Summary
Patients with Lyme disease often have pronounced TH17 inflammatory responses. Early in the infection, during erythema migrans, TH17 responses correlate with Borrelia burgdorferi antibodies, whereas late in the disease, in patients with antibiotic-refractory Lyme arthritis, these responses correlate with autoantibodies.
Keywords: Lyme disease, erythema migrans, Lyme arthritis, TH17, antibodies
Abstract
Background.
Control of Lyme disease is attributed predominantly to innate and adaptive T-helper 1 cell (TH1) immune responses, whereas the role of T-helper 17 cell (TH17) responses is less clear. Here we characterized these inflammatory responses in patients with erythema migrans (EM) or Lyme arthritis (LA) to elucidate their role early and late in the infection.
Methods.
Levels of 21 cytokines and chemokines, representative of innate, TH1, and TH17 immune responses, were assessed by Luminex in acute and convalescent sera from 91 EM patients, in serum and synovial fluid from 141 LA patients, and in serum from 57 healthy subjects. Antibodies to Borrelia burgdorferi or autoantigens were measured by enzyme-linked immunosorbent assay.
Results.
Compared with healthy subjects, EM patients had significantly higher levels of innate, TH1, and TH17-associated mediators (P ≤ .05) in serum. In these patients, the levels of inflammatory mediators, particularly TH17-associated cytokines, correlated directly with B. burgdorferi immunoglobulin G antibodies (P ≤ .02), suggesting a beneficial role for these responses in control of early infection. Late in the disease, in patients with LA, innate and TH1-associated mediators were often >10-fold higher in synovial fluid than serum. In contrast, the levels of TH17-associated mediators were more variable, but correlated strongly with autoantibodies to endothelial cell growth factor, matrix metalloproteinase 10, and apolipoprotein B-100 in joints of patients with antibiotic-refractory LA, implying a shift in TH17 responses toward an autoimmune phenotype.
Conclusions.
Patients with Lyme disease often develop pronounced TH17 immune responses that may help control early infection. However, late in the disease, excessive TH17 responses may be disadvantageous by contributing to autoimmune responses associated with antibiotic-refractory LA.
T-helper 17 cell (TH17) immune responses are important in the control of extracellular pathogens, but may also lead to autoimmune responses [1–4]. Exposure to interleukin (IL) 23 plays a critical role in the generation of pathogenic TH17 cells [1, 2]. Such responses, particularly in patients with polymorphisms in the IL23R gene, have been implicated in the pathogenesis of several rheumatic diseases, including rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis [1, 2, 5–8].
Lyme disease is caused by the tick-transmitted spirochete, Borrelia burgdorferi, a large extracellular pathogen [9, 10]. In untreated patients, the infection usually occurs in stages with different manifestations at each stage [11–13]. The initial sign of the infection is usually an expanding skin lesion, erythema migrans (EM), which is often accompanied by flulike symptoms such as myalgias, arthralgias, malaise, fever, or fatigue [11–13]. Months after disease onset, within the context of an expanded immune response to the spirochete, approximately 60% of untreated patients in the northeastern United States develop arthritis in one or a few large joints, most commonly the knee [14].
Most patients with Lyme arthritis (LA) respond well to oral and/or intravenous antibiotic therapy and their arthritis resolves, called antibiotic-responsive LA. However, a subset of patients has persistent proliferative synovitis for months to years after treatment with 2–3 months of oral and intravenous antibiotics and apparent spirochetal killing, termed antibiotic-refractory LA [15, 16]. Infection-induced autoimmunity is thought to be a contributing factor in this outcome [17–21]. To date, we have identified 4 autoantigens, endothelial cell growth factor (ECGF) [19], apolipoprotein B-100 (apoB-100) [18], annexin A2 [20], and matrix metalloproteinase 10 (MMP-10) [17], that are each targets of T- and B-cell responses in approximately 10%–35% of LA patients. Although antibodies to these autoantigens may appear early in the infection, they seem to be nonpathogenic at this stage, with little or no associated T-cell responses. However, late in the disease, these autoantibodies are often accompanied by T-cell responses, particularly in patients with antibiotic-refractory LA, in whom they are associated with specific pathologic findings in joints [17–20, 22].
Control of the B. burgdorferi infection in humans is attributed predominantly to innate and adaptive T-helper 1 cell (TH1) immune responses. However, excessive levels of inflammatory mediators, particularly those linked to TH1 responses, are associated with more severe disease, including more symptomatic early infection and antibiotic-refractory LA [23–25]. Although initial studies in animals and humans suggest a role for TH17 immunity in Lyme disease and in post–Lyme disease sequelae [26–30], these responses are incompletely characterized, particularly in human disease.
We have previously analyzed innate and TH1 adaptive immune responses in patients with early or late manifestations of Lyme disease and in tissue cell culture systems [23–25, 30–33]. Here we extend this work by characterizing TH17 immune responses in patients with Lyme disease. We found that a subset of patients have pronounced TH17 responses, which are associated with B. burgdorferi antibodies during early infection in patients with EM, and with autoantibodies in patients with LA, a late disease manifestation.
PATIENTS AND METHODS
Study Patients
All patients met the Centers for Disease Control and Prevention criteria for the diagnosis of Lyme disease [34] and were treated according to the guidelines recommended by the Infectious Diseases Society of America [35]. All patients provided written informed consent. The Human Investigation Committees at Tufts Medical Center (1988–2002) and Massachusetts General Hospital (2002–2016) approved the study.
For analysis of early infection, acute and convalescent serum samples and clinical information were available from 91 EM patients from the northeastern United States seen between 1998 and 2001, all of whom were culture positive for B. burgdorferi. In addition, serum and, in most instances, synovial fluid samples were available from 141 patients with LA who were referred to the Rheumatology Clinic at Tufts Medical Center or Massachusetts General Hospital from 1988 through 2014. These patients, were referred before, during, or after antibiotic treatment. For comparison, serum samples were obtained from 57 healthy laboratory and hospital donors. Although subsets of these samples were used in previous studies of innate and adaptive TH1 responses [23–25, 32], all of the samples were tested again here, along with new samples, so that variability between assays would not be a factor in data interpretation.
Laboratory Determinations
The levels of 21 cytokines and chemokines associated with innate (CCL2, CCL3, IL-1β, IL-6, IL-8, IL-10, tumor necrosis factor [TNF], interferon [IFN] α) and adaptive TH1 (IFN-γ, CXCL9, CXCL10, IL-12p40, IL-12p70, CCL19) or TH17 (IL-17A, IL-17F, IL-17E/IL-25, IL-21, IL-22, IL-23, IL-27) immune responses were assessed in acute and convalescent serum samples from EM patients, in serum and synovial fluid samples from LA patients, and in serum of healthy subjects using Luminex multiplex assays (EMD Millipore). Because patient sample volumes were limited, cytokine and chemokine levels in these samples were determined once.
Antibody responses (immunoglobulin G [IgG]) to B. burgdorferi, or to 3 human autoantigens specific for Lyme disease (ECGF, apoB-100, and MMP-10), were determined for this study in approximately 30–50 patients with EM or LA as previously described [17–20].
Statistical Analysis
Differences in cytokine and chemokine levels were assessed using Mann-Whitney rank-sum test. To show the range of values, differences among groups stratified according to EM-associated symptoms, or antibiotic-responsive vs antibiotic-refractory LA, were presented as box plots. Correlations involving inflammatory mediators and antibody responses were assessed using Pearson correlation test with Benjamini-Hochberg correction for multiple comparisons. Analyses were performed using SigmaStat software (SPSS). A P value ≤.05 with false discovery rate (FDR) ≤0.1 was considered statistically significant.
RESULTS
Inflammatory Responses in Patients With Erythema Migrans
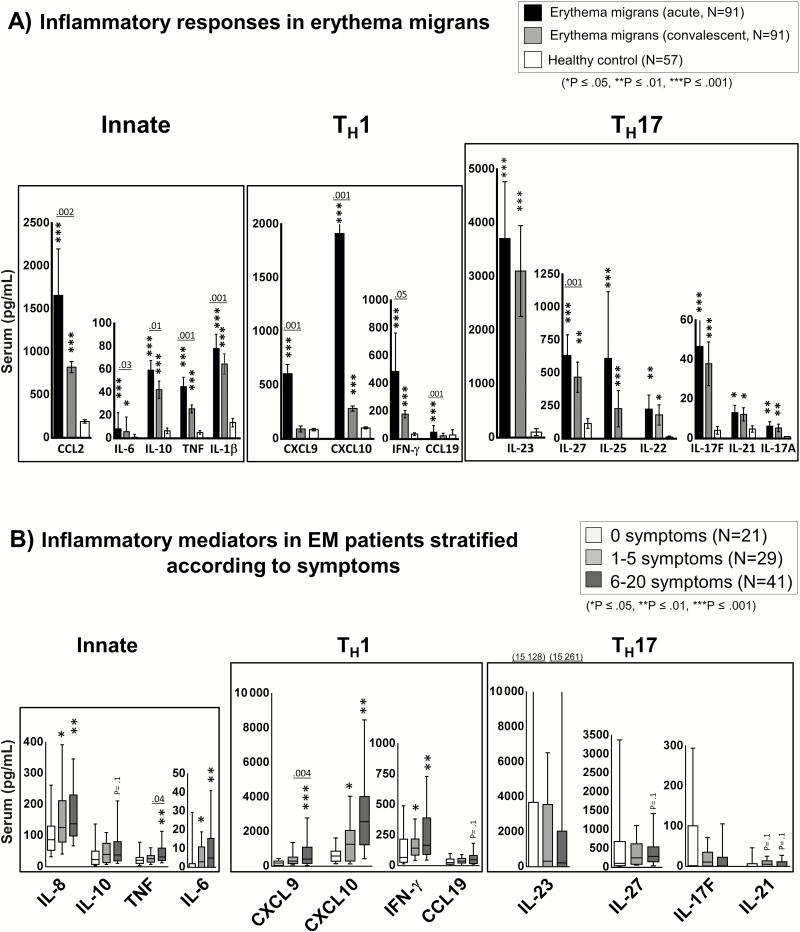
At study entry prior to antibiotic therapy, a median of 4 days after disease onset, the 91 B. burgdorferi culture–positive EM patients had significantly higher levels of 19 of the 21 mediators tested, including all TH17 mediators (IL-23, IL-27, IL-25, IL-22, IL-17F, IL-21, and IL-17A) compared with healthy subjects. Representative mediators of each type of immune response are shown in Figure 1A. During acute EM, most patients had robust innate and TH1 adaptive immune responses, with especially high levels of CCL2, CXCL9, and CXCL10, which are important chemoattractants for macrophages and CD4+ T-effector cells. Similarly, the levels of all TH17 mediators assessed were greater in patients than in healthy subjects, although the values among patients were quite variable. The most highly expressed TH17 mediator was IL-23. A subset of patients had both elevated TH1 and TH17 cytokine responses, but most had either high TH1 or TH17 responses, suggesting that some patients polarize toward a TH1 or TH17 immune response.
Figure 1.
Cytokine and chemokine levels in acute and convalescent serum samples from patients with erythema migrans (EM). Protein levels of 21 mediators associated with innate, T-helper 1 cell (TH1), or T-helper 17 cell (TH17) immune responses were assessed in 91 culture-positive patients with EM using bead-based Luminex assays. A, Comparison of cytokine and chemokine levels in matched acute and convalescent serum samples from patients with EM or in healthy controls. Black bars represent acute serum samples obtained prior to antibiotic therapy, a period of active infection. Gray bars represent convalescent samples obtained at the conclusion of antibiotic therapy, approximately 3 weeks after study entry. White bars represent values in healthy controls. The bars represent the mean values and I-bars represent the standard error of the mean. P values for comparison of acute and convalescent samples are indicated above the bars; P values for comparison with healthy controls are indicated by an asterisk (*P ≤ .05, **P ≤ .01, ***P ≤ .001). B, Cytokine and chemokine values in EM patients stratified according to the presence of symptoms: patients with no associated symptoms (n = 21, white box plots), 1–5 symptoms (n = 29, light gray box plots), or 6–20 symptoms (n = 41, dark-gray box plots) at first visit. The box represents 25th–75th percentiles, the line inside the box represents the median value, and I-bars represent the 10th–90th percentiles. P values for comparison of patients with 1–5 symptoms vs 6–12 symptoms are indicated above the box plots; P values for comparison of patients with 1–5 symptoms or 6–20 symptoms to those with no symptoms are indicated by an asterisk (*P ≤ .05, **P ≤ .01, ***P ≤ .001). Abbreviations: EM, erythema migrans; IFN, interferon; IL, interleukin; TH1, T-helper cell 1; TH17, T-helper cell 17; TNF, tumor necrosis factor.
Three weeks later, during the convalescent period, soon after the conclusion of antibiotic therapy, the levels of TH1-associated mediators had decreased dramatically, and in some cases approached the levels in healthy subjects (Figure 1A). Similarly, the levels of most innate mediators had decreased significantly during convalescence. In contrast, the levels of TH17 mediators declined less during convalescence, suggesting the potential for persistence of these responses after spirochetal killing [30].
Of the 91 EM patients, 70 (77%) had at least one associated symptom, including fatigue, malaise, headache, fever, neck stiffness, arthralgias, chills, or myalgias. Patients with no symptoms had the lowest levels of inflammatory mediators; those with moderate symptoms (1–5 symptoms) had intermediate levels, and those with many symptoms (6–20 symptoms) had the highest levels (Figure 1B). The greatest differences among groups were observed for the TH1-associated mediators IFN-γ, CXCL9, and CXCL10, and for the innate mediators IL-8, TNF, and IL-6. Similar trends were observed for TH17 mediators, but these did not reach statistical significance. Thus, appropriate innate and adaptive immune responses appear important for control of the infection, but the consequence of excessive inflammation is more symptomatic early infection.
Inflammatory Responses in Patients With Lyme Arthritis
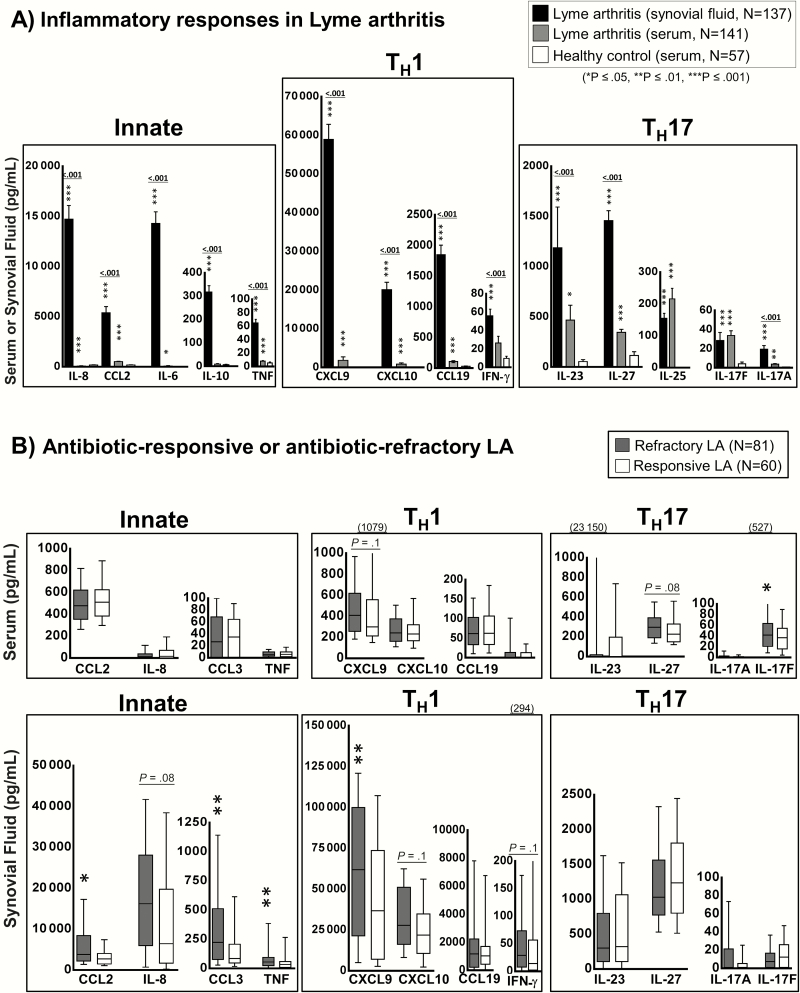
In patients with LA, which usually occurs months after initial spirochetal exposure, the disease was generally confined to one or a few large joints, particularly the knee. In these patients, the inflammatory responses, particularly innate and TH1 responses, were concentrated in synovial fluid of the affected joints (Figure 2A). The levels of IFN-γ–inducible chemokines CXCL9 and CXCL10, which are potent chemoattractants for CD4+ TH1-effector cells, were often approximately 100-fold higher in synovial fluid compared with serum (P < .001). In addition, synovial fluid in LA patients contained very high levels of several innate immune mediators, including CCL2, IL-8, and IL-6. The levels of TH17-associated mediators were also higher in LA patients compared with healthy subjects, but these responses were not uniformly concentrated in synovial fluid, suggesting that in some patients they may be occurring at extra-articular sites. Whereas the levels of IL-23, IL-27, and IL-17A were higher in synovial fluid compared with serum, the levels of IL-25, IL-22, and IL-17F were similar at both sites.
Figure 2.
Cytokine and chemokine levels in patients with Lyme arthritis (LA). Protein levels of 21 mediators associated with innate, T-helper 1 cell (TH1), or T-helper 17 cell (TH17) immune responses were assessed in serum and synovial fluid from 141 patients with LA using bead-based Luminex assays. A, Comparison of cytokine and chemokine values in serum or synovial fluid samples from patients with LA or in healthy controls. Black bars represent synovial fluid samples, gray bars represent serum samples, and white bars represent values in healthy subjects. The bars represent the mean values and I-bars represent the standard error of the mean. P values for comparison of synovial fluid and serum are indicated above the bars; P values for comparison with healthy controls are indicated by an asterisk (*P ≤ .05, **P ≤ .01, ***P ≤ .001). B, Cytokine and chemokine values in serum (upper panel) or synovial fluid (lower panel) in LA patients stratified according to antibiotic-responsive (white box plots, n = 60) or antibiotic-refractory (gray box plots, n = 81) course. The box represents the 25th–75th percentiles, the line inside the box represents median values, and I-bars represent the 10th–90th percentiles. Because of the large range in interferon-γ and interleukin 23 levels, the highest values for these mediators may be listed in parentheses above the graphs. P values for comparison of patients with antibiotic-responsive or refractory LA are indicated by an asterisk (*P ≤ .05, **P ≤ .01). Abbreviations: IFN, interferon; IL, interleukin; LA, Lyme arthritis; TH1, T-helper cell 1; TH17, T-helper cell 17; TNF, tumor necrosis factor.
When LA patients were subdivided into those with antibiotic-responsive arthritis or antibiotic-refractory arthritis, the refractory group had significantly higher synovial fluid levels of most innate-associated mediators, as well as higher levels of the TH1-associated mediators CXCL9, CXCL10, and IFN-γ (Figure 2B). In contrast, the levels of TH17 mediators in synovial fluid were similar in both the responsive and refractory groups. In serum, the generally low levels of innate and TH1-associated mediators were similar in patients with responsive or refractory arthritis, although there was a trend toward higher levels of the TH17-associated mediators IL-17F and IL-27 in the refractory group (Figure 2B). Thus, antibiotic-refractory LA was strongly associated with site-specific innate and TH1-adaptive immune responses in joints, whereas there was a trend toward higher TH17 responses in blood.
Borrelia Antibody Responses in Lyme Disease
To examine the associations between inflammatory mediators and antibody responses, the levels of innate, TH1, and TH17 mediators were correlated with B. burgdorferi–specific antibodies in 29 EM patients and 79 LA patients in whom sufficient sample volumes remained. Because no significant differences were observed in patients with refractory or responsive arthritis, these groups were combined for presentation here.
Early in the disease in EM patients, serum levels of one innate mediator (TNF), 4 TH1 mediators (CXCL9, CXCL10, IL-12p40, and CCL19), and 4 TH17 mediators (IL-17F, IL-23, IL-25, and IL-27) correlated directly with B. burgdorferi antibody levels (Table 1). In contrast, late in the disease in LA patients, these correlations were minimal; only one Th17 mediator, IL-17F, correlated with Borrelia antibodies in serum, and there was a negative correlation with IL-8 and Borrelia antibodies in synovial fluid. Thus, early in the infection, when large numbers of spirochetes were present and the immune response was developing, high levels of innate, TH1, and, in particular, TH17 mediators correlated with high antibody responses to the spirochete. In contrast, late in the disease when immune responses were fully matured and the levels of inflammatory mediators were generally high, these correlations were no longer observed.
Table 1.
Correlation of Inflammatory Mediators With Borrelia Immunoglobulin G Antibody Responses in Patients With Erythema Migrans or Lyme Arthritisa
| Mediator | Borrelia Antibody Responses (IgG) | ||
|---|---|---|---|
| EM Serum (n = 29) |
LA Serum (n = 79) |
LA Synovial Fluid (n = 44) |
|
| Innate responses | |||
| CCL2 | 0.4 | 0.1 | –0.1 |
| 0.06 | 0.5 | 0.3 | |
| CCL3 | 0.2 |
<–0.1 | –0.2 |
| 0.4 | 0.7 | 0.3 | |
| IL-1β | 0.1 | –0.1 | <–0.1 |
| 0.5 | 0.3 | 1 | |
| IL-6 | 0.2 | –0.1 | <0.1 |
| 0.3 | 0.2 | 1 | |
| IL-8 | –0.1 | <0.1 | r = –0.5 |
| 0.7 | 0.8 | P = .003 | |
| IL-10 | 0.2 | <–0.1 | <0.1 |
| 0.3 | 0.6 | 0.8 | |
| TNF | r = 0.4 | <–0.1 | -0.1 |
| P = .04 | 0.6 | 0.4 | |
| IFN-α | 0.3 | <0.1 | <0.1 |
| 0.08 | 0.6 | 0.9 | |
| TH1 adaptive responses | |||
| IFN-γ | 0.1 | <–0.1 | <0.1 |
| 0.7 | 0.7 | 1 | |
| CXCL9 | r = 0.4 | <0.1 | –0.2 |
| P = .03 | 1 | 0.3 | |
| CXCL10 | r = 0.4 | <–0.1 | <–0.1 |
| P = .02 | 0.5 | 0.6 | |
| IL-12p40 | r = 0.4 | –0.1 | <0.1 |
| P = .03 | 0.4 | 0.9 | |
| IL-12p70 | 0.3 | <0.1 | 0.2 |
| 0.1 | 0.7 | 0.3 | |
| CCL19 | r = 0.4 | 0.2 | –0.2 |
| P = .04 | 0.07 | 0.2 | |
| TH17 adaptive responses | |||
| IL-17A | r = 0.3 | <–0.1 | –0.2 |
| P = .07 | 0.6 | 0.2 | |
| IL-17F | r = .4 | r = 0.3 | 0.2 |
| P = .02 | P = .02 | 0.3 | |
| IL-23 | r = 0.4 | 0.2 | 0.2 |
| P = .02 | 0.2 | 0.4 | |
| IL-25 | r = 0.5 | <0.1 | 0.2 |
| P = .01 | 0.5 | 0.2 | |
| IL-27 | r = 0.4 | <0.1 | 0.2 |
| P = .02 | 0.5 | 0.2 | |
Abbreviations: EM, erythema migrans; IFN-y, interferon gamma; IgG, immunoglobulin G; IL, interleukin; LA, Lyme arthritis; TH1, T-helper cell 1; TH17, T-helper cell 17; TNF, tumor necrosis factor.
Correlations between the levels of inflammatory mediators (pg/mL) and Borrelia antibodies (optical density, OD450) were performed using a Pearson correlation test. For each mediator, r coefficient is presented in the first line, and P value in the second line. Values that did not reach significance are in plain text and do not contain "r" or "P" label. Values in bold reflect statistically significant correlations based on P ≤ .05 with Benjamini-Hochberg correction for multiple comparisons (false discovery rate ≤ 0.1).
Antibody Responses to Lyme Disease–Associated Autoantigens
The levels of inflammatory mediators were then correlated with autoantibody levels to 3 Lyme disease–specific autoantigens (ECGF, apoB-100, and MMP-10) in serum of 23 EM patients or in synovial fluid of 42 LA patients in whom sufficient sample volume was still available. In serum from EM patients, ECGF autoantibodies correlated strongly with most innate mediators and with 2 TH1 mediators, IFN-γ and IL-12p70 (Table 2). Only one innate mediator (CCL2) and 3 TH1 mediators (CXCL9, CXCL10, and CCL19) correlated with MMP-10 antibodies, and there were no correlations with apoB-100 autoantibodies. Moreover, early in the infection, there were no correlations between TH17 mediators and autoantibodies in serum.
Table 2.
Correlation of Inflammatory Mediators With Immunoglobulin G Antibody Responses to Autoantigens in Serum of Patients With Erythema Migrans or in Synovial Fluid of Patients With Lyme Arthritisa
| Mediator | EM Serum IgG Autoantibodies (N=23) | LA Synovial Fluid IgG Autoantibodies (N=42) | ||||
|---|---|---|---|---|---|---|
| ECGF | MMP-10 | apoB-100 | ECGF | MMP-10 | apoB-100 | |
| Innate responses | ||||||
| CCL2 | <0.1 | r = 0.7 | –0.2 | –0.1 | –0.2 | –0.2 |
| 1 | P = .0002 | 0.5 | 0.5 | 0.3 | 0.4 | |
| CCL3 | r = 0.7 | r = 0.4 | <0.1 | <0.1 | –0.2 | <–0.1 |
| P = .001 | P = .07 | 0.9 | 0.7 | 0.2 | 1 | |
| IL-1β | r = 0.8 | 0.3 | <0.1 | 0.1 | –0.3 | –0.2 |
| P = .0001 | 0.2 | 1 | 0.5 | 0.06 | 0.3 | |
| IL-6 | r = 0.6 | 0.1 | <–0.1 | <–0.1 | 0.1 | <–0.1 |
| P = .003 | 0.6 | 0.8 | 0.6 | 0.5 | 0.9 | |
| IL-8 | 0.3 | <–0.1 | 0.1 | <–0.1 | <–0.1 | –0.2 |
| 0.3 | 0.8 | 0.6 | 0.6 | 0.8 | 0.3 | |
| IL-10 | r = 0.8 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 |
| P = .00001 | 0.3 | 0.6 | 0.1 | 0.5 | 0.5 | |
| TNF | r = 0.6 | 0.3 | <0.1 | <–0.1 | –0.2 | –0.1 |
| P = .004 | 0.2 | 0.8 | 0.7 | 0.1 | 0.4 | |
| IFN-α | r = 0.5 | <0.1 | <0.1 | 0.2 | r = 0.4 | 0.3 |
| P = .02 | 0.8 | 1 | 0.1 | P = .02 | 0.1 | |
| TH1 adaptive responses | ||||||
| IFN-γ | r = 0.8 | 0.2 | <0.1 | <0.1 | –0.2 | –0.01 |
| P = .00001 | 0.3 | 0.7 | 0.9 | 0.3 | 0.5 | |
| CXCL9 | <0.01 | r = 0.6 | 0.2 | <0.1 | <–0.1 | <–0.1 |
| 1 | P = .002 | 0.4 | 0.8 | 1 | 0.6 | |
| CXCL10 | 0.2 | r = 0.6 | 0.4 | –0.1 | <–0.1 | <0.1 |
| 0.4 | P = .003 | 0.1 | 0.4 | 1 | 0.9 | |
| IL-12p40 | 0.4 | 0.2 | <–0.1 | r = 0.3 | –0.1 | <0.1 |
| 0.07 | 0.4 | 1 | P = .04 | 0.5 | 0.9 | |
| IL-12p70 | r = 0.5 | <0.1 | <–0.1 | 0.3 | r = 0.3 | 0.2 |
| P = .02 | 1 | 1 | 0.1 | P = .04 | 0.2 | |
| CCL19 | 0.1 | 0.5 | 0.4 | 0.2 | <–0.1 | <–0.1 |
| 0.7 | 0.01 | 0.1 | 0.3 | 0.8 | 1 | |
| TH17 adaptive responses | ||||||
| IL-17A | 0.4 | <0.1 | <0.1 | <0.1 | 0.3 | 0.2 |
| 0.08 | 1 | 1 | 0.6 | 0.08 | 0.3 | |
| IL-17F | 0.2 | <0.1 | <–0.1 | r = 0.8 | r = 0.4 | r = 0.6 |
| 0.4 | 0.9 | 1 | P = .00000007 | P = .01 | P = .00007 | |
| IL-23 | 0.3 | <0.1 | <–0.1 | r = 0.8 | r = 0.4 | r = 0.6 |
| 0.2 | 0.9 | 0.9 | P = .00000003 | P = .02 | P = .00003 | |
| IL-25 | 0.1 | <0.1 | <0.1 | r = 0.4 | r = 0.4 | r = 0.3 |
| 0.8 | 0.9 | 1 | P = .006 | P = .02 | P = .03 | |
| IL-27 | 0.2 | <0.1 | <–0.1 | r = 0.7 | r = 0.3 | r =0.5 |
| 0.3 | 1 | 0.9 | P = .000005 | P = .05 | P = .001 | |
Abbreviations: apoB-100, apolipoprotein B-100; ECGF, endothelial cell growth factor; EM, erythema migrans; IFN-y, interferon gamma; IgG, immunoglobulin G; IL, interleukin; LA, Lyme arthritis; MMP-10, matrix metalloproteinase 10; TH1, T-helper cell 1; TH17, T-helper cell 17; TNF, tumor necrosis factor.
Correlations between the levels of inflammatory mediators (pg/mL) and autoantibodies (optical density, OD450) were performed using a Pearson correlation test. For each mediator, r coefficient is presented in the first line, and P value in the second line. Values that did not reach significance are in plain text and do not contain "r" or "P" label. Values in bold reflect statistically significant correlations based on P ≤ .05 with Benjamini-Hochberg correction for multiple comparisons (false discovery rate ≤ 0.1).
In contrast, in LA patients, when the protein levels of these autoantigens were typically very high in synovial fluid [17–19], most TH17 mediators, including IL-17F, IL-23, IL-25, and IL-27, correlated directly with autoantibody levels to each of the 3 autoantigens in synovial fluid (Table 2). These correlations were not as pronounced in serum (data not shown). Moreover, when LA patients were stratified into antibiotic-responsive or antibiotic-refractory groups, IL-10, a prototypical anti-inflammatory cytokine, correlated with ECGF autoantibodies in patients with antibiotic-responsive LA. In contrast, TH17 mediators correlated strongly with autoantibodies only in patients with antibiotic-refractory LA (Table 3), suggesting a shift to an autoimmune phenotype in these patients. Thus, although innate and TH1-associated cytokines were often high in synovial fluid in LA patients, it was TH17 cytokine responses that correlated strongly with autoantibodies in patients with antibiotic-refractory LA, implicating TH17 responses in maladaptive immune processes in these patients.
Table 3.
Correlation of Inflammatory Mediators With Autoantibodies to Endothelial Cell Growth Factor, Matrix Metalloproteinase 10, or Apolipoprotein B-100 (Immunoglobulin G) in Synovial Fluid of Patients With Antibiotic-Responsive or Antibiotic-Refractory Lyme Arthritisa
| Mediator | Antibiotic-Responsive LA (n = 15) | Antibiotic-Refractory LA (n = 27) | ||||
|---|---|---|---|---|---|---|
| ECGF | MMP-10 | apoB-100 | ECGF | MMP-10 | apoB-100 | |
| Innate responses | ||||||
| IL-10 | r = 0.7 | 0.5 | 0.3 | <0.1 | <–0.1 | <0.1 |
| P = .005 | 0.1 | 0.3 | 0.6 | 0.8 | 0.8 | |
| IFN-α | –0.3 | 0.3 | <0.1 | r = 0.7 | r = 0.5 | r = 0.5 |
| 0.3 | 0.3 | 0.9 | P = .0002 | P = .02 | P = .008 | |
| TH17 adaptive responses | ||||||
| IL-17F | 0.1 | 0.2 | 0.1 | r = 0.9 | r = 0.4 | r = 0.6 |
| 0.7 | 0.5 | 0.7 | P = .00000003 | P = .03 | P = .0005 | |
| IL-23 | –0.1 | 0.2 | <–0.1 | r = 0.9 | r = 0.4 | r = 0.7 |
| 0.7 | 0.6 | 0.8 | P = .000000007 | P = .04 | P = .0002 | |
| IL-25 | … | … | … | r = 0.5 | r = 0.4 | r = 0.4 |
| … | … | … | P = .02 | P = .03 | P = .07 | |
| IL-27 | –0.4 | –0.4 | –0.5 | r = 0.8 | r = 0.4 | r = 0.6 |
| 0.2 | 0.2 | 0.08 | P = .000002 | P = .03 | P = .001 | |
Abbreviations: apoB-100, apolipoprotein B-100; ECGF, endothelial cell growth factor; IFN, interferon; IL, interleukin; LA, Lyme arthritis; MMP-10, matrix metalloproteinase 10; TH17, T-helper cell 17.
Correlations between the levels of inflammatory mediators (pg/mL) and autoantibodies (optical density, OD450) were performed in patients with antibiotic-responsive or refractory Lyme arthritis using a Pearson correlation test. For each mediator, r coefficient is presented in the first line, and P value in the second line. Values that did not reach significance are in plain text and do not contain "r" or "P" label. Values in bold reflect statistically significant correlations based on P ≤ .05 with Benjamini-Hochberg correction for multiple comparisons (false discovery rate ≤ 0.1). Only mediators with significant correlations are shown.
DISCUSSION
In this study, TH17 inflammatory responses correlated with B. burgdorferi antibody levels early in the infection in EM patients, and with autoantibody levels to self-antigens late in the illness in patients with antibiotic-refractory LA. The protective or pathogenic effector functions of TH17 cells are shaped by the composition of the local inflammatory milieu and the duration of exposure to such an environment. For example, transforming growth factor β and IL-6 appear to be essential for early TH17 lineage determination [1–4]. Such cells and their associated cytokines provide robust protection against extracellular pathogens, particularly bacteria and fungi, by rapid recruitment and activation of myeloid cells [1–3]. In contrast, prolonged or dysregulated exposure to IL-23 and IL-1β results in highly inflammatory TH17 cells, which recruit and activate myeloid cells with capacity to cause autoimmunity and severe local tissue pathology [2]. Moreover, IL-23–induced TH17 cells act directly on B cells and contribute to the generation of pathogenic autoantibodies that lead to onset of clinical inflammatory arthritis [36]. Studies in IL-23–deficient animals and in humans with IL-23R polymorphisms demonstrate that IL-23 is indispensible for the development of pathogenic TH17 cells that lead to chronic tissue injury and autoimmunity [1, 2, 5–8]. In human Lyme disease, which occurs in stages, one may observe both types of TH17 responses at different stages of the disease.
During early infection, the abundance of B. burgdorferi antigens in EM skin lesions leads to increased levels of innate, TH1, and TH17-associated inflammatory mediators that appear to help control the infection. Additionally, the infection triggers antibody responses to B. burgdorferi, and in some patients, nonpathogenic autoantibodies develop [17–20], presumably due to polyclonal activation of B cells [37, 38], or to close interactions between spirochetal and host lipids or proteins [39]. The fact that the 3 autoantigens studied here do not have sequence similarity with Borrelia proteins speaks against the T-cell epitope mimicry hypothesis [17–19]. Although some EM patients have pronounced TH17 responses with particularly high levels of IL-23, the responses at this stage appear to be protective and correlate directly with the levels of B. burgdorferi antibodies but not with autoantibodies. In contrast with innate and TH1 mediators, which decline markedly during convalescence, TH17 mediators decline more slowly, and this proclivity to remain elevated may become disadvantageous. In a prospective study of European patients with EM, high IL-23 levels sometimes persisted for many months after antibiotic therapy and were associated with post-Lyme symptoms and ECGF autoantibodies [30].
These responses appear to change in LA patients, particularly in those with refractory LA, presumably due to differences in the local inflammatory environment and a shift toward high levels of self-antigens associated with immune processes in joints. Patients with antibiotic-refractory LA have exceptionally high levels of IL-6 in synovial fluid (~15000 pg/mL vs ~10 pg/mL in EM sera), which is essential for TH17 lineage specification. In addition, most patients have elevated levels of IL-23, which would presumably polarize TH17 cells toward a pathogenic phenotype over the prolonged course of the disease, leading to recruitment of inflammatory myeloid cells that can induce local tissue injury [2, 3]. Moreover, emerging evidence demonstrates that IL-23–activated TH17 cells control the inflammatory activity of autoantibodies by regulating their glycosylation state on newly differentiating plasmablasts, thereby contributing to the shift from asymptomatic to clinical onset of autoimmune arthritis [36]. These findings demonstrate a unique linkage between TH17 immunity and pathogenic autoantibody responses, which appears to be absent with other mediators such as CCL19, CXCL9, and CXCL10, which have also been implicated in dysregulated immune responses after EM [40] or LA [23–25]. Thus, in some patients, the prolonged exposure to a chronic inflammatory environment, particularly high levels of IL-23, and the predominance of autoantigens in joints likely facilitates TH17-associated autoimmune responses.
As evidenced by recent work in rheumatoid arthritis, a prototypic chronic inflammatory arthritide, TH17 immunity, in the context of IL-23 stimulation, not only sets the stage for autoimmune responses, but also contributes to affinity maturation and posttranslational modifications of autoantibodies, both of which are necessary for pathogenicity and clinically evidenced autoimmune disease in joints [36]. We postulate that a similar scenario may occur in patients with Lyme disease, in whom autoantibodies may be present during several stages of disease but appear to become pathogenic primarily in the subgroup of patients with antibiotic-refractory LA. The correlation of autoantibody responses with elevated IL-10 levels in patients with antibiotic-responsive LA suggests that these patients have the ability to control autoreactive responses and their immune system regains homeostasis following the resolution of the infection. In contrast, in patients with antibiotic-refractory LA, the combination of genetic predisposition, abundance of autoantigens in joints, and prolonged exposure to high levels of TH17 mediators such as IL-23 leads to a shift toward immunoreactive autoantibody responses with pathogenic potential and persistent proliferative synovitis for months to years after antibiotic therapy.
Although an animal model of antibiotic-refractory LA is lacking, a unique mouse model supports a role for TH17 immune responses in severe Lyme arthritis. C57/BL/6 mice vaccinated with formalin-inactivated B. burgdorferi, followed 3 weeks later by infection with live spirochetes, developed severe destructive arthritis [26, 28, 29]. This manipulation appears to lead to the development of pathogenic TH17 cells, as the administration of neutralizing antibodies against IL-17 or IL-23 delayed the onset of joint swelling and ameliorated the histological changes associated with destructive arthritis. This was accompanied by a decrease in CD4+ IL-17–producing cells and an increase in CD4+CD25+ T cells, presumably of a regulatory phenotype [29].
In summary, our findings demonstrate that TH17 responses span the clinical spectrum of Lyme disease from host defense against the causative pathogen during early infection to autoimmunity late in the disease in patients with antibiotic-refractory LA. It will be important to learn whether similar dysregulated immune responses may play a role in other manifestations of Lyme disease or post-Lyme phenomena.
Notes
Author contributions. K. S. designed the study. K. S., K. B. S., A. P., and J. C. conducted the experiments and the data analyses for this study. A. C. S. and S. A. provided the patient samples and clinical information. K. B. S., J. C., and A. P. helped with design and interpretation of experiments involving autoantibodies. K. S., A. P., and A. C. S. wrote the manuscript. A. A. and R. S. performed the statistical analyses, contributed to generation of tables, and helped with the interpretation of the data. All authors reviewed and approved the manuscript.
Financial support. This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIH) (K01AR062098) and the Massachusetts General Hospital Executive Committee on Research (ECOR) Interim Support Fund (award number 2016A050303) to K. S.; and the National Institute of Allergy and Infectious Diseases, NIH (award number R01 A1-110175) to A. C. S.
Potential conflicts of interest. All authors: No potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 2008; 453:1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol 2014; 14:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol 2009; 27:485–517. [DOI] [PubMed] [Google Scholar]
- 4. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012; 11:763–76. [DOI] [PubMed] [Google Scholar]
- 5. Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 2006; 314:1461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reveille JD, Sims AM, Danoy P, et al. ; Australo-Anglo-American Spondyloarthritis Consortium (TASC) Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 2010; 42:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Helms C, Liao W, et al. A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet 2008; 4:e1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burton PR, Clayton DG, Cardon LR, et al. ; Wellcome Trust Case Control Consortium, Australo-Anglo-American Spondylitis Consortium. . Association scan of 14500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 2007; 39:1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benach JL, Bosler EM, Hanrahan JP, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med 1983; 308:740–2. [DOI] [PubMed] [Google Scholar]
- 10. Steere AC, Grodzicki RL, Kornblatt AN, et al. The spirochetal etiology of Lyme disease. N Engl J Med 1983; 308:733–40. [DOI] [PubMed] [Google Scholar]
- 11. Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet 2012; 379:461–73. [DOI] [PubMed] [Google Scholar]
- 12. Steere AC. Lyme disease. N Engl J Med 2001; 345:115–25. [DOI] [PubMed] [Google Scholar]
- 13. Steere AC. Lyme disease. N Engl J Med 1989; 321:586–96. [DOI] [PubMed] [Google Scholar]
- 14. Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med 1987; 107:725–31. [DOI] [PubMed] [Google Scholar]
- 15. Arvikar SL, Crowley JT, Sulka KB, Steere AC. Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthropathy, following Lyme disease. Arthritis Rheumatol 2017; 69:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 2006; 54:3079–86. [DOI] [PubMed] [Google Scholar]
- 17. Crowley JT, Strle K, Drouin EE, et al. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J Autoimmun 2016; 69:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crowley JT, Drouin DE, Pianta A, Wang Q, McHugh G, Costello CE, Steere AC. A highly expressed human protein, apolipoprotein B-100, serves as an autoantigen in a subgroup of patients with lyme disease. J Infect Dis 2015; 212:1841–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drouin EE, Seward RJ, Strle K, et al. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum 2013; 65:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pianta A, Drouin DE, Arvikar S, Costello CE, Steere AC. Identification of annexin A-2 as an autoantigen in rheumatoid arthritis and in Lyme arthritis. Arthritis Rheum 2014; 66:S437. [Google Scholar]
- 21. Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol 2004; 4:143–52. [DOI] [PubMed] [Google Scholar]
- 22. Londoño D, Cadavid D, Drouin EE, et al. Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory Lyme arthritis. Arthritis Rheumatol 2014; 66:2124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis. Arthritis Rheum 2007; 56:1325–35. [DOI] [PubMed] [Google Scholar]
- 24. Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol 2011; 178:2726–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum 2012; 64:1497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burchill MA, Nardelli DT, England DM, et al. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun 2003; 71:3437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henningsson AJ, Tjernberg I, Malmvall BE, Forsberg P, Ernerudh J. Indications of Th1 and Th17 responses in cerebrospinal fluid from patients with Lyme neuroborreliosis: a large retrospective study. J Neuroinflammation 2011; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotloski NJ, Nardelli DT, Peterson SH, et al. Interleukin-23 is required for development of arthritis in mice vaccinated and challenged with Borrelia species. Clin Vaccine Immunol 2008; 15:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nardelli DT, Burchill MA, England DM, Torrealba J, Callister SM, Schell RF. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged gamma interferon-deficient mice treated with anti-interleukin-17 antibody. Clin Diagn Lab Immunol 2004; 11:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strle K, Stupica D, Drouin EE, Steere AC, Strle F. Elevated levels of IL-23 in a subset of patients with post-Lyme disease symptoms following erythema migrans. Clin Infect Dis 2014; 58:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin JJ, Strle K, Glickstein LJ, Luster AD, Steere AC. Borrelia burgdorferi stimulation of chemokine secretion by cells of monocyte lineage in patients with Lyme arthritis. Arthritis Res Ther 2010; 12:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strle K, Drouin EE, Shen S, et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis 2009; 200:1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cerar T, Strle F, Stupica D, et al. Differences in genotype, clinical features, and inflammatory potential of Borrelia burgdorferi sensu stricto strains from Europe and the United States. Emerg Infect Dis 2016; 22:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Standardization of Lyme disease serodiagnosis. Lyme Disease Surveillance Summary. MMWR Morb Mortal Wkly Rep 1994; 5:1–3. [Google Scholar]
- 35. Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 36. Pfeifle R, Rothe T, Ipseiz N, et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol 2017; 18:104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hastey CJ, Elsner RA, Barthold SW, Baumgarth N. Delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J Immunol 2012; 188:5612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Steere AC, Hardin JA, Ruddy S, Mummaw JG, Malawista SE. Lyme arthritis: correlation of serum and cryoglobulin IgM with activity, and serum IgG with remission. Arthritis Rheum 1979; 22:471–83. [DOI] [PubMed] [Google Scholar]
- 39. Crowley JT, Toledo AM, LaRocca TJ, Coleman JL, London E, Benach JL. Lipid exchange between Borrelia burgdorferi and host cells. PLoS Pathog 2013; 9:e1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aucott JN, Soloski MJ, Rebman AW, et al. CCL19 as a chemokine risk factor for posttreatment Lyme disease syndrome: a prospective clinical cohort study. Clin Vaccine Immunol 2016; 23:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]