Abstract
SLE, a multisystem heterogeneous disease, is characterized by production of antibodies to cellular components, with activation of both the innate and the adaptive immune system. Decades of investigation of blood biomarkers has resulted in incremental improvements in the understanding of SLE. Owing to the heterogeneity of immune dysregulation, no single biomarker has emerged as a surrogate for disease activity or prediction of disease. Beyond identification of surrogate biomarkers, a multitude of clinical trials have sought to inhibit elevated SLE biomarkers for therapeutic benefit. Armed with new -omics technologies, the necessary yet daunting quest to identify better surrogate biomarkers and successful therapeutics for SLE continues with tenacity.
Keywords: systemic lupus erythematosus, autoimmunity, biomarkers, immunosuppressants, lupus nephritis, interleukin, cytokine, chemokine, complement, antibody.
Rheumatology key messages
Discovery and validation of serum and urine biomarkers continues to advance our understanding of SLE.
Numerous therapeutics targeting biomarkers have undergone clinical trials in SLE patients with varying success.
Large-scale proteomic screens yield additional biomarkers of potential clinical relevance that may become future therapeutic targets.
Introduction
SLE is a complex multisystem autoimmune disease commonly characterized by periods of flare and quiescence. Disease manifestations are heterogeneous, ranging from detectable laboratory abnormalities to multi-organ inflammation and failure. The clinical syndrome includes abnormal antibody production to cellular constituents, innate and adaptive immune alterations and dysregulated cytokine production. Several markers have been evaluated to classify diseased individuals and evaluate activity, or to determine treatment responses and predict therapeutic strategies.
Biomarkers
A biological marker, or biomarker, is ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention’ [1]. Biomarkers can be prognostic, diagnostic, predictive, pharmacodynamic and surrogate. Prognostic biomarkers identify a specific disease manifestation, individuals at risk for disease development or those likely to experience a flare. Diagnostic biomarkers confirm the presence or subtype of disease. Predictive biomarkers use baseline characteristics to predict therapeutic responses. Pharmacodynamic biomarkers assist in determining optimal therapeutic doses. Surrogate biomarkers are intended to substitute for a clinical end point.
Explorations for candidate biomarkers to date have targeted selected immune molecules or used multiple comprehensive screening approaches, examining genomics, transcriptomics, proteomics and metabolomics. Each of these techniques has been applied to SLE and has been reviewed elsewhere [2]. In addition, combining comprehensive and targeted studies reported in the literature with disease specific co-citation can potentially identify accepted and emerging biomarkers. A literature-mining program, Implicit Relationship IDEntification by in-Silico Construction of an Entity-based Network from Text (IRIDESCENT) [3–5] was used in this review (August 2015) to identify and rank entities in Medical Literature Analysis and Retrieval System Online, or MEDLARS Online (MEDLINE) related to SLE. Briefly, IRIDESCENT processes all electronically available peer-reviewed findings, as contained in MEDLINE records (>23 million as of this writing). Names and synonyms in the IRIDESCENT thesaurus are obtained from major publicly available databases to be able to recognize concepts and entities within text. The major contributing databases are Online Mendelian Inheritance in Man (OMIM) (diseases and phenotypes), Entrez (genes), CHEMical IDentification (CHEMID), US Food and Drug Administration (FDA; approved drugs) and Gene Ontology concepts (process, component and function). MEDLINE records were searched for concepts that co-occur within titles and abstracts, weighting their relative importance as conceptual pairs by how frequently they co-occur and by the distance between them in each record analysed (e.g. two terms in the same sentence receive higher weight than two terms within only the same abstract). IRIDESCENT identified key SLE biomarkers (followed by literature searches from August 2015 to August 2016) and quantified the strength of association of the biomarkers to each other and to SLE (Fig. 1 and Table 1). IRIDESCENT calculated values for literature strength (Lit. Str.), a measure of co-citation frequency and mutual information (MI), an information-theoretic measure that reflects the probability of seeing one term given the other weighted as an indication of specificity for various serum and urine biomarkers for both SLE and LN.
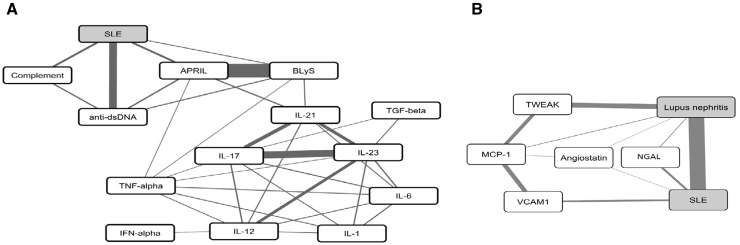
Fig. 1.
SLE biomarkers
(A) Serum biomarkers. (B) Urine biomarkers. Published relationships between SLE and lupus nephritis (grey fill) and selected biomarkers (white fill) are shown. Lines reflect the degree of mutual information (MI) between nodes (thicker indicates higher MI) as calculated by IRIDESCENT. MI reflects the relative specificity of nodes to each other. For example, there are many papers that mention IFN-α and SLE, but IFN-α is not specific to SLE, so it has a lower MI and therefore no direct connection in this graph. BLyS and APRIL, however, are closely related to each other and often mentioned together, giving them a high MI. As the MI scores are computed solely based on being co-mentioned in the published literature, these links do not necessarily imply a direct mechanistic relationship; the latter needs to be verified experimentally. APRIL: a proliferation-inducing ligand; BlyS: B lymphocyte stimulator; IRIDESCENT: Implicit Relationship IDEntification by in-Silico Construction of an Entity-based Network from Text; MCP-1: monocyte chemoattractant protein 1; TWEAK: TNF-like weak inducer of apoptosis.
Table 1.
An example of a data-mining method to identify potential SLE and LN biomarkers
| Biomarker | Lit Str. | MI | Lit Str. | MI | Years of cited manuscripts |
|---|---|---|---|---|---|
| SLE | LN | ||||
| Traditional clinical | |||||
| ANAs | 2083.2 | 7.2 | 12.5 | 14.9 | Reference |
| Anti-dsDNA | 308.3 | 20.2 | 70.8 | 62.5 | 1957–2016 |
| Complement | 243.6 | 16.5 | 35.2 | 36.5 | 1953–2015 |
| Non-traditional | |||||
| IFNα | 296.5 | 5.8 | 16.6 | 0.8 | 1979–2015 |
| BLyS | 254.2 | 3.9 | 13.9 | 3.7 | 2001–15 |
| APRIL | 139.4 | 1.9 | 13.2 | 2.9 | 2004–15 |
| Emerging | |||||
| TNFα | 1195.3 | 0.1 | 124.9 | 0.2 | 1996–2014 |
| IL-6 | 810.7 | 0.2 | 82.1 | 0.3 | 1991–2014 |
| IL-12 | 194.9 | 0.2 | 3 | 0.7 | 1995–2015 |
| IL-23 | 66.6 | 0.5 | 10.8 | 1.2 | 2008–15 |
| IL-1 | 303.3 | 0.1 | 45.5 | 0.2 | 1994–2016 |
| TGF-β | 8.6 | 0 | 79.9 | 0.2 | 2006–13 |
| IL-21 | 142.8 | 1.1 | 2.3 | 0.4 | 2008–13 |
| IL-17 | 236.3 | 0.5 | 2.5 | 1.5 | 2000–15 |
| Ferritin | 5.6 | 0.4 | 4.9 | 0 | 2012–16 |
| IGF binding proteins | 3.4 | 0 | 26.2 | 0.1 | 2016 |
| Urine | |||||
| TWEAK | 47.4 | 0.8 | 42.3 | 9.1 | 1997–2016 |
| MCP-1 | 194.6 | 0.1 | 3.3 | 1.8 | 1996–2015 |
| NGAL | 47.1 | 0.1 | 27.8 | 1.2 | 2007–15 |
| VCAM-1 | 82.7 | 0.2 | 29.3 | 0.9 | 2002–16 |
| Angiostatin | 3.7 | 0 | 3.7 | 0.3 | 2013–15 |
IRIDESCENT calculated values for literature strength (Lit. Str.), a measure of co-citation frequency, and mutual information (MI), an information-theoretic measure that reflects the probability of seeing one term given the other weighted as an indication of specificity for various serum and urine biomarkers for both SLE and LN. ANA is included for reference, as this biomarker is known to be sensitive for SLE (very high Lit Str.) but not very specific (MI lower than dsDNA and complement). Established biomarkers will have higher values than emerging biomarkers owing to the frequency in medical literature. The years of publication of the manuscripts cited within this review are included in the final column (please note that literature may exist outside of this date range, and the IRIDESCENT values were calculated in August 2015). APRIL: a proliferation-inducing ligand; BlyS: B lymphocyte stimulator; IRIDESCENT: Implicit Relationship IDEntification by in-Silico Construction of an Entity-based Network from Text; MCP-1: monocyte chemoattractant protein 1; NGAL: neutrophil gelatinase-associated lipocalcin; TWEAK: TNF-like weak inducer of apoptosis; VCAM-1: vascular cell adhesion molecule-1.
The goal of this review is to present an overview of blood and urine biomarkers that have been reported in the literature in relationship to SLE disease activity or therapeutics.
Traditional clinical biomarkers
More than half a century of research into SLE blood markers has generated few widely accepted biomarkers for routine clinical care. Although laboratory studies routinely obtained in the general patient population (blood count and urinalysis) can also assist in assessing disease activity and the presence of haematological and renal manifestations, discussion will focus on laboratory parameters from blood and urine that are more specific to SLE. Anti-dsDNA and complement deficiency are included in clinical instruments to assess SLE disease activity, including the SLEDAI [6], the Safety of Estrogens in Lupus Erythematosus National Assessment-SLEDAI [7] and the SLEDAI-2000(2K) [8].
Anti-dsDNA
The ACR [9, 10] and the SLICC [11] include ANA, anti-dsDNA, aPL and the anti-spliceosome antibody anti-Smith in their SLE classification criteria. However, the presence of anti-dsDNA is not unique to SLE. A Saudi Arabian study determined that 58.8% of patients with a positive dsDNA had SLE, yet 41.5% presented with other rheumatological diseases, malignancies, infections, hepatitis or endocrine disorders [12]. Additionally, Chinese patients with ovarian cancer, colon cancer and hepatocellular carcinoma were found to have 30, 40 and 50% dsDNA positivity, respectively [13]. Finally, 7.6% of healthy elderly Italian individuals had positive anti-dsDNA results [14].
Few biomarkers in SLE fulfil multiple biomarker categories; however, anti-dsDNA has been proposed to be a prognostic, predictive and partial surrogate biomarker of end-organ disease, although results are inconsistent. Nearly 60 years ago, antibodies to dsDNA were noted in sera from lupus patients [15–17]. Detectable levels precede clinical diagnosis by ∼2.2 years [18, 19]. Furthermore, elevation of anti-dsDNA correlates with the IFN signature, suggesting prolonged immune dysregulation and transition to SLE [20]. Moreover, patients with positive anti-dsDNA have increased antibody levels preceding disease exacerbation [21], and anti-dsDNA correlates with acute illness [22], disease activity [23] and complement levels in SLE patients [24]. Furthermore, anti-dsDNA was predictive of haematological or organ flare [25]. Analysis of patients in the placebo arms of two belimumab (a mAb that binds soluble B lymphocyte stimulator, BLyS) studies determined that high anti-dsDNA levels (>200 IU/ml) predicted SLE flare within 1 year; and anti-dsDNA normalization by 8 weeks was correlated with a reduced risk of severe flare [26]. Additionally, anti-dsDNA positivity predicted therapeutic benefit of belimumab [27], and treatment resulted in significant reduction of anti-dsDNA [28]. In another study, prednisone treatment was associated with reduced anti-dsDNA compared with placebo [29]. However, evaluation of anti-dsDNA levels in serologically active, clinically quiescent patients found no correlation with disease activity or subsequent flare [30].
An association of anti-dsDNA with LN has been described in multiple studies [31–34]. Yung and Chan [35] have previously detailed mechanisms for anti-dsDNA involvement in kidney pathogenesis, describing immune complex formation and renal binding. Anti-dsDNA levels have also been associated with disease activity and proliferative LN and were predictive of proliferative nephritis [36]. In patients with biopsy-proven proliferative nephritis, anti-dsDNA elevation occurred ∼2.7 years before diagnosis [37]. Rituximab, a mAb targeting B lymphocytes [38], resulted in reduction of anti-dsDNA in proliferative LN patients that correlated with reduced proteinuria [39].
More recently, hCDR1 (edratide), a synthesized 19 amino acid peptide of the heavy chain complementary determining region of human anti-DNA antibody, showed some improvements in clinical end points of SLE patients [40].
In summary, dsDNA autoantibodies are predictive of SLE and LN development, correlate with disease activity, are predictive of disease flare, are impacted by immunosuppressive therapeutics and correlate with improvement of proteinuria in LN. Anti-dsDNA is one of few SLE biomarkers measured longitudinally in routine clinical practice for assessment of disease activity.
Complement
The complement system is a key component of the innate immune system. Reduction of complement occurs in congenital complement deficiencies, infections, liver failure, acute pancreatitis, cryoglobulinaemia, thermal burns and SLE [41]. Early studies showed that SLE patients fixed complement, resulting in lower complement levels relative to controls using the total haemolytic complement assay (or CH50) [16, 42]. Complement fixation was present in kidney, liver, spleen and heart tissue of SLE patients and co-localized with antigen–antibody complexes [43]. C1q of the classical complement pathway bound directly to human keratinocytes (skin) [44] and apoptotic human Jurkat cells [45]. Decreases in C1q, C3 and C4 levels can precede a clinically evident flare and correlate with disease activity [24, 46].
Some of the strongest genetic risk factors for SLE result in deficiencies of complement proteins, including C1q, C1r, C4, C2 and C3 [47]. Furthermore, impaired clearance of apoptotic cells owing to complement pathway protein deficiency is a proposed mechanism for the development of SLE [48, 49]. C1q and mannose binding mediate apoptotic cell clearance by dendritic cells (DCs) and macrophages, with resultant increases in IL-6, TNF-α and IL-10 [50]. A recent review highlights multiple pathways for C1q in tolerance and autoimmunity [51]. Antibodies to C1q predicted renal flare in proliferative LN [52], and levels were correlated with renal disease activity [53]. Cell-bound complement activation products on erythrocytes and B cells were recently found to have higher diagnostic sensitivity for SLE than assessment of the standard complement components, C3 and C4 [54].
Eculizumab is a monoclonal antibody to C5 that is FDA approved for use in atypical haemolytic uraemic syndrome. In a small phase I clinical trial on SLE patients, it resulted in >80% reduction of CH50 in the highest dose, with no adverse effects [55]. In addition, SLE patient case reports have noted dramatic clinical improvements in thrombotic microangiopathy and proliferative LN [56, 57]. Likewise, patients with APS have been treated successfully with eculizumab [58–60]. Further evaluation of eculizumab in SLE may be warranted, potentially targeting subgroups with aPL and thrombotic microangiopathy.
Complement and the remaining biomarkers are categorized as prognostic biomarkers. Although most biomarkers are indicative of severity or flare or disease, some are predictive of flares, as noted in their respective section.
Non-traditional and emerging biomarkers
The majority of the non-traditional and emerging biomarkers are not routinely assessed in SLE patients, but are proteins involved in cellular signalling. Communication between various cells in the body, particularly immune cells, is fundamental for SLE pathogenesis. Cytokines, chemokines, growth factors and acute phase reactants allow adjacent and long-distance communication within the body.
IFNα
In the 1980s, studies noted that IFNα was elevated and correlated positively with clinical disease activity in SLE patients [61–64]. A variety of methods have been used to quantify serum (s) IFN, including WISH cells, immunoradiometric assay and ELISAs. IFN levels in serum or plasma can be transient because of localized expression and uptake; therefore, evaluation of IFN-regulated genes provides an additional method of quantification. Peripheral blood mononuclear cells (PBMCs) from SLE patients overexpress genes that are IFN regulated, and this is referred to as the IFN signature [65–67]. PBMCs of SLE patients and controls were compared for expression of type-I IFN-inducible genes and found to have higher levels in SLE that were correlated with disease activity levels, but not longitudinally [68, 69]. Interferon regulatory factor 5 (IRF5) [70–73] and IRF5-risk haplotype are associated with higher IFNα activity in SLE patients [74]. Autoimmunity, including lupus-like disease, has developed in patients treated with IFN for hepatitis C [75] and cancer [76–78]. DCs, key actors in SLE pathogenesis, differentiate from monocytes following IFNα induction [79]. DNA, RNA or immune complex binding to Toll-like receptor 7 or 9 can result in plasmacytoid DCs producing IFNα [80], and this has been proposed to occur locally in cutaneous lupus tissue [81, 82]. A further study confirmed the association of IFNα with SLE disease activity and noted that DC maturation is IFN dependent [83]. IFNα activity was correlated with the presence of autoantibodies rather than clinical features or ethnicity [84, 85]. In summary, IFNα plays a role in the pathogenesis of lupus through genetic susceptibility, a self-directed immune response resembling an antiviral response and chronic immune dysregulation [86].
Therefore, IFNα-directed pharmaceuticals are being developed and evaluated for therapeutic benefit in SLE. In a phase II trial, rontalizumab, a mAb targeting IFNα, reduced disease activity in a subgroup with low IFN; however, it did not meet the therapeutic target in the full group of patients [87].
Sifalimumab, another anti-IFNα monoclonal antibody, reduced the whole-blood IFNα gene signature in phase I studies [88, 89]. In phase II studies, anifrolumab, a type I IFN receptor antagonist, resulted in reduced disease activity in moderate to severe SLE as presented by R. Furie at the 2015 ACR Annual Meeting [90]. Two phase III trials are currently enrolling moderate to severe SLE patients for further evaluation, and a phase II study has begun enrolment of LN patients.
BLyS and APRIL
BLyS is a member of the TNF family which promotes B cell survival and antibody production. BLyS binds to three receptors (TACI, BCMA and BR3/BAFFR) present on the surface of B cells. A similar molecule, a proliferation-inducing ligand (APRIL), binds TACI and BCMA [91]. Elevated BLyS levels were found in SLE patients compared with normal controls, and BLyS was correlated with serum immunoglobulin and anti-dsDNA levels [92, 93]. Additional longitudinal studies from SLE patients confirmed these findings and showed a positive association with disease activity and a negative correlation between CS dosage and BLyS levels [94–97]. Classification criteria of discoid rash, renal disease, serositis and lymphopenia were associated with elevated BLyS levels, but levels failed to predict disease flare [96].
A phase II study of belimumab, a mAb that binds to soluble BLyS, found tolerability and efficacy in serologically active patients [98]. Two large phase III trials, BLISS-52 and BLISS-76, demonstrated sufficient efficacy for belimumab, leading to successful FDA approval for treatment of SLE [99, 100]. Phase I trials of blisibimod, a fusion polypeptide protein that binds both soluble and membrane-bound BLyS, in SLE patients noted a dose-dependent reduction in total B cells and naïve B cells and a relative increase in memory B cells, with favourable safety and tolerability [101]. The subsequent phase II study noted reduced disease activity, proteinuria and anti-dsDNA levels [102]. Furthermore, a phase III study has completed patient enrolment, with a second trial nearing initiation.
Although APRIL has many similarities to BLyS, reports noted a negative correlation of APRIL levels with disease activity and anti-dsDNA levels [103]. Furthermore, APRIL levels were inversely correlated with BLyS, potentially indicating a regulatory relationship or the result of ligand–receptor promiscuity [104]. Assessment of pre-flare and non-flare SLE patients found no significant difference in BLyS or APRIL levels, despite a difference between healthy controls (HCs) and disease activity state in SLE patients [105].
Atacicept is a fusion protein that includes the BLyS/APRIL binding site of the TACI receptor, potentially blocking BLyS and APRIL. TACI is involved in plasma cell survival, B cell proliferation and immunoglobulin production. A phase II/III study tested both a 75 and a 150 mg dose of atacicept against placebo. Concerns of infection risk, including two fatal infections, resulted in premature discontinuation of the 150 mg arm, whereas 75 mg lacked significant impact on disease flare [106]. However, additional phase II/III studies are in progress in SLE. Ultimately, BLyS and APRIL may prove beneficial in a subset of SLE patients.
Additional cytokine/chemokine biomarkers
TNFα
TNFα contributes to activation, differentiation, proliferation and antibody production in B cells and co-stimulates T cells. TNFα levels and soluble TNF receptors, TNFRI and TNFRII, are cleaved in response to inflammation [107, 108]. Levels were higher in SLE than in RA and spondyloarthritis [109]. TNFα was also correlated with disease activity in longitudinal specimens, with significantly higher levels in pre-flare vs non-flare patients [105, 110]. Contrary to this, blockade of TNFα can result in production of autoantibodies and drug-induced SLE [111]. An open-label study in six SLE patients on infliximab, a monoclonal antibody that binds TNFα, reported reduced arthritis and proteinuria, but higher levels of dsDNA and cardiolipin autoantibodies [112]. Anti-TNFα therapy, which has been extremely effective for RA, has shown some efficacy in SLE according to case reports. A more recent open-label study of 27 SLE patients randomized to infliximab or control reported improvement in disease activity and reduction in glucocorticoid use [113].
IL-6
IL-6, a pro-inflammatory cytokine produced by antigen-presenting cells, facilitates Th2 and Th17-type adaptive responses, and B cell activation, differentiation and antibody production. Both serum protein levels and PBMC IL-6 gene expression were significantly elevated in a study comparing SLE patients with HCs [114]. Furthermore, sIL-6 levels are correlated with disease activity, ESR and CRP in SLE patients [115, 116]. The IL-6 levels of pre-flare SLE patients are higher than those of non-flare SLE patients [105]. A phase I open-label, non-placebo-controlled study of 16 SLE patients noted that tocilizumab, an mAb directed at the IL-6 receptor, reduced autoantibody production and disease activity [117].
IL-12 and IL-23
IL-12, produced by antigen-presenting cells, facilitates the Th1 adaptive response by directly stimulating production of IFNγ and Th1 differentiation. sIL-12 levels were found to be higher in SLE patients than in HCs and to increase prior to flare in some patients [118]. In addition to elevated serum and urinary (u) IL-12, LN patients also exhibit IL-12 accumulation in renal glomerular mononuclear cells [119]. IL-12 levels were lower in patients treated with glucocorticoids or other immunosuppressants [120, 121]. IL-12p70 includes two subunits, p35 (also present in IL-23) and p40 (similar to IL-6 receptor α-chain), which is specific to IL-12. The p40 subunit can form a homodimer (IL-12p40/p40) that binds the IL-12 receptor, blocking signalling activity [122]. A study in 28 SLE patients found that the IL-12p40 monomer was positively correlated with SLE disease activity; however, they failed to detect the IL-12p70 heterodimer [123], contradicting other studies [116, 118]. The IL-12p70 heterodimer was significantly higher in pre-flare SLE patients compared with non-flare SLE patients [105]. Ustekinumab, an IL-12/IL-23 antagonist, is FDA approved for the treatment of moderate to severe psoriasis [124, 125], and a phase IIa study has been initiated for SLE to evaluate safety and efficacy.
Closely related to IL-12, IL-23 contains the IL-12 p40 subunit, and findings linking IL-12 to inflammation and autoimmune disease may also reflect a potential role of IL-23 in SLE pathogenesis. IL-23 levels are higher in SLE patients than HCs and result in significantly greater release of IL-17 in SLE PBMCs compared with HCs [126]. Longitudinal SLE samples were found to have significantly higher IL-23 in pre-flare compared with self non-flare or quiescent [105]. In LN patients, glomerular expression of IL-23 was much higher in proliferative disease and correlated with renal components of the SLEDAI and the histological activity index [127].
IL-1
A genetic polymorphism in IL-1 receptor antagonist was correlated with disease findings of discoid rash and photosensitivity in SLE patients [128]. Additionally, polymorphisms in IL-1 receptor-associated kinase, IRAK1, were associated with both childhood- and adult-onset SLE [129, 130]. IL-1 levels were found to be higher in SLE patients compared with controls and elevated in patients with high disease activity prior to treatment [131].
Anakinra, an IL-1 receptor antagonist, was evaluated in an open-label pilot study of four SLE patients with active arthritis and found to be safe and well tolerated, and resulted in a reduction in tender joint count [132].
TGF-β
TGF-β is a fibrotic cytokine involved in wound healing, angiogenesis and formation of scar tissue. Plasma levels of TGF-β were significantly elevated in both inactive and active SLE patients compared with controls; however, TGF-β mRNA from SLE lymphocytes was greatly reduced or absent [133]. A study evaluating microparticle proteins found that TGF-β was reduced in SLE, which the authors surmise is related to reduction of platelets, a key producer of TGF-β [134]. Urinary TGF-β mRNA levels were higher in diffuse proliferative LN and reduced in patients responsive to therapy [135]. Fresolimumab, an anti-TGF-β mAb, has been given orphan status by the FDA for treatment of primary focal segmental glomerulosclerosis and is being studied in idiopathic pulmonary fibrosis (NCT00125385) and systemic sclerosis (NCT01284322). All of these diseases are characterized by an increase in fibrosis of various organs, which also occurs in later stages of LN. Pirfenidone, an anti-fibrotic agent whose mechanism is not fully understood, results in reduction of TGF-β and collagen synthesis. It has been approved by the FDA for treatment of idiopathic pulmonary fibrosis, and studies have been planned in focal segmental glomerulosclerosis (NCT00001959) and chronic kidney disease (CKD) (NCT02408744). Use of TGF-β-inhibiting agents to prevent renal sclerosis in patients with LN may be beneficial, probably in combination with immunosuppression.
IL-21
IL-21 is a pro-inflammatory cytokine that plays a variety of roles in the activation of NK cells, DCs, T cells and B cells, where it contributes to the generation of (auto)antibody-secreting plasma cells [136]. There are increased IL-21-producing peripheral CD4+ T cells in SLE patients, correlating with a concurrent increase in memory B cells and Th17 cells and reduced Treg cells [137]. Genetic variants in the IL-21 gene are noted to be associated with SLE [138, 139]. A recent clinical trial of an IL-21 inhibitor, NNC0114-0006, has been terminated according to ClinicalTrials.Gov. Although there has been interest in the inhibition of IL-21 in SLE, the pleotropic effects of this cytokine might result in detrimental immunosuppressive and immunostimulatory effects.
IL-17
IL-17 is a pro-inflammatory cytokine produced by Th cells, γδT cells and Th17 cells [140]. IL-17-secreting CD4+ effector T cells are higher in SLE patients, and plasma IL-17 levels are correlated with disease activity in non-renal SLE patients [116, 126, 141]. IL-17 is significantly higher in pre-flare SLE patients than in non-flare SLE patients [105]. In LN, IL-17 was correlated with proteinuria and dsDNA and was significantly higher in active disease compared with remission [142]. Furthermore, renal glomerular IL-17 expression was elevated in diffuse proliferative disease compared with healthy kidneys, correlating with the histological activity index [127]. Secukinumab, brodalumab and ixekizumab block IL-17, but currently no SLE studies have been undertaken. Testing of these agents in PsA, RA and AS is ongoing, with recent FDA approval of secukinumab for psoriasis.
Other serum biomarkers discovered in proteomic screens
In addition to targeted studies, comprehensive screens of protein panels have uncovered potential disease biomarkers. Increased plasma levels of pro-inflammatory soluble mediators were found using a large panel in pre-flare SLE patients compared with non-flare patients, whereas regulatory mediators were increased during quiescent periods [105]. Axl, Fas, ferritin, intercellular adhesion molecule 1, insulin-like growth factor-binding protein 2 (IGFBP-2), sialic acid-binding Ig-like lectin 5 (Siglec-5) and sTNFRII were found to be promising SLE serum biomarkers from a 274-mediator panel [143]. The higher ferritin levels were confirmed in active compared with inactive SLE patients; a further correlation was identified with the histopathological response in LN patients [144, 145]. Furthermore, assessment of IGFBP-2 showed higher levels in LN, with a correlation with disease activity and clinical and histopathological response [146, 147]. Sera from 94 Chinese SLE patients and 49 HCs evaluated for Axl, sTNFRII, ferritin and IGFBP-2 by ELISA showed the highest levels in active SLE, followed by inactive SLE, then HCs; furthermore, all four biomarkers were correlated with disease activity [148]. Analysis of IGFBP-4, a biomarker in diabetic nephropathy, revealed increased levels in LN patients compared with non-lupus CKD and HCs [149]. These protein biomarkers initially discovered from proteomic screens may prove to be clinically informative in SLE and LN. However, some of these biomarkers are elevated in non-specific inflammation, cellular damage or fibrosis. Therefore, additional studies are warranted for further validation.
The traditional serum biomarkers discussed were anti-dsDNA and complement; non-traditional biomarkers discussed were IFNα, BLyS and APRIL. Assessment of the emerging SLE biomarkers found IL-17 and IL-23 to be the most promising prognostic biomarkers. This conclusion is based on the recentness of the biomarkers, the MI and literature strength scores (high despite their youth) and the available studies (e.g. cross-sectional, longitudinal, gene expression).
Urine biomarkers in LN
LN remains a major cause of morbidity and mortality for SLE patients. Urine is routinely evaluated for protein, red blood cells, white blood cells and cellular casts to screen for LN, guide treatment response and assess renal flare. Complete diagnosis of LN relies upon a kidney biopsy to determine classification and guide therapy. The invasiveness of biopsy encouraged investigators to seek serum and urine biomarkers with the ability to predict histopathology, renal flare and treatment response. Urine, easily collected by patients, may allow better assessment of the kidney microenvironment than peripheral blood.
TNF-like weak inducer of apoptosis (TWEAK) is a member of the TNF family capable of inducing IL-8 secretion, apoptosis and cell differentiation [150]. A multicentre longitudinal study found that uTWEAK was correlated with disease activity over time and was not elevated in other autoimmune disease and renal disease control groups [151]. Furthermore, TWEAK was found to activate TGF-β in kidney proximal tubule cells [152]. However, a pharmaceutical targeting TWEAK (BBIIB023) did not result in significant improvement in an LN proof-of-concept study [153]; therefore, development was recently terminated by Biogen (NCT01499355).
MCP-1/CCL2 recruits monocytes, memory T lymphocytes and NK cells to inflammatory sites. sMCP-1 and uMCP-1 were higher in LN patients compared with patients without LN and HCs, and MCP-1 was detected in the kidneys of LN patients [154, 155]. Longitudinal assessment of uMCP-1 found that levels increase a few months before renal flare and are correlated with urine protein and treatment response [156]. The anti-MCP-1, ABN912, showed no benefit in RA in a randomized, placebo-controlled trial [157]. It does not appear that additional studies in SLE or LN are planned at this time.
Kidney injury and inflammation results in increased secretion of neutrophil gelatinase-associated lipocalcin from epithelial cells and leucocytes. Levels are higher in SLE patients with LN than in SLE patients without LN and controls, and are correlated with disease activity [155, 158]. Urinary neutrophil gelatinase-associated lipocalcin levels on a preceding visit were correlated with renal flare, with an adjusted odds ratio of 1.7 [159].
Vascular cell adhesion molecule-1 is important for immune cell recruitment into tissues. SLE patients have increased levels compared with HCs, which are correlated with SLE disease activity [160]. Furthermore, patients with active renal disease have higher levels compared with non-renal and inactive SLE [143, 161].
Angiostatin, a proteolytic fragment of plasminogen, inhibits endothelial proliferation and angiogenesis. An initial array-based screen found urinary angiostatin to be higher in LN. A validation study of 100 SLE patients (∼80% with renal activity), 24 CKD patients and 21 HCs noted higher levels in SLE patients compared with HCs, but did not discriminate between SLE and CKD [162]. Additional mechanistic studies in human umbilical vein endothelial cells noted that angiostatin inhibited IL-1β-induced down-regulation of endothelial nitric oxide synthase through the nuclear factor-κB pathway [163]. Urinary angiostatin may be a non-specific marker for kidney damage and may be less likely to translate into beneficial therapeutics. However, urinary angiostatin could potentially serve as a biomarker to differentiate between active disease and prior damage in chronic LN patients.
Assessment of these and other urine biomarkers in clinical care may potentially predict development of LN, predict renal flares in those known to have LN and assist in determining therapeutic response and predicting histopathology.
Conclusion
The events leading to the development and continuation of SLE pathogenesis remain poorly understood. Patients continue to have active inflammation despite currently available treatments. Although the relationship between immune cells and biomarkers is complex, a simplified summary of the discussed blood biomarkers is included (Fig. 2), with a summary table of current therapeutics targeting the discussed biomarkers (Table 2). Combining currently available biomarkers, physical examination and patient history may allow better assessment of disease activity. The continuing growth in -omics technologies will continue to fuel the discovery of novel biomarker candidates, which will potentially guide therapeutic development and assist in assessment of disease activity. Assessment of biomarkers will be likely to provide clues to therapeutic regimen selection in the future. Here, we have summarized bedside to bench to bedside use of biomarkers to design novel therapeutics that will hopefully result in improved patient outcomes in SLE.
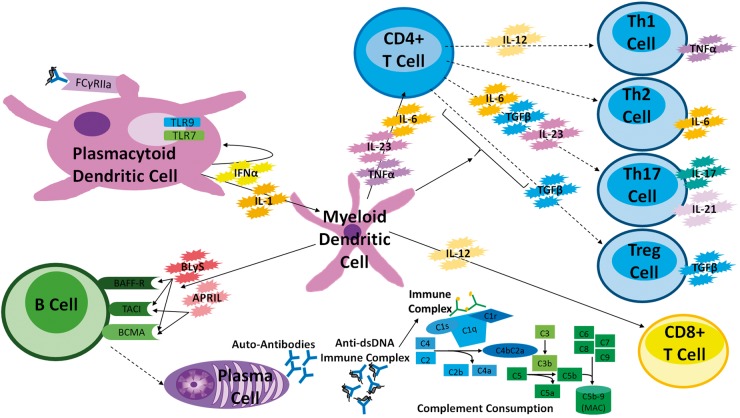
Fig. 2.
A schematic diagram of potential SLE biomarkers
Key biomarkers are included with examples of immune cells that produce them. Continuous lines indicate cytokines acting on a particular immune cell, whereas dashed lines indicate the differentiation of the CD4+ T cell into various subclasses of T cells. A plasmacytoid dendritic cell (pDC) binds an anti-dsDNA immune complex to the FCγRIIa receptor, also shown with an endosome containing Toll-like receptors (TLR) 7 and 9 that can bind DNA, RNA or immune complexes, inducing production of IFNα. Myeloid dendritic cells can produce BLyS and APRIL, which bind BAFF-R, TACI and BCMA or TACI and BCMA, respectively. The B cell differentiates into plasma cells, which produce antibodies, including autoantibodies. Anti-dsDNA binds to dsDNA, forming immune complexes, which bind and activate the complement. Immune complexes are also deposited in various organs and tissues; for example, kidneys. The myeloid dendritic cells also produce IL-6, IL-23 and TNFα, which can activate CD4+ T cells that then differentiate into T cell subclasses, dendritic cells and other cell types. IL-4 (data not shown) results in the differentiation of Th2 cells, which produce IL-6. APRIL: a proliferation-inducing ligand; BlyS: B lymphocyte stimulator; MCP-1: monocyte chemotactic protein 1; TWEAK: TNF-like weak inducer of apoptosis.
Table 2.
Summary of therapeutics targeting biomarkers
| Therapeutic | Target | ClinicalTrials.Gov ID | Highest trial phase |
|---|---|---|---|
| Edratide | Heavy chain of anti-dsDNA | NCT00203151 | Phase II |
| Eculizumab | C5 | Phase II | |
| Rontalizumab | IFNα | NCT00541749, NCT00962832 | Phase II |
| Sifalimumab | IFNα | NCT00299819, NCT00482989, NCT00657189, NCT00979654, NCT01031836, NCT01283139 | Phase II |
| Anifrolumab | Type I IFN receptor | NCT01438489, NCT01559090, NCT01753193, NCT02446912, NCT02446899, NCT02547922 | Phase III |
| Belimumab | Soluble BLyS | NCT00071487, NCT00724867, NCT00410384, NCT00424476, NCT00583362, NCT00657007, NCT00712933, NCT00732940, NCT01345253, NCT01484496, NCT01516450, NCT01597492, NCT01597622, NCT01632241, NCT01639339, NCT01649765, NCT01705977, NCT01729455, NCT01858792, NCT01914770, NCT02119156, NCT02260934, NCT02270970, NCT02284984 | Phase III |
| Blisibimod | Soluble and membrane-bound BLyS | NCT01162681, NCT01305746, NCT01395745, NCT02514967 | Phase III |
| Atacicept | TACI receptor (BLyS and APRIL) | NCT00573157, NCT00624338, NCT01369628, NCT01440231, NCT01972568, NCT02070978 | Phase II |
| Infliximab | TNFα | NCT00368264 | Phase III |
| Tocilizumab | IL-6 receptor | NCT00046774 | Phase I |
| Sirukumab | IL-6 | NCT01273389, NCT01702740 | Phase II |
| Ustekinumab | IL-12 and IL-23 | NCT02349061 | Phase II |
| Anakinra | IL-1 | ||
| Fresolimumab | TGF-β | ||
| Pirfenidone | Unknown antifibrotic, reduces TGF-β | ||
| NNC0114-0006 | IL-21 | NCT01689025 | Phase I |
| Secukinumab | IL-17 | ||
| Brodalumab | IL-17 | ||
| Ixekizumab | IL-17 | ||
| ABN912 | MCP-1/CCL-2 | ||
| BB11B023 | TWEAK | NCT01499355, NCT01930890 | Phase II |
The table includes names of therapeutics, the target of the drug and available clinical trial identification numbers through ClinicalTrials.gov, and the highest phase of study included. APRIL: a proliferation-inducing ligand; BlyS: B lymphocyte stimulator; MCP-1: monocyte chemoattractant protein 1; TWEAK: TNF-like weak inducer of apoptosis.
Acknowledgements
The authors would like to thank Anna-Marie Fairhurst for editorial assistance. All authors assisted in critical review and editing of the manuscript. Additionally, C.A. drafted and completed the manuscript, J.D.W. completed literature mining and contributed to figure and table preparation, M.E.M. contributed additional intellectual content to the manuscript and figures, and C.M. directed the content of the manuscript and contributed additional intellectual material to the manuscript, figures and tables.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: C.A. received support or services from the following while researching, drafting and completing this manuscript: Questcor/Mallinckrodt Pharmaceuticals Research Fellowship Award Program, US Department of Health and Human Services, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases T32 DK007257-32, US Department of Health and Human Services, National Institutes of Health, National Center for Advancing Translational Sciences UL1TR001105 (UTSW CTSA), US Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences U54 GM104938-02 (OU OSCTR). M.E.M. and J.D.W. are partially supported by US Department of Health and Human Services/National Institutes of Health/National Center for Research Resources P20 GM103636-02 and US Department of Health and Human Services/National Institutes of Health/National Institute of Allergy and Infectious Diseases U19 AI082714-07. The other author has declared no conflicts of interest.
References
- 1. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 2. Arriens C, Mohan C.. Systemic lupus erythematosus diagnostics in the ‘omics’ era. Int J Clin Rheumatol 2013;8:671–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wren JD. Extending the mutual information measure to rank inferred literature relationships. BMC Bioinformatics 2004;5:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wren JD, Bekeredjian R, Stewart JA, Shohet RV, Garner HR.. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics 2004;20:389–98. [DOI] [PubMed] [Google Scholar]
- 5. Wren JD, Garner HR.. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics 2004;20:191–8. [DOI] [PubMed] [Google Scholar]
- 6. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH.. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 7. Petri M, Kim MY, Kalunian KC. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 8. Gladman DD, Ibañez D, Urowitz MB.. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 9. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 11. Petri M, Orbai AM, Alarcón GS. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attar SM, Koshak EA.. Medical conditions associated with a positive anti-double-stranded deoxyribonucleic acid. Saudi Med J 2010;31:781–7. [PubMed] [Google Scholar]
- 13. Lv S, Zhang J, Wu J. et al. Origin and anti-tumor effects of anti-dsDNA autoantibodies in cancer patients and tumor-bearing mice. Immunol Lett 2005;99:217–27. [DOI] [PubMed] [Google Scholar]
- 14. Ruffatti A, Calligaro A, Del Ross T. et al. Anti-double-stranded DNA antibodies in the healthy elderly: prevalence and characteristics. J Clin Immunol 1990;10:300–3. [DOI] [PubMed] [Google Scholar]
- 15. Ceppellini R, Polli E, Celada F.. A DNA-reacting factor in serum of a patient with lupus erythematosus diffusus. Proc Soc Exp Biol Med 1957;96:572–4. [DOI] [PubMed] [Google Scholar]
- 16. Robbins WC, Holman HR, Deicher H, Kunkel HG.. Complement fixation with cell nuclei and DNA in lupus erythematosus. Proc Soc Exp Biol Med 1957;96:575–9. [DOI] [PubMed] [Google Scholar]
- 17. Seligman VA, Lum RF, Olson JL, Li H, Criswell LA.. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med 2002;112:726–9. [DOI] [PubMed] [Google Scholar]
- 18. Arbuckle MR, James JA, Kohlhase KF. et al. Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand J Immunol 2001;54:211–9. [DOI] [PubMed] [Google Scholar]
- 19. Arbuckle MR, McClain MT, Rubertone MV. et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 20. Munroe ME, Lu R, Zhao YD. et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis 2016;75:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG.. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum 1990;33:634–43. [DOI] [PubMed] [Google Scholar]
- 22. Casals SP, Friou GJ, Myers LL.. Significance of antibody to DNA in systemic lupus erythematosus. Arthritis Rheum 1964;7:379–90. [DOI] [PubMed] [Google Scholar]
- 23. Davis P, Percy JS, Russell AS.. Correlation between levels of DNA antibodies and clinical disease activity in SLE. Ann Rheum Dis 1977;36:157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Swaak AJ, Aarden LA, Statius van Eps LW, Feltkamp TE.. Anti-dsDNA and complement profiles as prognostic guides in systemic lupus erythematosus. Arthritis Rheum 1979;22:226–35. [DOI] [PubMed] [Google Scholar]
- 25. Petri M, Singh S, Tesfasyone H, Malik A.. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009;36:2476–80. [DOI] [PubMed] [Google Scholar]
- 26. Petri MA, van Vollenhoven RF, Buyon J. et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013;65:2143–53. [DOI] [PubMed] [Google Scholar]
- 27. van Vollenhoven RF, Petri MA, Cervera R. et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis 2012;71:1343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stohl W, Hiepe F, Latinis KM. et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012;64:2328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tseng CE, Buyon JP, Kim M. et al. The effect of moderate-dose corticosteroids in preventing severe flares in patients with serologically active, but clinically stable, systemic lupus erythematosus: findings of a prospective, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:3623–32. [DOI] [PubMed] [Google Scholar]
- 30. Steiman AJ, Urowitz MB, Ibañez D. et al. Anti-dsDNA and antichromatin antibody isotypes in serologically active clinically quiescent systemic lupus erythematosus. J Rheumatol 2015;42:810–6. [DOI] [PubMed] [Google Scholar]
- 31. Bastian HM, Roseman JM, McGwin G Jr. et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus 2002;11:152–60. [DOI] [PubMed] [Google Scholar]
- 32. Alba P, Bento L, Cuadrado MJ. et al. Anti-dsDNA, anti-Sm antibodies, and the lupus anticoagulant: significant factors associated with lupus nephritis. Ann Rheum Dis 2003;62:556–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Förger F, Matthias T, Oppermann M, Becker H, Helmke K.. Clinical significance of anti-dsDNA antibody isotypes: IgG/IgM ratio of anti-dsDNA antibodies as a prognostic marker for lupus nephritis. Lupus 2004;13:36–44. [DOI] [PubMed] [Google Scholar]
- 34. Font J, Cervera R, Ramos-Casals M. et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum 2004;33:217–30. [DOI] [PubMed] [Google Scholar]
- 35. Yung S, Chan TM.. Mechanisms of kidney injury in lupus nephritis – the role of anti-dsDNA antibodies. Front Immunol 2015;6:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cortés-Hernández J, Ordi-Ros J, Labrador M. et al. Antihistone and anti-double-stranded deoxyribonucleic acid antibodies are associated with renal disease in systemic lupus erythematosus. Am J Med 2004;116:165–73. [DOI] [PubMed] [Google Scholar]
- 37. Olson SW, Lee JJ, Prince LK. et al. Elevated subclinical double-stranded DNA antibodies and future proliferative lupus nephritis. Clin J Am Soc Nephrol 2013;8:1702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leandro MJ, Cambridge G, Edwards JC, Ehrenstein MR, Isenberg DA.. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology 2005;44:1542–5. [DOI] [PubMed] [Google Scholar]
- 39. Rovin BH, Furie R, Latinis K. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012;64:1215–26. [DOI] [PubMed] [Google Scholar]
- 40. Urowitz MB, Isenberg DA, Wallace DJ.. Safety and efficacy of hCDR1 (Edratide) in patients with active systemic lupus erythematosus: results of phase II study. Lupus Sci Med 2015;2:e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hebert LA, Cosio FG, Neff JC.. Diagnostic significance of hypocomplementemia. Kidney Int 1991;39:811–21. [DOI] [PubMed] [Google Scholar]
- 42. Elliott JA Jr, Mathieson DR.. Complement in disseminated (systemic) lupus erythematosus. AMA Arch Derm Syphilol 1953;68:119–28. [DOI] [PubMed] [Google Scholar]
- 43. Paronetto F, Koffler D.. Immunofluorescent localization of immunoglobulins, complement, and fibrinogen in human diseases. I. Systemic lupus erythematosus. J Clin Invest 1965;44:1657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korb LC, Ahearn JM.. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol 1997;158:4525–8. [PubMed] [Google Scholar]
- 45. Nauta AJ, Trouw LA, Daha MR. et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol 2002;32:1726–36. [DOI] [PubMed] [Google Scholar]
- 46. Swaak AJ, Groenwold J, Bronsveld W.. Predictive value of complement profiles and anti-dsDNA in systemic lupus erythematosus. Ann Rheum Dis 1986;45:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bryan AR, Wu EY.. Complement deficiencies in systemic lupus erythematosus. Curr Allergy Asthma Rep 2014;14:448. [DOI] [PubMed] [Google Scholar]
- 48. Botto M, Dell’Agnola C, Bygrave AE. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet 1998;19:56–9. [DOI] [PubMed] [Google Scholar]
- 49. Taylor PR, Carugati A, Fadok VA. et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med 2000;192:359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nauta AJ, Castellano G, Xu W. et al. Opsonization with C1q and mannose-binding lectin targets apoptotic cells to dendritic cells. J Immunol 2004;173:3044–50. [DOI] [PubMed] [Google Scholar]
- 51. Son M, Diamond B, Santiago-Schwarz F.. Fundamental role of C1q in autoimmunity and inflammation. Immunol Res 2015;63:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Coremans IE, Spronk PE, Bootsma H. et al. Changes in antibodies to C1q predict renal relapses in systemic lupus erythematosus. Am J Kidney Dis 1995;26:595–601. [DOI] [PubMed] [Google Scholar]
- 53. Moroni G, Trendelenburg M, Del Papa N. et al. Anti-C1q antibodies may help in diagnosing a renal flare in lupus nephritis. Am J Kidney Dis 2001;37:490–8. [DOI] [PubMed] [Google Scholar]
- 54. Putterman C, Furie R, Ramsey-Goldman R. et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med 2014;1:e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barilla-Labarca ML, Toder K, Furie R.. Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol 2013;148:313–21. [DOI] [PubMed] [Google Scholar]
- 56. Coppo R, Peruzzi L, Amore A. et al. Dramatic effects of eculizumab in a child with diffuse proliferative lupus nephritis resistant to conventional therapy. Pediatr Nephrol 2015;30:167–72. [DOI] [PubMed] [Google Scholar]
- 57. El-Husseini A, Hannan S, Awad A. et al. Thrombotic microangiopathy in systemic lupus erythematosus: efficacy of eculizumab. Am J Kidney Dis 2015;65:127–30. [DOI] [PubMed] [Google Scholar]
- 58. Bakhtar O, Thajudeen B, Braunhut BL. et al. A case of thrombotic microangiopathy associated with antiphospholipid antibody syndrome successfully treated with eculizumab. Transplantation 2014;98:e17–8. [DOI] [PubMed] [Google Scholar]
- 59. Kronbichler A, Frank R, Kirschfink M. et al. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine 2014;93:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zikos TA, Sokolove J, Ahuja N, Berube C.. Eculizumab induces sustained remission in a patient with refractory primary catastrophic antiphospholipid syndrome. J Clin Rheumatol 2015;21:311–3. [DOI] [PubMed] [Google Scholar]
- 61. Ytterberg SR, Schnitzer TJ.. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum 1982;25:401–6. [DOI] [PubMed] [Google Scholar]
- 62. Hooks JJ, Moutsopoulos HM, Geis SA. et al. Immune interferon in the circulation of patients with autoimmune disease. New Engl J Med 1979;301:5–8. [DOI] [PubMed] [Google Scholar]
- 63. Kim T, Kanayama Y, Negoro N. et al. Serum levels of interferons in patients with systemic lupus erythematosus. Clin Exp Immunol 1987;70:562–9. [PMC free article] [PubMed] [Google Scholar]
- 64. Preble OT, Black RJ, Friedman RM, Klippel JH, Vilcek J.. Systemic lupus erythematosus: presence in human serum of an unusual acid-labile leukocyte interferon. Science 1982;216:429–31. [DOI] [PubMed] [Google Scholar]
- 65. Baechler EC, Batliwalla FM, Karypis G. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA 2003;100:2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirou KA, Lee C, George S. et al. Coordinate overexpression of interferon-α-induced genes in systemic lupus erythematosus. Arthritis Rheum 2004;50:3958–67. [DOI] [PubMed] [Google Scholar]
- 67. Bennett L, Palucka AK, Arce E. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Feng X, Wu H, Grossman JM. et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:2951–62. [DOI] [PubMed] [Google Scholar]
- 69. Petri M, Singh S, Tesfasyone H. et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus 2009;18:980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Graham RR, Kozyrev SV, Baechler EC. et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet 2006;38:550–5. [DOI] [PubMed] [Google Scholar]
- 71. Sigurdsson S, Nordmark G, Göring HH. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Human Genet 2005;76:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN), Harley JB, Alarcón-Riquelme ME. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 2008;40:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Han JW, Zheng HF, Cui Y. et al. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 2009;41:1234–7. [DOI] [PubMed] [Google Scholar]
- 74. Niewold TB, Kelly JA, Flesch MH. et al. Association of the IRF5 risk haplotype with high serum interferon-α activity in systemic lupus erythematosus patients. Arthritis Rheum 2008;58:2481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Okanoue T, Sakamoto S, Itoh Y. et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol 1996;25:283–91. [DOI] [PubMed] [Google Scholar]
- 76. Rönnblom LE, Alm GV, Öberg KE.. Possible induction of systemic lupus erythematosus by interferon-α treatment in a patient with a malignant carcinoid tumour. J Int Med 1990;227:207–10. [DOI] [PubMed] [Google Scholar]
- 77. Schilling PJ, Kurzrock R, Kantarjian H, Gutterman JU, Talpaz M.. Development of systemic lupus erythematosus after interferon therapy for chronic myelogenous leukemia. Cancer 1991;68:1536–7. [DOI] [PubMed] [Google Scholar]
- 78. Gota C, Calabrese L.. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity 2003;36:511–8. [DOI] [PubMed] [Google Scholar]
- 79. Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J.. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 2001;294:1540–3. [DOI] [PubMed] [Google Scholar]
- 80. Barrat FJ, Meeker T, Gregorio J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 2005;202:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL.. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol 2001;159:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Blomberg S, Eloranta ML, Cederblad B. et al. Presence of cutaneous interferon-alpha producing cells in patients with systemic lupus erythematosus. Lupus 2001;10:484–90. [DOI] [PubMed] [Google Scholar]
- 83. Dall’era MC, Cardarelli PM, Preston BT, Witte A, Davis JC Jr.. Type I interferon correlates with serological and clinical manifestations of SLE. Ann Rheum Dis 2005;64:1692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Weckerle CE, Franek BS, Kelly JA. et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum 2011;63:1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ko K, Koldobskaya Y, Rosenzweig E, Niewold TB.. Activation of the interferon pathway is dependent upon autoantibodies in African-American SLE patients, but not in European-American SLE patients. Front Immunol 2013;4:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Crow MK. Type I interferon in the pathogenesis of lupus. J Immunol 2014;192:5459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kalunian KC, Merrill JT, Maciuca R. et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis 2016;75:196–202. [DOI] [PubMed] [Google Scholar]
- 88. Yao Y, Richman L, Higgs BW. et al. Neutralization of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-interferon-α monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum 2009;60:1785–96. [DOI] [PubMed] [Google Scholar]
- 89. Petri M, Wallace DJ, Spindler A. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum 2013;65:1011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. 2015 ACR/ARHP Annual Meeting Abstract Supplement. Arthritis Rheumatol 2015;67 (Suppl 10):1–4046. [DOI] [PubMed] [Google Scholar]
- 91. Stohl W. Biologic differences between various inhibitors of the BLyS/BAFF pathway: should we expect differences between belimumab and other inhibitors in development? Curr Rheumatol Rep 2012;14:303–9. [DOI] [PubMed] [Google Scholar]
- 92. Cheema GS, Roschke V, Hilbert DM, Stohl W.. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum 2001;44:1313–9. [DOI] [PubMed] [Google Scholar]
- 93. Zhang J, Roschke V, Baker KP. et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol 2001;166:6–10. [DOI] [PubMed] [Google Scholar]
- 94. Stohl W, Metyas S, Tan SM. et al. B lymphocyte stimulator overexpression in patients with systemic lupus erythematosus: longitudinal observations. Arthritis Rheum 2003;48:3475–86. [DOI] [PubMed] [Google Scholar]
- 95. Petri M, Stohl W, Chatham W. et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum 2008;58:2453–9. [DOI] [PubMed] [Google Scholar]
- 96. Ritterhouse LL, Crowe SR, Niewold TB. et al. B lymphocyte stimulator levels in systemic lupus erythematosus: higher circulating levels in African American patients and increased production after influenza vaccination in patients with low baseline levels. Arthritis Rheum 2011;63:3931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Becker-Merok A, Nikolaisen C, Nossent HC.. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus 2006;15:570–6. [DOI] [PubMed] [Google Scholar]
- 98. Wallace DJ, Stohl W, Furie RA. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum 2009;61:1168–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Navarra SV, Guzmán RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:721–31. [DOI] [PubMed] [Google Scholar]
- 100. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011;63:3918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stohl W, Merrill JT, Looney RJ. et al. Treatment of systemic lupus erythematosus patients with the BAFF antagonist “peptibody” blisibimod (AMG 623/A-623): results from randomized, double-blind phase 1a and phase 1b trials. Arthritis Res Ther 2015;17:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Furie RA, Leon G, Thomas M. et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis 2015;74:1667–75. [DOI] [PubMed] [Google Scholar]
- 103. Stohl W, Metyas S, Tan SM. et al. Inverse association between circulating APRIL levels and serological and clinical disease activity in patients with systemic lupus erythematosus. Ann Rheum Dis 2004;63:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morel J, Roubille C, Planelles L. et al. Serum levels of tumour necrosis factor family members a proliferation-inducing ligand (APRIL) and B lymphocyte stimulator (BLyS) are inversely correlated in systemic lupus erythematosus. Ann Rheum Dis 2009;68:997–1002. [DOI] [PubMed] [Google Scholar]
- 105. Munroe ME, Vista ES, Guthridge JM. et al. Proinflammatory adaptive cytokine and shed tumor necrosis factor receptor levels are elevated preceding systemic lupus erythematosus disease flare. Arthritis Rheumatol 2014;66:1888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Isenberg D, Gordon C, Licu D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 2015;74:2006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Scheller J, Chalaris A, Garbers C, Rose-John S.. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol 2011;32:380–7. [DOI] [PubMed] [Google Scholar]
- 108. Richter C, Messerschmidt S, Holeiter G. et al. The tumor necrosis factor receptor stalk regions define responsiveness to soluble versus membrane-bound ligand. Mol Cell Biol 2012;32:2515–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gabay C, Cakir N, Moral F. et al. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol 1997;24:303–8. [PubMed] [Google Scholar]
- 110. Studnicka-Benke A, Steiner G, Petera P, Smolen JS.. Tumour necrosis factor alpha and its soluble receptors parallel clinical disease and autoimmune activity in systemic lupus erythematosus. Br J Rheumatol 1996;35:1067–74. [DOI] [PubMed] [Google Scholar]
- 111. Shakoor N, Michalska M, Harris CA, Block JA.. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet 2002;359:579–80. [DOI] [PubMed] [Google Scholar]
- 112. Aringer M, Graninger WB, Steiner G, Smolen JS.. Safety and efficacy of tumor necrosis factor α blockade in systemic lupus erythematosus: an open-label study. Arthritis Rheum 2004;50:3161–9. [DOI] [PubMed] [Google Scholar]
- 113. Uppal SS, Hayat SJ, Raghupathy R.. Efficacy and safety of infliximab in active SLE: a pilot study. Lupus 2009;18:690–7. [DOI] [PubMed] [Google Scholar]
- 114. Linker-Israeli M, Deans RJ, Wallace DJ. et al. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol 1991;147:117–23. [PubMed] [Google Scholar]
- 115. Chun HY, Chung JW, Kim HA. et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007;27:461–6. [DOI] [PubMed] [Google Scholar]
- 116. Talaat RM, Mohamed SF, Bassyouni IH, Raouf AA.. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: correlation with disease activity. Cytokine 2015;72:146–53. [DOI] [PubMed] [Google Scholar]
- 117. Illei GG, Shirota Y, Yarboro CH. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum 2010;62:542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tokano Y, Morimoto S, Kaneko H. et al. Levels of IL-12 in the sera of patients with systemic lupus erythematosus (SLE)—relation to Th1- and Th2-derived cytokines. Clin Exp Immunol 1999;116:169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tucci M, Lombardi L, Richards HB, Dammacco F, Silvestris F.. Overexpression of interleukin-12 and T helper 1 predominance in lupus nephritis. Clin Exp Immunol 2008;154:247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Qiu F, Song L, Yang N, Li X.. Glucocorticoid downregulates expression of IL-12 family cytokines in systemic lupus erythematosus patients. Lupus 2013;22:1011–6. [DOI] [PubMed] [Google Scholar]
- 121. Koenig KF, Groeschl I, Pesickova SS. et al. Serum cytokine profile in patients with active lupus nephritis. Cytokine 2012;60:410–6. [DOI] [PubMed] [Google Scholar]
- 122. Ling P, Gately MK, Gubler U. et al. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol 1995;154:116–27. [PubMed] [Google Scholar]
- 123. Lauwerys BR, Van Snick J, Houssiau FA.. Serum IL-12 in systemic lupus erythematosus: absence of p70 heterodimers but presence of p40 monomers correlating with disease activity. Lupus 2002;11:384–7. [DOI] [PubMed] [Google Scholar]
- 124. Leonardi CL, Kimball AB, Papp KA. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–74. [DOI] [PubMed] [Google Scholar]
- 125. Papp KA, Langley RG, Lebwohl M. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet 2008;371:1675–84. [DOI] [PubMed] [Google Scholar]
- 126. Wong CK, Lit LC, Tam LS. et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol 2008;127:385–93. [DOI] [PubMed] [Google Scholar]
- 127. Chen DY, Chen YM, Wen MC. et al. The potential role of Th17 cells and Th17-related cytokines in the pathogenesis of lupus nephritis. Lupus 2012;21:1385–96. [DOI] [PubMed] [Google Scholar]
- 128. Blakemore AI, Tarlow JK, Cork MJ. et al. Interleukin-1 receptor antagonist gene polymorphism as a disease severity factor in systemic lupus erythematosus. Arthritis Rheum 1994;37:1380–5. [DOI] [PubMed] [Google Scholar]
- 129. Jacob CO, Zhu J, Armstrong DL. et al. Identification of IRAK1 as a risk gene with critical role in the pathogenesis of systemic lupus erythematosus. Proc Natl Acad Sci USA 2009;106:6256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alarcón-Riquelme ME, Ziegler JT, Molineros J. et al. Genome-wide association study in an amerindian ancestry population reveals novel systemic lupus erythematosus risk loci and the role of European admixture. Arthritis Rheumatol 2016;68:932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cigni A, Pileri PV, Faedda R. et al. Interleukin 1, interleukin 6, interleukin 10, and tumor necrosis factor α in active and quiescent systemic lupus erythematosus. J Investig Med 2014;62:825–9. [DOI] [PubMed] [Google Scholar]
- 132. Ostendorf B, Iking-Konert C, Kurz K. et al. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis 2005;64:630–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kohut E, Hajdu M, Gergely P. et al. Expression of TGFβ1 and its signaling components by peripheral lymphocytes in systemic lupus erythematosus. Pathol Oncol Res 2009;15:251–6. [DOI] [PubMed] [Google Scholar]
- 134. Ostergaard O, Nielsen CT, Iversen LV. et al. Unique protein signature of circulating microparticles in systemic lupus erythematosus. Arthritis Rheum 2013;65:2680–90. [DOI] [PubMed] [Google Scholar]
- 135. Avihingsanon Y, Phumesin P, Benjachat T. et al. Measurement of urinary chemokine and growth factor messenger RNAs: a noninvasive monitoring in lupus nephritis. Kidney Int 2006;69:747–53. [DOI] [PubMed] [Google Scholar]
- 136. Nakou M, Papadimitraki ED, Fanouriakis A. et al. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin Exp Rheumatol 2013;31:172–9. [PubMed] [Google Scholar]
- 137. Terrier B, Costedoat-Chalumeau N, Garrido M. et al. Interleukin 21 correlates with T cell and B cell subset alterations in systemic lupus erythematosus. J Rheumatol 2012;39:1819–28. [DOI] [PubMed] [Google Scholar]
- 138. Sawalha AH, Kaufman KM, Kelly JA. et al. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis 2008;67:458–61. [DOI] [PubMed] [Google Scholar]
- 139. Qi JH, Qi J, Xiang LN, Nie G.. Association between IL-21 polymorphism and systemic lupus erythematosus: a meta-analysis. Genet Mol Res 2015;14:9595–603. [DOI] [PubMed] [Google Scholar]
- 140. Jin W, Dong C.. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect 2013;2:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wong CK, Ho CY, Li EK, Lam CW.. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000;9:589–93. [DOI] [PubMed] [Google Scholar]
- 142. Abdel Galil SM, Ezzeldin N, El-Boshy ME.. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine 2015;76:280–7. [DOI] [PubMed] [Google Scholar]
- 143. Wu T, Ding H, Han J. et al. Antibody array based proteomic screening of serum markers in systemic lupus erythematosus: a discovery study. Proteomics 2016;15:2102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Vanarsa K, Ye Y, Han J. et al. Inflammation associated anemia and ferritin as disease markers in SLE. Arthritis Res Therap 2012;14:R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Parodis I, Ding H, Zickert A. et al. Serum ferritin as a biomarker of clinical and histopathological response to treatment in lupus nephritis. Ann Rheum Dis - Eur League Against Rheum 2016;75(2):1063. [Google Scholar]
- 146. Ding H, Kharboutli M, Saxena R, Wu T.. Insulin-like growth factor binding protein-2 as a novel biomarker for disease activity and renal pathology changes in lupus nephritis. Clin Exp Immunol 2016;184:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Parodis I, Ding H, Zickert A. et al. Serum insulin-like growth factor-binding protein 2 (IGFBP2) as a biomarker of clinical and histopathological treatment response in lupus nephritis. Ann Rheum Dis - European League Against Rheumatism 2016;75(2):547. [Google Scholar]
- 148. Mok CC, Ding HH, Kharboutli M, Mohan C.. Axl, ferritin, insulin-like growth factor binding protein 2 and tumor necrosis factor receptor type II as biomarkers in systemic lupus erythematosus. Arthritis Care Res 2016;68:1303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wu T, Xie C, Han J. et al. Insulin-like growth factor binding protein-4 as a marker of chronic lupus nephritis. PLoS One 2016;11:e0151491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chicheportiche Y, Bourdon PR, Xu H. et al. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 1997;272:32401–10. [DOI] [PubMed] [Google Scholar]
- 151. Schwartz N, Rubinstein T, Burkly LC. et al. Urinary TWEAK as a biomarker of lupus nephritis: a multicenter cohort study. Arthritis Res Ther 2009;11:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Liu Z, Xue L, Liu Z. et al. Tumor necrosis factor-like weak inducer of apoptosis accelerates the progression of renal fibrosis in lupus nephritis by activating SMAD and p38 MAPK in TGF-β1 signaling pathway. Mediators Inflamm 2016;2016:8986451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Galluppi GR, Wisniacki N, Stebbins C.. Population pharmacokinetic and pharmacodynamic analysis of BIIB023, an anti-TNF-like weak inducer of apoptosis (anti-TWEAK) monoclonal antibody. Br J Clin Pharmacol 2016;82:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wada T, Yokoyama H, Su SB. et al. Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int 1996;49:761–7. [DOI] [PubMed] [Google Scholar]
- 155. Susianti H, Iriane VM, Dharmanata S. et al. Analysis of urinary TGF-β1, MCP-1, NGAL, and IL-17 as biomarkers for lupus nephritis. Pathophysiology 2015;22:65–71. [DOI] [PubMed] [Google Scholar]
- 156. Rovin BH, Song H, Birmingham DJ. et al. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol 2005;16:467–73. [DOI] [PubMed] [Google Scholar]
- 157. Haringman JJ, Gerlag DM, Smeets TJ. et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:2387–92. [DOI] [PubMed] [Google Scholar]
- 158. Pitashny M, Schwartz N, Qing X. et al. Urinary lipocalin-2 is associated with renal disease activity in human lupus nephritis. Arthritis Rheum 2007;56:1894–903. [DOI] [PubMed] [Google Scholar]
- 159. Rubinstein T, Pitashny M, Levine B. et al. Urinary neutrophil gelatinase-associated lipocalin as a novel biomarker for disease activity in lupus nephritis. Rheumatology 2010;49:960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Molad Y, Miroshnik E, Sulkes J. et al. Urinary soluble VCAM-1 in systemic lupus erythematosus: a clinical marker for monitoring disease activity and damage. Clin Exp Rheumatol 2002;20:403–6. [PubMed] [Google Scholar]
- 161. Wu T, Xie C, Wang HW. et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol 2007;179:7166–75. [DOI] [PubMed] [Google Scholar]
- 162. Wu T, Du Y, Han J. et al. Urinary angiostatin – a novel putative marker of renal pathology chronicity in lupus nephritis. Mol Cell Proteomics 2013;12:1170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Kawasaki Y, Yokobayashi E, Sakamoto K. et al. Angiostatin prevents IL-1β-induced down-regulation of eNOS expression by inhibiting the NF-κB cascade. J Pharmacol Sci 2015;129:200–4. [DOI] [PubMed] [Google Scholar]