Summary
This meta-analysis, which systematically appraises the performance of single drugs and combination regimens across early clinical endpoints in trials of treatment of pulmonary tuberculosis, demonstrates that the existing evidence base supporting phase 2 methodology in tuberculosis is highly incomplete.
Keywords: tuberculosis, efficacy, meta-analysis
Abstract
Background
A systematic review of early clinical outcomes in tuberculosis was undertaken to determine ranking of efficacy of drugs and combinations, define variability of these measures on different endpoints, and to establish the relationships between them.
Methods
Studies were identified by searching PubMed, Medline, Embase, LILACS (Latin American and Caribbean Health Sciences Literature), and reference lists of included studies. Outcomes were early bactericidal activity results over 2, 7, and 14 days, and the proportion of patients with negative culture at 8 weeks.
Results
One hundred thirty-three trials reporting phase 2A (early bactericidal activity) and phase 2B (culture conversion at 2 months) outcomes were identified. Only 9 drug combinations were assessed on >1 phase 2A endpoint and only 3 were assessed in both phase 2A and 2B trials.
Conclusions
The existing evidence base supporting phase 2 methodology in tuberculosis is highly incomplete. In future, a broader range of drugs and combinations should be more consistently studied across a greater range of phase 2 endpoints.
(See the Editorial Commentary by Phillips on pages 55–6.)
First-line tuberculosis therapy has remained unchanged for 40 years. Whereas “short-course” treatment is effective in clinical trials, in practice the 6 months required for successful cure is burdensome for patients and tuberculosis programs. Identifying new and ultra-short regimens will require identification of suitable surrogate outcomes to facilitate progression of novel treatment regimens through phase 2 to phase 3 trials and de-risk drug development [1].
The current “gold standard” phase 3 endpoint is a composite of treatment failure and relapse up to 24 months following treatment completion. Use of this binary outcome, which is rare in the comparator arm (<5% with standard short-course regimens), mandates large sample sizes to adequately power clinical trials. The prolonged follow-up that is required further adds to trial costs, making the definitive outcome unsuitable for extensive evaluation of drug combinations or dose-finding.
Numerous surrogate outcomes have been used for these purposes in phase 2 studies. Phase 2A studies of early bactericidal activity (EBA) based on quantitative sputum bacteriology enroll small patient numbers for up to 2 weeks [2]. While the original rationale for such studies was dose-finding for single agents, more recent studies have evaluated drug combinations [3, 4]. This concept has been extended into larger phase 2B studies with combination therapy lasting up to 2 months [5]. The most studied phase 2B outcome has been sputum culture conversion at fixed time-points, usually 2 months [6–8]. This endpoint is supported by regulators for conditional approval of novel drugs [9], but there remains a lack of consensus among trialists as to the utility of EBA studies and of other approaches to intermediate bacteriological data such as time-to-event and regression modeling [10].
A complete understanding of the performance of tuberculosis treatment regimens in early-phase clinical trials is critical to understanding their usefulness in predicting phase 3 trial results and in calibrating preclinical models of treatment. Although the goals of historical phase 2A and 2B regimens are distinct, with the former focusing on proof-of-concept for individual drugs and the latter on identifying the best combinations of drugs, it seems important to understand whether this information can be transmitted rationally through these phases. We undertook a systematic review of early clinical outcomes in tuberculosis (within the first 2 months of treatment), focusing on the key drugs comprising modern and historical first-line treatment regimens, to determine the overall ranking of efficacy of drugs and combinations, to define the variability of these measures of effect on the different endpoints used, and to establish the relationships between them.
METHODS
The review included randomized clinical trials (RCTs) including patients with smear- and culture-positive pulmonary tuberculosis, being treated for the first time or with known isoniazid-monoresistant organisms, and including regimens containing any combination of historic or novel drugs used or proposed for use in first-line treatment regimens. Predefined outcomes of interest were EBA over 2 (EBA0-2), 7 (EBA0-7), and 14 days (EBA0-14), and the proportion of patients with negative culture results at 8 weeks. A systematic search of databases was conducted on 12 December 2016 (see Supplementary Appendix 1). Risk of bias was considered [11]. Pooled estimates of each outcome for each drug or combination were obtained. Meta-regression was used to examine the impact of clinical covariates on the effect size of culture results at 8 weeks. Analyses were performed using R version 2.14.1 [12]. Full methodology is detailed in the Supplementary Appendix 1.
RESULTS
Included Studies
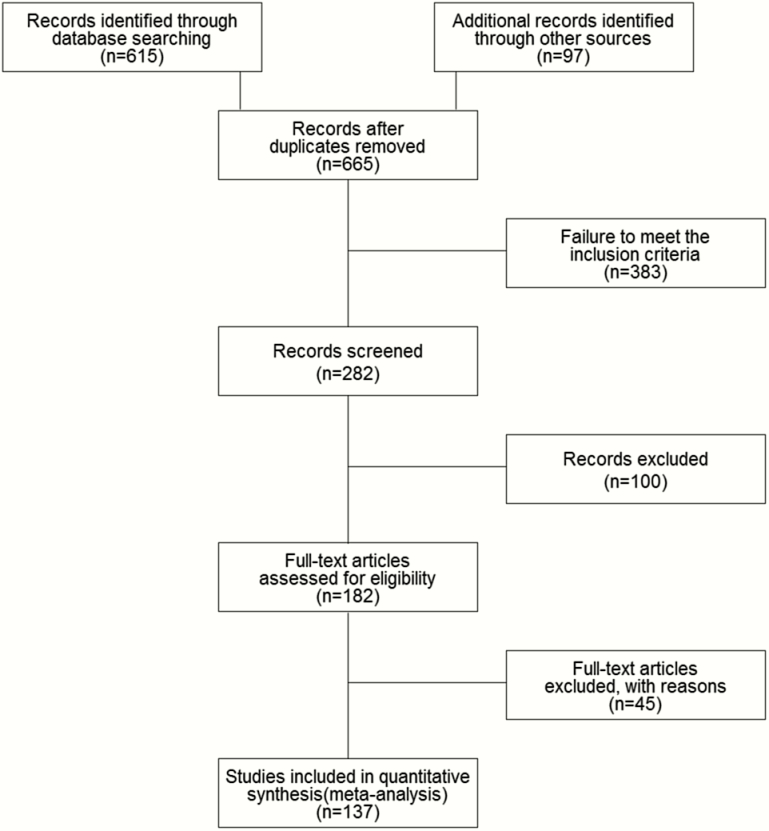
Figure 1 shows the number of studies included at each stage of the review. The main reasons for exclusion were failure to meet the inclusion criteria, specifically previously treated or drug- resistant patients, and study design other than RCT. One hundred thirty-three relevant studies were identified that reported outcomes of interest. Of these, 37 were phase 2 studies and 96 were phase 3 studies reporting intermediate bacteriological outcomes. All 96 phase 3 studies contributed to the phase 2B outcome only—no phase 3 studies contributed data to the phase 2A outcomes. Together these studies provide data relating to 37173 patients and 67 drug combinations.
Figure 1.
Literature review process.
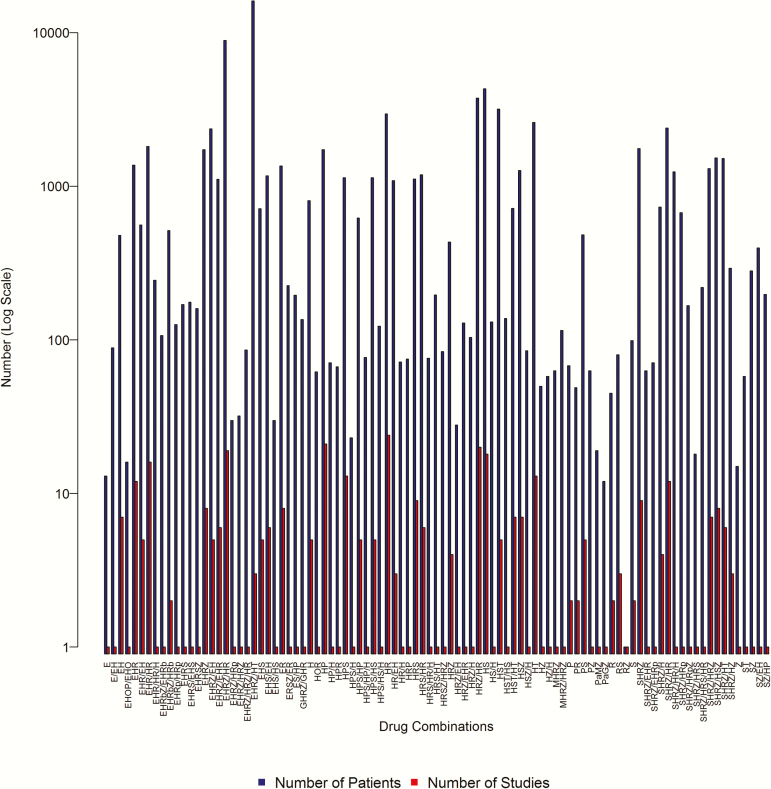
Figure 2 summarizes numbers of studies and patients pertaining to each drug combination across all the outcomes of interest. Drug abbreviations are as follows: E, ethambutol; G, gatifloxacin; H, isoniazid; J, bedaquiline; L, levofloxacin; M, moxifloxacin; O, ofloxacin; P, para-aminosalicylic acid; Pa, PA-824/pretomanid; R, rifampicin; Rb, rifabutin; Rp, rifapentine; S, streptomycin; T, thiacetazone; Z, pyrazinamide. Because only combinations including drugs of interest to the review are summarized, in some cases only data concerning the control arms of trials are presented. Additionally, studies with ≥2 trial arms were analyzed separately. The composition of each drug combination refers only to the period preceding the endpoint of interest. Therefore, for the 8-week culture outcome, usually only drugs used in the initiation phase of treatment are reported without the associated continuation-phase drugs. Where the initiation phase was <2 months, however, continuation drugs are also listed. The regimens for which most data were available were HRZE, SHRZ, and HRZ combination therapy.
Figure 2.
Drug combinations of included studies. Abbreviations: E, ethambutol; G, gatifloxacin; H, isoniazid; J, bedaquiline; L, levofloxacin; M, moxifloxacin; O, ofloxacin; P, para-aminosalicylic acid; Pa, PA-824/pretomanid; R, rifampicin; Rb, rifabutin; Rp, rifapentine; S, streptomycin; T, thiacetazone; Z, pyrazinamide.
Twenty-four studies reported phase 2A outcomes. EBA0-2 was reported in all 24 studies (141 trial arms, 35 drug combinations, 1424 patients). In some cases, studies considered the same drug combination but different treatment strategies and dosing intervals. Others considered single-formulation treatments vs combined formulations, and some considered multiple dosages of a drug. EBA0-7 and EBA0-14 were reported in only 6 (23 trial arms, 14 drug combinations, 296 patients) and 8 studies (46 trial arms, 27 drug combinations, 449 patients), respectively.
The proportion of patients who were culture negative by 8 weeks was reported in 104 studies considering phase 2B outcomes. These studies investigated 45 different drug combinations in 34418 patients. One study reported both phase 2A and phase 2B outcomes [13].
Forest plots for each drug combination and outcome and associated numerical results are presented in the Supplementary Appendices 2 and 3. Results shown in Figures 3–8 are graphical summaries based on standard doses recommended in treatment guidelines in the case of historic drugs, or doses going into phase 3 trials in the case of novel drugs.
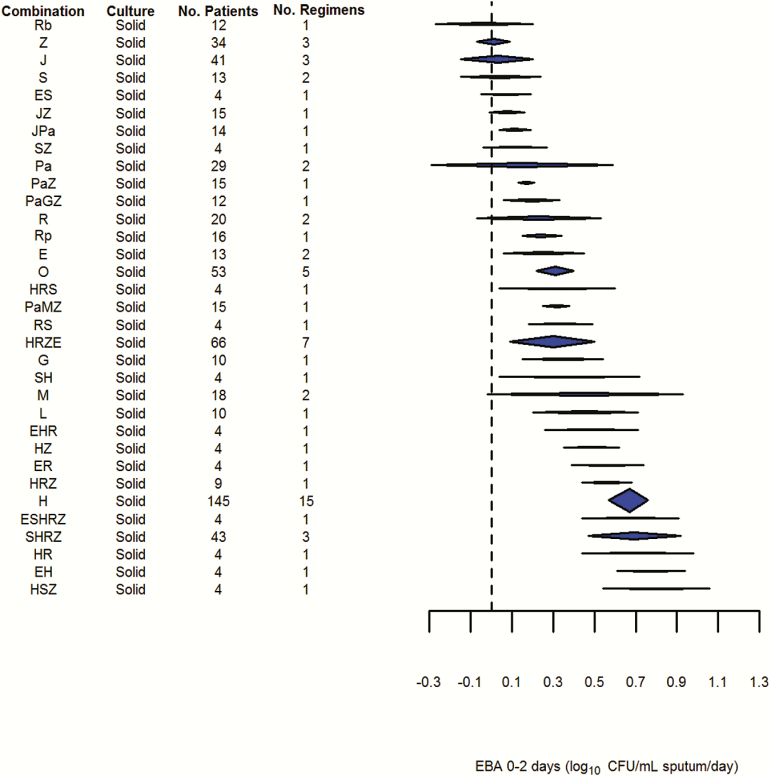
Figure 3.
Results of early bactericidal activity over 2 days (fixed effects, generalized inverse variance method). Abbreviations: CFU, colony-forming units; E, ethambutol; EBA, early bactericidal activity; G, gatifloxacin; H, isoniazid; J, bedaquiline; L, levofloxacin; M, moxifloxacin; O, ofloxacin; P, para-aminosalicylic acid; Pa, PA-824/pretomanid; R, rifampicin; Rb, rifabutin; Rp, rifapentine; S, streptomycin; T, thiacetazone; Z, pyrazinamide.
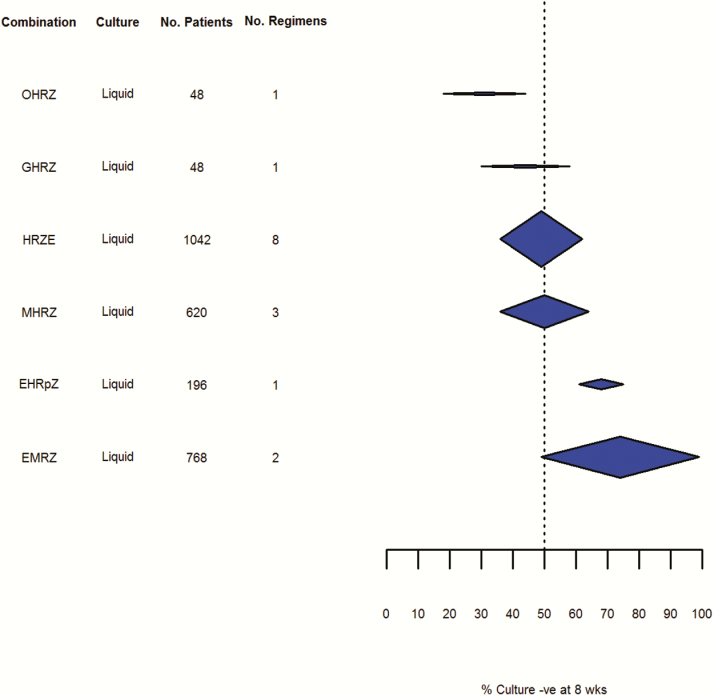
Figure 7.
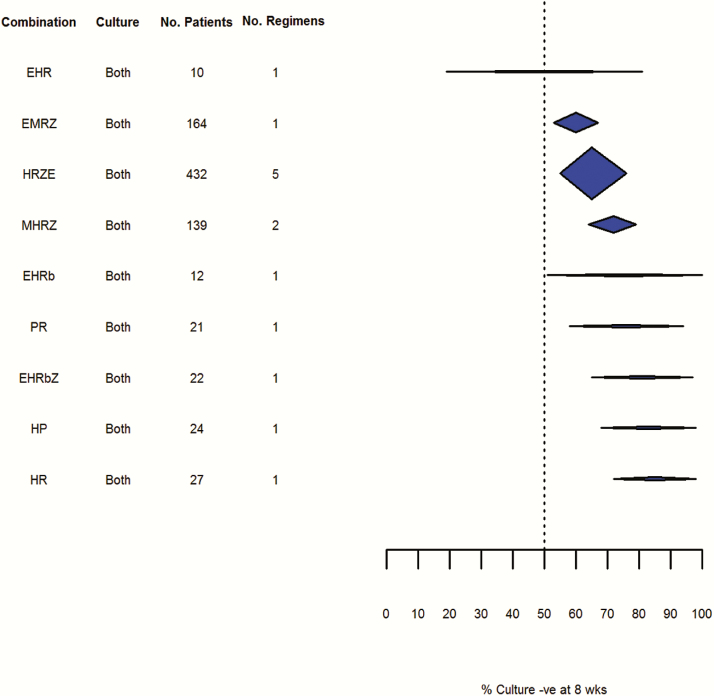
Culture negativity at 8 weeks: liquid culture (random effects, DerSimonian and Laird method). Abbreviations: -ve, negative; E, ethambutol; G, gatifloxacin; H, isoniazid; M, moxifloxacin; O, ofloxacin; R, rifampicin; Rp, rifapentine; Z, pyrazinamide.
Figure 8.
Culture negativity at 8 weeks: solid and liquid culture (random effects, DerSimonian and Laird method). Abbreviations: -ve, negative; E, ethambutol; H, isoniazid; M, moxifloxacin; P, para-aminosalicylic acid; R, rifampicin; Rb, rifabutin; Z, pyrazinamide.
Risk of Bias
Eighty-seven (65%) studies provided information on sequence generation. In most cases (94%) patients were “randomly allocated,” so studies were classified as unclear risk of bias. Some publications mentioned stratifying by factors such as severity, or used permuted block designs, random tables, or similar and were classified as low risk of bias. Five studies referred to quasi-randomization and were therefore classified as high risk of bias.
Only 30 (23%) studies mentioned allocation concealment. Of these, 28 (93%) studies used sealed envelopes and were classified as low risk. Ninety-seven (73%) studies either reported that the study was unblinded, or did not specify blinding procedures and were classified as high risk of bias. Fifteen included studies were of a double-blinded nature including the use of telephone randomization, or prearranged lists, although 1 study stated that it was double-blind during the maintenance phase of treatment only [14]. Most other blinded studies mentioned that radiographers or laboratory staff were blinded to treatment; these were considered as single-blinded designs and classified as low risk of bias.
Ninety-seven (73%) studies were published before the Consolidated Standards of Reporting Trials (CONSORT) when selective reporting had not been raised as a possible source of bias. In all studies published post-CONSORT, the risk of bias is unclear as there is insufficient information to determine whether the published reports include all expected outcomes, including those prespecified. Eighty-five (64%) studies reported reasons for exclusions, or numbers lost to follow-up.
Due to the limited number of high-quality studies, sensitivity analysis assessing the impact of risk of bias was not performed.
Phase 2A Studies
EBA0-2
Pooled results for the EBA0-2 outcome can be seen in Figure 3. Of the 32 drugs and combinations, only 5 were studied in >30 patients. Hence, the confidence intervals on pooled estimates of effect are wide and frequently overlap. Some drugs (Rb, Z, J, and S) do not demonstrate any significant efficacy on this endpoint and can also clearly be distinguished from H, the most commonly studied and precisely estimated drug. However, even quite commonly studied combinations containing H such as HRZ, HRZE, and SHRZ do not appear significantly different from H monotherapy using the EBA0-2 endpoint. Similarly, it does not appear to be possible to separate the effect of HRZE from any of its component drugs, with the exception of Z.
EBA0-7 and EBA0-14
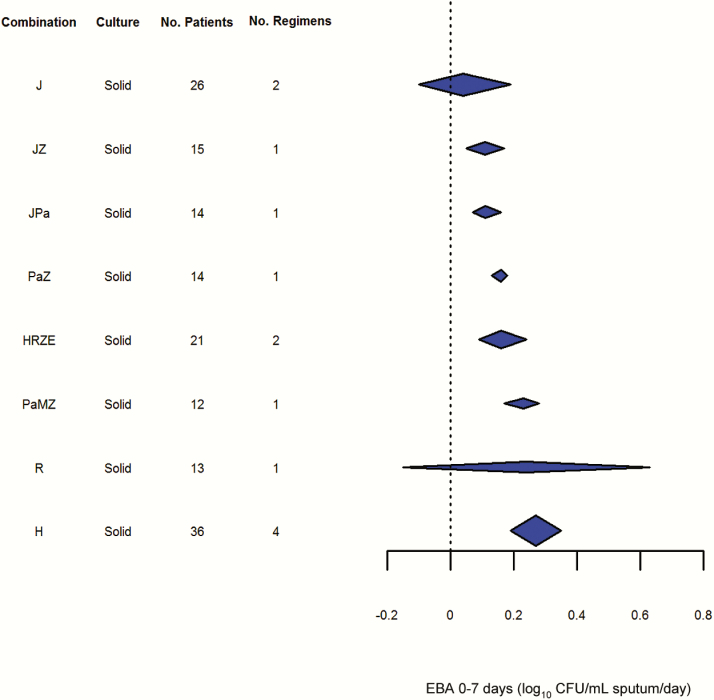
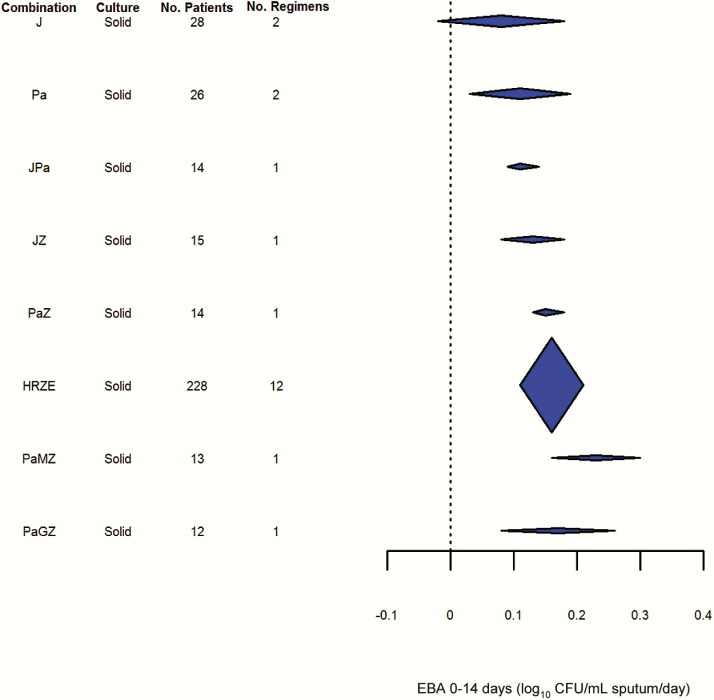
Pooled results for the EBA0-7 and EBA0-14 outcomes can be seen in Figures 4 and 5, respectively. Although the number of studies reporting these endpoints was fewer, variability of these endpoints appeared lower than for EBA0-2. Even so, it did not appear possible to distinguish statistically between the drugs and regimens studied, including combinations such as HRZE and drugs as diverse as H, Pa, and J.
Figure 4.
Results of early bactericidal activity over 7 days (fixed effects, generalized inverse variance method). Abbreviations: CFU, colony-forming units; E, ethambutol; EBA, early bactericidal activity; H, isoniazid; J, bedaquiline; M, moxifloxacin; Pa, PA-824/pretomanid; R, rifampicin; Z, pyrazinamide.
Figure 5.
Results of early bactericidal activity over 14 days (fixed effects, generalized inverse variance method). Abbreviations: CFU, colony-forming units; E, ethambutol; EBA, early bactericidal activity; G, gatifloxacin; H, isoniazid; J, bedaquiline; M, moxifloxacin; Pa, PA-824/pretomanid; R, rifampicin; Z, pyrazinamide.
Phase 2B Studies
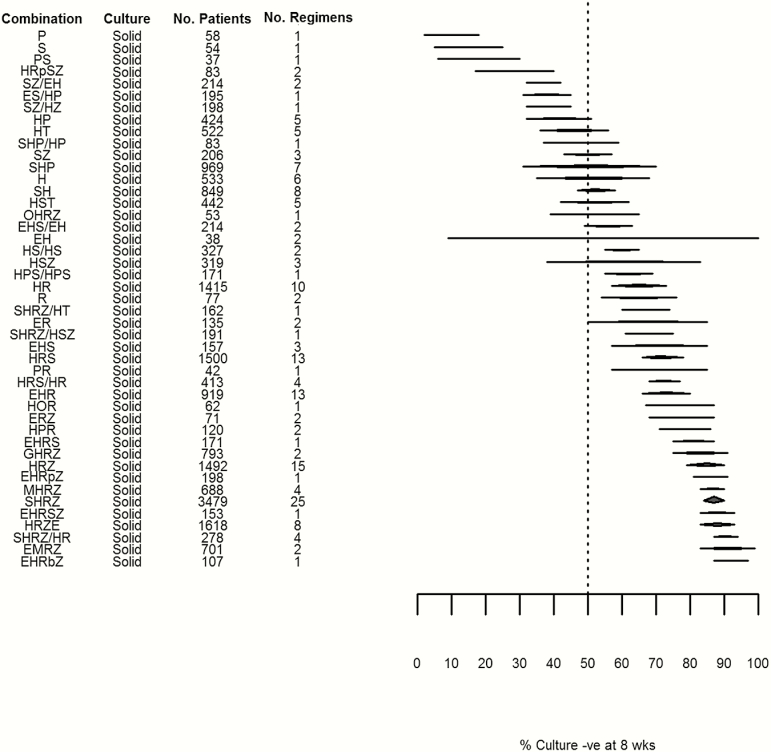
Figures 6–8 summarize the pooled estimates of the proportion of patients who were culture negative at 8 weeks, grouped by culture method. The overall point estimates of culture conversion for the most effective rifampicin-containing regimens on this endpoint (HRZ, SHRZ, and HRZE) exceeded 85%, whereas for most nonrifampicin regimens this estimate was no better than 50%. Though relatively precise estimates were obtained for frequently studied modern short-course regimens such as HRZ, SHRZ, and HRZE, the performance of these regimens was not statistically distinguishable from historical regimens comprising similar numbers of trials and patients, such as HS, HPS, or SHR. This appeared to reflect high intertrial variability within regimens as measured by I2 and τ2 estimated using 2 approaches (see Supplementary Appendices 2 and 3).
Figure 6.
Culture negativity at 8 weeks: solid culture (random effects, DerSimonian and Laird method). Abbreviations: -ve, negative; E, ethambutol; G, gatifloxacin; H, isoniazid; J, bedaquiline; L, levofloxacin; M, moxifloxacin; O, ofloxacin; P, para-aminosalicylic acid; Pa, PA-824/pretomanid; R, rifampicin; Rb, rifabutin; Rp, rifapentine; S, streptomycin; T, thiacetazone; Z, pyrazinamide.
Fewer data were available relating to culture conversion as measured by liquid culture. Although variability appeared lower than with solid culture, this could be due to the smaller number of studies included in this analysis. Variability increased when pooled results based on solid and liquid culture methods were reported and confidence intervals for all the regimens tested using liquid culture results overlapped.
Meta-regression Analyses
The results of meta-regression analyses can be seen in (Table 1). For the selected drug combinations HRZE, HRZ, and SHRZ, neither year of publication nor geographical location were statistically significant. Human immunodeficiency virus (HIV) coinfection could only be examined for the drug combination HRZE as there was insufficient data for HRZ and SHRZ. It was also not significantly associated with the proportion of patients who were culture negative at either weeks. The inclusion of R and Z in a regimen independently explained significant heterogeneity among drug combinations (see Supplementary Appendix 4).
Table 1.
Meta-regression Results for the Impact of Selected Variables on the Proportion of Patients Who Were Culture Negative at 8 Weeks
| Drug Combinationsa | Covariate: β Regression Coefficient (P Values) | ||
|---|---|---|---|
| Year of Publication | Proportion of Patients With HIV Coinfection |
Geographical Location (Africa or Not) |
|
| HRZE | –0.00 (.27) | 0.00 (.79) | 0.01 (.87) |
| HRZ | 0.01 (.24) | Insufficient datab | –0.12 (.45) |
| SHRZ | 0.00 (.57) | Insufficient datac | –0.08 (.17) |
β coefficients describe how the outcome variable changes with a unit increase in the explanatory variable.
Abbreviation: HIV, human immunodeficiency virus.
aDrug abbreviations: E, ethambutol; H, isoniazid; R, rifampicin; S, streptomycin; Z, pyrazinamide.
bOnly 1 study presented proportion of patients with HIV coinfection.
cNo studies presented proportion of patients with HIV coinfection.
Ranking
Because it was difficult to discriminate between regimens in terms of formal statistical inference, we evaluated whether the rank order of regimens was consistent between different endpoints. The ranking, however, was highly constrained by the limited number of drugs and regimens studied in both phases, principally because of the ethical unacceptability of prolonged monotherapy in 2-month studies and the lack of historical combination EBA studies.
Nine distinct regimens (HRZE, H, J, JPa, JZ, Pa, PaMZ, PaZ, and R) were considered on at least 2 phase 2A endpoints. All 9 were considered when examining EBA0-2, and all except Pa were considered when examining EBA0-7. Seven were considered (H, HPa, HZ, Pa, PaMZ, and PaZ) when examining EBA0-14. Only 3 of these regimens (HRZE, H, and R) were considered for 2-month culture conversion on solid media.
Using the relative order among the drugs in the common sets and basing results only on solid culture, the rankings are shown in (Table 2). Though qualitative rankings for the available regimens were reasonably consistent, the dataset was too small to be able to draw conclusions about their usefulness for decision making.
Table 2.
Ranking of Drugs Across Outcomes Based on a Subset of Regimens for Which at Least 2 of the Early Bactericidal Activity Results Were Available
| Ranking and No. of Patients (No. of Regimens) | ||||
|---|---|---|---|---|
| Drug Combinationsa | EBA0–2 | EBA0–7 | EBA0–14 | 2 Months |
| H | 1 149 (16) |
1 36 (4) |
… | 3 533 (6) |
| HRZE | 2 51 (6) |
4 21 (2) |
2 50 (6) |
1 1618 (8) |
| PaMZ | 3 15 (1) |
3 12 (1) |
1 13 (1) |
… |
| R | 4 28 (3) |
2 13 (1) |
… | 2 77 (2) |
| PaZ | 5 15 (1) |
5 14 (1) |
3 14 (1) |
… |
| Pa | 6 29 (2) |
… | 6 26 (2) |
… |
| JPa | 7 14 (1) |
6 14 (1) |
5 14 (1) |
… |
| JZ | 8 15 (1) |
7 15 (1) |
4 15 (1) |
… |
| J | 9 41 (3) |
8 26 (2) |
7 28 (2) |
… |
Abbreviations: EBA0–2, early bactericidal activity over 2 days; EBA0–7, early bactericidal activity over 7 days; EBA0–14, early bactericidal activity over 14 days.
aDrug abbreviations: E, ethambutol; H, isoniazid; J, bedaquiline; M, moxifloxacin; Pa, PA-824/pretomanid; R, rifampicin; Z, pyrazinamide.
DISCUSSION
This review is the first to systematically appraise the performance of single drugs and combination regimens across early clinical endpoints in trials of treatment of pulmonary tuberculosis. Though we focussed only on the set of drugs most relevant to historical and modern first-line therapy, we identified 133 trials reporting phase 2A and 2B outcomes comprising >37000 patients and 67 drug combinations. However, the diversity of treatment regimens represented in 14-day phase 2A studies was much lower, with only 9 drug combinations assessed on >1 phase 2A endpoint and only 3 of these combinations assessed in both phase 2A and 2B trials. While these findings partly reflect the history, development, and differing goals of such trials, the narrowness of this evidence base is concerning and suggests a potentially serious gap in rational translation between these 2 critical phases of development. While rankings of the efficacy of treatment appeared reasonably consistent on different phase 2A endpoints, the existing dataset does not provide convincing support to current practices, and intertrial variability was high in many cases.
We selected 4 outcome measures for our review based on those most commonly reported in the included studies [15]. However, there was large variation in reporting, particularly of EBA measures, with many unique to a single study. Overall the quality of reporting, particularly of phase 2A studies, made data extraction and synthesis challenging and imposes limitations in interpretation of the data. The striking feature of the available dataset is the variability of pooled estimates of effect for all the endpoints examined. For EBA0-2 and 2-month culture conversion, this variability was particularly marked, with overlapping confidence intervals for the majority of regimens. Though there were appreciable differences between the best-performing regimens on these endpoints (H, HRZ, HRZE, and SHRZ) and the worst (Z, S, SH, and SHP), this suggests that such trials may lack the power to formally discriminate between regimens where differences in treatment effect are more modest but still clinically relevant. The reasons for this variability were difficult to explore using the data available, given the quality and consistency of reporting.
We used the data as reported—some studies adopted an intention-to-treat approach to analysis and included patients with missing or contaminated culture results, while others used a per-protocol approach and excluded these patients. Poor-quality reporting meant it was mostly impossible to distinguish these situations. This may account for some of the observed heterogeneity.
The variance of the pooled estimates for EBA endpoints may be inflated by the regression coefficients being based on different numbers of observations. There were no such methodological problems for the 2-month culture conversion results, suggesting that the observed heterogeneity is likely to be a real clinical effect. Among the most likely sources of this within-regimen heterogeneity are pharmacological (ethnic differences in absorption, elimination, and clearance), bacteriological (differences in initial bacterial burden, and virulence), and patient factors (disease stage such as presence of cavities; comorbidities such as malnutrition, diabetes, and HIV). Our meta-regression analysis was able to explore a very limited subset of such variables for a few of the most common regimens on a single endpoint.
Because the review incorporated all reported trials over the last 60 years, evolution in the efficiency and standardization even of solid culture methods may have contributed to variation in 2-month culture results, with older studies tending to produce numerically higher rates (not statistically significant) of culture conversion due to lower assay sensitivity compared to more modern methods. We tested this via meta-regression for drug combinations HRZE, HRZ, and SHRZ and in all 3 cases, year of publication was not significant. Isoniazid resistance, whether known or undetected, may have tended to increase heterogeneity in outcomes, but this is difficult to assess due to small numbers of patients. While it is known that, at least for modern regimens such as HRZE, the risk of poor outcome for patients with H-resistant organisms is only modestly increased [16, 17], this could be more important for older regimens, although there were insufficient data to test this in a meta-regression. Finally, patient factors—in particular, chronicity of disease, geographical location, and HIV coinfection—could also have increased intertrial variability within regimens, although there were insufficient data to test this for chronicity of disease. It has been observed that culture conversion at 2 months may vary widely even between study sites in individual trials [18] and may be influenced by the lower sputum bacillary load observed in HIV-infected patients.
We propose 3 approaches that could help to overcome some of the limitations this review identifies in the existing evidence base for phase 2 trials in tuberculosis. First, assembly of a database of individual patient data relating to the trials identified would facilitate reanalysis of the trials and also enable computation of endpoints not reported in the original study publication, which may help to address the lack of diversity of regimens on each endpoint. Such an effort is currently in progress by the model-based preclinical development of anti-tuberculosis drug combinations (PreDiCT-TB) consortium, and we anticipate an update of this review based on individual patient data when that process has been completed.
Second, development of a core outcome set for tuberculosis trials that could be applied to new studies in the field would likely assist both investigators and systematic reviewers in choosing and reporting endpoints in such a way that the contribution of each trial to the overall evidence base is maximized [19]. This would provide a minimum but not exhaustive set of clearly defined outcomes to be reported in each study.
Finally, we also suggest wider use of novel, more efficient, adaptive screening trial designs that would enable a broader range of regimens to be studied in phase 2 than was previously possible. However, such trials may also impact any meta-analysis to which they contribute in terms of the endpoints that they prioritize and any bias they might introduce due to early termination.
Our review shows that the existing evidence base supporting phase 2 methodology in tuberculosis is highly incomplete. To truly understand and improve drug development in tuberculosis, it is desirable that a broader range of drugs and combinations be more consistently studied across a greater range of phase 2 endpoints than is currently available and that these regimens be rigorously compared in a cumulative meta-analytic framework. Although this review forms an initial contribution, achieving this goal will require a coordinated and multidisciplinary effort by the tuberculosis trials community.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. The authors thank Sarah Donegan for her review of this manuscript.
Financial support. The research leading to these results has received funding from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115337, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations companies’ in kind contribution.
Potential conflicts of interest. G. C. K. W. K. is an employee of and holds shares in GlaxoSmithKline. No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lienhardt C, Raviglione M, Spigelman M, et al. New drugs for the treatment of tuberculosis: needs, challenges, promise, and prospects for the future. J Infect Dis 2012; 205(suppl 2):S241–9. [DOI] [PubMed] [Google Scholar]
- 2. Donald PR, Diacon AH. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis (Edinb) 2008; 88(suppl 1):S75–83. [DOI] [PubMed] [Google Scholar]
- 3. Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis 1980; 121:939–49. [DOI] [PubMed] [Google Scholar]
- 4. Diacon AH, Dawson R, von Groote-Bidlingmaier F, et al. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 2012; 380:986–93. [DOI] [PubMed] [Google Scholar]
- 5. Rustomjee R, Lienhardt C, Kanyok T, et al. ; Gatifloxacin for TB (OFLOTUB) study team A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2008; 12:128–38. [PubMed] [Google Scholar]
- 6. Phillips PP, Fielding K, Nunn AJ. An evaluation of culture results during treatment for tuberculosis as surrogate endpoints for treatment failure and relapse. PLoS One 2013; 8:e63840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wallis RS, Wang C, Meyer D, Thomas N. Month 2 culture status and treatment duration as predictors of tuberculosis relapse risk in a meta-regression model. PLoS One 2013; 8:e71116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallis RS, Peppard T, Hermann D. Month 2 culture status and treatment duration as predictors of recurrence in pulmonary tuberculosis: model validation and update. PLoS One 2015; 10:e0125403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Center for Drug Evaluation and Research. Pulmonary tuberculosis: developing drugs for treatment. Silver Spring, MD: US Food and Drug Administration, 2013. [Google Scholar]
- 10. Davies GR, Phillips PP, Jaki T. Adaptive clinical trials in tuberculosis: applications, challenges and solutions. Int J Tuberc Lung Dis 2015; 19:626–34. [DOI] [PubMed] [Google Scholar]
- 11. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions Available at: www.cochrane-handbook.org: Cochrane Collaboration, 2011. [Google Scholar]
- 12. R Cran Team. R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing, 2015. [Google Scholar]
- 13. Kennedy N, Fox R, Kisyombe GM, et al. Early bactericidal and sterilizing activities of ciprofloxacin in pulmonary tuberculosis. Am Rev Respir Dis 1993; 148(6 pt 1):1547–51. [DOI] [PubMed] [Google Scholar]
- 14. Snider DE, Graczyk J, Bek E, Rogowski J. Supervised six-months treatment of newly diagnosed pulmonary tuberculosis using isoniazid, rifampin, and pyrazinamide with and without streptomycin. Am Rev Respir Dis 1984; 130:1091–4. [DOI] [PubMed] [Google Scholar]
- 15. Bonnett LJ, Davies GR. Quality of outcome reporting in phase II studies in pulmonary tuberculosis. Trials 2015; 16:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aber VR, Nunn AJ. Factors affecting relapse following short-course chemotherapy. Bull Int Union Tuberc Lung Dis 1978; 53:260–4. [PubMed] [Google Scholar]
- 17. Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA 2000; 283:2537–45. [DOI] [PubMed] [Google Scholar]
- 18. Burman WJ, Goldberg S, Johnson JL, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med 2006; 174:331–8. [DOI] [PubMed] [Google Scholar]
- 19. Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007; 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.