Patients reporting a penicillin allergy had a 50% increased odds of developing a surgical site infection, due to receipt of non–beta-lactam perioperative antibiotics. Clarifying reported penicillin allergies preoperatively may improve perioperative antibiotic prophylaxis choice and decrease surgical site infection risk.
Keywords: prophylaxis, antibiotic, healthcare-associated infections, surgical site infections, allergy
Abstract
Background
A reported penicillin allergy may compromise receipt of recommended antibiotic prophylaxis intended to prevent surgical site infections (SSIs). Most patients with a reported penicillin allergy are not allergic. We determined the impact of a reported penicillin allergy on the development of SSIs.
Methods
In this retrospective cohort study of Massachusetts General Hospital hip arthroplasty, knee arthroplasty, hysterectomy, colon surgery, and coronary artery bypass grafting patients from 2010 to 2014, we compared patients with and without a reported penicillin allergy. The primary outcome was an SSI, as defined by the Centers for Disease Control and Prevention’s National Healthcare Safety Network. The secondary outcome was perioperative antibiotic use.
Results
Of 8385 patients who underwent 9004 procedures, 922 (11%) reported a penicillin allergy, and 241 (2.7%) had an SSI. In multivariable logistic regression, patients reporting a penicillin allergy had increased odds (adjusted odds ratio, 1.51; 95% confidence interval, 1.02–2.22) of SSI. Penicillin allergy reporters were administered less cefazolin (12% vs 92%; P < .001) and more clindamycin (49% vs 3%; P < .001), vancomycin (35% vs 3%; P < .001), and gentamicin (24% vs 3%; P < .001) compared with those without a reported penicillin allergy. The increased SSI risk was entirely mediated by the patients’ receipt of an alternative perioperative antibiotic; between 112 and 124 patients with reported penicillin allergy would need allergy evaluation to prevent 1 SSI.
Conclusions
Patients with a reported penicillin allergy had a 50% increased odds of SSI, attributable to the receipt of second-line perioperative antibiotics. Clarification of penicillin allergies as part of routine preoperative care may decrease SSI risk.
(See the Editorial Commentary by Dellinger et al on pages 337–8.)
Surgical site infections (SSIs) account for 40% of all healthcare-associated infections among hospitalized patients [1]. Surgical site infections result in substantial morbidity and mortality [2] and an estimated attributable cost exceeding $25000 (2017 US dollars) per case [1, 3, 4]. Surgical site infections additionally influence perceptions of hospital quality; the Centers for Medicare and Medicaid Services Inpatient Prospective Payment System publicly reports SSIs through the Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN), with data made available to patients on Hospital Compare [1, 5]. For these reasons, reducing SSIs is a national healthcare priority [6–9].
Appropriate use of perioperative antibiotics can decrease the incidence of SSIs [10–12]. For most surgical procedures, a beta-lactam antibiotic is the preferred perioperative antibiotic [13]. Cefazolin, a first-generation cephalosporin antibiotic, is often the recommended drug due to its spectrum of coverage (ie, activity against common skin flora, including methicillin-sensitive Staphylococcus aureus [MSSA] and Streptococcus species), bactericidal activity, and favorable pharmacokinetics whereby the drug rapidly reaches optimal antibacterial concentrations in tissues [13–15]. For the 10% of patients who report a prior penicillin allergy [16, 17], non–beta-lactam antibiotics (eg, clindamycin, vancomycin) may be administered [18–21]. This is despite evidence demonstrating that 90%–99% of patients with a reported penicillin allergy are not truly allergic (ie, there is no immediate hypersensitivity) and <3% of patients with an allergy to penicillin will also react to cefazolin [22–25].
A reported penicillin allergy has been associated with increased healthcare-associated infections, including 23% increased odds of Clostridium difficile, 14% increased odds of methicillin-sensitive Staphylococcus aureus [MRSA] colonization or infection, and 30% increased odds of vancomycin-resistant Enterococcus (VRE) colonization or infection [26]. Few studies have assessed risk of SSIs in patients reporting drug allergies [27–29]. Our objective was to assess SSI risk among surgical patients reporting a penicillin allergy.
METHODS
Data Source
We performed a retrospective cohort study of patients who underwent hip arthroplasty (HPRO), knee arthroplasty (KPRO), hysterectomy (HYST), colon surgery (COLO), or coronary artery bypass grafting (CABG) from 2010 through 2014 at the Massachusetts General Hospital (MGH). All data were identified electronically from the Enterprise Data Warehouse, a comprehensive electronic health record database for Partners HealthCare System [30]. We additionally used MGH procedure and SSI data, which were collected, compiled, and reported to NHSN by the MGH Infection Control Unit in accordance with NHSN guidance.
Exposure
The exposure was a reported penicillin allergy, defined as a historically documented allergy to any penicillin antibiotic at the time of surgery, regardless of stated reaction (ie, hypersensitivities, side effects, and intolerances were all included). Allergies included both those reactions that were clinician-observed and those self-reported by patients. For patients who underwent multiple surgical procedures, we used penicillin allergy status at the time of their first procedure.
Reactions to penicillin antibiotics were identified using both coded and free-text reaction data, and categorized as (1) an allergic or hypersensitivity reaction; (2) a side effect, intolerance, or toxicity; or (3) an unknown reaction. We identified reactions representing contraindications to receiving beta-lactam antibiotics and those that would have been amenable to standard penicillin allergy evaluation methods (ie, penicillin skin testing and/or test dose challenge procedures) [31].
Outcomes
The primary outcome was SSI, as defined and reported to NHSN for each procedure, including all SSI types: superficial, deep, and organ space. At MGH, SSIs are determined through prospective surveillance by 8 infection control practitioners, registered nurses certified in infection prevention and control with 9–30 years of experience. Surgical site infections were identified through chart review of 100% of procedures and application of standardized NHSN definitions at the time of reporting [32].
The secondary outcome was perioperative antibiotic use, defined as antibiotics administered from 120 minutes prior to surgery until the end of surgery. Given the importance of antibiotic timing to development of SSI [13], we additionally assessed a preoperative antibiotic timing window, with initiation of antibiotics from 120 minutes prior to surgery until the time of incision, and separately assessed a stringent timing window for vancomycin (initiation of antibiotics 60–120 minutes prior to incision), given its pharmacokinetics and guideline recommendation for earlier administration [13]. During the study period, there were institutional guidelines for periprocedural antimicrobial prophylaxis with recommendations for agent choice and administration timing.
Covariates and Confounders
Age, sex, and race were identified from patient demographic tables. Body mass index (BMI) was calculated from height and weight assessments during the study period. American Society of Anesthesiologists (ASA) class was identified from the preoperative anesthesiology visit and documented according to established guidelines [33]. Medical comorbidities (ie, diabetes, cardiovascular disease, malignancy, asthma, chronic renal impairment) were identified using codes from the International Classification of Diseases, Ninth Edition billed during the surgical procedure or subsequent hospitalization; chosen codes were informed by prior studies, when available [34–37]. Resistant organism colonization was identified using electronic health record “flags” for patients with MRSA and VRE. Cephalosporin allergy, vancomycin allergy, and number of other allergies were identified from the allergy record in the Enterprise Data Warehouse.
Surgery type was specified as HPRO, KPRO, HYST, COLO, or CABG and identified as per NHSN criteria [38]. Hip arthroplasty included all surgical procedures involving the partial or total placement or replacement of a hip prosthesis; KPRO included all surgical procedures involving partial or total placement or replacement of a knee prosthesis; HYST included all abdominal and vaginal hysterectomies, including those performed by laparoscope; COLO included all incisions, resections, or anastomosis of the large intestine, including large-to-small and small-to-large bowel anastomoses; and CABG included all coronary artery bypass grafts with chest or chest and donor incisions to perform direct revascularization of the heart or internal thoracic artery [32, 38]. Emergency procedures, those related to trauma, wound class, and procedure duration (minutes) were identified from prospectively collected data reported to NHSN. Length of hospital stay (days) was calculated by subtracting the discharge date/time from the admission date/time.
Statistical Analysis
We compared patient and procedure characteristics in patients with and without a reported penicillin allergy using Wilcoxon rank sum test for continuous variables and χ2 test or Fisher’s exact test, as appropriate, for binary variables. To determine the adjusted impact of the reported penicillin allergy on SSIs for patients’ demographic, clinical, and surgery characteristics, we built a multivariable logistics model. We first selected important covariates by using both a priori knowledge and a univariate test with P < .10 as the criteria for model entry. We forced the variables included in NSHN risk stratification (ASA score, procedure duration, and wound class) into the model [14, 39]. We added additional candidate variables one at a time, and assessed their impact on the model through change in the quasi-likelihood under the independence model criterion goodness of fit and the P value of the model parameter. Given the low SSI event rate and concern for model overfitting, we removed candidate variables from the model with P > .05 and those that were collinear with other variables in the model (eg, cardiovascular disease and BMI are considered in the ASA score [33]). When precedent existed, we reduced the class-level sizes of the categorical variables into fewer numbers (eg, grouping ASA scores of IV and V together) [40, 41]. The model estimation was performed using generalized estimating equations to consider the intrapatient clustered outcomes that arose from repeated surgical procedures. We reported odds ratios (ORs) with 95% confidence intervals (CIs).
The final multivariable model was used for a mediation analysis to determine whether the association between penicillin allergy and SSI was mediated by perioperative antibiotic use. We grouped antibiotics into beta-lactam antibiotics and beta-lactam alternative antibiotics and assessed receipt of only alternative antibiotics as the potential mediator. We used a marginal structural model to assess the amount of mediation, or indirect effect, while controlling for confounders in the multivariable model [42].
The final multivariable logistic model was used to calculate the expected number of patients who would require allergy evaluation, or number needed to evaluate (NNE), to prevent 1 SSI. In this calculation, we used prior knowledge that patients reporting penicillin allergy are not truly penicillin allergic, with the plausible range of 90%–100%, and assumed that without a reported penicillin allergy, patients would be administered a first-line beta-lactam antibiotic [43–45].
We used SAS version 9.4 for all analyses, and a 2-sided P value of < .05 was considered statistically significant.
RESULTS
Cohort Characteristics
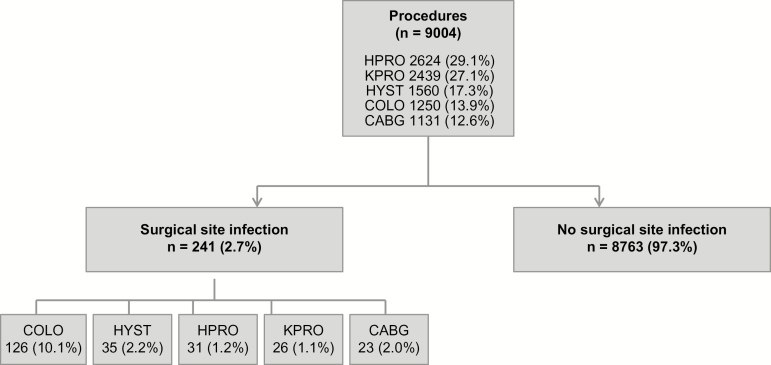
The cohort included 8385 patients who underwent 9004 procedures: 2624 HPRO, 2439 KPRO, 1560 HYST, 1250 COLO, and 1131 CABG (Figure 1).
Figure 1.
Retrospective cohort of 8385 patients undergoing 9004 surgical procedures from 2010 through 2014 at the Massachusetts General Hospital. Abbreviations: CABG, coronary artery bypass grafting; COLO, colon surgery; HPRO, hip arthroplasty; HYST, hysterectomy; KPRO, knee arthroplasty.
Nine hundred twenty-two patients (11.0%) reported a penicillin allergy. There were 1042 reactions documented to penicillin among the 922 patients (Table 1). Most reactions were possibly allergic, or hypersensitivity, reactions (n = 718, 68.9%), with rash (n = 346, 37.5%), urticaria (n = 166, 18.0%), and angioedema (n = 82, 8.9%) most common. There were 89 reactions (8.5%) that were side effects or toxicities, most commonly gastrointestinal symptoms (n = 51, 5.5%); 235 (25.5%) had unknown reactions. There were 5 hypersensitivity reactions (acute interstitial nephritis: n = 2; blister: n = 2; and Stevens-Johnson syndrome or toxic epidermal necrolysis: n = 1) that possibly represented true contraindications to receiving beta-lactam class antibiotics. There were 953 reactions (92.0%; 718 hypersensitivity and 235 unknown reactions) amenable to traditional penicillin allergy evaluation methods of skin testing and/or drug challenges (Table 1).
Table 1.
Reactions Identified for the 922 Patients Reporting Penicillin Allergy (n = 1042)
| Hypersensitivity Reactions,a n = 718 (68.9%) | |
|---|---|
| Rashb | 346 (37.5) |
| Urticariab | 166 (18.0) |
| Angioedema or swellingb | 82 (8.9) |
| Anaphylaxisb | 42 (4.6) |
| Itchingb | 41 (4.5) |
| Shortness of breathb | 19 (2.1) |
| Flushingb | 12 (1.3) |
| Hypotensionb | 5 (0.5) |
| Acute interstitial nephritisc | 2 (0.2) |
| Blisterc | 2 (0.2) |
| Stevens-Johnson syndrome or toxic epidermal necrolysisc | 1 (0.1) |
| Side Effects and Intolerances, n = 89 (8.5%) | |
| Gastrointestinal symptoms | 51 (5.5) |
| Renal damage | 2 (0.2) |
| Headache | 4 (0.4) |
| Fever | 2 (0.2) |
| Mental status change | 4 (0.4) |
| Musculoskeletal symptoms | 7 (0.8) |
| Other adverse reactions | 19 (2.1) |
| Unknown Reactions, n = 235 (25.5%)b | |
aTwenty-one patients had both hypersensitivity reactions and side effects to penicillin.
bReactions amenable to penicillin allergy evaluation (ie, penicillin skin testing and/or test dose challenges).
cReactions that are potential contraindications to beta-lactam antibiotic administrations.
Patients reporting penicillin allergy were older (65 y vs 64 y; P = .03) and more likely to be female (72.0% vs 56.8%; P < .001 (Table 2). Race was imbalanced between allergy groups, with penicillin allergy reporters more likely to be white (87.9% vs 84.7%; P = .02). Body mass index (P = .15) and ASA scores (P = .06) were slightly imbalanced between allergy groups; comorbidities were balanced between penicillin allergy groups, except cardiovascular disease, which was less common in patients reporting penicillin allergy (19.0% vs 23.4%; P = .003), and asthma, which was more common in patients with a penicillin allergy (12.4% vs 9.4%; P = .004). Vancomycin-resistant Enterococcus colonization was uncommon (n = 85), but patients reporting penicillin allergy had a higher frequncy of VRE colonization or infection history (1.7% vs 0.9%; P = .02). Patients with penicillin allergy histories had more cephalosporin allergy, vancomycin allergy, and more allergies overall (P < .001).
Table 2.
Characteristics of Cohort Patients and Procedures
| PATIENTS | ||||
|---|---|---|---|---|
| Characteristica | All (n = 8385) |
Reported penicillin allergy (n = 922) |
No reported penicillin allergy (n = 7463) |
P valueb |
| Age, median (IQR) | 64 (54–73) | 65 (56–73) | 64 (54–73) | .03 |
| Female sex | 4900 (58.4) | 664 (72.0) | 4236 (56.8) | <.001 |
| Race | .02 | |||
| White | 7130 (85.0) | 810 (87.9) | 6320 (84.7) | |
| Black | 284 (3.4) | 23 (2.5) | 261 (3.5) | |
| Hispanic | 327 (3.9) | 31 (3.4) | 296 (4.0) | |
| Asian | 192 (2.3) | 11 (1.2) | 181 (2.4) | |
| Other | 43 (0.5) | 1 (0.1) | 42 (0.6) | |
| Unknown | 409 (4.9) | 46 (5.0) | 363 (4.9) | |
| Body mass index | .15 | |||
| <18.5 | 108 (1.3) | 17 (1.8) | 91 (1.3) | |
| 18.5–24.9 | 2003 (23.9) | 225 (24.4) | 1778 (23.8) | |
| 25.0–29.9 | 2745 (23.7) | 283 (30.7) | 2462 (33.0) | |
| >30.0 | 3239 (38.6) | 380 (41.2) | 2859 (38.3) | |
| Missing | 290 (3.5) | 17 (1.8) | 273 (3.7) | |
| ASA class | .10 | |||
| I | 302 (3.6) | 19 (2.1) | 283 (3.8) | |
| II | 4913 (58.6) | 561 (60.9) | 4352 (58.3) | |
| III | 2779 (33.1) | 299 (32.4) | 2480 (33.2) | |
| IV | 383 (4.6) | 42 (4.6) | 341 (4.6) | |
| V | 8 (0.1) | 1 (0.1) | 7 (0.1) | |
| Medical comorbidities | ||||
| Diabetes | 1298 (15.5) | 136 (14.8) | 1162 (15.6) | .52 |
| Cardiovascular disease | 1922 (22.9) | 175 (19.0) | 1747 (23.4) | .003 |
| Malignancy | 1160 (13.8) | 140 (15.2) | 1020 (13.7) | .21 |
| Asthma | 812 (9.7) | 114 (12.4) | 698 (9.4) | .004 |
| Chronic renal impairment | 537 (6.4) | 53 (5.8) | 484 (6.5) | .39 |
| Resistant organism colonization | ||||
| MRSA flag | 50 (0.6) | 5 (0.5) | 45 (0.6) | >.99 |
| VRE flag | 85 (1.0) | 16 (1.7) | 69 (0.9) | .02 |
| Drug allergies | ||||
| Cephalosporin allergy | 187 (2.2) | 62 (6.7) | 125 (1.7) | <.001 |
| Vancomycin allergy | 44 (0.5) | 14 (1.5) | 30 (0.4) | <.001 |
| Total number of other allergies | <.001 | |||
| 0 | 7536 (89.9) | 786 (85.3) | 6750 (90.5) | |
| 1–2 | 668 (8.0) | 102 (11.1) | 566 (7.6) | |
| 3–4 | 118 (1.4) | 23 (2.5) | 95 (1.3) | |
| >5 | 63 (0.8) | 11 (1.2) | 52 (0.7) | |
| PROCEDURES | ||||
| Characteristica | All (n = 9004) |
Reported penicillin allergy (n = 978) |
No reported penicillin allergy (n = 8026) |
P valueb |
| Surgery type | <.0001 | |||
| HPRO | 2624 (29.1) | 282 (28.8) | 2342 (29.2) | |
| KPRO | 2439 (27.1) | 267 (27.3) | 2172 (27.1) | |
| HYST | 1560 (17.3) | 230 (23.5) | 1330 (16.6) | |
| COLO | 1250 (13.9) | 120 (12.3) | 1130 (14.1) | |
| CABG | 1131 (12.6) | 79 (8.1) | 1052 (13.1) | |
| Emergency | 148 (1.6) | 13 (1.3) | 135 (1.7) | .41 |
| Trauma | 282 (3.1) | 41 (4.2) | 241 (3.0) | .04 |
| Wound class | .01 | |||
| Clean | 6212 (69.0) | 637 (65.1) | 5575 (69.5) | |
| Clean-contaminated | 2692 (29.9) | 325 (33.2) | 2367 (29.5) | |
| Contaminated | 43 (0.5) | 9 (0.9) | 34 (0.4) | |
| Dirty | 57 (0.6) | 7 (0.7) | 50 (0.6) | |
| Procedure duration, min, median (IQR) | 120 (81–199) | 121 (81–185) | 119 (81–201) | .24 |
| Hospital length of stay, d, median (IQR) | 3.3 (2.4–5.4) | 3.3 (2.3–5.2) | 3.3 (2.4–5.6) | .01 |
Abbreviations: ASA, American Society of Anesthesiologists; CABG, coronary artery bypass grafting; COLO, colon surgery; HPRO, hip arthroplasty; HYST, hysterectomy; IQR, interquartile range; KPRO, knee arthroplasty; MRSA, methicillin-resistant Staphyloccoccus aureus; VRE, vancomycin-resistant Enterococcus.
aData are no. (%) unless otherwise specified.
bWilcoxon rank sum test, χ2 test, or Fisher’s exact test, as appropriate considering the sample size.
Surgery type was imbalanced between allergy groups (P < .001), with patients reporting penicillin allergy less likely to have had CABG and COLO and more likely to have had HYST. Although emergency surgical procedures were generally similar between penicillin allergy groups (P = .41), trauma was more frequent in the patients reporting penicillin allergy (4.2% vs 3.0%; P = .04). Wound class was imbalanced between penicillin allergy groups (P = .01), with patients reporting penicillin allergy less frequently having a clean wound and more frequently having clean-contaminated, contaminated, or dirty wounds. Procedure duration was relatively similar between penicillin allergy groups (P = .24), and hospital length of stay was slightly shorter for patients reporting penicillin allergy, with a median of 3 days (interquartile range [IQR], 2–5 d) compared with those without (median, 3 d; IQR, 2–6 d; P = .01).
Primary Outcome: Surgical Site Infections
Among 9004 procedures, there were 241 SSIs (2.7%): 126 SSIs for COLO (10.1%), 35 SSIs for HYST (2.2%), 31 SSIs for HPRO (1.2%), 26 SSIs for KPRO(1.1%), and 23 SSIs for CABG (2.0%) (Figure 1, Supplementary Table 1). In the univariable analysis, patients who reported a penicillin allergy history more frequently had an SSI (3.5% vs 2.6%; P = .10). In the multivariable logistic regression model adjusting for age, sex, race, surgery type, ASA class, procedure duration, and wound class, a reported penicillin allergy was associated with increased odds of SSI (adjusted OR [aOR], 1.51; 95% CI, 1.02–2.22) (Table 3).
Table 3.
Impact of a Reported Penicillin Allergy on Surgical Site Infection
| Adjustment | Odds ratio (95% confidence interval) | P value |
|---|---|---|
| None (univariable) | 1.36 (.94–1.97) | .10 |
| Surgery type | 1.45 (1.00–2.12) | .051 |
| Surgery type, age, sex, and race | 1.49 (1.02–2.18) | .04 |
| Surgery type, age, sex, race, American Society of Anesthesiologists class, procedure duration, and wound class | 1.51 (1.02–2.22) | .04 |
In the multivariable marginal structural model, the effect of penicillin allergy reporting on SSI development was entirely mediated through receipt of a beta-lactam alternative antibiotic. Although the indirect effect of reported penicillin allergy on SSI through perioperative antibiotic choice demonstrated significantly increased odds of SSI for penicillin allergy reporters (aOR, 2.05; 95% CI, 1.40–3.00), the direct effect of a reported penicillin allergy on SSI was not significant (aOR, 1.00; 95% CI, .36–2.78).
Secondary Outcome: Perioperative Antibiotic Choice and Timing
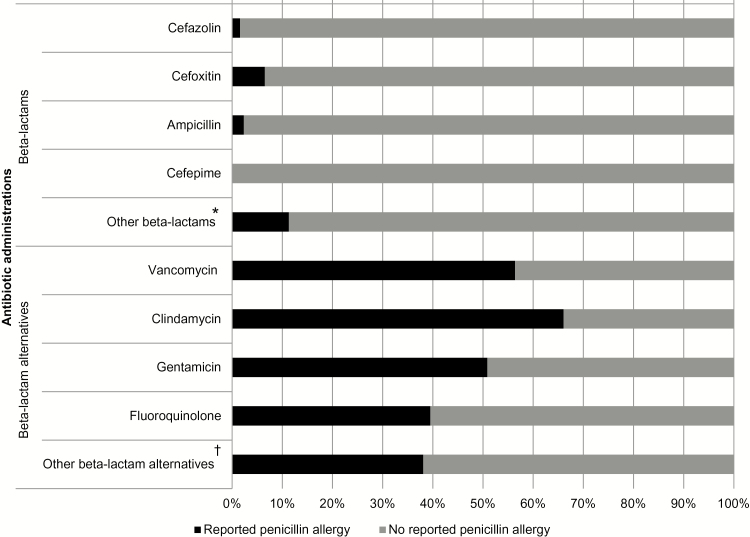
Overall, the most commonly used perioperative antibiotics were cefazolin (n = 7534, 83.7%), metronidazole (n = 1083, 12.0%), clindamycin (n = 722, 8.0%), and vancomycin (n = 601, 6.7%). Patients with a reported penicillin allergy were significantly less likely to receive cefazolin (12.2% vs 92.4%; P < .001) and more likely to receive clindamycin (48.8% vs 3.1%; P < .001), vancomycin (34.7% vs 3.3%; P < .001), gentamicin (24.0% vs 2.8%; P < .001), and fluoroquinolones (6.8% vs 1.3%; P < .001) (Figure 2).
Figure 2.
Perioperative antibiotic use among patients with and without a reported penicillin allergy. *Includes ceftriaxone (n = 4), ertapenem (n = 1), and piperacillin/tazobactam (n = 1) (reported penicillin allergy) and amoxicillin (n = 1), ceftazidime (n = 2), ceftriaxone (n = 20), nafcillin (n = 3), penicillin G (n = 3), piperacillin/tazobactam (n = 9), ertapenem (n = 4), imipenem (n = 4), and meropenem (n = 1) (no reported penicillin allergy). †Includes linezolid (n = 4) and other antibiotics (n = 4) (reported penicillin allergy), and azithromycin (n = 2), aztreonam (n = 1), daptomycin (n = 6), linezolid (n = 2), and other antibiotics (n = 3) (no reported penicillin allergy).
When restricting antibiotics to preoperative timing (ie, initiation from 120 minutes prior to incision until surgical incision), similar differences in antibiotic choice were observed, although 209 patients (2.3%) received the antibiotic outside of the timing window (Supplementary Table 2). Among procedures that did not use both a beta-lactam antibiotic and beta-lactam alternative antibiotic during surgery (n = 7893), beta-lactam antibiotics were more commonly initiated in the preoperative time window (98.2% vs 96.0%; P < .001).
Among patients receiving cefazolin or vancomycin (n = 8085), 97.5% did not receive vancomycin in the guideline-recommended time frame for administration (ie, 60–120 minutes before incision), whereas only 1.7% of patients did not receive cefazolin in the recommended time-frame (P < .001). Vancomycin initiation occurred a median of 24.0 minutes (IQR, 13.0–35.0 min) prior to incision.
Number Needed to Evaluate
Between 112 and 124 patients with reported penicillin allergy would need allergy evaluation to prevent 1 SSI.
DISCUSSSION
In this retrospective cohort study of more than 8000 patients and 9000 procedures, including hip and knee arthroplasty, hysterectomy, colon surgery, and coronary artery bypass grafting, patients who reported a prior penicillin allergy had a 50% increased odds of developing a SSI, using established definitions from NHSN and after controlling for important confounders. The differential SSI risk was attributable to receipt of alternative antimicrobial prophylaxis in the patient population reporting a prior history of penicillin allergy. Most patients had reported penicillin reactions eligible for standard allergy evaluation procedures that can distinguish true penicillin allergies and identify >95% of them as false. Based on published incidence of true penicillin allergy in this population, between 112 and 124 patients would need penicillin allergy evaluation to avert 1 SSI.
Although prior studies suggested that a penicillin allergy may be associated with increased infection risk generally, previous reports have not used NHSN SSI criteria and have focused on a specific surgical procedure (eg, head and neck free tissue transfer [27], infections after dental implants [29]) or infection type (eg, septic arthritis after reconstruction of the anterior cruciate ligament) [28]. The 50% increased odds of SSI among patients reporting a penicillin allergy in this cohort was entirely due to the use of beta-lactam alternative perioperative antibiotics [46] because the association between a reported penicillin allergy and SSI was an indirect effect in the marginal structural model. This implies that the increased SSI risk is potentially modifiable if patients with a reported penicillin allergy could receive first-line beta-lactam antibiotic prophylaxis after a negative penicillin allergy evaluation [12].
Although approximately 85% of procedures used the first-line recommended antibiotic cefazolin, patients reporting a penicillin allergy rarely received cefazolin and instead were 8–16 times more likely than those without a penicillin allergy history to receive clindamycin, vancomycin, or gentamicin. Prior studies reported similar patterns of antibiotic prophylaxis use among those with a reported penicillin allergy [18–21]. Patients with reported penicillin allergy in this study were not only less likely to receive the most effective perioperative antibiotic, they were also less likely to receive prophylaxis in the recommended time-frame for optimal tissue concentration, especially when vancomycin was the alternative antibiotic administered. Administration of beta-lactam alternative antibiotics, even perioperatively, increases risk of antibiotic adverse effects, such as Clostridium difficile colitis from clindamycin [47–50] or nephrotoxicity from vancomycin or gentamicin [51].
Current antimicrobial prophylaxis guidelines delineate recommended agent(s) and alternative agents for patients with immunoglobulin E–mediated penicillin allergy [13]. Although this guideline encourages taking an allergy history, no guidance on methods to clarify allergies or risk stratify patients is provided [13]. With limited direction and comfort with drug allergy assessment [52–54], providers understandably choose alternative agents for patients with prior reported penicillin allergy [25]. From the penicillin reaction histories in this study, all but 5 patients should have been able to either tolerate cefazolin directly (eg, the patients with nonimmunologic reactions or mild, nonspecific immunologic reactions) or would be eligible for outpatient penicillin allergy evaluation by an allergist/immunologist (ie, penicillin skin testing and/or test dose challenges) prior to surgery [55, 56]. Approaches to preoperative patients with reported penicillin allergy histories have been well documented and include outpatient specialist consultations and skin testing at preoperative visits [43]. Alternative approaches may include implementation of perioperative test dose challenges to cefazolin for patients with mild and/or remote penicillin allergy histories, an approach successfully used among hospitalized [57, 58] and ambulatory [59, 60] patients. Assuming all patients without true penicillin allergy would receive first-line perioperative prophylaxis, only 112–124 patients would need an allergy assessment to prevent 1 SSI.
This analysis, although limited by its retrospective design, used prospectively collected data (eg, procedure data, SSI) and manually validated data (eg, penicillin allergy) for key variables. Although the process for determining an SSI is not standard across institutions [61–67], differential surveillance methodologies would not be related to the exposure (penicillin allergy) and would therefore not affect our conclusions. Because SSIs occurred in only 241 patients, we could not account for all possible confounders in the relationship between penicillin allergy and SSI simultaneously. However, we were able to adjust for the most important confounders or a variable collinear with an important confounder. Further, the small number of SSIs left us underpowered to perform subgroup analyses by surgical procedure. Finally, these data represent the experience of 1 academic medical center, whose patients, providers, and practices may differ from those of other institutions. However, given the breadth of surgical disciplines included in this study and standardized implementation of Centers for Disease Control and Prevention NHSN criteria, these findings are likely generalizable to many other US and international hospitals. Further, given that a penicillin allergy label conferred a 50% increased risk of SSI at a hospital with low SSI prevalence, rigorous infection control and prevention, and ready access to allergy specialists, the problem is likely to be greater in other settings.
CONCLUSIONS
We identified that patients with a reported penicillin allergy are at 50% increased odds of developing SSI and that this increased risk is attributable to the inferior antibiotic prophylaxis such patients receive in terms of drug choice and/or administration time. Only 5 reactions to penicillin represented contraindications to receiving a beta-lactam; the vast majority of patients would have tolerated first-line recommended cephalosporin prophylaxis had allergy evaluation been pursued. Systematic, preoperative penicillin allergy evaluations in surgical patients may not only improve antibiotic choice but also decrease SSI risk.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by NIH K01AI125631, the American Academy of Allergy Asthma and Immunology Foundation. E.S.S. was supported by NIH K01AI110524 and the Massachusetts General Hospital-Massachusetts Institute of Technology Grand Challenge. This work was supported by Harvard Catalyst I Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers.
Acknowledgments. The authors thank Yuqing Zhang, DSc, for guidance and review of the mediation analysis; Lauren West, MPH, Winston Ware, MS, Christopher Fusco, Kenneth H. Lai, MS, and Li Zhou, MD, PhD, for assistance with data extraction; and David C. Hooper, MD, and Thoralf M. Sundt, MD, for their thoughtful review of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of neither the National Institutes of Health (NIH) nor the American Academy of Allergy Asthma and Immunology.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zimlichman E, Henderson D, Tamir O et al. Health care–associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013; 173:2039–46. [DOI] [PubMed] [Google Scholar]
- 2. Awad SS. Adherence to surgical care improvement project measures and post-operative surgical site infections. Surg Infect (Larchmt) 2012; 13:234–7. [DOI] [PubMed] [Google Scholar]
- 3. de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009; 37:387–97. [DOI] [PubMed] [Google Scholar]
- 4. Schweizer ML, Cullen JJ, Perencevich EN, Vaughan Sarrazin MS. Costs associated with surgical site infections in veterans affairs hospitals. JAMA Surg 2014; 149:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medicare.gov. Hospital Compare https://www.medicare.gov/hospitalcompare/search.html. Accessed 23 May 2017.
- 6. Kahn KL, Mendel P, Weinberg DA, Leuschner KJ, Gall EM, Siegel S. Approach for conducting the longitudinal program evaluation of the US Department of Health and Human Services National Action Plan to prevent healthcare-associated infections: roadmap to elimination. Med Care 2014; 52:S9–16. [DOI] [PubMed] [Google Scholar]
- 7. The Joint Commission. Surgical Site Infections https://www.jointcommission.org/topics/hai_ssi.aspx. Accessed 23 May 2017.
- 8. Centers for Medicare & Medicaid Services. Resources: Surgical Site Infections https://partnershipforpatients.cms.gov/p4p_resources/tsp-surgicalsiteinfections/toolsurgicalsiteinfections.html. Accessed 23 May 2017.
- 9. Anderson DJ, Podgorny K, Berríos-Torres SI et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(suppl 2):S66–88. [DOI] [PubMed] [Google Scholar]
- 10. Bratzler DW, Dellinger EP, Olsen KM et al. American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm 2013; 70:195–283. [DOI] [PubMed] [Google Scholar]
- 11. Alexander JW, Solomkin JS, Edwards MJ. Updated recommendations for control of surgical site infections. Ann Surg 2011; 253:1082–93. [DOI] [PubMed] [Google Scholar]
- 12. Bull AL, Worth LJ, Richards MJ. Impact of vancomycin surgical antibiotic prophylaxis on the development of methicillin-sensitive staphylococcus aureus surgical site infections: report from Australian Surveillance Data (VICNISS). Ann Surg 2012; 256:1089–92. [DOI] [PubMed] [Google Scholar]
- 13. Bratzler DW, Dellinger EP, Olsen KM et al. ; American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA) Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 2013; 14:73–156. [DOI] [PubMed] [Google Scholar]
- 14. Berríos-Torres SI, Yi SH, Bratzler DW et al. Activity of commonly used antimicrobial prophylaxis regimens against pathogens causing coronary artery bypass graft and arthroplasty surgical site infections in the United States, 2006–2009. Infect Control Hosp Epidemiol 2014; 35:231–9. [DOI] [PubMed] [Google Scholar]
- 15. Bratzler DW, Houck PM; Surgical Infection Prevention Guidelines Writers Workgroup; American Academy of Orthopaedic Surgeons; American Association of Critical Care Nurses; American Association of Nurse Anesthetists; American College of Surgeons; American College of Osteopathic Surgeons; American Geriatrics Society; American Society of Anesthesiologists; American Society of Colon and Rectal Surgeons; American Society of Health-System Pharmacists; American Society of PeriAnesthesia Nurses; Ascension Health; Association of periOperative Registered Nurses; Association for Professionals in Infection Control and Epidemiology; Infectious Diseases Society of America; Medical Letter; Premier; Society for Healthcare Epidemiology of America; Society of Thoracic Surgeons; Surgical Infection Society Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Clin Infect Dis 2004; 38:1706–15. [DOI] [PubMed] [Google Scholar]
- 16. Zhou L, Dhopeshwarkar N, Blumenthal KG et al. Drug allergies documented in electronic health records of a large healthcare system. Allergy 2016; 71:1305–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee CE, Zembower TR, Fotis MA et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med 2000; 160:2819–22. [DOI] [PubMed] [Google Scholar]
- 18. Beltran RJ, Kako H, Chovanec T, Ramesh A, Bissonnette B, Tobias JD. Penicillin allergy and surgical prophylaxis: cephalosporin cross-reactivity risk in a pediatric tertiary care center. J Pediatr Surg 2015; 50:856–9. [DOI] [PubMed] [Google Scholar]
- 19. Ponce B, Raines BT, Reed RD, Vick C, Richman J, Hawn M. Surgical site infection after arthroplasty: comparative effectiveness of prophylactic antibiotics: do surgical care improvement project guidelines need to be updated?J Bone Joint Surg Am 2014; 96:970–7. [DOI] [PubMed] [Google Scholar]
- 20. Tan TL, Springer BD, Ruder JA, Ruffolo MR, Chen AF. Is vancomycin-only prophylaxis for patients with penicillin allergy associated with increased risk of infection after arthroplasty?Clin Orthop Relat Res 2016; 474:1601–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Epstein RH, Jacques PS, Wanderer JP, Bombulie MR, Agarwalla N. Prophylactic antibiotic management of surgical patients noted as “allergic” to penicillin at two academic hospitals. A A Case Rep 2016; 6:263–7. [DOI] [PubMed] [Google Scholar]
- 22. Sagar PS, Katelaris CH. Utility of penicillin allergy testing in patients presenting with a history of penicillin allergy. Asia Pac Allergy 2013; 3:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract 2013; 1:258–63. [DOI] [PubMed] [Google Scholar]
- 24. Arroliga ME, Radojicic C, Gordon SM et al. A prospective observational study of the effect of penicillin skin testing on antibiotic use in the intensive care unit. Infect Control Hosp Epidemiol 2003; 24:347–50. [DOI] [PubMed] [Google Scholar]
- 25. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics 2005; 115:1048–57. [DOI] [PubMed] [Google Scholar]
- 26. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol 2014; 133:790–6. [DOI] [PubMed] [Google Scholar]
- 27. Pool C, Kass J, Spivack J et al. Increased surgical site infection rates following clindamycin use in head and neck free tissue transfer. Otolaryngol Head Neck Surg 2016; 154:272–8. [DOI] [PubMed] [Google Scholar]
- 28. Ristić V, Maljanović M, Harhaji V, Milankov M. Infections after reconstructions of anterior cruciate ligament. Med Pregl 2014; 67:11–5. [PubMed] [Google Scholar]
- 29. French D, Noroozi M, Shariati B, Larjava H. Clinical retrospective study of self-reported penicillin allergy on dental implant failures and infections. Quintessence Int 2016; 47:861–70. [DOI] [PubMed] [Google Scholar]
- 30. Kuperman GJ, Marston E, Paterno M et al. Creating an enterprise-wide allergy repository at Partners HealthCare System. AMIA Annu Symp Proc 2003:376–80. [PMC free article] [PubMed] [Google Scholar]
- 31. Solensky R, Khan D. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105:259–73. [DOI] [PubMed] [Google Scholar]
- 32. Centers for Disease Control and Prevention. Surgical Site Infection (SSI) Event; 2017. https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf. Accessed 22 September 2017. [Google Scholar]
- 33. American Society of Anesthesiologists. ASA Physical Status Classification System http://www.asahq.org/resources/clinical-information/asa-physical-status-classification-system. Accessed 23 May 2017.
- 34. Wilke RA, Berg RL, Peissig P et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res 2007; 5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kern EF, Maney M, Miller DR et al. Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res 2006; 41:564–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med 2006; 119:60–8. [DOI] [PubMed] [Google Scholar]
- 37. Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma—United States, 1980–1999. MMWR Surveill Summ 2002; 51:1–13. [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention. Procedure Code Mapping to NHSN Operative Procedure Codes https://www.cdc.gov/nhsn/acute-care-hospital/ssi/index.html. Accessed 23 May 2017.
- 39. Mu Y, Edwards JR, Horan TC, Berrios-Torres SI, Fridkin SK. Improving risk-adjusted measures of surgical site infection for the national healthcare safety network. Infect Control Hosp Epidemiol 2011; 32:970–86. [DOI] [PubMed] [Google Scholar]
- 40. Kaafarani HM, Smith TS, Neumayer L, Berger DH, Depalma RG, Itani KM. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am J Surg 2010; 200:32–40. [DOI] [PubMed] [Google Scholar]
- 41. Glatz T, Kulemann B, Marjanovic G, Bregenzer S, Makowiec F, Hoeppner J. Postoperative fluid overload is a risk factor for adverse surgical outcome in patients undergoing esophagectomy for esophageal cancer: a retrospective study in 335 patients. BMC Surg 2017; 17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol 2012; 176:190–5. [DOI] [PubMed] [Google Scholar]
- 43. Sacco KA, Bates A, Brigham TJ, Imam JS, Burton MC. Clinical outcomes following inpatient penicillin allergy testing: a systematic review and meta-analysis. Allergy 2017; 72:1288–96. [DOI] [PubMed] [Google Scholar]
- 44. Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2006; 97:681–7. [DOI] [PubMed] [Google Scholar]
- 45. Frigas E, Park MA, Narr BJ et al. Preoperative evaluation of patients with history of allergy to penicillin: comparison of 2 models of practice. Mayo Clin Proc 2008; 83:651–62. [DOI] [PubMed] [Google Scholar]
- 46. Murphy J, Isaiah A, Dyalram D, Lubek JE. Surgical site infections in patients receiving osteomyocutaneous free flaps to the head and neck. Does choice of antibiotic prophylaxis matter?J Oral Maxillofac Surg 2017. In press. [DOI] [PubMed] [Google Scholar]
- 47. Yee J, Dixon CM, McLean AP, Meakins JL. Clostridium difficile disease in a department of surgery. The significance of prophylactic antibiotics. Arch Surg 1991; 126:241–6. [DOI] [PubMed] [Google Scholar]
- 48. Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile–associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol 2008; 29:44–50. [DOI] [PubMed] [Google Scholar]
- 49. Palmore TN, Sohn S, Malak SF, Eagan J, Sepkowitz KA. Risk factors for acquisition of Clostridium difficile–associated diarrhea among outpatients at a cancer hospital. Infect Control Hosp Epidemiol 2005; 26:680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Southern WN, Rahmani R, Aroniadis O et al. Postoperative Clostridium difficile–associated diarrhea. Surgery 2010; 148:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Warkentin D, Ippoliti C, Bruton J, Van Besien K, Champlin R. Toxicity of single daily dose gentamicin in stem cell transplantation. Bone Marrow Transplant 1999; 24:57–61. [DOI] [PubMed] [Google Scholar]
- 52. Stukus DR, Green T, Montandon SV, Wada KJ. Deficits in allergy knowledge among physicians at academic medical centers. Ann Allergy Asthma Immunol 2015; 115:51–55.e1. [DOI] [PubMed] [Google Scholar]
- 53. Prematta T, Shah S, Ishmael FT. Physician approaches to beta-lactam use in patients with penicillin hypersensitivity. Allergy Asthma Proc 2012; 33:145–51. [DOI] [PubMed] [Google Scholar]
- 54. Blumenthal KG, Shenoy ES, Hurwitz S, Varughese CA, Hooper DC, Banerji A. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers’ antibiotic prescribing knowledge. J Allergy Clin Immunol Pract 2014; 2:407–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McDanel DL, Azar AE, Dowden AM et al. Screening for beta-lactam allergy in joint arthroplasty patients to improve surgical prophylaxis practice. J Arthroplasty 2017; 32:101–8. [DOI] [PubMed] [Google Scholar]
- 56. Cook DJ, Barbara DW, Singh KE, Dearani JA. Penicillin skin testing in cardiac surgery. J Thorac Cardiovasc Surg 2014; 147:1931–5. [DOI] [PubMed] [Google Scholar]
- 57. Blumenthal KG, Shenoy ES, Varughese CA, Hurwitz S, Hooper DC, Banerji A. Impact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergy. Ann Allergy Asthma Immunol 2015; 115:294–300.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blumenthal KG, Wickner PG, Hurwitz S et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017; 140:154–161.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tucker MH, Lomas CM, Ramchandar N, Waldram JD. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract 2017; 5:813–5. [DOI] [PubMed] [Google Scholar]
- 60. Mill C, Primeau MN, Medoff E et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr 2016; 170:e160033. [DOI] [PubMed] [Google Scholar]
- 61. Cruse PJ, Foord R. The epidemiology of wound infection. A 10-year prospective study of 62,939 wounds. Surg Clin North Am 1980; 60:27–40. [DOI] [PubMed] [Google Scholar]
- 62. Petherick ES, Dalton JE, Moore PJ, Cullum N. Methods for identifying surgical wound infection after discharge from hospital: a systematic review. BMC Infect Dis 2006; 6:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Niedner MF; 2008 National Association of Children’s Hospitals and Related Institutions Pediatric Intensive Care Unit Patient Care FOCUS Group The harder you look, the more you find: catheter-associated bloodstream infection surveillance variability. Am J Infect Control 2010; 38:585–95. [DOI] [PubMed] [Google Scholar]
- 64. Haut ER, Pronovost PJ. Surveillance bias in outcomes reporting. JAMA 2011; 305:2462–3. [DOI] [PubMed] [Google Scholar]
- 65. Baker C, Luce J, Chenoweth C, Friedman C. Comparison of case-finding methodologies for endometritis after cesarean section. Am J Infect Control 1995; 23:27–33. [DOI] [PubMed] [Google Scholar]
- 66. Cardo DM, Falk PS, Mayhall CG. Validation of surgical wound surveillance. Infect Control Hosp Epidemiol 1993; 14:211–5. [DOI] [PubMed] [Google Scholar]
- 67. Ming DY, Chen LF, Miller BA, Anderson DJ. The impact of depth of infection and postdischarge surveillance on rate of surgical-site infections in a network of community hospitals. Infect Control Hosp Epidemiol 2012; 33:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.