Abstract
Aedes aegypti (L.) is one of the most important arboviral vectors worldwide. Vector control is targeted at immature and adult stages; however, eggs are resistant to desiccation and may repopulate treated areas long after treatment ceases. We investigated the effect of age on Ae. aegypti egg hatching rates using newly colonized populations (F2) from an arid region. We found a strongly negative association where older eggs had lower hatch rates. The capacity of eggs to survive for long periods of time has implications on mosquito control. In addition, the accumulation of eggs in containers should be accounted for in abundance modeling efforts where populations may grow rapidly early in the season.
Keywords: Aedes aegypti, hatch rate, egg viability
Aedes aegypti (L.) is an important arboviral disease vector with an established presence on in all human-inhabited continents (Kraemer et al. 2015). Successful elimination efforts of the 1950–1960’s reduced the range of this vector throughout the Americas (Gubler 2002). However, cessation of those the coordinated efforts, travel, trade, and climate have allowed this vector to reestablish (Gubler 2002, Kearney et al. 2009).
The capacity to resist desiccation in the egg stage has been suggested as a mechanism for between-season survival (Trpis 1972). In the absence of rain-filling containers and submerging eggs, the number of eggs in a container may accumulate as additional eggs are laid along the receding water line. The onset of seasonal precipitation may refill the containers, submerging accumulated eggs, which may result in rapid population growth (Trpis 1972). This early season population boom as well as the long period of viability in a protected state requires prolonged vector control efforts (Fox 1974).
The purpose of this study was to assess the effect of age on egg viability in Ae. aegypti mosquitoes stored for long periods of time (up to 298 days) and which experienced only limited adaptation to the artificial laboratory environment (F2). We interpret our findings with respect to vector control and dynamic models of mosquito abundance.
Materials and Methods
Study Area
Immature Ae. aegypti were collected from a flowerpot saucer in Tucson, Arizona AZ. Despite that AedesAe.aegypti is established in this area, the pathogens it transmits are not (Fink et al. 1998).
Data Collection
Wild-caught (F0) Ae. aegypti larvae and pupae were transported to 0.02-cubic meter tabletop incubators (Quincy Lab Inc., Chicago, IL) maintained at a median temperature of 26.5°C (inter quartile range [(IQR] )= 3°C) and median 97.5% relative humidity (IQR = 15% RH). Immatures were raised to adults, a subset identified to confirm species, and used to establish a colony. Adults were allowed carbohydrates in the form of 10% sucrose-soaked cotton balls ad libitum. Colony females were offered a 2-ml blood meal twice weekly, supplied using a water bath and bell jars with either Parafilm M (Bemis NA, Neehah, WI) or HOG sausage casing (Dewied International Inc., San Antonio, TX). The blood meal consisted of whole blood collected in citrate phosphate dextrose anticoagulant (∼7 ml whole blood: 1 ml anticoagulant) from volunteer allogeneic donors meeting all FDA mandated criteria, supplied by the American Red Cross Biomedical Services (ARC IRB #2016-005). Seed germination papers were placed in oviposition cups with distilled water and rabbit chow as an attractant. After 2 to 3 days, the papers were removed, wrapped in a paper towel and stored in the incubator in unsealed zip-lock bags. Each paper is considered a batch for this analysis. Batches were hatched between 3 and 298 days later by submerging egg papers for at least 48 hours in distilled water with rabbit chow. The papers were then removed, left to dry, and the percent hatched assessed using a Stereo Microscope (VWR International, Randor, PA) under 20× x magnification (number hatched/total number of eggs×*100).
Data Analysis
The date when egg paper was placed in the oviposition cup, date of submersion, number of eggs hatched, and total eggs were recorded in an Excel spreadsheet. Egg papers with 10 or fewer eggs were not considered for the analysis (n = 9) and papers where no eggs hatched were dropped from analysis (n = 5 additional egg papers). Linear regression was used to describe the association between age and hatch rate, and residuals tested for outliers and to confirm validity. Statistical analyses were completed using Stata v12 (StataCorp, College Station, TX).
Results
A total of 67 batches (65 with outliers removed) yielded 7,564 (7,478 with outliers removed) eggs. With and without the outliers, the median number of eggs per batch was 19 (range 16–646) and the percent hatching ranged from 0.3–100% (median 52.7%).
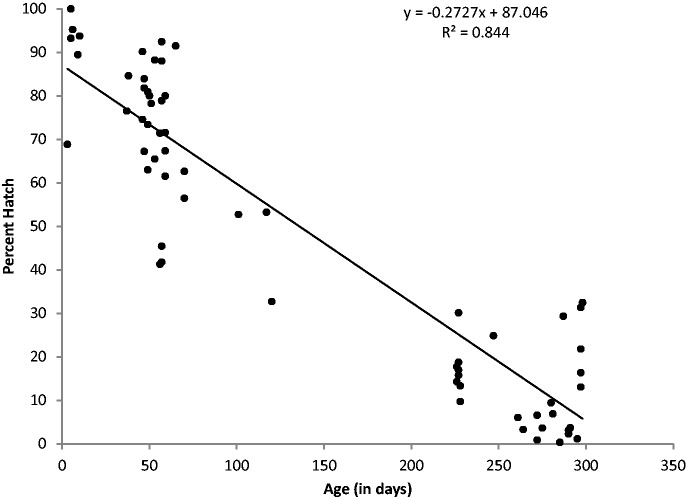
We found a significant linear association between age of eggs and percent hatching (Fig. 1). The final model, after removing two statistical outliers (age 287 d with 50% hatch and 298 d with 77% hatch), included 65 observations. Age of the eggs explained 84.4% of the variance in percent hatch (RMSE = 13, P < 0.001). The final model was %Hatch = 87.0 – 0.27 × *AgeofEggs where for every additional day eggs are left in the incubator, hatching decreases by 0.27%. After removing the outliers, the model residuals were confirmed to be normally distributed both visually using kernel density and quantile norm plots, as well as statistically using Shapiro-Wilk W test for normality (P = 0.28).
Fig. 1.
Scatterplot of the percent hatching by age for Aedes aegypti F2 eggs maintained in the laboratory.
Discussion
We found age was strongly, negatively associated with the percent of eggs hatching (R2 = 0.844). While the incubator conditions were cooler and more humid than where they were collected, these experiments have implications for vector control, given how long eggs may remain viable. Our study only assesses the effect of egg storage in an incubator on hatch rate. Predation, mold, and reduced larval survival have been reported as negative consequences of long-term storage (Meola 1964, Russell et al. 2001). We did not experience predation or mold, and did not assess larval fitness.
We use F2 mosquitoes and storage times of 3–298 d. Similar to the experiments of Meola (1964) using the Rockefeller strain mosquitoes maintained at 27 °C and 80% RH, we found a strong negative association between age and egg viability. The effect of age on survival is limited among younger mosquitoes. A study using offspring from wild-captured mosquitoes kept in the laboratory under three temperature conditions (22, 24, and 26 °C) and four RH conditions (25, 55, 75, and 95%) found no effect of temperature or RH and survival near 90% at one month (Juliano et al. 2002). The average hatch rate estimated using our linear model for 30-d-old eggs maintained at 26.5 °C and 97% RH was near 78.9% and at 37.5 d (n = 2 batches; 101 eggs/batch), we observed 80.6% hatching. Laboratory and wild-type colonies maintained for 56 d under simulated origin conditions of 17.6–2.35 °C temperature and 51.5–76.4% RH remained viable (>88% across all condition and population combinations; Faull and Williams 2015). At 56 d, we observed 67.8% (average across 11 batches [mean = 110.4 eggs/batch] aged 53–57 d) and estimate 71.9% survival with the eggs stored in incubators maintained at higher temperatures and RH. Among eggs stored for longer periods, temperature and RH has been shown to more strongly effect survival. Our linear model estimates 54.6% survival at 120 d in the incubator at 26 °C and 97% RH, and we observed (2 batches aged an average 118.5 days, 376.5 eggs/batch) 43% hatching. This compares with estimates of >33% survival of Ae. aegypti eggs from Dar es Salaam maintained at 25 °C and 80% RH for 120 days (Trpis 1972). While survival of offspring of field collected Ae. aegypti eggs aged 3 months was near 0% at 25 °C and 25% RH, it was near 70% when stored at 26 °C and 95% RH (Juliano et al. 2002). Between 290 and 298 days, we observed survival near 16% (10 batches, 77.9 eggs/batch) and the model predicts 7.5%.
While generally the patterns observed are similar to others, variability between experiments was observed. Geographic differences in egg viability among wild-type and colony populations have been previously observed (Trpis 1972, Faull and Williams 2015), which has been suggested to be the result of local adaptation (Faull and Williams 2015). We used second-generation offspring of mosquitoes from a hot (average summer daytime temperatures exceed 38 °C), arid (average annual rainfall = 288.7 mm; summer RH near 21%, while monsoon season RH near 45%) climate and stored the eggs under cooler, more humid conditions (26.5 °C and 97% RH).
The previous studies and our results here indicate that the observed resistance to desiccation of Ae. aeypgti eggs may allow eggs to survive for long periods of time–whether in climates closely resembling their origin or not. As suggested by Fox (1974), this association can be used to guide elimination efforts by recognizing that eggs may survive for long periods of time at high RH even in the absence of water. While these experiments and the literature suggest variability in egg viability across climate conditions and populations, this accumulation of eggs and resultant seasonal onset population bursts may be important in attenuating dynamic simulation models. That is, it may be important to capture the effect of egg survival over dry periods, which may explain rapid population growth after dry spells (Trpis 1972).
Acknowledgments
Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K01AI101224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Caitlin Smith is an Office of Research and Discovery Fellow in the University of Arizona Undergraduate Biology Research Program.
References Cited
- Faull K. J., Williams C. R. 2015. Intraspecific variation in desiccation survival time of Aedes aegypti (L.) mosquito eggs of Australian origin. J. Vector Ecol. 40: 292–300. [DOI] [PubMed] [Google Scholar]
- Fink M. T., Hau B., Baird B. L., Palmer S., Kaplan S., Ramberg F. B., Mead D. G., Hagedorn H. 1998. Aedes aegypti in Tucson, Arizona. Emerg. Infect. Dis. 4: 703.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. 1974. Viability of Puerto Rican Aedes aegypti eggs after long periods of storage. Mosq. News 34: 274–275. [Google Scholar]
- Gubler D. J. 2002. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. TRENDS Microbiol. 10: 100–103. [DOI] [PubMed] [Google Scholar]
- Juliano S. A., O'Meara G. F., Morrill J. R., Cutwa M. M. 2002. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 130: 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M., Porter W. P., Williams C., Ritchie S., Hoffmann A. A. 2009. Integrating biophysical models and evolutionary theory to predict climatic impacts on species' ranges: The dengue mosquito Aedes aegypti in Australia. Funct. Ecol. 23: 528–538. [Google Scholar]
- Kraemer M.U.G., Sinka M. E., Duda K. A., Mylne A.Q.N., Shearer F. M., Barker C. M., Moore C. G., Carvalho R. G., Coelho G. E., Van Bortel W., et al. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 4: e08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola R. 1964. The influence of temperature and humidity on embryonic longevity in Aedes aegypti. Ann. Entomol. Soc. Am. 57: 468–472. [Google Scholar]
- Russell B. M., Kay B. H., Shipton W. 2001. Survival of Aedes aegypti (Diptera: Culicidae) eggs in surface and subterranean breeding sites during the northern Queensland dry season. J. Med. Entomol. 38: 441–445. [DOI] [PubMed] [Google Scholar]
- Trpis M. 1972. Dry season survival of Aedes aegypti eggs in various breeding sites in the Dar es Salaam area, Tanzania. Bull. World Health Organ. 47: 433–437. [PMC free article] [PubMed] [Google Scholar]