We enrolled 34581 individuals newly diagnosed with human immunodeficiency virus infection with CD4 cell counts >500 cells/μL and found that initiation of antiretroviral therapy within 30 days of diagnosis was associated with a 63% reduction in overall mortality at 12 months of follow-up.
Keywords: HIV/AIDS, antiretroviral therapy, treatment initiation, CD4 count, mortality
Abstract
Background
Clinical trials have demonstrated that immediate initiation of antiretroviral therapy (ART) reduces AIDS-related morbidity and mortality. We tested the hypothesis that initiating ART ≤30 days after human immunodeficiency virus (HIV) diagnosis would be associated with reduced mortality among people living with HIV (PLWH) with CD4 counts >500 cells/μL.
Methods
PLWH enrolled in the Chinese National HIV Information System between January 2012 and June 2014 with CD4 counts >500 cells/μL were followed for 12 months. Cox proportional hazards model was used to determine hazard ratios (HRs) for PLWH who initiated ART after HIV diagnosis. ART initiation was treated as a time-dependent variable.
Results
We enrolled 34581 PLWH with CD4 >500 cells/μL; 1838 (5.3%) initiated ART ≤30 days after diagnosis (immediate ART group), and 19 deaths were observed with a mortality rate of 1.04 per 100 person-years (PY). Fifty-eight deaths were documented among the 5640 PLWH in the delayed ART group with a mortality rate of 2.25 per 100 PY. There were 713 deaths among the 27103 PLWH in the no ART group with a mortality rate of 2.39 per 100 PY. After controlling for potential confounding factors, ART initiation at ≤30 days (adjusted HR, 0.37 [95% confidence interval, .23–.58]) was a statistically significant protective factor.
Conclusions
We found that immediate ART is associated with a 63% reduction in overall mortality among PLWH with CD4 counts >500 cells/μL in China, supporting the recommendation to initiate ART immediately following HIV diagnosis.
Over the past 2 decades, combination antiretroviral therapy (ART) has been a cornerstone of the response to the epidemic of human immunodeficiency virus (HIV) infection that causes AIDS [1–3]. The scale-up of ART has resulted in substantial reductions in AIDS-related mortality and morbidity, as well as reduced HIV transmission [4–8]. Nevertheless, less than half of the estimated 36.7 million people living with HIV (PLWH) were receiving ART in 2015 [9].
Historically, there has been an early consensus that PLWH who had an AIDS-defining illness should initiate ART immediately. For those who had not yet progressed to AIDS, the World Health Organization (WHO) has recommended that CD4+ T-lymphocyte thresholds guide the timing of ART initiation [10]. However, observational studies, predominantly carried out in resource-rich settings [11], have found that ART initiation among PLWH with CD4 counts >500 cells/μL was associated with substantial clinical benefits, and this has now been confirmed by prospective randomized trials [12–15]. Taken together with the results of the HPTN 052 trial [7, 8], showing the sustained ability of ART to prevent sexual transmission of HIV, these findings led the WHO to revise their recommendation in September 2015, now completely removing the CD4 count threshold, such that ART is globally recommended for all PLWH [16].
Standard of care for all those diagnosed with HIV infection in China includes free CD4 testing each year, with the first CD4 test offered soon after diagnosis. From 2008 to 2014, the primary criterion for entry into the Chinese National Free Antiretroviral Treatment Program (NFATP) was CD4 count ≤350 cells/μL. During this time, exceptions to the CD4 threshold of ≤350 cells/μL in the NFATP included patients with coinfections, and prevention of mother-to-child transmission [17]. In 2011, the NFATP added a recommendation that ART be offered to all PLWH in serodiscordant relationships, regardless of CD4 cell count [17]. In 2012, a special national project for the “Prevention and Treatment of Major Infectious Diseases” was launched, wherein the National Center for AIDS/STD Control and Prevention (NCAIDS) of the Chinese Center for Disease Control and Prevention (China CDC) implemented a series of cluster-randomized trials among key populations, including men who have sex with men, serodiscordant couples, people who inject drugs, and commercial sex workers. The targeted outcome was early identification of HIV infection and immediate ART initiation regardless of CD4 cell count. Thus, PLWH participating in the study were exempt from the standard-of-care CD4 threshold for ART initiation.
The primary aim of the present study was to examine mortality and its determinants among a cohort of newly diagnosed PLWH with first CD4 count >500 cells/μL. Participants were followed for 1 year to examine the impact of immediate ART initiation on mortality among PLWH with high CD4 counts, compared to those with delayed ART initiation and to those who remained ART naive. Secondarily, we also sought to describe causes of death within our cohort.
METHODS
Study Design and Setting
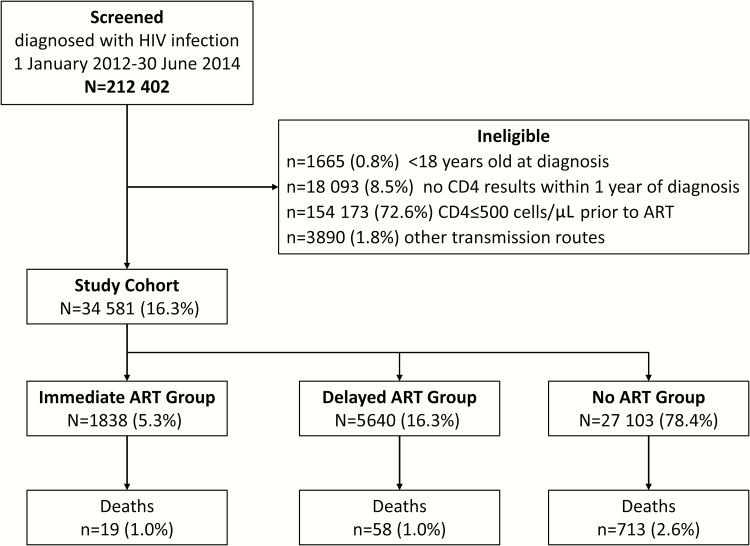
This nationwide, observational cohort study was designed to examine mortality among patients who received confirmed diagnoses of HIV infection between 1 January 2012 and 30 June 2014 and had a CD4 count >500 cells/μL. Mortality and factors associated with mortality were assessed at 1 year. A flow diagram illustrating the study design is shown in Figure 1. The treatment setting was China’s NFATP. This program, as well as treatment regimens (eg, drug combinations used, dosage, and administration) and follow-up protocols for enrolled patients, have been described previously [17, 18].
Figure 1.
Flow diagram depicting study design and development of the cohort. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
Data Source
Data were extracted from China’s Web-based national database for real-time collection and maintenance of information related to the HIV epidemic. This system, called the HIV/AIDS Comprehensive Response Information Management System (CRIMS), has been described elsewhere [19–21]. Because screening for inclusion was nationwide and included all identified cases, sample size was not initially defined.
Data Collection and Inclusion Criteria
All CRIMS records of persons with a confirmed diagnosis of HIV infection between 1 January 2012 and 30 June 2014 were extracted and then further screened for study eligibility criteria. In addition to a confirmed new HIV diagnosis, PLWH were included if they were 18 years or older at the time of diagnosis, had a CD4 test result within 1 year of diagnosis, had baseline (pre-ART) CD4 count of >500 cells/μL, and had reported having acquired HIV through heterosexual contact, male-to-male sexual contact, or injection drug use. These transmission routes accounted for >90% of newly diagnosed HIV infections in China during this time period [22].
All eligible records were extracted and de-identified. Each study participant was followed from the date of their first CD4 test for 12 months afterward or the date of death, whichever came first. As illustrated in Figure 1, participants were stratified by timing of ART initiation relative to the date of confirmed HIV diagnosis (≤30 days after HIV diagnosis referred to as the immediate ART group; >30 days after HIV diagnosis referred to as the delayed ART group; and PLWH who did not start ART within 1 year referred to as the no ART group).
Mortality and Causes of Death
The main outcome measure was all-cause mortality. Dates and causes of death were obtained from clinical records. AIDS-related causes of death were defined by China CDC medical guidelines [23]. Non-AIDS-related causes of death included all other causes.
Statistical Analysis
Continuous variables are presented using median and interquartile range (IQR), and categorical variables are presented using number and percentage. Because of the very large number of patients in our cohort, even very small differences between subgroups would likely result in P values reaching statistical significance. However, these small differences would not necessarily be meaningful. Therefore, we focused only on differences of ≥5% between groups being compared.
Observed time, in person-years (PY), was calculated for participants who contributed to the study observation. As the median time interval between confirmed HIV diagnosis and first CD4 test was only 14 days (IQR, 3–57 days), CD4 test date was selected as the start of observed time. Thus, observed time was calculated as the difference between the date of first CD4 test and the date of death or the end of the follow-up period, whichever came first. Mortality rate was calculated as the sum of deaths divided by the sum of observed time in PY. Due to survival bias, patients who accepted ART were always alive before ART initiation. Thus, ART status was treated as a time-dependent variable, meaning that those who accepted ART were a part of the no ART group until ART initiation. Individuals contributed person-time (and deaths) to the immediate ART group after they began ART, provided ART initiation occurred within 30 days of the start. Individuals contributed person-time to the delayed ART group after they began ART, provided ART initiation occurred after 30 days from the start but before 1 year. A multivariate Cox proportional hazards regression model was constructed to assess hazard ratios (HRs) for the study variable ART status, and for demographic variables and HIV clinical variables. The immediate ART variable in the regression model was considered time dependent. That is, it was set to 0 before ART initiation, and to 1 after ART initiation, provided it occurred before the end of day 30. Similarly, the delayed ART variable was set to 0 before ART initiation, and to 1 after ART initiation, provided it occurred after day 30, but within 1 year. Adjusted HRs for the study variable were generated to identify prognostic risk of death by controlling potential confounding bias caused by demographic variables and HIV clinical variables.
All P values presented are 2-sided, and P < .05 was considered statistically significant. All analyses were performed using SAS software version 9.1.3 (SAS Institute).
Ethical Considerations
The study protocol was reviewed and approved by the Institutional Review Board of NCAIDS, China CDC. As patients entering CRIMS sign informed consent at time of enrollment, no further informed consent was required for this study. All patient records were de-identified prior to data analysis.
RESULTS
A full 212402 individual records with a date of confirmed HIV diagnosis between 1 January 2012 and 30 June 2014 were available for the study. A total of 34581 individuals (16.3%) met the eligibility criteria and were included in the final study cohort. Development of the cohort is illustrated in Figure 1.
Characteristics of the Cohort
Characteristics of the entire study cohort, as well as individual subgroups thereof, are shown in Table 1. The median age of patients in the cohort was 32 years (IQR, 25–42 years). A majority were male (75.0%), Han Chinese (72.2%), had junior middle school education or less (63.7%), were single, divorced, or widowed (60.8%), and self-reported acquisition of HIV infection via heterosexual contact (59.9%). Median baseline CD4 count was 616 cells/μL (IQR, 549–732 cells/μL).
Table 1.
Baseline Characteristics of the Entire Study Cohort and of Each Subgroup Based on Antiretroviral Therapy Timing After Confirmed Human Immunodeficiency Virus Diagnosis
| Characteristics | Entire Study Cohort | ART Timing After Confirmed HIV Diagnosis | ||
|---|---|---|---|---|
|
Immediate ART
(≤30 d) |
Delayed ART
(>30 d) |
No ART
(>1 y) |
||
| Total | 34581 (100.0) | 1838 (100.0) | 5640 (100.0) | 27103 (100.0) |
| Age, y | ||||
| Median (IQR) | 32 (25–42) | 34 (27–46) | 33 (26–45) | 31 (25–41) |
| 18–29 | 14771 (42.7) | 653 (35.5) | 2110 (37.4) | 12008 (44.3) |
| 30–49 | 14976 (43.3) | 816 (44.4) | 2565 (45.5) | 11595 (42.8) |
| ≥50 | 4834 (14.0) | 369 (20.1) | 965 (17.1) | 3500 (12.9) |
| Sex | ||||
| Male | 25927 (75.0) | 1160 (63.1) | 4058 (72.0) | 20709 (76.4) |
| Female | 8654 (25.0) | 678 (36.9) | 1582 (28.1) | 6394 (23.6) |
| Ethnicity | ||||
| Han | 24969 (72.2) | 1176 (64.0) | 4029 (71.4) | 19764 (72.9) |
| Other | 9612 (27.8) | 662 (36.0) | 1611 (28.6) | 7339 (27.1) |
| Education level | ||||
| Junior middle school or less | 22039 (63.7) | 1323 (72.0) | 3630 (64.4) | 17086 (63.0) |
| Senior middle school or higher | 12542 (36.3) | 515 (28.0) | 2010 (35.6) | 10017 (37.0) |
| Marital status | ||||
| Single, divorced, or widowed | 21019 (60.8) | 783 (42.6) | 2829 (50.2) | 17407 (64.2) |
| Married or cohabiting | 13562 (39.2) | 1055 (57.4) | 2811 (49.8) | 9696 (35.8) |
| Transmission route | ||||
| Heterosexual contact | 20713 (59.9) | 1409 (76.7) | 3615 (64.1) | 15689 (57.9) |
| Male-to-male sexual contact | 10167 (29.4) | 348 (18.9) | 1674 (29.7) | 8145 (30.1) |
| Injecting drug use | 3701 (10.7) | 81 (4.4) | 351 (6.2) | 3269 (12.1) |
| Baseline CD4 count, cells/μL | ||||
| Median (IQR) | 616 (549–732) | 596 (538–694) | 594 (540–692) | 623 (552–744) |
| Time from diagnosis to CD4 test, d | ||||
| Median (IQR) | 14 (3–57) | 6 (0–20) | 11 (1–39) | 18 (4–65) |
| Time from CD4 to ART, d | ||||
| Median (IQR) | 10 (4–18) | 197 (109–283) | ||
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral treatment; HIV, human immunodeficiency virus; IQR, interquartile range.
Characteristics of Groups Stratified by Timing of ART Initiation
Among 34581 PLWH, 1838 (5.3%) initiated ART within 30 days of diagnosis and were included in the immediate ART group and 5640 (16.3%) initiated ART >30 days after diagnosis and were included in the delayed ART group. The remaining 27103 PLWH (78.4%) formed the no ART group. A larger proportion of PLWH in the immediate ART group were female (36.9% vs 28.1% for the delayed ART group and 23.6% for the no-ART group), ethnic minorities (36.0% vs 28.6% and 27.1%), less well educated (junior middle school or less: 72.0% vs 64.4% and 63.0%), married or cohabitating (57.4% vs 49.8% and 35.8%), and reported heterosexual contact as their mode of HIV acquisition (76.7% vs 64.1% and 57.9%).
Mortality and Causes of Death
Deaths and mortality rates are shown in Table 2. The 34581 PLWH in the study cohort were followed from the date of their first CD4 test until 1 year after or death, whichever was first, resulting in a total observed time of 34188 PY. Seven hundred ninety deaths of 34581 (2.3%) were documented, for an overall mortality rate of 2.31 per 100 PY.
Table 2.
Cox Proportional Hazards Regression Models for Mortality Rates and Associated Risk Factors
| Characteristic | Entire Study Cohort, No. (%) |
Deaths,
No. (%) |
Observed Time a , PY |
Mortality
Rate per 100 PY |
Unadjusted
HR (95% CI) |
P Value |
Adjusted
HR (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|---|
| Total | 34581 (100.0) | 790 (100.0) | 34188 | 2.31 | ||||
| Age, y | ||||||||
| 18–29 | 14771 (42.7) | 122 (15.4) | 14718 | 0.83 | 1.00 | 1.00 | ||
| 30–49 | 14976 (43.3) | 334 (42.3) | 14809 | 2.26 | 2.72 (2.21–3.35) | <.001 | 2.03 (1.64–2.52) | <.001 |
| ≥50 | 4834 (14.0) | 334 (42.3) | 4661 | 7.17 | 8.65 (7.03–10.64) | <.001 | 6.10 (4.87–7.64) | <.001 |
| Sex | ||||||||
| Male | 25927 (75.0) | 629 (79.6) | 25616 | 2.46 | 1.31 (1.10–1.56) | .002 | 1.90 (1.59–2.27) | <.001 |
| Female | 8654 (25.0) | 161 (20.4) | 8573 | 1.88 | 1.00 | 1.00 | ||
| Ethnicity | ||||||||
| Han | 24969 (72.2) | 543 (68.7) | 24692 | 2.20 | 1.00 | 1.00 | ||
| Other | 9612 (27.8) | 247 (31.3) | 9497 | 2.60 | 1.18 (1.02–1.38) | .03 | 1.09 (.93–1.28) | .30 |
| Education level | ||||||||
| Junior middle school or less | 22039 (63.7) | 688 (87.1) | 21691 | 3.17 | 3.89 (3.16–4.78) | <.001 | 1.85 (1.48–2.31) | <.001 |
| Senior middle school or higher | 12542 (36.3) | 102 (12.9) | 12498 | 0.82 | 1.00 | 1.00 | ||
| Marital status | ||||||||
| Single, divorced, or widowed | 21019 (60.8) | 411 (52.0) | 20814 | 1.97 | 1.00 | 1.00 | ||
| Married or cohabitating | 13562 (39.2) | 379 (48.0) | 13375 | 2.83 | 1.44 (1.25–1.65) | <.001 | 0.88 (.76–1.02) | .928 |
| Transmission route | ||||||||
| Heterosexual contact | 20713 (59.9) | 623 (78.9) | 20394 | 3.05 | 7.04 (5.19–9.56) | <.001 | 4.16 (2.99–5.77) | <.001 |
| Male-to-male sexual contact | 10167 (29.4) | 44 (5.6) | 10145 | 0.43 | 1.00 | 1.00 | ||
| Injection drug use | 3701 (10.7) | 123 (15.6) | 3649 | 3.37 | 7.77 (5.51–10.97) | <.001 | 5.07 (3.49–7.35) | <.001 |
| ART statusb | ||||||||
| Immediate ART | 1838 (5.3) | 19 (2.4) | 1828 | 1.04 | 0.44 (.28–.69) | <.001 | 0.37 (.23–.58) | <.001 |
| Delayed ART | 5640 (16.3) | 58 (7.3) | 2583 | 2.25 | 0.80 (.61–1.05) | .11 | 0.74 (.57–.98) | .04 |
| No ART | 27103 (78.4) | 713 (90.3) | 29778 | 2.39 | 1.00 | 1.00 | ||
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HR, hazard ratio; PY, person-years.
aObserved time, in PY, was calculated as beginning on the date of first CD4 test, and ending at 1 year or on the date of death, whichever came first.
bART was treated as the time-dependent variable; immediate ART means ART was initiated within 30 days of confirmed human immunodeficiency virus (HIV) diagnosis; delayed ART means ART was initiated >30 days after diagnosis, but within 1 year; no ART means that ART was never initiated in the 1 year after HIV diagnosis.
Among the 1838 PLWH in the immediate ART group, 19 deaths were observed within 1828 PY of observed time, for a mortality rate of 1.04 per 100 PY. Fifty-eight deaths were documented among the 5640 PLWH in the delayed ART group within 2583 PY of observed time, for a mortality rate of 2.25 per 100 PY. Finally, 713 deaths were documented among the 27103 PLWH in the no ART group within 29778 PY of observed time, for a mortality rate of 2.39 per 100 PY.
The causes of death are presented in Table 3. Causes of death for a majority of the 790 PLWH were documented as non-AIDS-related complications (76.3%). A further 8.6% of PLWH had no cause of death documented or an unclassified cause of death listed in their record. AIDS-related complications were identified as the cause of death for 15.1%. In the immediate ART group, no AIDS-related causes of death were documented. The most common non-AIDS cause was cardiovascular disease (36.8%). Among those in the delayed ART group, 41.4% died from AIDS-related causes, whereas 55.1% died from non-AIDS-related causes. In the no ART group, a majority of deaths were documented as having non-AIDS-related causes (77.6%), the most frequent of which were other diseases (13.9%), respiratory disease (13.1%), and non-disease-related deaths/accidents (12.9%).
Table 3.
Causes of Death During Follow-up
| Cause of Death | Entire Study Cohort | ART Timing After Confirmed HIV Diagnosis | ||
|---|---|---|---|---|
|
Immediate ART
(≤30 d) |
Delayed ART
(>30 d) |
No ART
(>1 y) |
||
| Total | 790 (100.0) | 19 (100.0) | 58 (100.0) | 713 (100.0) |
| AIDS-related | 119 (15.1) | 0 (0.0) | 24 (41.4) | 95 (13.3) |
| AIDS-defining cancer | 10 (1.3) | 1 (1.7) | 9 (1.3) | |
| CNS infection | 3 (0.4) | 1 (1.7) | 2 (0.3) | |
| Tuberculosis | 15 (1.9) | 2 (3.5) | 13 (1.8) | |
| Cryptococcal meningitis | 4 (0.5) | 1 (1.7) | 3 (0.4) | |
| Herpes virus infection | 4 (0.5) | 4 (0.6) | ||
| Wasting syndrome | 6 (0.8) | 2 (3.5) | 4 (0.6) | |
| Candidiasis | 5 (0.6) | 2 (3.5) | 3 (0.4) | |
| Recurrent pneumonia | 5 (0.6) | 1 (1.7) | 4 (0.6) | |
| Pneumocystis pneumonia | 14 (1.8) | 3 (5.2) | 11 (1.5) | |
| Cryptosporidiosis | 2 (0.3) | 1 (1.7) | 1 (0.1) | |
| Other infections | 12 (1.5) | 12 (1.6) | ||
| Other | 39 (4.9) | 10 (17.2) | 29 (4.1) | |
| Non-AIDS-related | 603 (76.3) | 18 (94.7) | 32 (55.1) | 553 (77.6) |
| Non-AIDS-defining cancer | 80 (10.1) | 4 (6.9) | 76 (10.7) | |
| Cardiovascular disease | 94 (11.9) | 7 (36.8) | 6 (10.3) | 81 (11.4) |
| Overdose of illicit drugs | 45 (5.7) | 1 (5.3) | 1 (1.7) | 43 (6.0) |
| Suicide | 35 (4.4) | 2 (10.5) | 2 (3.5) | 31 (4.3) |
| Hepatitis B or C virus | 12 (1.5) | 1 (5.3) | 1 (1.7) | 10 (1.4) |
| Respiratory disease | 98 (12.4) | 3 (15.8) | 2 (3.5) | 93 (13.1) |
| Metabolic disease | 10 (1.3) | 2 (10.5) | 2 (3.5) | 6 (0.8) |
| Gastrointestinal disease | 22 (2.8) | 1 (1.7) | 21 (3.0) | |
| Medicine-related events | 1 (0.1) | 1 (0.1) | ||
| Other diseases | 107 (13.6) | 2 (10.5) | 6 (10.3) | 99 (13.9) |
| Non-disease-related death/accident | 99 (12.5) | 7 (12.0) | 92 (12.9) | |
| Unknown | 68 (8.6) | 1 (5.3) | 2 (3.5) | 65 (9.1) |
| Unclassified | 66 (8.3) | 1 (5.3) | 2 (3.5) | 63 (8.8) |
| Missing | 2 (0.3) | 0 (0.0) | 0 (0.0) | 2 (0.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; CNS, central nervous system; HIV, human immunodeficiency virus.
Factors Associated With Death
Factors associated with death within 1 year are presented in Table 2. Higher risk of death was associated with older age (30–49 years: adjusted HR, 2.03, P < .001; ≥50 years: adjusted HR, 6.10, P < .001), being male (adjusted HR, 1.90, P < .001), having only a junior middle school education or less (adjusted HR, 1.85, P < .001), and having been infected via heterosexual contact (adjusted HR, 4.16, P < .001) or injection drug use (adjusted HR, 5.07, P < .001). As far as the timing of ART initiation, immediate ART (initiating within 30 days of diagnosis) provided the strongest protection against death (adjusted HR, 0.37, P < .001), while delayed ART (initiating after 30 days of diagnosis, but within 1 year) provided more modest protection (adjusted HR, 0.74, P = .040) relative to not receiving ART within the first year.
DISCUSSION
Our results demonstrate that PLWH with a CD4 count >500 cells/μL who initiated ART within 30 days of diagnosis (immediate ART group) experienced a 63% decrease in mortality. Additional risk factors for mortality in this study were older age, being male, having a lesser education, and becoming infected via injection drug use or heterosexual contact. A majority of deaths observed were attributed to non-AIDS-related complications. Our results confirm those from the recent START (strategic timing of antiretroviral treatment) and TEMPRANO (early antiretroviral treatment and/or early isoniazid prophylaxis against tuberculosis in HIV-infected adults – ANRS 12136 TEMPRANO) studies [12, 15], and further indicate that these results are also generalizable to China.
The push for earlier ART initiation has recently been formalized through policy recommendations [16]. Arguments to delay ART initiation based on the use of a CD4 cell count threshold include concerns about drug resistance, side effects, resource allocation, and adherence [24–29]. However, research studies have definitively concluded that the potential clinical and public health benefits of immediate ART initiation far outweigh the risks. Our results highlight the urgency of immediate ART initiation among PLWH with high CD4 cell counts.
HIV-infected, ART-naive patients with high CD4 cell counts still experience elevated mortality compared with the general population [30, 31]. Non-AIDS events remain common among this group [32, 33], and non-AIDS-related mortality can exceed AIDS-related mortality [34]. In settings outside of China, a high proportion of non-AIDS mortality has been attributed to illicit drug use, suicide, unintentional injury, and violence [35, 36]. A cohort study of ART-naive PLWH with CD4 count >350 cells/μL in Europe and North America found that only 14.6% of deaths were categorized as AIDS-related, whereas 44.9% were categorized as non-AIDS-related (40.6% of deaths in the study were due to unknown causes) [30]. In our study, 94.7% of deaths in the immediate ART group, 55.1% in the delayed ART group, and 77.6% in the no ART group were classified as non-AIDS-related deaths [34]. Consistent with other studies, the most common causes of death observed were cardiovascular disease, liver disease, non-AIDS-defining malignancies, overdose, suicide, and accidental deaths [33, 35–38].
In addition to the direct benefit of ART for survival, it is also likely that regular follow-up and comprehensive care services associated with ART use contributed to the decreased mortality observed. After ART initiation, patients entered into the stable care system and received multidisciplinary services including regular medical visits as well as psychosocial support [39, 40]. ART clinics provided chronic disease management and cancer and cardiovascular disease screening, which may have contributed to the improvement in health outcomes observed [41–43]. Our study design did not allow us to discriminate the relative contribution of ART vs close medical monitoring. However, the fact that a similar benefit in clinical outcomes was observed in randomized clinical trials [12, 15], where clinical follow-up was standardized between study arms, suggests that our conclusion regarding the benefit of ART at high CD4 cell counts is valid.
Other risk factors for mortality in this study included older age, being male, acquiring HIV through injection drug use or heterosexual transmission, and low educational attainment. In China, HIV incidence has been increasing among older males, particularly in rural areas, who are mainly infected through engaging in commercial sex [44]. Older HIV-infected individuals are more likely to present with comorbidities that increase their risk of death [45]. Mortality was highest among injection drug users, which is related to a variety of factors including delayed ART initiation, poor ART adherence, concomitant drug use, and comorbidities such as depression and hepatitis C virus infection [46].
In contrast to other studies that have examined ART initiation at high CD4 counts [14], an important strength of this study was that all participants who initiated ART in our cohort did so when their CD4 count was >500 cells/μL. Nevertheless, our study had several limitations. As with any observational study, our data should be interpreted with caution, as participants may have had individual circumstances that could have influenced their treatment initiation decisions, including medical comorbidities. Also, because we used routine care data, we did not have access to some potentially informative variables, such as viral load results, details on complications, and, in some cases, causes of death. Moreover, the delayed ART group and no ART group were subject to a survival bias, as the immediate ART group always included persons who lived long enough to initiate ART. To partially avoid this bias, we treated ART as a time-dependent variable. Finally, as the start of follow-up time was date of CD4 test, and those in the delayed ART group tended to have longer median durations between CD4 testing and ART initiation, the shorter time on ART during follow-up may have contributed to poorer outcomes in this group.
CONCLUSIONS
Our results demonstrate that immediate ART initiation within 30 days among PLWH with CD4 counts >500 cells/μL is associated with a 63% reduction in overall mortality. Our results highlight the significant negative impact of delays in ART initiation in a real-world setting in China. Our findings are consistent with the results of recent randomized controlled trials and demonstrate that these results are generalizable. Furthermore, our results support the urgent need to increase the number of PLWH identified early and started on effective, long-term ART immediately [13, 47], as predicated by the United Nations 90-90-90 targets [38, 48, 49].
Notes
Author contributions. Y. Z. and Z. W. designed the study. Y. Z. performed the statistical analysis. Y. Z., Z. W., J. M. M., C. X. S., R. B., J. S. G. M., and R. D. interpreted the results and developed the initial draft of manuscript. All authors contributed to manuscript revisions and approved the final version for publication. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Acknowledgments. The authors thank Chunming Li for his advice on the data analyses.
Disclaimer. The views and opinions expressed herein belong to the authors alone and do not represent the official policy or endorsement of their affiliated institutions. The funding organization had no role in the development of study design, collection, analysis, and interpretation of data, the writing of the report, or the final decision to submit the manuscript for publication.
Financial support. This study was supported by the China National AIDS Program, the National Science and Technology Major Project on Prevention and Treatment of Major Infectious Diseases including AIDS and Viral Hepatitis (grant number 2012ZX10001-007). J. S. G. M. is supported with grants paid to his institution by the British Columbia Ministry of Health and by the US National Institutes of Health (grant number R01DA036307).
Potential conflicts of interest. J. S. G. M. has received limited unrestricted funding, paid to his institution, from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hammer SM, Squires KE, Hughes MD et al. . A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997; 337:725–33. [DOI] [PubMed] [Google Scholar]
- 2. Gulick RM, Mellors JW, Havlir D et al. . Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 1997; 337:734–9. [DOI] [PubMed] [Google Scholar]
- 3. Palmisano L, Vella S. A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann Ist Super Sanita 2011; 47:44–8. [DOI] [PubMed] [Google Scholar]
- 4. Mocroft A, Vella S, Benfield TL et al. . Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998; 352:1725–30. [DOI] [PubMed] [Google Scholar]
- 5. Kitahata MM, Gange SJ, Abraham AG et al. . NA-ACCORD Investigators Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science 2013; 339:966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen MS, Chen YQ, McCauley M et al. . HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen MS, Chen YQ, McCauley M et al. . HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joint United Nations Programme on HIV/AIDS. AIDS by the numbers 2016 Available at: http://www.unaids.org/en/resources/documents/2016/AIDS-by-the-numbers. Accessed 13 November 2016.
- 10. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 11. Lodi S, Phillips A, Logan R et al. . HIV-CAUSAL Collaboration Comparative effectiveness of immediate antiretroviral therapy versus CD4-based initiation in HIV-positive individuals in high-income countries: observational cohort study. Lancet HIV 2015; 2:e335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lundgren JD, Babiker AG, Gordin F et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olubajo B, Mitchell-Fearon K, Ogunmoroti O. A comparative systematic review of the optimal CD4 cell count threshold for HIV treatment initiation. Interdiscip Perspect Infect Dis 2014; 2014:625670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lima VD, Reuter A, Harrigan PR et al. . Initiation of antiretroviral therapy at high CD4+ cell counts is associated with positive treatment outcomes. AIDS 2015; 29:1871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danel C, Moh R, Gabillard D et al. . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 16. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 17. Ministry of Health Working Group on Clinical AIDS Treatment. China free antiretroviral therapy manual [in Chinese]. 3rd ed Beijing: People’s Medical Publishing House, 2012. [Google Scholar]
- 18. Zhang F, Dou Z, Ma Y et al. . Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis 2011; 11:516–24. [DOI] [PubMed] [Google Scholar]
- 19. Mao Y, Wu Z, Poundstone K et al. . Development of a unified Web-based national HIV/AIDS information system in China. Int J Epidemiol 2010; 39(Suppl 2):79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma Y, Zhang F, Zhao Y et al. . Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol 2010; 39:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang F, Haberer JE, Wang Y et al. . The Chinese free antiretroviral treatment program: challenges and responses. AIDS 2007; 21(Suppl 8):S143–8. [DOI] [PubMed] [Google Scholar]
- 22. Wu Z. Achievement of HIV/AIDS program in the past 30 years and challenges in China. Zhonghua Liu Xing Bing Xue Za Zhi 2015; 36:1329–31. [PubMed] [Google Scholar]
- 23. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992; 41(RR-17):1–19. [PubMed] [Google Scholar]
- 24. Jose S, Quinn K, Hill T et al. . UK CHIC Steering Committee Laboratory adverse events and discontinuation of therapy according to CD4(+) cell count at the start of antiretroviral therapy. AIDS 2014; 28:1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundgren JD, Babiker AG, Gordin FM, Borges ÁH, Neaton JD. When to start antiretroviral therapy: the need for an evidence base during early HIV infection. BMC Med 2013; 11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katz IT, Essien T, Marinda ET et al. . Antiretroviral therapy refusal among newly diagnosed HIV-infected adults in Soweto, South Africa. AIDS 2011; 25:2177–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59(Suppl 1):S21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallant JE, Mehta SH, Sugarman J. Universal antiretroviral therapy for HIV infection: should US treatment guidelines be applied to resource-limited settings?Clin Infect Dis 2013; 57:884–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ford N, Meintjes G, Pozniak A et al. . The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015; 15:241–7. [DOI] [PubMed] [Google Scholar]
- 30. Lodwick RK, Sabin CA, Porter K et al. . Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet 2010; 376:340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alejos B, Hernando V, López-Aldeguer J et al. . Overall and cause-specific mortality in HIV-positive subjects compared to the general population. J Int AIDS Soc 2014; 17:19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mocroft A, Reiss P, Gasiorowski J et al. . Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr 2010; 55:262–70. [DOI] [PubMed] [Google Scholar]
- 33. Cowell A, Shenoi SV, Kyriakides TC, Friedland G, Barakat LA. Trends in hospital deaths among human immunodeficiency virus–infected patients during the antiretroviral therapy era, 1995 to 2011. J Hosp Med 2015; 10:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr 2007; 44:179–87. [DOI] [PubMed] [Google Scholar]
- 35. McManus H, Petoumenos K, Franic T et al. . Australian HIV Observational Database Determinants of suicide and accidental or violent death in the Australian HIV Observational Database. PLoS One 2014; 9:e89089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mathers BM, Degenhardt L. Examining non-AIDS mortality among people who inject drugs. AIDS 2014; 28(Suppl 4):S435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domingues CS, Waldman EA. Causes of death among people living with AIDS in the pre- and post-HAART eras in the city of São Paulo, Brazil. PLoS One 2014; 9:e114661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lartey M, Asante-Quashie A, Essel A, Kenu E, Ganu V, Neequaye A. Causes of death in hospitalized HIV patients in the early anti-retroviral therapy era. Ghana Med J 2015; 49:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sutton R, Lahuerta M, Abacassamo F et al. . Feasibility and acceptability of health communication interventions within a combination intervention strategy for improving linkage and retention in HIV care in Mozambique. J Acquir Immune Defic Syndr 2017; 74(Suppl 1):S29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horberg MA, Hurley LB, Towner WJ et al. . Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. J Acquir Immune Defic Syndr 2012; 60:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tu D, Belda P, Littlejohn D, Pedersen JS, Valle-Rivera J, Tyndall M. Adoption of the chronic care model to improve HIV care: in a marginalized, largely aboriginal population. Can Fam Physician 2013; 59:650–7. [PMC free article] [PubMed] [Google Scholar]
- 42. Burkholder GA, Tamhane AR, Appell LE et al. . Short communication: viral suppression is associated with increased likelihood of colorectal cancer screening among persons living with HIV/AIDS. AIDS Res Hum Retroviruses 2015; 31:519–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhodes CM, Chang Y, Regan S, Triant VA. Non-communicable disease preventive screening by HIV care model. PLoS One 2017; 12:e0169246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu H, Tang Z, Shen Z et al. . Sildenafil use and relevant risk factors among middle-aged or elderly male clients of female commercial sex workers in the central areas of Guangxi, China. Zhonghua Liu Xing Bing Xue Za Zhi 2014; 35:1218–22. [PubMed] [Google Scholar]
- 45. O’Brien D, Spelman T, Greig J et al. . Risk factors for mortality during antiretroviral therapy in older populations in resource-limited settings. J Int AIDS Soc 2016; 19:20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy 2007; 18:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mallitt KA, Grigoryan SR, Papoyan AS, Wand HC, Wilson DP. Access to antiretroviral therapy and survival in Eastern Europe and Central Asia: a case study in Armenia. J Int AIDS Soc 2014; 17:18795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Abdool Karim SS. Overcoming impediments to global implementation of early antiretroviral therapy. N Engl J Med 2015; 373:875–6. [DOI] [PubMed] [Google Scholar]
- 49. Joint United Nations Programme on HIV/AIDS. 90-90-90 an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]