ABSTRACT
Lipopolysaccharide (LPS), a cell-associated glycolipid that makes up the outer leaflet of the outer membrane of Gram-negative bacteria, is a canonical mediator of microbe-host interactions. The most prevalent Gram-negative gut bacterial taxon, Bacteroides, makes up around 50% of the cells in a typical Western gut; these cells harbor ~300 mg of LPS, making it one of the highest-abundance molecules in the intestine. As a starting point for understanding the biological function of Bacteroides LPS, we have identified genes in Bacteroides thetaiotaomicron VPI 5482 involved in the biosynthesis of its lipid A core and glycan, generated mutants that elaborate altered forms of LPS, and used matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry to interrogate the molecular features of these variants. We demonstrate, inter alia, that the glycan does not appear to have a repeating unit, and so this strain produces lipooligosaccharide (LOS) rather than LPS. This result contrasts with Bacteroides vulgatus ATCC 8482, which by SDS-PAGE analysis appears to produce LPS with a repeating unit. Additionally, our identification of the B. thetaiotaomicron LOS oligosaccharide gene cluster allowed us to identify similar clusters in other Bacteroides species. Our work lays the foundation for developing a structure-function relationship for Bacteroides LPS/LOS in the context of host colonization.
KEYWORDS: Bacteroides, lipopolysaccharide, microbiome
IMPORTANCE
Much is known about the bacterial species and genes that make up the human microbiome, but remarkably little is known about the molecular mechanisms through which the microbiota influences host biology. A well-known mechanism by which bacteria influence the host centers around lipopolysaccharide (LPS), a component of the Gram-negative bacterial outer membrane. Pathogen-derived LPS is a potent ligand for host receptor Toll-like receptor 4, which plays an important role in sensing bacteria as part of the innate immune response. Puzzlingly, the most common genus of human gut bacteria, Bacteroides, produces LPS but does not elicit a potent proinflammatory response. Previous work showing that Bacteroides LPS differs structurally from pathogen-derived LPS suggested the outlines of an explanation. Here, we take the next step, elucidating the biosynthetic pathway for Bacteroides LPS and generating mutants in the process that will be of great use in understanding how this molecule modulates the host immune response.
INTRODUCTION
High-abundance cell-associated molecules are of great interest to understanding microbiota-host interactions at the level of molecular mechanism. Unlike high-abundance diffusible molecules, which are the characteristic products of amino acid and sugar metabolism, high-abundance cell-associated molecules are often lipids and glycolipids (1). These molecules tend to be structural components of the cell membrane or cell wall that are architecturally similar although chemically different among bacterial taxa—lipopolysaccharides (LPSs), lipoteichoic and wall teichoic acids, mycolic acids, and muramyl dipeptides are key examples. Their ubiquity on the cell surface makes them excellent targets for bacterial detection by innate immune receptors, including Toll-like receptors and NOD proteins (2). However, a longstanding question remains: how do innate immune cells “know” whether the bacterial cell that they encounter is a mutualist or a pathogen and “decide” how to respond appropriately? Part of the answer likely involves unique strain-specific chemical signatures within these cell-associated molecules.
Lipopolysaccharide (LPS) is a canonical cell-associated glycolipid. The interaction between LPS and host Toll-like receptor 4 (TLR4) is a paradigm for immunologic sensing of Gram-negative bacteria. LPS is generally composed of a lipid anchor (termed lipid A), a core oligosaccharide region, and a polysaccharide repeating unit called the O antigen. The core oligosaccharide and O antigen are typically biosynthesized from separate gene clusters, while lipid A biosynthetic genes are distributed throughout the genome (3). The chemical structure of LPS varies considerably among species, and these differences in structure are relevant to function. For example, Yersinia pestis deacylates its lipid A when infecting humans, thus avoiding detection by TLR4 (4). Helicobacter pylori elaborates its O antigen with Lewis antigens to mimic host glycans (5, 6). More drastic changes in overall structure have also been observed. Species of Neisseria produce an LPS variant, known as lipooligosaccharide (LOS), which has a more elaborate core oligosaccharide in place of the conventional O antigen (7, 8). Notably, almost everything known about the biosynthesis, structure, and function of LPS comes from studies of “conventional” pathogens. Remarkably little is known about LPS from commensal organisms and its importance to host innate immunity.
Among the glycolipids found in the gut microbiome, Bacteroides LPS is of particular interest. Bacteroides and, in ~10% of humans, its relative Prevotella are the only high-abundance Gram-negative bacterial genera in the gut. Bacteroides as a genus makes up ~50% of the typical Western gut community (9). Notably, the species distribution within that 50% is highly variable between individuals (10). Different Bacteroides species have been reported to produce LPS molecules with distinct architectures based on their banding pattern on an SDS-PAGE gel, suggesting that each Bacteroides species has the potential to influence innate immunity in its own way (11, 12). Bacteroides LPS is already known to have a different lipid A structure than “pathogenic” LPS: Bacteroides thetaiotaomicron, Bacteroides fragilis, and Bacteroides dorei produce penta-acylated, monophosphorylated lipid A, in contrast to the hexa-acylated, diphosphorylated lipid A from Escherichia coli (13–16). With a recent exception reporting a B. thetaiotaomicron lipid A phosphatase, very little is known about the biosynthetic genes involved in Bacteroides LPS biogenesis (17).
It takes as little as 50 ng of E. coli LPS injected intravenously into a mouse to cause septic shock (18). In contrast, given our laboratory purification yield of approximately 10 mg B. thetaiotaomicron LPS per 1 liter of confluent culture and assuming ~7 × 1011 bacteria per liter in vitro and 20 trillion Bacteroides cells per individual, we estimate that a typical Western human gut contains ~300 mg of Bacteroides LPS, likely making it one of the highest-abundance bacterially derived molecules present (19). We set out to better define the biosynthesis and structure of Bacteroides LPS as a starting point for understanding and manipulating the interaction between Bacteroides and the mammalian immune system.
RESULTS AND DISCUSSION
Characterization of the Bacteroides lipid A core.
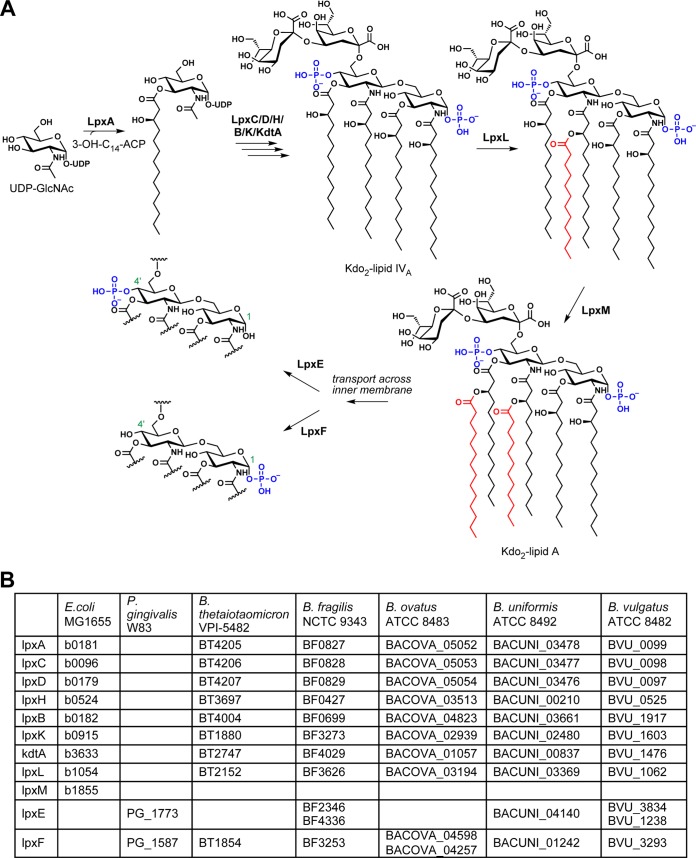
In order to identify candidate biosynthetic genes for Bacteroides lipid A, we performed BLAST searches against Bacteroides genomes using, as queries, the E. coli MG1655 lipid A biosynthesis genes (20). E. coli normally produces a lipid A molecule that has six acyl chains and two phosphate groups, as shown in Fig. 1A labeled “Kdo2-lipid A.” As expected, orthologs of each Raetz pathway enzyme were identified, except that the Bacteroides species had only one ortholog of the acyltransferases LpxL and LpxM; we refer to this ortholog as LpxL for simplicity. LpxL and LpxM are responsible for adding the fifth and sixth acyl chains to E. coli lipid A, so the presence of only one of these acyltransferases in Bacteroides genomes is consistent with published reports that B. thetaiotaomicron, B. fragilis, and Bacteroides dorei lipid A is penta-acylated rather than hexa-acylated (13–15). Bacteroides vulgatus was the only surveyed species to have a second LpxL/LpxM homolog, BVU_1014. Previous work indicates that this gene is part of an aryl polyene gene cluster, indicating that it likely transfers an acyl chain to a non-LPS substrate (21). We next used BLAST to predict lipid A phosphorylation by sequence homology to the lipid A 1- and 4′-phosphatases discovered in Porphyromonas gingivalis (22, 23) (Fig. 1). While this search resulted in only one candidate for some species like B. thetaiotaomicron, for others there were multiple candidates, and experimental validation will be necessary to conclude which, if any, perform the predicted function.
FIG 1 .
The Raetz pathway for lipid A biosynthesis in E. coli and its homologs in Bacteroides. (A) Abbreviated schematic of the Raetz pathway, where starting material UDP-GlcNAc is acylated, glycosylated, and phosphorylated by a series of nine biosynthetic enzymes to produce 3-deoxy-d-manno-octulosonic acid 2 (Kdo2)-lipid A. E. coli produces lipid A that is phosphorylated at both the 1 and 4′ positions on the diglucosamine backbone, but the lipid A 1- and 4′-phosphatases LpxE and LpxF have been identified in other bacteria and are thought to act after biosynthesis of Kdo2-lipid A is complete. (B) Locus tags of homologs of the Raetz pathway genes in E. coli MG1655 and lpxE and lpxF in Porphyromonas gingivalis W83 from a selection of Bacteroides species. Bacteroides has homologs for every gene in the pathway except that it has only one secondary acyltransferase, suggesting that its lipid A is predominantly penta-acylated. The species vary more in their putative homologs of the P. gingivalis phosphatases.
In order to determine the lipid A profile of each species, we isolated lipid A from five common Bacteroides species using the TRI reagent method and characterized their lipid A profile by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) (24). Consistent with previous reports, the structures of B. thetaiotaomicron VPI 5482 and B. fragilis NCTC 9343 lipid A are penta-acylated and monophosphorylated, with their MALDI spectra showing a cluster of peaks around 1,688 m/z (13, 14). Moreover, Bacteroides uniformis ATCC 8492, B. vulgatus ATCC 8482, and Bacteroides ovatus ATCC 8483 produce lipid A with virtually identical mass spectra (Fig. 2). Because the bacteria were grown in rich medium under normal anaerobic growth conditions, we cannot be certain that the structure of their lipid A is the same under conditions of host colonization, nor do we know whether it can change in response to stresses encountered in the host.
FIG 2 .
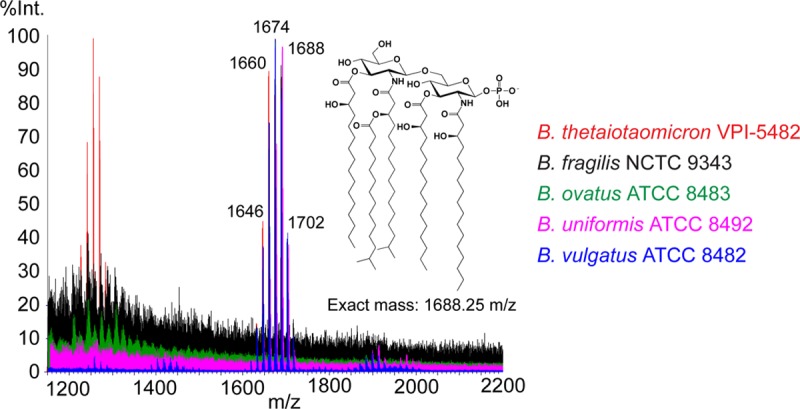
MALDI-TOF MS analysis of lipid A isolated from five Bacteroides species. Lipid A was purified from B. thetaiotaomicron VPI 5482, B. fragilis NCTC 9343, B. ovatus ATCC 8343, B. uniformis ATCC 8492, and B. vulgatus ATCC 8482 by the TRI reagent method, dissolved in 3:1 chloroform-methanol, spotted on a 5-chloro-2-mercaptobenzothiazole (CMBT) matrix, and analyzed on a Waters Corporation Synapt G2 HDMS 32k MALDI-TOF instrument in reflectron negative-ion mode. All five have as their dominant lipid A species a cluster of peaks around 1,688 m/z corresponding to the published structure of B. thetaiotaomicron lipid A. The peaks in the cluster are separated by 14 m/z (methylene group, CH2), likely caused by heterogeneity in the number of carbons in each acyl chain of lipid A.
Characterization of late biosynthetic genes in B. thetaiotaomicron lipid A biosynthesis: acylation and dephosphorylation.
Because lipid A is typically an essential component of the outer membrane of Gram-negative bacteria, deletion of genes in the lipid A biosynthetic pathway is frequently lethal to bacteria (25–30). Interestingly, the later biosynthetic genes such as those for the acyltransferases (lpxL and lpxM) and phosphatases (lpxE and lpxF) can often be deleted (31). Working in B. thetaiotaomicron VPI 5482 Δtdk, our background strain for genetic knockouts lacking the thymidine kinase gene tdk, we made scarless single deletions of the putative lpxL and lpxF orthologs that we had identified by BLAST search (BT2152 and BT1854, respectively) and isolated lipid A from the resulting mutants. MALDI-TOF analysis showed a loss of 224 Daltons in the ΔlpxL mutant, consistent with the loss of a 15-carbon acyl chain, and a gain of 80 Daltons in the ΔlpxF mutant, indicating the addition of a phosphate group (Fig. 3). The assignment of BT1854 as the B. thetaiotaomicron lipid A 4′-phosphatase represents independent confirmation of a result first reported by Goodman and coworkers (17).
FIG 3 .
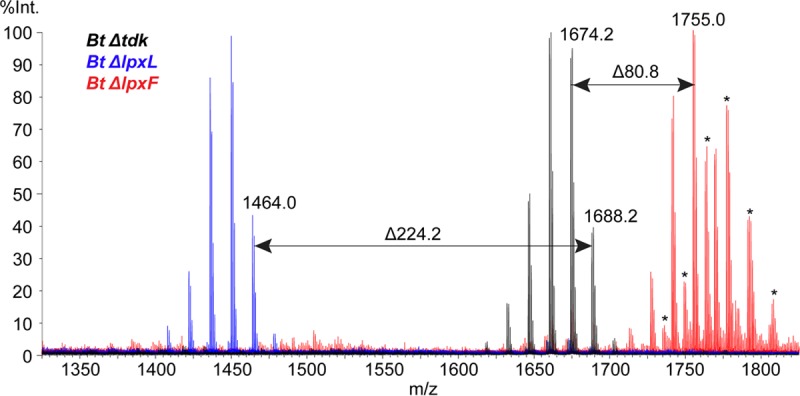
MALDI-TOF mass spectra of lipid A from B. thetaiotaomicron lipid A mutants. Lipid A was isolated from B. thetaiotaomicron ΔlpxL and B. thetaiotaomicron ΔlpxF strains by the TRI reagent method, dissolved in 3:1 chloroform-methanol, spotted on a CMBT matrix, and analyzed on a Waters Corporation Synapt G2 HDMS 32k MALDI-TOF instrument in reflectron negative-ion mode. The B. thetaiotaomicron ΔlpxL strain produced a spectrum where the cluster of peaks characteristic of lipid A has decreased in mass by 224 Daltons, the same mass as the 15-carbon secondary acyl chain from the published B. thetaiotaomicron structure. The B. thetaiotaomicron ΔlpxF strain lipid A increased by 80 Daltons over that in wild-type B. thetaiotaomicron, indicating the presence of a phosphate group as expected. Sodiated peaks are marked with an asterisk.
Lipid A acylation and phosphorylation are important both for bacterial membrane physiology and for the interaction between LPS and host TLR4. An ΔlpxL ΔlpxM double mutant of E. coli MG1655—which lacks the fifth and sixth acyl chains on lipid A, yielding a tetra-acylated, diphosphorylated molecule referred to as lipid IVA—cannot grow above 32°C (Fig. 1A) (32). The crystal structure of the TLR4/MD-2 complex with E. coli lipid A suggests that the number of acyl chains and the number and position of phosphate groups on the molecule may affect binding affinity to the receptor and possibly receptor dimerization (33). E. coli lipid IVA is capable of inhibiting TLR4 activation by wild-type E. coli lipid A (34). This tetra-acylated diphosphorylated scaffold has been used in the design of eritoran, a lipid A mimic developed as a TLR4 antagonist as a potential treatment for sepsis (35). Additionally, lipid A mutants in E. coli differentially stimulate NF-κB production in a THP-1 reporter cell line (36). We hypothesize that identifying lipid A biosynthesis genes in Bacteroides will allow us to make mutants that may have different immunostimulatory abilities and could be used to control innate immune responses in a host. Toward this goal, we have generated a B. thetaiotaomicron ΔlpxL ΔlpxF double mutant that elaborates tetra-acylated, diphosphorylated lipid A, which we anticipate will be a TLR4 antagonist (see Fig. S1 in the supplemental material).
The double knockout of lpxL and lpxF in B. thetaiotaomicron produces tetra-acylated diphosphorylated lipid A. Lipid A was isolated from the B. thetaiotaomicron ΔlpxL ΔlpxF strain using the TRI reagent method. The resulting material was dissolved in 3:1 chloroform-methanol, mixed in a 1:1 ratio with saturated 5-chloro-2-mercaptobenzothiazole in 3:1 chloroform-methanol, and spotted on a Waters Corporation MALDI target. The sample was analyzed on a Waters Synapt G2 MALDI-TOF mass spectrometer in reflectron negative-ion mode. The mass of the resulting cluster of peaks is around 1,544 m/z, which is 144 m/z less than wild-type B. thetaiotaomicron lipid A at 1,688 m/z. That 144 Daltons decrease is equivalent to the loss of 224 Daltons observed in the B. thetaiotaomicron ΔlpxL mutant plus the addition of 80 Daltons as observed in the B. thetaiotaomicron ΔlpxF mutant, indicating that we have made a tetra-acylated diphosphorylated lipid A molecule in B. thetaiotaomicron. B. thetaiotaomicron ΔlpxL ΔlpxF strain peaks are accented in blue for clarity, and sodiated B. thetaiotaomicron ΔlpxL ΔlpxF strain peaks are in black and marked with an asterisk. Download FIG S1, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
SDS-PAGE analysis of Bacteroides LPS.
In contrast to the level of structural detail already available about B. thetaiotaomicron lipid A, relatively little is known about the oligosaccharide component of the Bacteroides LPS molecule. Purified LPS molecules can be visualized by SDS-PAGE to gain a general idea of the number of molecules present in the preparation and their relative sizes. We purified LPS from B. thetaiotaomicron and B. vulgatus ATCC 8482 (B. vulgatus) and compared them to commercially available E. coli O55:B5 and E. coli MG1655 LPS to confirm the previous observation that B. vulgatus produces LPS exhibiting a “laddered” pattern on a gel like E. coli O55:B5 does, whereas B. thetaiotaomicron LPS does not (11, 12). The laddering pattern is of note because it indicates the presence of an O antigen; the number of repeating units added to the core oligosaccharide is variable, so the result is a population of LPS molecules of different sizes. As shown in Fig. 4, B. vulgatus LPS appears to have an O antigen based on the laddered pattern of its LPS, but B. thetaiotaomicron instead appears to synthesize a small number of structures that we propose are more likely to be lipooligosaccharides (LOSs) due to their apparent lack of an O antigen. Here, we will refer to the B. thetaiotaomicron outer membrane glycolipid as a LOS rather than LPS.
FIG 4 .
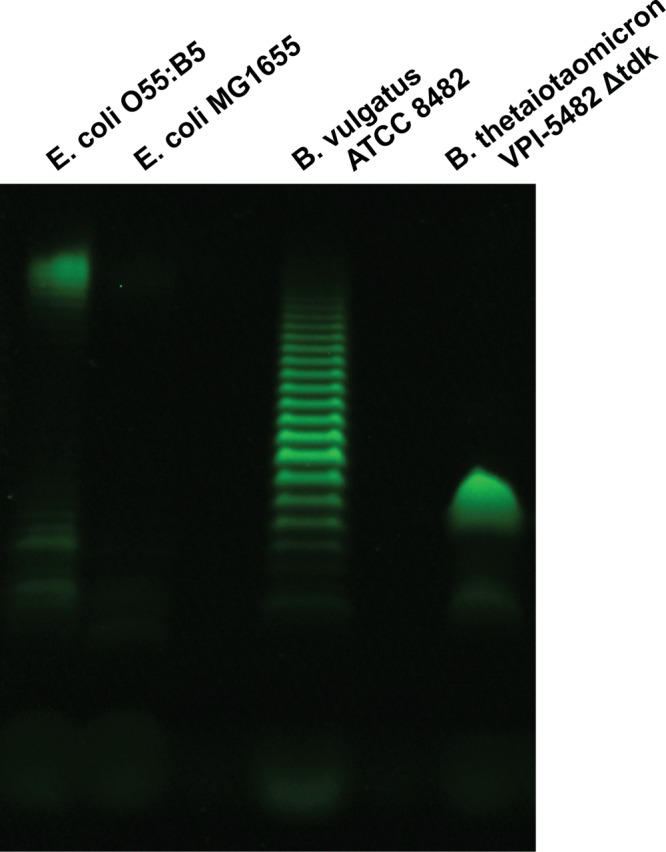
B. thetaiotaomicron LPS analyzed by SDS-PAGE does not appear to have an O antigen. Purified LPS from E. coli O55:B5, E. coli MG1655, B. vulgatus ATCC 8482, and the B. thetaiotaomicron Δtdk mutant were run on a 16% Tricine SDS-PAGE gel. B. vulgatus LPS has a laddered pattern similar to that of LPS from E. coli O55:B5, suggesting the presence of an O-antigen repeating unit, but B. thetaiotaomicron LPS appears to lack a repeating unit and is therefore better characterized as lipooligosaccharide (LOS). B. vulgatus LPS was extracted using the microscale LPS method, and B. thetaiotaomicron LOS was extracted using the large-scale LPS method. LPS from E. coli O55:B5 and E. coli MG1655 was purchased from InvivoGen.
Recent work on the human-associated bacteria H. pylori and Hafnia alvei indicates that the glycan portion of LPS from these strains can interact with C-type lectin receptors DC-SIGN and dectin-2, respectively, to influence a dendritic cell’s cytokine output (37, 38). Engagement of this class of receptors by a human-associated bacterium lacking an O antigen has not been investigated, however, and so we sought to identify the biosynthetic route for the oligosaccharide component of B. thetaiotaomicron LOS to explore its apparent lack of an O antigen and provide tools for manipulating the glycan structure.
Identification of the gene cluster for LOS oligosaccharide biosynthesis in B. thetaiotaomicron.
Previous work analyzing biosynthetic gene clusters from the NIH Human Microbiome Project data indicated that the phylum Bacteroidetes contains the largest number of predicted saccharide-producing gene clusters (39). B. thetaiotaomicron alone is known to harbor eight gene clusters responsible for making different capsular polysaccharides (CPSs) (40, 41). We first wanted to determine whether any of these CPS gene clusters influenced the assembly of LOS. We isolated LOS from a B. thetaiotaomicron mutant constructed by Martens and coworkers in which all eight of its CPS clusters have been deleted (labeled the ΔCPS strain), as well as eight additional strains that each possess only one CPS cluster (CPS1-only, CPS2-only, and so on) (42). By SDS-PAGE analysis, we determined that neither deletion nor expression of CPS clusters affects the banding pattern of B. thetaiotaomicron LOS, indicating that these gene clusters do not encode the biosynthetic machinery for synthesis of B. thetaiotaomicron LOS (Fig. S2).
Capsular polysaccharide gene clusters do not appear to be involved in LOS biosynthesis. LOS was isolated from all 10 of these strains using the microscale extraction method and analyzed by SDS-PAGE and staining with the Pro-Q Emerald 300 lipopolysaccharide gel stain kit (Thermo Fisher). CPS bands can be seen for certain samples like CPS2-only and CPS4-only near the top of the gel. Whether the strains contain the gene clusters for all the CPSs (Δtdk), none of the CPSs (ΔCPS), or one CPS at a time (CPS1-only, CPS2-only, and so on), the LOS bands appear unchanged, suggesting that the CPS gene clusters do not contribute to LOS biosynthesis. Download FIG S2, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
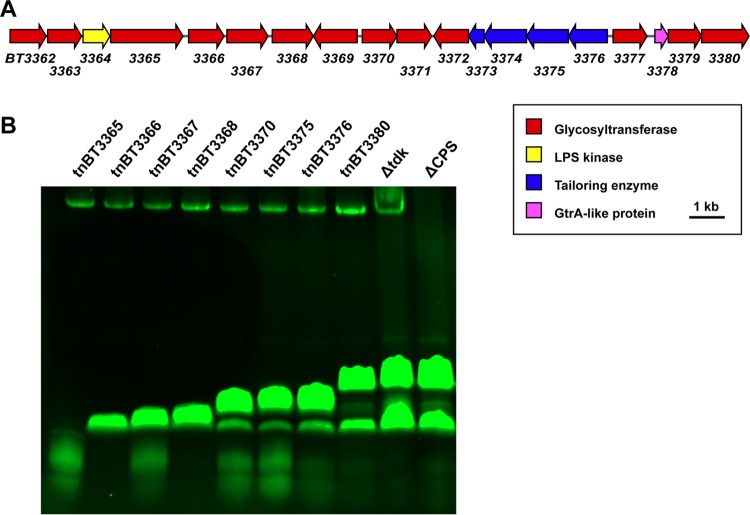
An independent lead came from a recently published report: by screening a B. thetaiotaomicron transposon library using an antibody that binds the bacterial cell surface of B. thetaiotaomicron, Peterson et al. identified a gene cluster that they predicted might be responsible for the biosynthesis of the B. thetaiotaomicron LPS O antigen, due to a lack of antibody binding when the cluster was disrupted and the annotated functions of several of the genes within the cluster (43). Surprisingly, nine out of the 13 transposon mutants that did not bind the antibody had insertions in genes in the same gene cluster, BT3362 to BT3380 (Fig. 5A). No transposon insertions were obtained in the first three genes of the cluster, indicating that these genes might be essential or their deletion might lead to the accumulation of a toxic intermediate. Intrigued, we obtained a subset of these transposon mutants and analyzed LOS isolated from each mutant by SDS-PAGE (Fig. 5B). Each mutant produced LOS with a banding pattern that appeared different from that of the wild type, except for the mutant with an insertion in the final gene in the cluster, BT3380. These data suggest that BT3362 to BT3380 encode the biosynthesis of the B. thetaiotaomicron LOS oligosaccharide. Additionally, they support our hypothesis that bands observed on the SDS-PAGE gel are glycans that do not have a single polymerized repeating unit but rather are variants of a heterogeneous oligosaccharide.
FIG 5 .
The gene cluster BT3362 to BT3380 is responsible for the biosynthesis of the B. thetaiotaomicron LOS oligosaccharide region. (A) Schematic of the gene cluster BT3362 to BT3380, which includes genes predicted to encode 13 glycosyltransferases shown in red, those for an LPS kinase shown in yellow, those for four tailoring enzymes shown in blue, and one for a putative GtrA-like protein shown in magenta. (B) LOS was isolated from the eight B. thetaiotaomicron transposon mutants, the B. thetaiotaomicron Δtdk strain, and the B. thetaiotaomicron ΔCPS strain by the microscale extraction method, and the resulting material was run on a 16% Tricine SDS-PAGE gel. The transposon mutants are labeled “tn” followed by the locus tag of the gene in which the transposon has inserted. For example, “tnBT3365” indicates a B. thetaiotaomicron strain with a transposon inserted in BT3365. All transposon mutants are in the wild-type Bacteroides thetaiotaomicron VPI 5482 background with the tdk gene present and are erythromycin resistant. The LOS migrates on the gel differently for almost all of the transposon mutants compared to B. thetaiotaomicron Δtdk and B. thetaiotaomicron ΔCPS strains; only the B. thetaiotaomicron tnBT3380 mutant, with the transposon inserted in the last gene in the cluster, has the same banding pattern on the gel as the two strains with wild-type LOS. The band at the top of the gel is CPS, which is present in all of the transposon mutants and the B. thetaiotaomicron Δtdk strain but not the B. thetaiotaomicron ΔCPS strain.
Furthermore, the predicted function of the genes within the BT3362 to BT3380 cluster also supports this conclusion. This cluster bears some resemblance to the waa core oligosaccharide gene clusters characterized in E. coli, with the first gene, BT3362, sharing homology with the genes for heptosyltransferases WaaC and WaaQ (44). Overall, the cluster possesses 13 predicted glycosyltransferases (BT3362-BT3363, BT3365 to BT3372, BT3377, and BT3379-BT3380), a putative LPS kinase (BT3363), four tailoring enzymes (BT3373 to BT3376), and a GtrA-like protein (BT3378). It is unclear what specific structural modifications BT3373 to BT3376 might make based solely on sequence homology. Proteins in the GtrA-like family are typically integral membrane proteins that are thought to play a role in the transport of cell surface polysaccharides (45, 46).
Intact LPS MALDI-TOF MS as a diagnostic tool for LOS mutants.
While the SDS-PAGE analysis of LOS from the transposon mutants implicates BT3362 to BT3380 in B. thetaiotaomicron LOS oligosaccharide biosynthesis, we wanted to increase the resolution of our analysis using mass spectrometry (MS). The LPS/LOS bands on an SDS-PAGE gel are approximations of the sizes of molecules in a sample, and using mass spectrometry would allow us to gain a clearer picture of the molecules made by the transposon mutants. Although it is easier to analyze LPS/LOS by MALDI-TOF after removing the O- and/or N-linked acyl chains via hydrazine or hydrogen fluoride treatment, we chose to analyze intact LOS molecules that were not subjected to chemical degradation or derivatization. We reasoned that LOS from B. thetaiotaomicron may contain important functional groups in the oligosaccharide chain that could be removed by these treatments, further complicating our efforts to elucidate detailed structural information about B. thetaiotaomicron LOS.
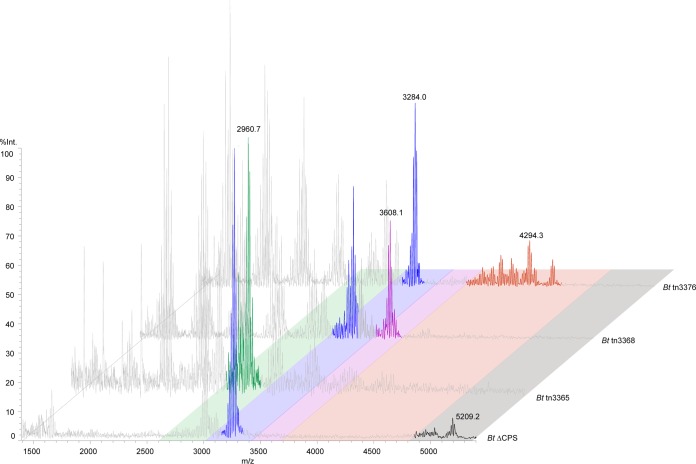
We adapted a previously published strategy for analyzing intact LPS/LOS by MALDI-TOF (47, 48). LPS/LOS analysis is typically challenging due to difficulties in inducing the glycolipid to ionize because of its size and polarity. We chose three transposon mutants that appeared by SDS-PAGE analysis to be truncated to various degrees (B. thetaiotaomicron tn3365, B. thetaiotaomicron tn3368, and B. thetaiotaomicron tn3376), along with LOS isolated from the B. thetaiotaomicron ΔCPS strain. The ΔCPS strain has wild-type LOS biosynthetic genes, but its lack of CPS yielded mass spectra with a cleaner background. The transposon mutants were created in the background of wild-type B. thetaiotaomicron, and so CPS is present in those preparations. In LOS from the ΔCPS strain, we observed a cluster of peaks around 5,209 m/z, the largest mass detected for any of the samples (Fig. 6). Although instrument-specific limitations prevented us from obtaining a resolution as high as others have observed, the limited degree of resolution that we achieved was sufficient for confirming the approximate masses of LOS molecules in the sample and comparing them to those of the truncated mutants (8, 48).
FIG 6 .
MALDI-TOF MS analysis of intact LOS indicates that the transposon mutants make truncated LOS compared to wild type. LOSs from B. thetaiotaomicron ΔCPS, B. thetaiotaomicron tnBT3365, B. thetaiotaomicron tnBT3368, and B. thetaiotaomicron tnBT3376 strains were isolated using the large-scale LPS/LOS extraction method. The resulting material was desalted and spotted on a THAP-nitrocellulose matrix for analysis on a Shimadzu Axima Performance MALDI-TOF mass spectrometer in linear negative-ion mode. LOS peaks of interest have been colored, with each color representing a different LOS species present in the different strains. Peaks colored gray are likely derived from capsular polysaccharide (those that do not appear in the B. thetaiotaomicron ΔCPS spectrum) or lipid A in the case of the peak clusters around 1,650 to 1,700 m/z.
In addition to the peak that we propose corresponds to full-length LOS at 5,209 m/z, the ΔCPS sample has additional peaks at 3,284 and 3,017 m/z, which are likely intermediate species created by partial completion of the biosynthetic pathway. The most truncated transposon mutant in our set, tn3365, has a single predominant peak at 2,961 m/z. LOS from tn3368 does not have this 2,961 m/z peak but instead has peaks at 3,284 m/z (as in the ΔCPS sample) and 3,608 m/z. Interestingly, 2,961, 3,284, and 3,608 m/z are separated from one another by ~324 Daltons. The expected mass of a hexose is ~162 Daltons, and so we predict that tn3368 makes two LOS molecules that are two and four hexose units longer than the molecule made by tn3365. Finally, LOS from the least truncated transposon mutant that we assayed, tn3376, has its largest peaks around 4,497 and 4,295 m/z, as well as the 3,284 m/z peak that is common to both the tn3368 and the ΔCPS samples. The mass differences between tn3368 and tn3376, as well as between tn3376 and ΔCPS, do not suggest a structural difference as straightforward as the addition of hexoses between tn3365 and tn3368. Given the presence of genes encoding tailoring enzymes in the LOS biosynthetic cluster, we expect that the longer LOS species have modifications like phosphorylation, acetylation, or carbamoylation. All of the transposon mutants have additional peaks between 1,500 and 3,000 m/z that presumably derive from CPS, since these peaks are absent in the ΔCPS sample but present in LOS isolated from the B. thetaiotaomicron Δtdk mutant (Fig. S3). Additionally, every sample has a cluster of peaks around 1,688 m/z representing lipid A, which likely derives from in-source fragmentation (48).
Capsular polysaccharide is likely the cause of contaminating peaks in samples in the Δtdk wild-type background. LOS from the B. thetaiotaomicron Δtdk strain was purified by the large-scale extraction method, and the intact molecule was analyzed on a Shimadzu Axima Performance MALDI-TOF MS instrument in linear negative-ion mode. The peaks in black are those species that are also present in the ΔCPS intact LOS sample, but the rest of the peaks in red are unique to the Δtdk sample despite its differing from the ΔCPS mutant only in that it contains capsule. Because similar peaks are noted in the intact LOS MALDI-TOF spectra of the transposon mutants, which also contain capsule, we conclude that the peaks in red are due to the presence of CPS in the LOS preparation. Download FIG S3, EPS file, 1.6 MB (1.7MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
With the goal of confirming these results using clean deletion mutants rather than the transposon mutants, we deleted as much of the cluster as we could—BT3363 and BT3365 to BT3380 (we could not obtain mutants of BT3362 and BT3364, consistent with the lack of transposon insertions in these genes) (43). Surprisingly, when we compared LOSs purified from this strain and from the tn3365 mutant on an SDS-PAGE gel, the banding pattern of B. thetaiotaomicron ΔBT3363 ΔBT3365–BT3380 LOS appeared to resemble that of wild-type LOS. However, when we subjected the B. thetaiotaomicron ΔBT3365 ΔBT3365–BT3380 LOS to MALDI-TOF analysis, the mass of the intact glycolipid was around 4,870 Daltons, smaller than the 5,209 Daltons seen in wild-type LOS (Fig. S4). These data suggest that there might be a compensatory mechanism in which the bacterium is able to glycosylate truncated forms of the LOS molecule. Peterson et al. similarly predicted that B. thetaiotaomicron may have the ability to produce an O antigen when this gene cluster is disrupted because the colony morphology of their transposon mutants did not differ from that of wild-type B. thetaiotaomicron (43). However, by our intact LOS MALDI-TOF analysis, we see evidence for new oligosaccharide production only when the cluster is cleanly deleted, rather than when single genes are disrupted by transposon insertion. Our result highlights the limitations of SDS-PAGE analysis in determining structural differences between LOS samples (Fig. S5). Further studies need to be conducted to understand whether B. thetaiotaomicron has an alternative lipid A glycosylation pathway that is unmasked in the absence of most of the LOS oligosaccharide gene cluster.
B. thetaiotaomicron produces a molecule with a different mass when the LOS oligosaccharide biosynthesis cluster is deleted. LOS was isolated from the B. thetaiotaomicron ΔCPS ΔBT3363 ΔBT3365–BT3380 (red) and B. thetaiotaomicron ΔBT3363 ΔBT3365–BT3380 (black) strains by the large-scale extraction method and analyzed on a Shimadzu Axima Performance MALDI-TOF MS instrument in linear negative-ion mode. These mutants have as much of the LOS oligosaccharide biosynthesis cluster deleted as possible and should not be able to make glycosylated LOS, and yet they appear to make new molecules in its stead. Lipid A is still present in both samples, suggesting that a new oligosaccharide may be getting attached to the truncated LOS left over after the cluster deletion. Download FIG S4, EPS file, 1.7 MB (1.7MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LOSs from a transposon insertion strain and a clean deletion strain do not have the same banding pattern on an LOS gel. LOS was isolated from B. thetaiotaomicron ΔBT3365 and B. thetaiotaomicron tnBT3365 strains by the large-scale LPS/LOS extraction method. The resulting material was analyzed by SDS-PAGE and stained using the Pro-Q Emerald 300 lipopolysaccharide gel stain kit (Thermo Fisher). While a transposon insertion in BT3365 results in what appears to be a truncated LOS structure, a clean deletion of the same gene looks like it has a wild-type banding pattern. Download FIG S5, EPS file, 0.5 MB (502.9KB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Predicting other Bacteroides LOS oligosaccharide gene clusters.
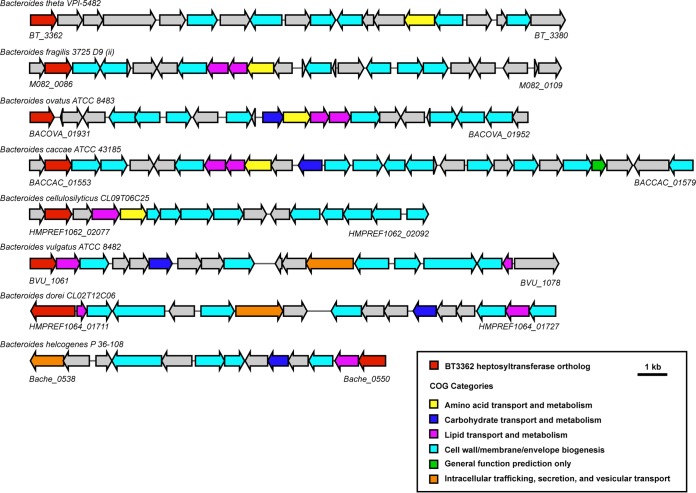
Having identified the probable B. thetaiotaomicron LOS biosynthetic gene cluster, we hypothesized that it could be used to identify candidate LOS and LPS gene clusters in other Bacteroides species. We used the two essential genes in the cluster, BT3362 (a putative heptosyltransferase) and BT3364 (a putative LPS kinase), as queries in BLAST searches against other Bacteroides genomes and were able to identify similar clusters in many Bacteroides species (Fig. 7). Given that a homologous cluster is found in B. vulgatus, which elaborates a laddered LPS, we expect that B. vulgatus harbors an additional cluster encoding the biosynthesis of the O antigen repeating unit. This would likely be attached to the product of the B. thetaiotaomicron-like core oligosaccharide.
FIG 7 .
Clusters orthologous to the B. thetaiotaomicron LOS oligosaccharide gene cluster in additional Bacteroides species. Orthologs of the BT3362 to BT3380 cluster in B. thetaiotaomicron were identified using the “Show neighborhood ortholog regions with the same top COG hit” function within the DOE Joint Genome Institute’s IMG portal, using BT3362, the putative B. thetaiotaomicron LOS heptosyltransferase, as the query gene. We have estimated the start and end of each gene cluster by inspecting the surrounding genomic area of each ortholog and comparing it to the B. thetaiotaomicron cluster. Our list shows a representative sample of the clusters resulting from this search.
Our results are a first step in characterizing what we expect will be a large amount of biosynthetic and structural heterogeneity among Bacteroides LPS or LOS molecules. Given our understanding of the privileged role that glycolipids play in communicating with the mammalian immune system and the sheer quantity of LPS in the gut, Bacteroides LPS/LOS molecules are likely to be critical mediators in the interaction between commensal microbes and the host. By understanding how these molecules are made, we gain the possibility of manipulating their structure and by extension the host’s immune response.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and reagents.
See Table S1 in the supplemental material for a full list of bacterial strains and plasmids used in this study. All Bacteroides strains were cultured anaerobically at 37°C in peptone-yeast extract-glucose (PYG) liquid medium or brain heart infusion (BHI) agar (BD Biosciences) with defibrinated horse blood (Hardy Diagnostics) added to 10% vol/vol. The B. thetaiotaomicron transposon mutants were grown in liquid and agar media supplemented with 25 μg/ml erythromycin. B. thetaiotaomicron strains needed for large-scale LPS extraction were grown in BHI broth (BD Biosciences) supplemented with 5 μg/ml hemin and 500 μg/ml l-cysteine hydrochloride (Sigma-Aldrich). The gas mix for the anaerobic chamber (Coy Laboratory Products) was 5% hydrogen and 20% carbon dioxide, balanced with nitrogen (Airgas). The E. coli strains used for cloning the pExchange-tdk knockout constructs were cultured in Luria broth (LB) or agar supplemented with carbenicillin. LPS from E. coli O55:B5 and E. coli MG1655 was purchased from InvivoGen (LPS-B5 Ultrapure and LPS-K12 Ultrapure, respectively).
Strains, plasmids, and primers used in this study. (A) List of strains and plasmids. (B) List of primers and their sequences used for cloning and sequencing. Download TABLE S1, EPS file, 1 MB (1MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Construction of B. thetaiotaomicron clean deletion mutants.
See Table S1 for a list of all primers used for making scarless gene deletions in B. thetaiotaomicron. To make clean deletions as described by Koropatkin et al., approximately 1,000 bp upstream and downstream of the desired gene to be deleted, including the start and stop codons, respectively, were amplified by PCR (49). For BT2152 and BT1854, the two fragments were stitched together by PCR, taking advantage of the overlapping regions in the original primers. For BT2152, the pExchange-tdk vector (gift from Justin Sonnenburg, Stanford University) and the fused insert were digested with SalI and NotI and ligated together. For BT1854, only the pExchange-tdk vector was digested with SalI and XbaI, and the insert was assembled with the vector by circular polymerase extension cloning (CPEC). For BT3363, BT3365, and BT3365 to BT3380, Gibson assembly was used to combine the upstream and downstream fragments with SpeI/NotI-digested pExchange-tdk. Ligation, CPEC, and Gibson assembly products were transformed into electrocompetent E. coli S17-1 λ pir (Bio-Rad MicroPulser; 18 kV in 0.1-cm cuvettes), selected for on LB agar plus carbenicillin, and conjugated into B. thetaiotaomicron VPI 5482 Δtdk, which serves as the background strain for all the clean deletions. Single recombinants were selected for on BHI-blood agar supplemented with 200 μg/ml gentamicin and 25 μg/ml erythromycin, picked, cultured overnight in PYG medium, and plated on BHI-blood agar supplemented with 200 μg/ml 5-fluoro-2-deoxyuridine. Colonies were screened for success of the gene deletion by PCR, and deletion was confirmed by DNA sequencing.
The TRI reagent method for lipid A extraction.
To purify lipid A from Bacteroides species, we used the TRI reagent method (24). Briefly, bacterial cells were grown in 10-ml liquid cultures to mid-log phase, harvested by centrifugation at 3,270 × g for 10 min, resuspended in 1 ml TRI reagent (Molecular Research Center), vortexed vigorously, and left at room temperature for 10 min. One milliliter of chloroform was added, and the mixture was vortexed and left at room temperature for another 10 min. The mixture was centrifuged at 15,000 × g for 10 min, and the aqueous layer was removed to a clean tube. The sample was extracted again with 200 μl deionized water, and the resulting aqueous layer was removed to the same tube as described above. The aqueous extraction was repeated two more times, and the collected aqueous material was pooled and lyophilized (Labconco FreeZone). A mild acid hydrolysis was used to separate the lipid A from its poly- or oligosaccharide chain. The lyophilized material was resuspended in 1 ml 10 mM sodium acetate (pH 4.5) and 1% sodium dodecyl sulfate (SDS). Samples were boiled at 100°C for 1 h and then lyophilized again. To remove SDS from the lipid A, the lyophilized material was washed with 1 ml ice-cold acidified ethanol (20 mM hydrochloric acid in 95% ethanol) once followed by three washes each with 500 μl ice-cold 95% ethanol, centrifuging the mixture at 3,270 × g at 4°C for 5 min each time. A Bligh-Dyer extraction was used to separate the hydrolyzed lipid A from the saccharides, and the organic and interface layers were removed to a small glass vial. Solvent was removed with a rotary evaporator, and the material was stored at −20°C.
MALDI-TOF mass spectrometry analysis of lipid A. (i) Sample and matrix preparation.
Three to five drops of 3:1 chloroform-methanol was added to a thawed vial of dried lipid A, with gentle rocking to help the lipid A dissolve. The matrix was a saturated solution of 5-chloro-2-mercaptobenzothiazole (CMBT) in 3:1 chloroform-methanol. To spot the sample on the target, 3 μl of dissolved sample was mixed with 3 μl matrix, and 1 μl of the resulting mixture was spotted within an inscribed circle on the MALDI target (Waters Corporation) and allowed to dry (50).
(ii) Negative-ion MALDI-TOF MS.
MALDI-TOF MS analysis was carried out on a Waters Corporation Synapt G2 high-definition mass spectrometer with a 355-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser in reflectron negative-ion mode. The instrument was calibrated using a mixture of angiotensin II, renin substrate, insulin chain B, and bovine insulin (Sigma-Aldrich), with monoisotopic [M-H]− ion masses of 1,044.5267, 1,756.9175, 3,492.6357, and 5,728.5931 m/z, respectively (51). The standards were dissolved together in a solution of 0.1% trifluoroacetic acid in water. Because the standard mixture was not in the same solvent as the CMBT matrix mentioned above, 1 μl of CMBT matrix was spotted on the target and allowed to dry before 1 μl of the standard mixture was spotted on top of the matrix. MS data were collected between 400 and 5,000 m/z, and the resulting spectra were smoothed and baseline corrected using MassLynx software.
Large-scale LPS/LOS extraction.
LPS or LOS was isolated from whole bacteria using the hot phenol-water method (52). Briefly, bacteria were grown overnight in a 10-ml culture and then expanded to 2 liters. Cells were harvested once cultures reached an optical density (OD) of at least 0.7 and pelleted by centrifugation at 6,000 × g for 30 min at 4°C. The entire wet cell pellet from the 2-liter culture was suspended in 20 ml water. Separately, the cell suspension and 20 ml of 90% phenol solution in water were each brought up to 68°C with stirring. Once at temperature, the phenol solution was slowly added to the cell suspension. The mixture was stirred vigorously for 30 min at 68°C and then cooled rapidly in an ice water bath for 10 min. The sample was centrifuged at 15,000 × g for 45 min, and the upper aqueous layer was transferred into 1,000-molecular-weight-cutoff (MWCO) dialysis tubing. The sample was dialyzed against 4 liters of water for 4 days, changing the water twice per day. LPS/LOS was pelleted out of the dialysate by ultracentrifugation at 105,000 × g for 4 h. The pellet was resuspended in water and treated with RNase A (Thermo Fisher), DNase I (New England Biolabs), and proteinase K (Thermo Fisher) before repeating the ultracentrifugation step. The pellet was resuspended in water, lyophilized, and stored at −20°C. LPS/LOS samples that were prepared by this method include the B. thetaiotaomicron Δtdk strain in Fig. 4, all samples in Fig. 6, and all samples in Fig. S3, S4, and S5.
Microscale LPS/LOS extraction.
The microscale LPS/LOS extraction was used when a large number of samples was needed for SDS-PAGE analysis. In this method, adapted from the work of Marolda and coworkers, bacteria were grown to mid-log phase in 5 ml of medium and pelleted (53). Cell pellets were resuspended in 150 μl lysis buffer (0.5 M Tris-hydrochloride, pH 6.8, 2% SDS, 4% β-mercaptoethanol) and boiled at 100°C for 10 min. Proteinase K was added to each sample before incubation at 60°C for 1 h. The sample temperature was raised to 70°C, and 150 μl prewarmed 90% phenol in water was added. Samples were vortexed three times at 5-min intervals during a 15-min incubation. The samples were immediately cooled on ice for 10 min and centrifuged at 10,000 × g for 1 min. The aqueous layer (~100 μl) was pipetted into a clean tube, and 5 volumes of ethyl ether saturated with 10 mM Tris-hydrochloride (pH 8.0) and 1 mM EDTA was added. The samples were vortexed and centrifuged, and the aqueous layer was removed to a clean tube. An appropriate amount of 3× loading dye (0.187 M Tris-hydrochloride, pH 6.8, 6% SDS, 30% glycerol, 0.03% bromophenol blue, 15% β-mercaptoethanol) was added, and the samples were stored at −20°C. LPS/LOS samples that were prepared by this method include B. vulgatus in Fig. 4, all samples in Fig. 5, and all samples in Fig. S2.
SDS-PAGE analysis of LPS.
To visualize LPS/LOS on an SDS-PAGE gel, we used Novex 16% Tricine protein gels (1.0 mm, 12 wells) and 10× Novex Tricine SDS running buffer (Thermo Fisher) (12). For samples prepared by the LPS/LOS microscale extraction, 15 μl of the resulting aqueous layer mixed with 3× loading dye was added to each lane. For samples prepared by LPS/LOS large-scale extraction or purchased from InvivoGen, 2.5 μg of material was resuspended in 15 μl 1× loading dye and the whole volume was added to a lane. Gels were run at 125 V for 90 min at room temperature, stained with Pro-Q Emerald 300 lipopolysaccharide gel stain (Thermo Fisher) per the manufacturer’s instructions, and imaged on a Bio-Rad Gel Doc EZ Imager using the SYBR green filter.
MALDI-TOF mass spectrometry analysis of intact LOS. (i) Sample and matrix preparation.
To detect intact LPS by MALDI-TOF MS, we closely followed the technique developed by Phillips et al. adapted to study Neisseria lipooligosaccharides (48). One milligram of lyophilized LOS was dissolved in 100 μl 1:3 methanol-water with 5 mM EDTA. Cation exchange beads (Dowex 50WX8, 200 to 400 mesh) were converted to the ammonium form and deposited into 1.5-ml tubes before desalting the LOS. Each sample suspension was added to the beads, vortexed, and centrifuged briefly to pellet the beads. The sample was removed to a clean tube and mixed 9:1 with 100 mM dibasic ammonium citrate before spotting on the target. The matrix was made by mixing a 15-mg/ml solution of nitrocellulose membrane in 1:1 isopropanol-acetone with a 200-mg/ml solution of 2′,4′,6′-trihydroxyacetophenone (THAP) in methanol in a 1:3 ratio. The matrix was deposited by pipetting 1 μl within an inscribed circle on the target (Shimadzu) and allowed to dry. Once the matrix had dried completely, 1 μl of the sample preparation was added on top of the matrix and allowed to dry.
(ii) Negative-ion MALDI-TOF MS.
MALDI-TOF MS analysis was performed on a Shimadzu Axima Performance mass spectrometer with an N2 laser in linear negative-ion mode. It was calibrated using the same solution of four standards that was used to calibrate the Waters Synapt G2 for lipid A analysis—angiotensin II, renin substrate, insulin chain B, and bovine insulin in 0.1% trifluoroacetic acid—and the standards were spotted as described above except on the THAP-nitrocellulose matrix. MS data were collected between 700 and 7,000 m/z, and the resulting spectra were smoothed and baseline corrected using Shimadzu Biotech Launchpad software.
ACKNOWLEDGMENTS
We are deeply indebted to Colleen O’Loughlin and members of the Fischbach group for helpful comments on the manuscript, Daniel Peterson for providing the B. thetaiotaomicron transposon mutants, and Nancy Phillips and Constance John for their guidance on mass spectrometry analysis of lipid A and LOS. Intact LOS mass spectrometry analysis was completed using the Shimadzu Axima Performance MALDI-TOF instrument in the laboratory of William DeGrado at the University of California, San Francisco. Lipid A mass spectrometry analysis on the Waters Synapt G2 HDMS 32k instrument was performed at the University of California, San Francisco Sandler-Moore Mass Spectrometry Core Facility.
This work was supported by NIH grants R01 DK110174 (M.A.F.) and DP1 DK113598 (M.A.F.), a research award from Pfizer, an HHMI-Simons Faculty Scholars Award (M.A.F.), an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Foundation (M.A.F.), and a National Science Foundation Graduate research fellowship grant, no. 1144247 (A.N.J.).
Footnotes
Citation Jacobson AN, Choudhury BP, Fischbach MA. 2018. The biosynthesis of lipooligosaccharide from Bacteroides thetaiotaomicron. mBio 9:e02289-17. https://doi.org/10.1128/mBio.02289-17.
REFERENCES
- 1.Donia MS, Fischbach MA. 2015. Small molecules from the human microbiota. Science 349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 3.Raetz CRH, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 5.Wirth HP, Yang M, Sanabria-Valentín E, Berg DE, Dubois A, Blaser MJ. 2006. Host Lewis phenotype-dependent Helicobacter pylori Lewis antigen expression in rhesus monkeys. FASEB J 20:1534–1536. doi: 10.1096/fj.05-5529fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozniewski A, Haristoy X, Rasko DA, Hatier R, Plenat F, Taylor DE, Angioi-Duprez K. 2003. Influence of Lewis antigen expression by Helicobacter pylori on bacterial internalization by gastric epithelial cells. Infect Immun 71:2902–2906. doi: 10.1128/IAI.71.5.2902-2906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahler CM, Datta A, Tzeng Y-L, Carlson RW, Stephens DS. 2005. Inner core assembly and structure of the lipooligosaccharide of Neisseria meningitidis: capacity of strain NMB to express all known immunotype epitopes. Glycobiology 15:409–419. doi: 10.1093/glycob/cwi018. [DOI] [PubMed] [Google Scholar]
- 8.John CM, Liu M, Jarvis GA. 2009. Profiles of structural heterogeneity in native lipooligosaccharides of Neisseria and cytokine induction. J Lipid Res 50:424–438. doi: 10.1194/jlr.M800184-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraal L, Abubucker S, Kota K, Fischbach MA, Mitreva M. 2014. The prevalence of species and strains in the human microbiome: a resource for experimental efforts. PLoS One 9:e97279-11. doi: 10.1371/journal.pone.0097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskell JP. 1991. The resolution of Bacteroides lipopolysaccharides by polyacrylamide gel electrophoresis. J Med Microbiol 34:253–257. doi: 10.1099/00222615-34-5-253. [DOI] [PubMed] [Google Scholar]
- 12.Maskell JP. 1994. Electrophoretic analysis of the lipopolysaccharides of Bacteroides spp. Antonie Van Leeuwenhoek 65:155–161. doi: 10.1007/BF00871756. [DOI] [PubMed] [Google Scholar]
- 13.Berezow AB, Ernst RK, Coats SR, Braham PH, Karimi-Naser LM, Darveau RP. 2009. The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses. Microb Pathog 47:68–77. doi: 10.1016/j.micpath.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weintraub A, Zähringer U, Wollenweber H-W, Seydel U, Rietschel ET. 1989. Structural characterization of the lipid A component of Bacteroides fragilis strain NCTC 9343 lipopolysaccharide. Eur J Biochem 183:425–431. doi: 10.1111/j.1432-1033.1989.tb14945.x. [DOI] [PubMed] [Google Scholar]
- 15.Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen A-M, Peet A, Tillmann V, Uibo R, Mokurov S, Dorshakova N, Ilonen J, Virtanen SM, Szabo SJ, Porter JA, Lähdesmäki H, Huttenhower C, Gevers D, Cullen TW, Knip M, Xavier RJ, DIABIMMUNE Study Group . 2016. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165:842–853. doi: 10.1016/j.cell.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brozek KA, Raetz CRH. 1990. Biosynthesis of lipid A in Escherichia coli. J Biol Chem 265:15410–15417. [PubMed] [Google Scholar]
- 17.Cullen TW, Schofield WB, Barry NA, Putnam EE, Rundell EA, Trent MS, Degnan PH, Booth CJ, Yu H, Goodman AL. 2015. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347:170–175. doi: 10.1126/science.1260580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T. 2009. Protection from lethal Gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A 106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sender R, Fuchs S, Milo R. 2016. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell 164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Raetz CRH, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem 76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cimermancic P, Medema MH, Claesen J, Kurita K, Wieland Brown LC, Mavrommatis K, Pati A, Godfrey PA, Koehrsen M, Clardy J, Birren BW, Takano E, Sali A, Linington RG, Fischbach MA. 2014. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters. Cell 158:412–421. doi: 10.1016/j.cell.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. 2009. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4′-phosphatase activities. Cell Microbiol 11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coats SR, Berezow AB, To TT, Jain S, Bainbridge BW, Banani KP, Darveau RP. 2011. The lipid A phosphate position determines differential host toll-like receptor 4 responses to phylogenetically related symbiotic and pathogenic bacteria. Infect Immun 79:203–210. doi: 10.1128/IAI.00937-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi EC, Hackett M. 2000. Rapid isolation method for lipopolysaccharide and lipid A from Gram-negative bacteria. Analyst 125:651–656. doi: 10.1039/b000368i. [DOI] [PubMed] [Google Scholar]
- 25.Nishijima M, Bulawa CE, Raetz CRH. 1981. Two interacting mutations causing temperature-sensitive phosphatidylglycerol synthesis in Escherichia coli membranes. J Bacteriol 145:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowell DN, Anderson MS, Raetz CRH. 1986. Molecular cloning of the genes for lipid A disaccharide synthase and UDP-N-acetylglucosamine acyltransferase in Escherichia coli. J Bacteriol 168:152–159. doi: 10.1128/jb.168.1.152-159.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galloway SM, Raetz CRH. 1990. A mutant of Escherichia coli defective in the first step of endotoxin biosynthesis. J Biol Chem 265:6394–6402. [PubMed] [Google Scholar]
- 28.Meredith TC, Aggarwal P, Mamat U, Lindner B, Woodard RW. 2006. Redefining the requisite lipopolysaccharide structure in Escherichia coli. ACS Chem Biol 1:33–42. doi: 10.1021/cb0500015. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds CM, Raetz CRH. 2009. Replacement of lipopolysaccharide with free lipid A molecules in Escherichia coli mutants lacking all core sugars. Biochemistry 48:9627–9640. doi: 10.1021/bi901391g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaras AP, McPherson CJ, Kuhn M, Carifa A, Mullins L, George D, Desbonnet C, Eidem TM, Montgomery JI, Brown MF, Reilly U, Miller AA, O’Donnell JP. 2014. LpxC inhibitors as new antibacterial agents and tools for studying regulation of lipid A biosynthesis in gram-negative pathogens. mBio 5:e01551-14. doi: 10.1128/mBio.01551-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein G, Lindner B, Brabetz W, Brade H, Raina S. 2009. Escherichia coli K-12 suppressor-free mutants lacking early glycosyltransferases and late acyltransferases: minimal lipopolysaccharide structure and induction of envelope stress response. J Biol Chem 284:15369–15389. doi: 10.1074/jbc.M900490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorachek-Warren MK, Ramirez S, Cotter RJ, Raetz CRH. 2002. A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J Biol Chem 277:14194–14205. doi: 10.1074/jbc.M200409200. [DOI] [PubMed] [Google Scholar]
- 33.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. 2009. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 34.Golenbock DT, Hampton RY, Qureshi N, Takayama K, Raetz CRH. 1991. Lipid A-like molecules that antagonize the effects of endotoxins on human monocytes. J Biol Chem 266:19490–19498. [PubMed] [Google Scholar]
- 35.Mullarkey M, Rose JR, Bristol J, Kawata T, Kimura A, Kobayashi S, Przetak M, Chow J, Gusovsky F, Christ WJ, Rossignol DP. 2003. Inhibition of endotoxin response by E5564, a novel toll-like receptor 4-directed endotoxin antagonist. J Pharmacol Exp Ther 304:1093–1102. doi: 10.1124/jpet.102.044487. [DOI] [PubMed] [Google Scholar]
- 36.Needham BD, Carroll SM, Giles DK, Georgiou G, Whiteley M, Trent MS. 2013. Modulating the innate immune response by combinatorial engineering of endotoxin. Proc Natl Acad Sci U S A 110:1464–1469. doi: 10.1073/pnas.1218080110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TBH. 2009. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol 10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 38.Wittmann A, Lamprinaki D, Bowles KM, Katzenellenbogen E, Knirel YA, Whitfield C, Nishimura T, Matsumoto N, Yamamoto K, Iwakura Y, Saijo S, Kawasaki N. 2016. Dectin-2 recognizes mannosylated O-antigens of human opportunistic pathogens and augments lipopolysaccharide activation of myeloid cells. J Biol Chem 291:17629–17638. doi: 10.1074/jbc.M116.741256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donia MS, Cimermancic P, Schulze CJ, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. 2014. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell 158:1402–1414. doi: 10.1016/j.cell.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 41.Martens EC, Roth R, Heuser JE, Gordon JI. 2009. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J Biol Chem 284:18445–18457. doi: 10.1074/jbc.M109.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers TE, Pudlo NA, Koropatkin NM, Bell JSK, Moya Balasch M, Jasker K, Martens EC. 2013. Dynamic responses of Bacteroides thetaiotaomicron during growth on glycan mixtures. Mol Microbiol 88:876–890. doi: 10.1111/mmi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson DA, Planer JD, Guruge JL, Xue L, Downey-Virgin W, Goodman AL, Seedorf H, Gordon JI. 2015. Characterizing the interactions between a naturally primed immunoglobulin A and its conserved Bacteroides thetaiotaomicron species-specific epitope in gnotobiotic mice. J Biol Chem 290:12630–12649. doi: 10.1074/jbc.M114.633800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amor K, Heinrichs DE, Frirdich E, Ziebell K, Johnson RP, Whitfield C. 2000. Distribution of core oligosaccharide types in lipopolysaccharides from Escherichia coli. Infect Immun 68:1116–1124. doi: 10.1128/IAI.68.3.1116-1124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan S, Bastin DA, Verma NK. 1999. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology 145:1263–1273. doi: 10.1099/13500872-145-5-1263. [DOI] [PubMed] [Google Scholar]
- 46.Samuel G, Reeves P. 2003. Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly. Carbohydr Res 338:2503–2519. doi: 10.1016/j.carres.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Sturiale L, Garozzo D, Silipo A, Lanzetta R, Parrilli M, Molinaro A. 2005. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun Mass Spectrom 19:1829–1834. doi: 10.1002/rcm.1994. [DOI] [PubMed] [Google Scholar]
- 48.Phillips NJ, John CM, Jarvis GA. 2016. Analysis of bacterial lipooligosaccharides by MALDI-TOF MS with traveling wave ion mobility. J Am Soc Mass Spectrom 27:1263–1276. doi: 10.1007/s13361-016-1383-3. [DOI] [PubMed] [Google Scholar]
- 49.Koropatkin NM, Martens EC, Gordon JI, Smith TJ. 2008. Starch catabolism by a prominent human gut symbiont is directed by the recognition of amylose helices. Structure 16:1105–1115. doi: 10.1016/j.str.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schilling B, McLendon MK, Phillips NJ, Apicella MA, Gibson BW. 2007. Characterization of lipid A acylation patterns in Francisella tularensis, Francisella novicida, and Francisella philomiragia using multiple-stage mass spectrometry and matrix-assisted laser desorption/ionization on an intermediate vacuum source linear ion trap. Anal Chem 79:1034–1042. doi: 10.1021/ac061654e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips NJ, Schilling B, McLendon MK, Apicella MA, Gibson BW. 2004. Novel modification of lipid A of Francisella tularensis. Infect Immun 72:5340–5348. doi: 10.1128/IAI.72.9.5340-5348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westphal O, Jann K. 1965. Bacterial lipopolysaccharides extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem 5:83–91. [Google Scholar]
- 53.Marolda CL, Lahiry P, Vinés E, Saldïas S, Valvano MA. 2006. Micromethods for the characterization of lipid A-core and O-antigen lipopolysaccharide. Methods Mol Biol 347:237–252. doi: 10.1385/1-59745-167-3:237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The double knockout of lpxL and lpxF in B. thetaiotaomicron produces tetra-acylated diphosphorylated lipid A. Lipid A was isolated from the B. thetaiotaomicron ΔlpxL ΔlpxF strain using the TRI reagent method. The resulting material was dissolved in 3:1 chloroform-methanol, mixed in a 1:1 ratio with saturated 5-chloro-2-mercaptobenzothiazole in 3:1 chloroform-methanol, and spotted on a Waters Corporation MALDI target. The sample was analyzed on a Waters Synapt G2 MALDI-TOF mass spectrometer in reflectron negative-ion mode. The mass of the resulting cluster of peaks is around 1,544 m/z, which is 144 m/z less than wild-type B. thetaiotaomicron lipid A at 1,688 m/z. That 144 Daltons decrease is equivalent to the loss of 224 Daltons observed in the B. thetaiotaomicron ΔlpxL mutant plus the addition of 80 Daltons as observed in the B. thetaiotaomicron ΔlpxF mutant, indicating that we have made a tetra-acylated diphosphorylated lipid A molecule in B. thetaiotaomicron. B. thetaiotaomicron ΔlpxL ΔlpxF strain peaks are accented in blue for clarity, and sodiated B. thetaiotaomicron ΔlpxL ΔlpxF strain peaks are in black and marked with an asterisk. Download FIG S1, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Capsular polysaccharide gene clusters do not appear to be involved in LOS biosynthesis. LOS was isolated from all 10 of these strains using the microscale extraction method and analyzed by SDS-PAGE and staining with the Pro-Q Emerald 300 lipopolysaccharide gel stain kit (Thermo Fisher). CPS bands can be seen for certain samples like CPS2-only and CPS4-only near the top of the gel. Whether the strains contain the gene clusters for all the CPSs (Δtdk), none of the CPSs (ΔCPS), or one CPS at a time (CPS1-only, CPS2-only, and so on), the LOS bands appear unchanged, suggesting that the CPS gene clusters do not contribute to LOS biosynthesis. Download FIG S2, EPS file, 2.3 MB (2.3MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Capsular polysaccharide is likely the cause of contaminating peaks in samples in the Δtdk wild-type background. LOS from the B. thetaiotaomicron Δtdk strain was purified by the large-scale extraction method, and the intact molecule was analyzed on a Shimadzu Axima Performance MALDI-TOF MS instrument in linear negative-ion mode. The peaks in black are those species that are also present in the ΔCPS intact LOS sample, but the rest of the peaks in red are unique to the Δtdk sample despite its differing from the ΔCPS mutant only in that it contains capsule. Because similar peaks are noted in the intact LOS MALDI-TOF spectra of the transposon mutants, which also contain capsule, we conclude that the peaks in red are due to the presence of CPS in the LOS preparation. Download FIG S3, EPS file, 1.6 MB (1.7MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
B. thetaiotaomicron produces a molecule with a different mass when the LOS oligosaccharide biosynthesis cluster is deleted. LOS was isolated from the B. thetaiotaomicron ΔCPS ΔBT3363 ΔBT3365–BT3380 (red) and B. thetaiotaomicron ΔBT3363 ΔBT3365–BT3380 (black) strains by the large-scale extraction method and analyzed on a Shimadzu Axima Performance MALDI-TOF MS instrument in linear negative-ion mode. These mutants have as much of the LOS oligosaccharide biosynthesis cluster deleted as possible and should not be able to make glycosylated LOS, and yet they appear to make new molecules in its stead. Lipid A is still present in both samples, suggesting that a new oligosaccharide may be getting attached to the truncated LOS left over after the cluster deletion. Download FIG S4, EPS file, 1.7 MB (1.7MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LOSs from a transposon insertion strain and a clean deletion strain do not have the same banding pattern on an LOS gel. LOS was isolated from B. thetaiotaomicron ΔBT3365 and B. thetaiotaomicron tnBT3365 strains by the large-scale LPS/LOS extraction method. The resulting material was analyzed by SDS-PAGE and stained using the Pro-Q Emerald 300 lipopolysaccharide gel stain kit (Thermo Fisher). While a transposon insertion in BT3365 results in what appears to be a truncated LOS structure, a clean deletion of the same gene looks like it has a wild-type banding pattern. Download FIG S5, EPS file, 0.5 MB (502.9KB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains, plasmids, and primers used in this study. (A) List of strains and plasmids. (B) List of primers and their sequences used for cloning and sequencing. Download TABLE S1, EPS file, 1 MB (1MB, eps) .
Copyright © 2018 Jacobson et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.