antibiotic-resistant or antibiotic-sensitive [42]. We were not able to assess whether RCTs considered confounding variables between antibiotic-resistant and antibiotic-sensitive infections except for 1 RCT [42] because baseline characteristics of the study population were not reported according to whether participants had an antibiotic-resistant or antibiotic-sensitive infection.
For the 13 observational studies, participants were representative of the defined population except for 1 study [34] and generally clearly defined. Antibiotic exposure was accurately measured (eg, secure medical records) in 10 studies [8, 32–34, 36, 39, 41, 48–50]. Only 6 observational studies attempted to address potential confounders, and measurement of outcome was only satisfactorily blinded in 2 studies [8, 35].
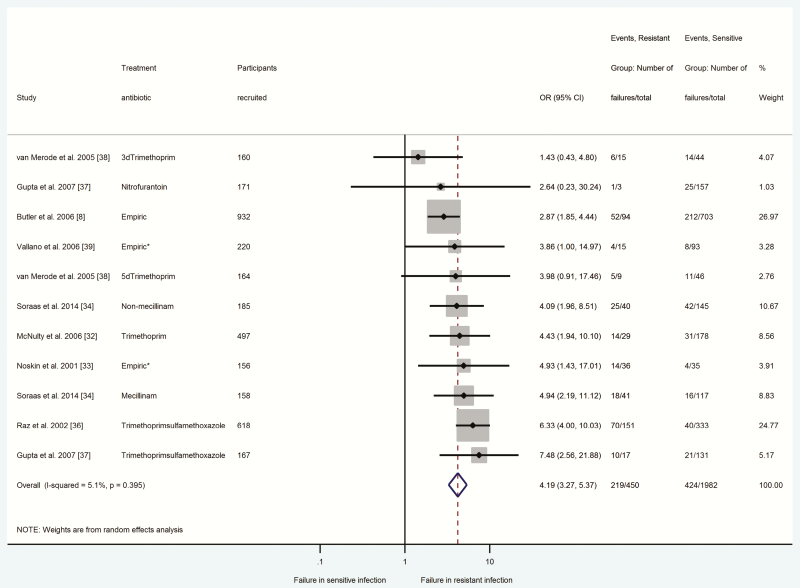
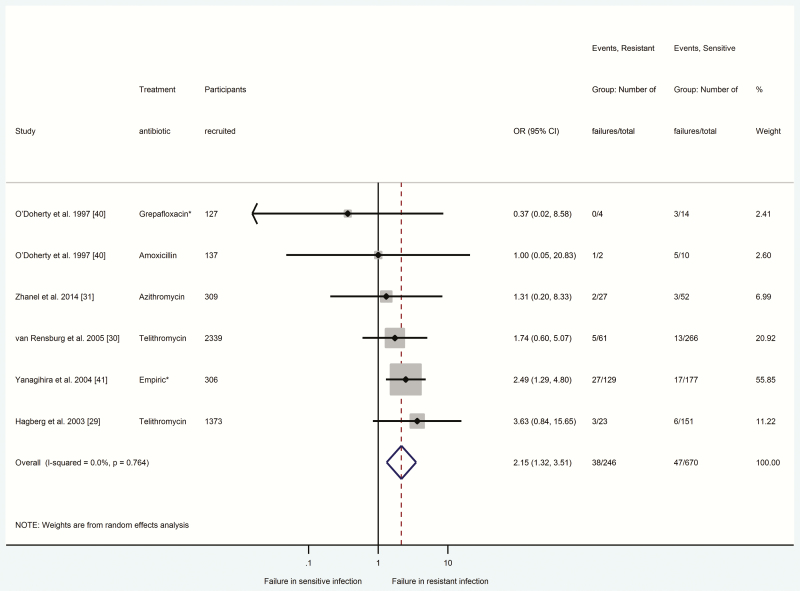
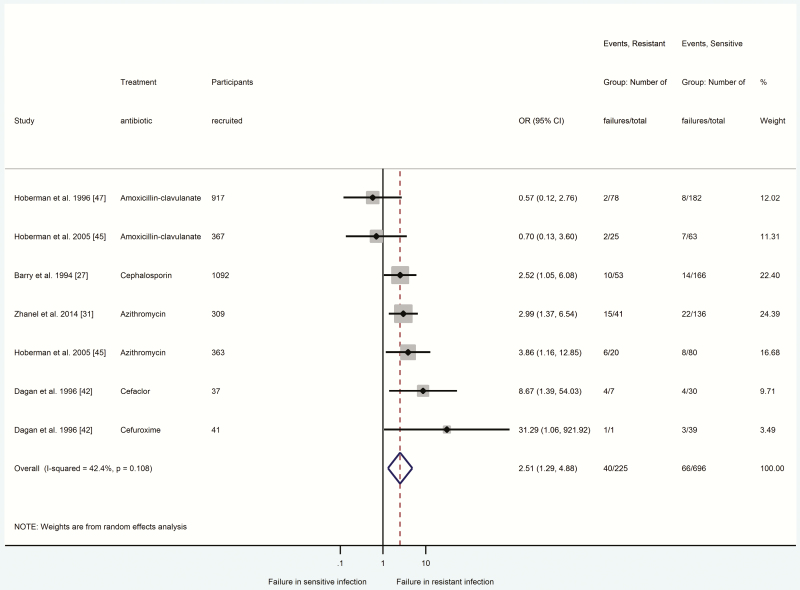
Figures 2–4 summarize odds ratios with 95% confidence intervals for participants with antibiotic-resistant E. coli UTIs (Figure 2), S. pneumoniae CAP (Figure 3), and S. pneumoniae AOM (Figure 4) in relation to clinical response failure. Clinical response failure was significantly more likely in antibiotic-resistant than antibiotic-sensitive E. coli UTIs (OR = 4.19; 95% CI = 3.27–5.37; P < .001; n = 2432 participants, 8 studies) [8, 32–34, 36–39]. Antibiotic-resistant S. pneumoniae CAP and AOM were also associated with significantly greater odds of clinical response failure (CAP: OR = 2.15, 95% CI = 1.32–3.51, P < .002, n = 916 participants, 5 studies [29–31, 40, 41]; AOM: OR = 2.51, 95% CI = 1.29–4.88, P < .007, n = 921 participants, 5 studies [27, 31, 42, 45, 47]).
Figure 2.
Comparison between antibiotic-resistant and antibiotic-sensitive (Escherichia coli) urinary tract infections in relation to response failure. Odds ratio > 1 indicated higher odds of response failure in the presence of antibiotic-resistant infection. *indicates there was no agreement between susceptibility and treatment antibiotic, or the study did not report the type of antibiotic prescribed. Abbreviations: 3d, 3-day regimen; 5d, 5-day regimen; CI, confidence interval; OR, odds ratio.
Figure 3.
Comparison between antibiotic-resistant and antibiotic-sensitive (Streptococcus pneumoniae) community-acquired pneumonia in relation to response failure. Odds ratio > 1 indicated higher odds of response failure in the presence of antibiotic-resistant infection. *indicates there was no agreement between susceptibility and treatment antibiotic, or the study did not report the type of antibiotic prescribed. Abbreviations: CI, confidence interval; OR, odds ratio.
Figure 4.
Comparison between antibiotic-resistant and antibiotic-sensitive (Streptococcus pneumoniae) acute otitis media in relation to response failure. Odds ratio > 1 indicated higher odds of response failure in the presence of antibiotic-resistant infection. *indicates there was no agreement between susceptibility and treatment antibiotic, or the study did not report the type of antibiotic prescribed. Abbreviations: CI, confidence interval; OR, odds ratio.
Clinical heterogeneity precluded meta-analysis for skin or soft-tissue infections because data were only available from 2 studies [43, 44], of which 1 involved children with impetigo and the other involved adults and adolescents with a range of different infections, including cellulitis, simple abscesses, and wound infections (Supplementary Data File 5). Likewise for sore throat, there was uncertainty regarding similarity of study population characteristics between the 2 studies [46, 48], and for sinus infections [28, 31], 1 study [28] had only 1 patient with an antibiotic-resistant infection.
Reconsultation was significantly more likely in patients with antibiotic-resistant E. coli UTIs (Supplementary Data File 6A) (OR = 5.07; 95% CI = 2.17–11.82; n = 1283 participants, 3 studies) [8, 32, 35]. Data on patient reconsultations were not available for other infections. Two studies involving patients with M. pneumoniae CAP reported data on further antibiotic prescriptions (Supplementary File 6B) [49, 51]. However, meta-analysis was not performed because 1 study did not report which antibiotic was used to treat participants [49], and there were no outcome events among patients with antibiotic-sensitive infections in the other study [51]. Two studies involving patients with E. coli UTIs also reported data on further antibiotic prescriptions [8, 34]. However, treatment antibiotic was not reported in 1 study [8], and the other study focused specifically on extended-spectrum beta-lactamases (ESBL) E. coli infections [34].
Antibiotic-resistant infections were associated with longer duration of symptoms in 2 [32, 50] of 3 E. coli UTI studies (Supplementary Data File 7) [8, 32, 50], but not in the 1 M. pneumoniae CAP study [49]. Only 1 study compared symptom severity between antibiotic-resistant and antibiotic-sensitive E. coli UTIs and found that patients with resistant infections had significantly greater symptom severity between days 2 and 4 (antibiotic-resistant: 2.01, standard deviation = 0.89 vs antibiotic-sensitive: 1.47, SD = 0.88; P < .001; n = 264 participants; severity grading 0 = no symptoms, 6 = as bad as it could be) (Supplementary Data File 8) [50].
Increased odds of clinical response failure in antibiotic-resistant E. coli UTIs were demonstrated in both observational studies (OR = 4.28; 95% CI = 3.31–5.54) and RCTs (OR = 3.49; 95% CI = 1.53–7.97).Odds of clinical response failure were also increased among participants recruited from both hospital outpatient (OR = 5.42; 95% CI = 3.87–7.61) and primary-care settings (OR = 3.29; 95% CI = 2.38–4.56).
For E. coli UTIs, post hoc sensitivity analysis was conducted excluding studies conducted in areas where the prevalence of antibiotic-resistant infections was reported to be high [36], studies that examined highlyspecific antibiotic-resistant bacteria (eg, ESBL E. coli) [34], studies where the reported susceptibility did not match the treatment antibiotic class [29, 30, 40, 41], and studies where the treatment antibiotic was not specified [33, 39]. This did not change the overall findings (OR = 3.27; 95% CI = 2.32–4.60; n = 1426 participants, 5 studies) [8, 32, 37, 38].
For S. pneumoniae CAP, the findings were no longer statistically significant (OR = 1.22; 95% CI = 0.25–5.91; n = 91 participants, 2 studies) [31, 40], after excluding studies where the reported susceptibility did not match the prescribed treatment antibiotic class [29, 30] or where the treatment antibiotic was not reported [41]. For S. pneumoniae AOM, the overall findings did not change (OR = 3.37; 95% CI = 2.04–5.56; n = 573 participants, 4 studies) [27, 31, 42, 45], after excluding 1 study conducted in an inpatient/outpatient setting [47].
DISCUSSION
Main Findings
Our findings demonstrate that patients who present in community healthcare settings with antibiotic-resistant UTIs and RTIs are more likely to experience clinical response failures than patients with antibiotic-sensitive infections. Patients with antibiotic-resistant E. coli UTIs are also more likely to reconsult a healthcare professional and experience prolonged and more severe symptoms than patients with antibiotic-sensitive infections. This challenges the perception that patients in the community are at little additional personal risk from the impact of antibiotic resistance for common infections.
Comparison With Existing Literature
Previous systematic reviews have demonstrated a clear association between commonly prescribed antibiotics in the community and carriage of antibiotic-resistant bacteria [22, 23, 52]. Our estimates are consistent with estimates of clinical response failure rates in community populations for UTIs (14%–38%) [53, 54], CAP (11%–24%) [55], and AOM (7%–24%) [56, 57]. These earlier studies did not, however, determine the specific contribution (or association) of antibiotic resistance to response failure.
We were only able to estimate reconsultation rates for E. coli UTIs, with our results (28%; n = 357/1283) being comparable with those of other studies (26%–55%) [58, 59].
The prevalence of resistant E. coli in the UTI studies we included for our primary outcome (10.4%; n = 357/3428) falls within the lower end of the spectrum compared with most community-based population estimates (5%–53%) because this depends on the antibiotic susceptibility measured, the clinical criteria used for obtaining urine samples and diagnosing UTIs [60, S61–S63], and study population characteristics [52]. However, when examining resistance to the same antibiotic in community populations, our prevalence of E. coli resistant to nitrofurantoin (1.75%; n = 3/171), for example, is similar to that of other studies (<2%) [S61, S63]. Similarly, the prevalence of resistant S. pneumoniae in CAP and AOM in our included studies are lower than population estimates (5.4%, n = 246/4591 vs 8%–33% for CAP [S64, S65]; 0.4%, n = 353/3407 vs 1%–48% for AOM [S66, S67]).
Strengths and Limitations
Our search strategy used validated search filters, and we included both RCTs and observational studies conducted in community healthcare settings. We identified studies that may have collected but did not publish relevant data, and we contacted a sample of the authors to request unpublished and/or additional data (Supplementary Data File 2).
We focussed on more practical, clinically relevant outcomes for patients and clinicians, moving beyond a laboratory-focused, microbiological outcome. Because most of our included studies specifically excluded patients with known medical conditions [8, 27, 28, 30, 32–40, 43–50], we may be underestimating the impact of antibiotic-resistant infections in patients with multimorbidity. Individual patient data were not available to allow us to adjust for potential confounders.
An important limitation is that antibiotic resistance is just 1 explanation for clinical response failure, which could also be due to factors such as coinfection or reinfection. We cannot say what the relative contribution of antibiotic resistance was compared with other factors that could potentially influence the likelihood of clinical response failure. Such factors may also explain why a significant proportion of patients with sensitive infections failed to respond to antibiotics. Previous studies of failure from antibiotic treatment have been criticized because many patients probably had viral infections and would not have been expected to recover with antibiotic treatment [S68]. All included patients in our review had laboratory-confirmed bacterial infections. That said, this may limit generalizability of findings to clinical practice, given that treatment decisions in the community are based on clinical findings without knowledge of the causative pathogen and where most respiratory infections, for example, are viral.
Clinical criteria for diagnosing infections varied between studies, which could impact on clinical outcome. This was particularly evident for E. coli UTIs, for which criteria for obtaining urine samples and diagnostic thresholds varied. Using a lower reference standard of ≥102 CFU/mL and of ≥103 CFU/mL and combining nitrite dipstick test results with clinical symptoms and signs improves diagnostic accuracy for UTI [S69] and, therefore, earlier treatment initiation and improved outcome [S70].
Although we applied a consistent approach associating resistance and sensitivity data to a specific antibiotic class, the class of treatment antibiotic was not always consistent with the class of antibiotic against which resistance was measured. This potentially overestimates clinical response failure associated with resistance to the specific antibiotic being used for treatment. Clinical response failures were more likely in both the main analysis and sensitivity analysis for E. coli UTIs and S. pneumoniae AOM but not sustained for the sensitivity analysis for S. pneumoniae CAP. We therefore cannot reach a robust conclusion that there was there was no greater likelihood of failure in resistant S. pneumoniae CAP compared with sensitive S. pneumoniae. Potential reasons for this may be the limited number of participants with CAP (n = 91), the low number of outcome events overall (n = 11), or that clinical criteria for CAP diagnosis were not reported in 1 of the 2 studies [31]. Data were limited for some infections (eg, skin or soft tissue) and secondary outcomes. It remains unclear whether other infections or bacteria have similar implications on patients’ illness burden.
Implications for Practice, Policy, and Future Research
Clinically, our findings support the need to better identify patients who might need an antibiotic. By testing for antibiotic resistance through promoting and evaluating rapid diagnostics, we can avoid or reduce the risk of clinical response failure. Early evidence suggests that rapid diagnostics used in a community setting can guide antibiotic prescribing for CAP [S71], and trials are underway for UTIs [S72, S73].
Given that at least 1 in 3 women will experience a UTI during their lifetime [4] and that the incidence of UTI is approximately 0.5–0.7 per person-year [S74], our findings show that antibiotic resistance significantly impacts on patients’ illness burden. We estimate that clinical response failure is almost 3 times more likely in patients with antibiotic-resistant E. coli UTIs and around 2 times more likely in patients with antibiotic-resistant S. pneumoniae CAP and AOM than in patients whose infections are antibiotic-sensitive based on our odds ratio estimate and median clinical response failure rate (E. coli UTI: relative risk = 2.96, OR = 4.19, median failure rate = 13%, range = 9%–32% [8, 32–34, 36–39]; S.pneumoniae CAP: relative risk = 1.97, OR = 2.15, median failure rate = 8%, range = 4%–50% [29–31, 40, 41]; and S.pneumoniae AOM: relative risk = 2.18, OR = 2.51, median failure rate = 10%, range = 4%–16% [27, 31, 42, 45, 47]). Expressing the consequences of antibiotic-resistant infections in terms that are more meaningful to patients, among whom the concept of antibiotic resistance has been shown to be misunderstood [2], is important, especially where decisions about whether to start antibiotics may not be clear cut.
This impact may be much greater where the prevalence of antibiotic-resistant E. coli is higher (eg, in children with UTIs) [52]. Recent evidence reports that the global pooled prevalence of trimethoprim resistance used as first-line antibiotic treatment for E.coli UTI in children is 23.6% (range = 17.9%–30.3%) [52]. For more common illnesses like RTIs, the impact of antibiotic-resistant S.pneumoniae CAP in adults may be considerable because estimates vary considerably across European countries where approximately 1%–50% of S.pneumoniae isolates have been recorded as nonsusceptible to penicillin or macrolides [S75, S76].
A better grasp of the implications of antibiotic resistance on tangible outcomes may help curb patients’ expectations for antibiotics [S77], facilitate shared decision making [S78], and inform more appropriate antibiotic prescribing behavior [S79] by informing guidelines, campaigns, and interventions to help healthcare professionals explain the potential implications of antibiotic-resistant infections in relation to outcomes that matter to patients.
More research is needed on the socioeconomic burden associated with antibiotic-resistant infections in the community, both in relation to direct healthcare resource utilization and indirect costs (eg, days off work) [S80]. Future work needs to develop a better understanding of the relationship between antibiotic prescribing levels and development of clinically significant antibiotic resistance in the community.
CONCLUSIONS
Antibiotic resistance has worse implications for patients’ illness burden in the community. These findings could usefully inform better dialogue between clinician and patient, guidelines and campaigns about the benefits and risks of antibiotic treatment.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. All authors have read the approved manuscript and agree to be answerable to all aspects of the work. O. V. H., K. W., and C. C. B. designed and led the research that contributed to the manuscript and were responsible for the main analysis. N. W., O. V. H. and K. W. designed and completed the literature search. O. V. H. and J. J. L. collected and extracted data. O. V. H., K. W., J. J. L., and C. C. B. were involved in appraising and interpreting the data, and all authors subsequently contributed to successive drafts of the paper.The authors are grateful to Associate Professor Ann Van den Bruel for her helpful suggestions and critical reading of the manuscript, and Constantinos Koshiaris for his statistical advice.
Disclaimer. The funding source was not involved in study design, data collection, analysis or interpretation, report writing, or submission for publication. The views expressed are those of the author(s) and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
Financial support. C. C. B. is supported in part by the Oxford NIHR Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance. The ARCHIE research programme (The early use of Antibiotics for “at Risk” CHildren with InfluEnza) is funded by the National Institute for Health Research’s (NIHR) Applied Research Programme. This publication summarizes independent research funded by the NIHR under its Programme Grants for Applied Research Programme (grant RP-PG-1210-12012).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Simpson SA, Wood F, Butler CC. General practitioners’ perceptions of antimicrobial resistance: a qualitative study. J Antimicrob Chemother 2007; 59:292–6. [DOI] [PubMed] [Google Scholar]
- 2. McCullough AR, Parekh S, Rathbone J, Del Mar CB, Hoffmann TC. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2016; 71:27–33. [DOI] [PubMed] [Google Scholar]
- 3. Brookes-Howell L, Elwyn G, Hood K, et al. “The body gets used to them”: patients’ interpretations of antibiotic resistance and the implications for containment strategies. J Gen Intern Med 2012; 27:766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract 2015; 65:e702–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wood F, Simpson S, Butler CC. Socially responsible antibiotic choices in primary care: a qualitative study of GPs’ decisions to prescribe broad-spectrum and fluroquinolone antibiotics. Fam Pract 2007; 24:427–34. [DOI] [PubMed] [Google Scholar]
- 6. de Kraker ME, Davey PG, Grundmann H; BURDEN study group Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 2011; 8:e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Kraker ME, Wolkewitz M, Davey PG, et al. Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother 2011; 66:398–407. [DOI] [PubMed] [Google Scholar]
- 8. Butler CC, Hillier S, Roberts Z, Dunstan F, Howard A, Palmer S. Antibiotic-resistant infections in primary care are symptomatic for longer and increase workload: outcomes for patients with E. coli UTIs. Br J Gen Pract 2006; 56:686–92. [PMC free article] [PubMed] [Google Scholar]
- 9. Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health (Oxf) 2004; 26:268–74. [DOI] [PubMed] [Google Scholar]
- 10. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA 2009; 302:758–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69:234–40. [DOI] [PubMed] [Google Scholar]
- 12. MeReC. Urinary tract infection. MeReC Bulletin 1995; 6:1–7. [Google Scholar]
- 13. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13 2011; 1–38. [PubMed] [Google Scholar]
- 14. Hippisley-Cox J, Vinogradova Y.Trends in consultation rates in general practice 1995/1996 to 2008/2009: analysis of the QResearch® database. Final report to the NHS Information Centre and Department of health. Leeds, UK: NHS Information Centre for Health and Social Care, 2009. [Google Scholar]
- 15. McCormick A, Fleming D, Charlton J.Morbidity statistics from general practice. Fourth National Study 1991–1992. London, 1995. [Google Scholar]
- 16. Pallin DJ, Espinola JA, Leung DY, Hooper DC, Camargo CA., Jr Epidemiology of dermatitis and skin infections in United States physicians’ offices, 1993–2005. Clin Infect Dis 2009; 49:901–7. [DOI] [PubMed] [Google Scholar]
- 17. National Center for Health Statistics (US). Health, United States, 2010: with special feature on death and dying. Hyattsville, MD: Centers for Disease Control and Prevention, 2011. [PubMed] [Google Scholar]
- 18. Public Health England. English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) Report 2014. London: Public Health England, 2014. [Google Scholar]
- 19. Cals JW, Ament AJ, Hood K, et al. C-reactive protein point of care testing and physician communication skills training for lower respiratory tract infections in general practice: economic evaluation of a cluster randomized trial. J Eval Clin Pract 2011; 17:1059–69. [DOI] [PubMed] [Google Scholar]
- 20. Hollinghurst S, Gorst C, Fahey T, Hay AD. Measuring the financial burden of acute cough in pre-school children: a cost of illness study. BMC Fam Pract 2008; 9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenberg M. Pharmacoeconomics of treating uncomplicated urinary tract infections. Int J Antimicrob Agents 1999; 11:247–51; discussion 261–4. [DOI] [PubMed] [Google Scholar]
- 22. Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340:c2096. [DOI] [PubMed] [Google Scholar]
- 24. Gill PJ, Roberts NW, Wang KY, Heneghan C. Development of a search filter for identifying studies completed in primary care. Fam Pract 2014; 31:739–45. [DOI] [PubMed] [Google Scholar]
- 25. The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions version 5.1.0. Higgins JPT, Green S, eds. The Cochrane Collaboration, 2011. Available at: www.handbook.cochrane.org. Accessed March 2011. [Google Scholar]
- 26. Critical Appraisals Skills Programme (CASP). CASP checklists 2013. Available at: http://www.casp-uk.net/ Accessed 22 December 2015.
- 27. Barry B, Gehanno P, Blumen M, Boucot I. Clinical outcome of acute otitis media caused by pneumococci with decreased susceptibility to penicillin. Scand J Infect Dis 1994; 26:446–52. [DOI] [PubMed] [Google Scholar]
- 28. Buchanan P, Roos K, Tellier G, Rangaraju M, Leroy B. Bacteriological efficacy of 5-day therapy with telithromycin in acute maxillary sinusitis. Int J Antimicrob Agents 2005; 25:237–46. [DOI] [PubMed] [Google Scholar]
- 29. Hagberg L, Carbon C, van Rensburg DJ, Fogarty C, Dunbar L, Pullman J. Telithromycin in the treatment of community-acquired pneumonia: a pooled analysis. Respir Med 2003; 97:625–33. [DOI] [PubMed] [Google Scholar]
- 30. van Rensburg DJ, Fogarty C, Kohno S, Dunbar L, Rangaraju M, Nusrat R. Efficacy of telithromycin in community-acquired pneumonia caused by pneumococci with reduced susceptibility to penicillin and/or erythromycin. Chemotherapy 2005; 51:186–92. [DOI] [PubMed] [Google Scholar]
- 31. Zhanel GG, Wolter KD, Calciu C, et al. Clinical cure rates in subjects treated with azithromycin for community-acquired respiratory tract infections caused by azithromycin-susceptible or azithromycin-resistant Streptococcus pneumoniae: analysis of phase 3 clinical trial data. J Antimicrob Chemother 2014; 69:2835–40. [DOI] [PubMed] [Google Scholar]
- 32. McNulty CA, Richards J, Livermore DM, et al. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother 2006; 58:1000–8. [DOI] [PubMed] [Google Scholar]
- 33. Noskin GA, Zembower T, Chmielewski J, Tang P, La Rosa M, Peterson LR. Disappearance of the “uncomplicated” urinary tract infection—the impact of emerging resistance. Clin Drug Invest 2001; 21:13–20. [Google Scholar]
- 34. Søraas A, Sundsfjord A, Jørgensen SB, Liestøl K, Jenum PA. High rate of per oral mecillinam treatment failure in community-acquired urinary tract infections caused by ESBL-producing Escherichia coli. PLoS One 2014; 9:e85889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown PD, Freeman A, Foxman B. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin Infect Dis 2002; 34:1061–6. [DOI] [PubMed] [Google Scholar]
- 36. Raz R, Chazan B, Kennes Y, et al. ; Israeli Urinary Tract Infection Group. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin Infect Dis 2002; 34:1165–9. [DOI] [PubMed] [Google Scholar]
- 37. Gupta K, Hooton TM, Roberts PL, Stamm WE. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med 2007; 167:2207–12. [DOI] [PubMed] [Google Scholar]
- 38. van Merode T, Nys S, Raets I, Stobberingh E. Acute uncomplicated lower urinary tract infections in general practice: clinical and microbiological cure rates after three- versus five-day treatment with trimethoprim. Eur J Gen Pract 2005; 11:55–8. [DOI] [PubMed] [Google Scholar]
- 39. Vallano A, Rodríguez D, Barceló ME, et al. ; Grupo de Estudio de las Infecciones del Tracto Urinario en Atención Primaria. Antimicrobial susceptibility of uropathogens and outcome following antibiotic treatment for urinary tract infections in primary health care. Enferm Infecc Microbiol Clin 2006; 24:418–25. [DOI] [PubMed] [Google Scholar]
- 40. O’Doherty B, Dutchman DA, Pettit R, Maroli A. Randomized, double-blind, comparative study of grepafloxacin and amoxycillin in the treatment of patients with community-acquired pneumonia. J Antimicrob Chemother 1997; 40:73–81. [DOI] [PubMed] [Google Scholar]
- 41. Yanagihara K, Otsu Y, Ohno H, et al. Clinical characteristics of pneumonia caused by penicillin resistant and sensitive Streptococcus pneumoniae in Japan. Intern Med 2004; 43:1029–33. [DOI] [PubMed] [Google Scholar]
- 42. Dagan R, Abramson O, Leibovitz E, et al. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr Infect Dis J 1996; 15:980–5. [DOI] [PubMed] [Google Scholar]
- 43. Dagan R, Bar-David Y. Double-blind study comparing erythromycin and mupirocin for treatment of impetigo in children: implications of a high prevalence of erythromycin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 1992; 36:287–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giordano PA, Elston D, Akinlade BK, et al. Cefdinir vs. cephalexin for mild to moderate uncomplicated skin and skin structure infections in adolescents and adults. Curr Med Res Opin 2006; 22:2419–28. [DOI] [PubMed] [Google Scholar]
- 45. Hoberman A, Dagan R, Leibovitz E, et al. Large dosage amoxicillin/clavulanate, compared with azithromycin, for the treatment of bacterial acute otitis media in children. Pediatr Infect Dis J 2005; 24:525–32. [DOI] [PubMed] [Google Scholar]
- 46. Quinn J, Ruoff GE, Ziter PS. Efficacy and tolerability of 5-day, once-daily telithromycin compared with 10-day, twice-daily clarithromycin for the treatment of group A beta-hemolytic streptococcal tonsillitis/pharyngitis: a multicenter, randomized, double-blind, parallel-group study. Clin Ther 2003; 25:422–43. [DOI] [PubMed] [Google Scholar]
- 47. Hoberman A, Paradise JL, Block S, Burch DJ, Jacobs MR, Balanescu MI. Efficacy of amoxicillin/clavulanate for acute otitis media: relation to Streptococcus pneumoniae susceptibility. Pediatr Infect Dis J 1996; 15:955–62. [DOI] [PubMed] [Google Scholar]
- 48. Seppälä H, Nissinen A, Järvinen H, et al. Resistance to erythromycin in group A streptococci. N Engl J Med 1992; 326:292–7. [DOI] [PubMed] [Google Scholar]
- 49. Cao B, Zhao CJ, Yin YD, et al. High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin Infect Dis 2010; 51:189–94. [DOI] [PubMed] [Google Scholar]
- 50. Little P, Merriman R, Turner S, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ 2010; 340:b5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kawai Y, Miyashita N, Yamaguchi T, et al. Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology 2012; 17:354–62. [DOI] [PubMed] [Google Scholar]
- 52. Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ 2016; 352:i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gagyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ 2015; 351:h6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferry SA, Holm SE, Stenlund H, Lundholm R, Monsen TJ. Clinical and bacteriological outcome of different doses and duration of pivmecillinam compared with placebo therapy of uncomplicated lower urinary tract infection in women: the LUTIW project. Scand J Prim Health Care 2007; 25:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pakhale S, Mulpuru S, Verheij TJ, Kochen MM, Rohde GG, Bjerre LM. Antibiotics for community-acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2014; 10:Cd002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tähtinen PA, Laine MK, Huovinen P, Jalava J, Ruuskanen O, Ruohola A. A placebo-controlled trial of antimicrobial treatment for acute otitis media. N Engl J Med 2011; 364:116–26. [DOI] [PubMed] [Google Scholar]
- 57. Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015; CD000219. doi:10.1002/14651858.CD000219.pub4. [DOI] [PubMed] [Google Scholar]
- 58. Fahey T, Webb E, Montgomery AA, Heyderman RS. Clinical management of urinary tract infection in women: a prospective cohort study. Fam Pract 2003; 20:1–6. [DOI] [PubMed] [Google Scholar]
- 59. Little P, Turner S, Rumsby K, et al. Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study. Health Technol Assess 2009; 13:iii–iv, ix–xi, 1–73. [DOI] [PubMed] [Google Scholar]
- 60. Farrell DJ, Morrissey I, De Rubeis D, Robbins M, Felmingham D. A UK multicentre study of the antimicrobial susceptibility of bacterial pathogens causing urinary tract infection. J Infect 2003; 46:94–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.