Summary
SWIFT-C is an open-label, 2-cohort clinical trial that is assessing the safety and efficacy of 12 weeks of sofosbuvir plus ribavirin for treatment of acute hepatitis C virus in human immunodeficiency virus–infected patients. The relapse rate was high (41%), suggesting a need for better therapeutic options.
Keywords: hepatitis, human immunodeficiency virus, direct-acting antivirals, early infection, interferon-free
Abstract
Background.
Historically, acute hepatitis C virus (HCV) infection was treated with shorter durations of interferon-containing therapies. In the era of direct-acting antivirals (DAAs), it is unclear whether the efficacy of treatment achieved in chronic infection can be maintained with abbreviated courses of therapy during the acute phase.
Methods.
The sofosbuvir-containing regimens without interferon for treatment of acute HCV in HIV-1 infected individuals (SWIFT-C) is an open-label, 2-cohort clinical trial in which the first cohort assessed for the safety and efficacy of 12 weeks of sofosbuvir plus ribavirin for the treatment of acute HCV infection in participants with chronic human immunodeficiency virus type 1 (HIV-1) infection. This is a preplanned analysis of the first cohort, which had a planned accrual of 17 participants.
Results.
Seventeen men (11 Hispanic, 6 white, median age 45 years) were enrolled. Most (88%) had HCV genotype-1 infection and few (24%) had the favorable IL28B CC genotype. Median baseline HCV RNA was 2 280 000 IU/mL (interquartile range, 272 000–4 230 000). Ten participants (59%) achieved the primary outcome of SVR12 (90% confidence interval, 36%–78%), failing to establish noninferiority. All treatment failures were due to viral relapse (41%). There were no premature treatment discontinuations. The only factor that differed between participants who achieved SVR vs those who relapsed was ribavirin concentration at the end of treatment.
Conclusion.
Sofosbuvir-ribavirin for 12 weeks for the treatment of acute HCV genotype-1 infection in HIV-1–infected persons results in a high relapse rate. Preliminary studies of DAA combination therapies suggest improved response rates, although the adequate duration of therapy remains unclear.
Clinical Trials Registration.
Hepatitis C virus (HCV) infection affects more than 4 million persons annually in the United States [1]. The Centers for Disease Control and Prevention estimate that there are at least 30 000 new cases of acute HCV infection each year, which is likely an underestimate [2, 3]. In the United States, 2 populations that are especially vulnerable to acute HCV infection are young suburban residents due to increased injection heroin use and men who have sex with men [4, 5]. Historically, early identification and treatment of HCV during the acute phase resulted in significantly higher response rates and shorter durations of interferon-containing therapies [6–9]. However, in the era of direct-acting antivirals (DAAs), it is unclear whether the exceptional efficacy of treatment achieved in chronic infection can be maintained with abbreviated courses of therapy during the acute phase.
Sofosbuvir is a pan-genotypic nucleotide (NS5B) analogue of the HCV polymerase that was approved in combination with ribavirin by the US Food and Drug Administration (FDA) in December 2013 for the treatment of chronic HCV genotypes 2 and 3 infection [10]. Sofosbuvir in combination with ribavirin for 12-weeks duration has been shown to be safe and effective in participants with HCV genotype 2 infection, while for genotype 3 infection, extension to 24 weeks is required to achieve acceptable response rates [10]. Sofosbuvir was approved in combination with pegylated-interferon and ribavirin for genotypes 1 and 4 but was also used clinically without the interferon. The pan-genotypic nature of the sofosbuvir-ribavirin regimen, limited drug interaction profile, and lack of impact of baseline resistance makes this an attractive regimen for the treatment of acute hepatitis C, in particular for human immunodeficiency virus (HIV)/HCV–coinfected persons who may be on antiretrovirals with risk of drug interactions.
In this phase 1, open-label, 2-cohort clinical trial, we investigated the safety and efficacy of a 12-week sofosbuvir-ribavirin regimen in HIV-1–coinfected individuals with acute HCV infection or reinfection regardless of HCV genotype. The planned interim analysis of the first cohort presented here sought to determine if the course of sofosbuvir-ribavirin therapy could be further shortened to 8 weeks of treatment based on a noninferiority comparison to the historical control sustained virologic response (SVR) rate of 60% [7, 8, 11, 12].
METHODS
Study Design
A5327 is an ongoing, open-label, 2-cohort clinical trial in which cohort 1 was assessed for the safety and efficacy of 12 weeks of 400 mg of sofosbuvir in combination with weight-based ribavirin (RBV) dosed twice daily (1200 mg daily in participants with body weight ≥75 kg and 1000 mg daily in participants with body weight <75 kg) for the treatment of acute HCV infection in participants with chronic HIV-1 infection. The sample size of 17 participants for the first cohort was chosen to provide 90% power to show that the underlying true SVR12 rate is greater than the study-defined historical SVR rate of 60%, based on a 2-sided 90% confidence interval (CI) [7, 8, 11, 12]. An interim efficacy analysis was conducted to determine the appropriate length of therapy for the second cohort. The second cohort was planned to open for an 8-week treatment of sofosbuvir-RBV if the SVR rate was concluded to be noninferior to the study-defined historical SVR rate of 60%. The final analysis of data from cohort 1 is presented here.
Participant Population
Adults (aged ≥18 years) with chronic HIV-1 infection and documented confirmation of acute HCV infection or HCV reinfection within 6 months prior to entry were recruited from 8 AIDS clinical research sites in the United States. Acute HCV infection was defined per the European AIDS Treatment Network (NEAT) Acute Hepatitis C Infection Consensus Panel [11] and with exclusion of other causes of acute hepatitis as follows:
new (<24 weeks prior to study entry) alanine aminotransferase (ALT) elevation to ≥5× upper limit of normal (ULN), or >250 U/L in participants with documented normal ALT in the preceding 12 months, or ≥10× ULN, or >500 U/L in participants with abnormal, or no measured ALT baseline in the preceding 12 months with detectable HCV RNA excluding those with any prior positive anti-HCV
or
detectable HCV RNA with prior negative anti-HCV antibody or undetectable HCV RNA within the preceding 6 months.
Reinfection was defined by meeting the definition of acute infection and having documentation of clearance of prior infection (as evidenced by positive anti-HCV Ab) either spontaneously or after treatment with 2 negative HCV RNA a minimum of 6 months apart. All HCV genotypes were included.
Participants were not required to be on antiretroviral (ARV) therapy (ART), but those who were on ARVs were required to have evidence of HIV RNA suppression (HIV-1 RNA <50 copies/mL or < lower limit of quantification (LLOQ) if local assay LLOQ was >50 copies/mL). The ARVs that were excluded included didanosine, zidovudine, stavudine, and ritonavir-boosted tipranavir [10, 13]. Other inclusion criteria were hemoglobin ≥9 g/dL and creatinine clearance ≥60 mL/min, as calculated using by the Cockcroft-Gault equation. Full eligibility criteria are provided in the study protocol, available with the full text of this article on line.
We obtained ethics committee approval at all participating centers in accordance with the principles of the 2008 Declaration of Helsinki. All participants provided written informed consent before undergoing any protocol-specified procedures. An independent study monitoring committee reviewed progress of the study.
Study Endpoints and Procedures
The primary efficacy endpoint was the rate of SVR, which was defined as HCV RNA undetectable (< LLOQ TND [target not detected]) (Roche COBAS Taqman HCV Test 2.0 with LLOQ of 15 IU/mL) 12 weeks after date of last dose of study treatment (SVR12). The primary safety endpoint was the occurrence during treatment or within 28 days after treatment discontinuation of grade ≥2 adverse events (AEs) as defined by the Division of AIDS [14], including clinical and laboratory, serious AEs according to International Conference on Harmonisation (ICH) criteria, or treatment-limiting AEs. Secondary endpoints included viral kinetics, treatment emergent NS5B resistance, ribavirin pharmacokinetics, and baseline predictors of viral clearance.
Study visits after screening occurred at baseline (entry); on-treatment weeks 1, 2, 4, 8, and 12; and post-treatment weeks 2, 4, 8, 12, and 24. On-study HCV and HIV RNA testing was done centrally at Quest Diagnostics. HIV RNA was analyzed using Abbott RealTime HIV-1. Whole blood was obtained at screening or baseline for IL28B and ITPA genotyping (Roche Taqman). Self-reported adherence was assessed by participant self-report of missed dose during the 4 days prior to each visit.
Ribavirin was measured in plasma samples at weeks 1 or 2, 4, 8, and 12 of treatment. Ribavirin concentrations were determined using a validated liquid chromatography–tandem mass spectrometry assay at the Colorado Antiviral Pharmacology Laboratory. The assay was linear in the range of 10 to 10 000 ng/mL. The minimum quantifiable limit of the method was 10.0 ng/mL when 0.2 mL of human plasma was analyzed.
Sequencing of the NS5B region was completed at baseline and at the time of virologic failure for all participants exhibiting confirmed virologic relapse. The QIAamp Viral RNA Mini Kit (Qiagen) was used to isolate and measure the HCV RNA levels. HCV RNA was amplified using Illumina deep-sequencing technology (NS5B forward primer: TCTCAGCGACGGGTCWTGGTC, reverse primer: CCTGCAGMAAGYAGGAGTAGGC). Consensus assemblies were constructed using VICUNA and V-FAT programs. The classification of HCV genotypes and subtypes was confirmed comparing participant NS5B sequences to previously reported reference consensus sequences in the literature. The reference sequences used were 1a: H77, accession number AF009606, and 1b: Con1, accession number AJ238799. A phylogenetic tree was constructed using SplitsTree software.
Statistical Analyses
The primary efficacy objective was assessed by estimating the proportion of participants who started study treatment and achieved SVR12 in an intent-to-treat analysis. A 2-sided 90% CI was calculated for the proportion using the Blyth-Still-Casella (BSC) method for binomial outcomes. If this CI was entirely above 60%, then it would be concluded that there was reasonable evidence that the underlying true SVR12 rate was greater than 60% (ie, noninferior to 60%). Independent predictors of response (eg, HCV genotype, host IL28B genotype, ribavirin concentrations) were compared in those who achieved SVR vs those who relapsed using the Mann-Whitney U test (or Fisher exact). The primary safety objective was addressed by estimating the proportion of participants who had 1 of the defined AEs in the same way as the primary efficacy endpoint.
RESULTS
Participants
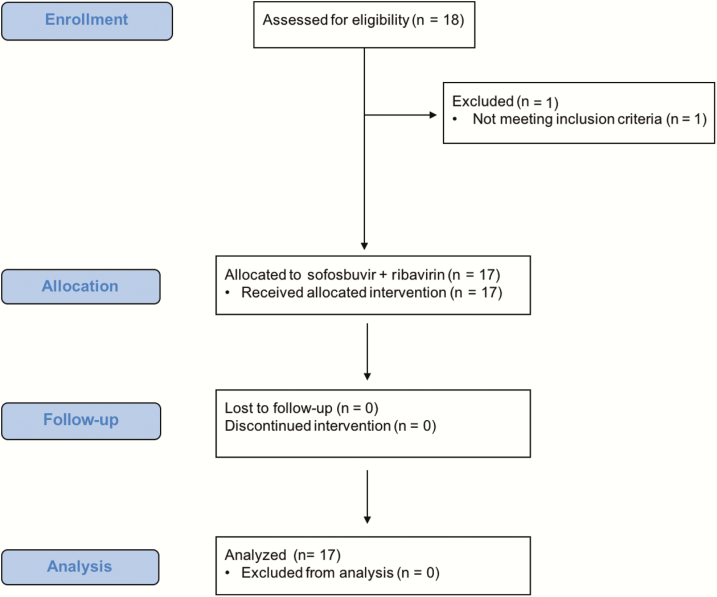
Seventeen participants were enrolled into cohort 1 between May 2014 and October 2014 (Figure 1). One participant was screened and deemed ineligible due to low hemoglobin outside of the inclusion criteria. All participants were male, the median age was 45 years (interquartile range [IQR], 41–47 years), 11 (65%) were Hispanic, and 6 (35%) were white, non-Hispanic (Table 1). All 17 participants in cohort 1 had new acute HCV infections. The median time from first laboratory evidence of acute HCV infection until study entry was 140 days (IQR, 121–151 days, and range 91–172 days). The majority of participants (88%) had HCV genotype 1 infection. Few participants (24%) had the favorable IL28B CC genotype. One participant had an HCV RNA <LLOQ TND at entry (the screening HCV RNA was detectable).
Figure 1.
SWIFT-C study Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Table 1.
Demographics Characteristics of Patients at Baseline (Entry)
| Characteristic |
Sofosbuvir-Ribavirin for 12 Weeks
(N = 17) |
|---|---|
| Male, n (%) | 17 (100) |
| Median age, y (IQR) | 45 (41–47) |
| White, n (%) | 6 (35) |
| Hispanic or Latino, n (%) | 11 (65) |
| Intravenous drug use ever, n (%) | 4 (24) |
| Reported history of sexually transmitted infection, n (%) | 8 (47) |
| IL28B CC favorable genotype, n (%) | 4 (24) |
| HCV genotype, n (%) | |
| 1a | 11 (65) |
| 1b | 2 (12) |
| 1, subtype unknown | 2 (12) |
| 2 | 1 (6) |
| First HCV infection, n (%) | 17 (100) |
| Median HCV RNA, IU/mL (IQR) | 2 280 000 (272 000–4 230 000) |
| Median HCV RNA, log10 IU/mL (IQR) | 6.36 (5.43–6.63) |
| HCV RNA ≥6 million IU/mL, n (%) | 2 (12) |
| Median time (days) from first evidence of acute infection | 140 (121, 151) |
| Median CD4, cells/µL (IQR) | 498 (387–612) |
| Human immunodeficiency virus type 1 RNA <50 copies/mL, n (%) | 15a (88) |
| Median alanine aminotransferase, mg/dL (IQR) | 181 (165–284) |
| Median aspartate aminotransferase, mg/dL (IQR) | 106 (69–159) |
| Median total bilirubin, mg/dL (IQR) | 0.70 (0.60–0.80) |
Abbreviations: HCV, hepatitis C virus; IQR, interquartile range.
aOne sample missing at baseline.
All but 1 participant was receiving ART (Table 2) prior to entry; that participant began ART at entry (Table 2). The median baseline CD4+ cell count was 498 cells/mm3 (IQR, 387–612 cells/mm3).
Table 2.
Antiretroviral Regimens
| Antiretroviral Regimens |
Sofosbuvir-Ribavirin for 12 Weeks
(N = 17) |
|---|---|
| Receiving human immunodeficiency virus treatment at screen, n (%) | 16 (94) |
| Protease inhibitor, n (%) | 4 (24) |
| Darunavir-ritonavir | 3 (18) |
| Lopinavir-ritonavir | 1 (6) |
| Nonnucleoside reverse transcriptase inhibitor, n (%) | 7 (41) |
| Efavirenz | 4 (24) |
| Rilpivirine | 3 (18) |
| Integrase inhibitor, n (%) | 7 (41) |
| Raltegravir | 3 (18) |
| Dolutegravir | 1 (6) |
| Elvitegravir | 3 (18) |
| Nucleoside reverse transcriptase inhibitor, n (%) | 17 (100) |
| Tenofovir-emtricitabine | 15 (88) |
| Abacavir-lamivudine | 2 (12) |
Efficacy
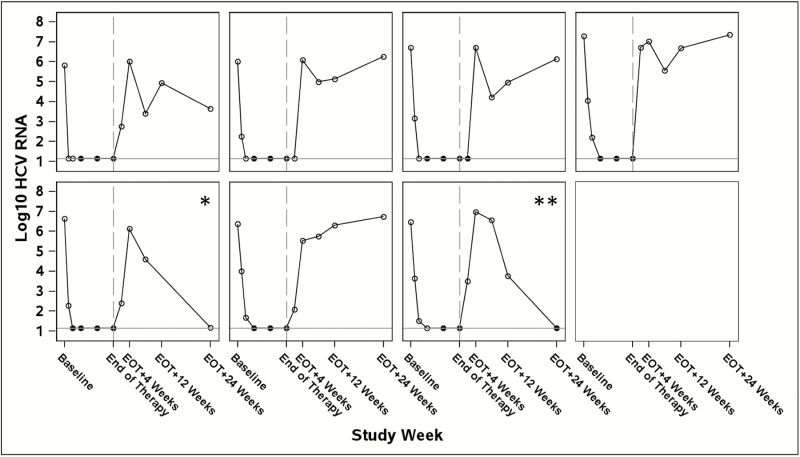
Among the 17 participants who were enrolled and treated, 10 (59%) achieved the primary outcome of SVR12 (90% CI, 36%–78%; Table 3), which failed to achieve noninferiority. All participants achieved undetectable HCV RNA while on therapy, and all treatment failures were consistent with HCV viral relapse (41%; Table 3). All relapses occurred by post-treatment week 4 (Figure 2). All participants completed the planned course of treatment; there were no premature treatment discontinuations. There were no ribavirin dose changes or premature ribavirin discontinuations.
Table 3.
Virologic Response During and After Therapy
| Response |
Sofosbuvir-Ribavirin for 12 Weeks
(N = 17) |
| HCV RNA < LLOQ TND | |
| On therapy, n (%) | |
| Week 1 | 2 (12) |
| Week 2 | 5 (29) |
| Week 4 | 12 (71) |
| Week 8 | 17 (100) |
| End of therapy (week 12), n (%) | 17 (100) |
| After end of therapy, n (%) | |
| Week 2 (SVR2) | 8 (57)a |
| Week 4 (SVR4) | 10 (59) |
| Week 12 (SVR12) | 10 (59)b |
| Virologic breakthrough during treatment | 0 |
| Relapse in patients with HCV RNA < LLOQ TND at end of therapy | 7 (41) |
Abbreviations: HCV, hepatitis C virus; LLOQ, lower limit of quantification; SVR, sustained virologic response; TND, target not detected.
aThree participants missed the week 2 visit (N = 14).
bOne participant missed the end-of-therapy week 12 visit but had detectable HCV RNA at the end of treatment week 4 visit and thus is counted as a relapse based on preceding result.
Figure 2.
Hepatitis C virus (HCV) RNA viral kinetic plots for those who did not achieve sustained virologic response (SVR) 12 (N = 7). HCV RNA (in log10 IU/mL) is shown on the y-axis and study follow-up visits are shown on the x-axis.
Legend: open markers = detectable HCV RNA; closed markers = undetectable HCV RNA log10 HCV RNA; reference line = 1.15 log10 IU/mL = undetectable HCV RNA; study week reference line = HCV end of therapy. Abbreviations: EOT, end of treatment; HCV, hepatitis C virus.
*Participant with EOT+24 weeks HCV RNA < LLOQ (lower limit of quantification) began ledipasvir-sofosbuvir 28 days after EOT+12 weeks visit and received 56 days of ledipasvir-sofosbuvir before going off study.
**Participant with EOT+24 weeks HCV RNA < LLOQ target not detected began ledipasvir-sofosbuvir 32 days after EOT+12 weeks visit and received 52 days of ledipasvir-sofosbuvir before going off study.
There were no differences between participants who achieved SVR vs those who relapsed in factors traditionally associated with virologic response including race/ethnicity, IL28B genotype, baseline HCV RNA, baseline ALT or total bilirubin, change in HCV RNA from screening to entry, or time from first laboratory evidence of acute infection to study entry (Supplemental Table 1A–D). Similarly, on-treatment viral kinetics (time to HCV RNA undetectable) were not predictive of treatment response.
Ribavirin Pharmacokinetics
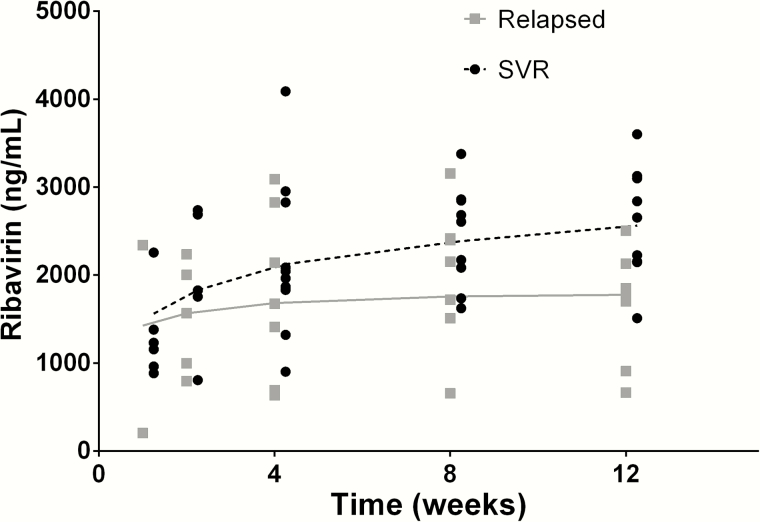
Ribavirin concentrations were similar at weeks 1 and 2 of treatment in those who achieved SVR vs those who relapsed but began to separate at week 4 and were significantly different at the end of treatment (Figure 3). Median (IQR) ribavirin concentrations at week 12 were 2655 ng/mL (2156, 3101) in those who achieved SVR vs 1745 ng/mL (908, 2131) in those who relapsed, P = .01.
Figure 3.
Ribavirin concentrations in participants who achieved sustained virologic response (SVR; black) vs those who experienced viral relapse (gray). Ribavirin concentration (in nanogram per milliliter) is shown on the y-axis and time on study (in weeks) is shown on the x-axis. The median (interquartile range) ribavirin concentration in those who achieved SVR was 2655 (2156–3101) ng/mL vs 1745 (908–2131) ng/mL in those who relapsed, P = .01. Smooth lines of the ribavirin concentrations are shown (dashed for SVR, solid for relapse). Abbreviation: SVR, sustained virologic response.
Viral Sequencing
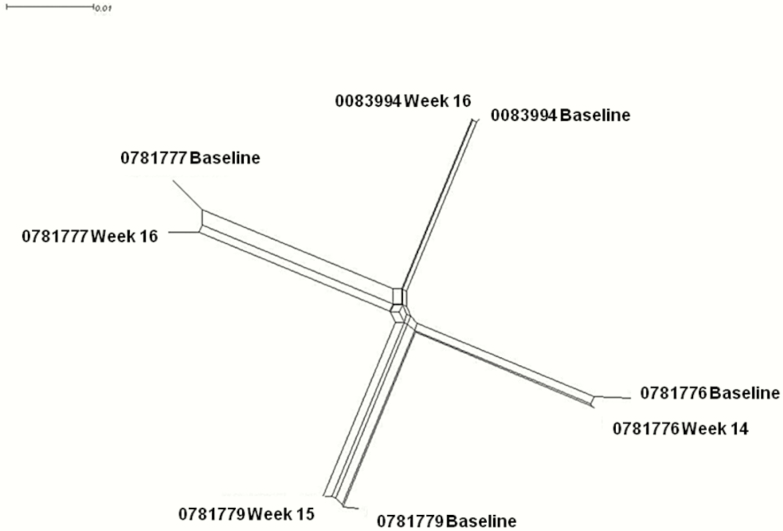
For the 7 participants who relapsed, HCV RNA was extracted from plasma samples at baseline and at the time of confirmed relapse. NS5B amplification was attempted on all 14 samples; however, 3 failed to generate NS5B product during amplification, likely due to low HCV RNA amount in these samples. NS5B sequences were amplified and sequenced from 7 relapsers: for 4 both pre- and post-HCV treatment, for 2 pre-treatment, and for 1 post-treatment. No S282T mutations were identified at any time point. Six treatment-emergent substitutions surfaced (G62N, F352L, S460P, Q578L, V421A, and K212R). The phylogenetic tree illustrated nearly identical viral NS5B sequences after HCV therapy (Figure 4) for the 4 participants with pre- and post-viremic samples, suggesting that post-therapy viremia was attributable to relapse rather than acute reinfection.
Figure 4.
Phylogenetic tree of paired hepatitis C virus NS5B sequences for 4 participants with relapse and available sequences pre- and post-therapy.
Safety
Eight of the participants (47%; 90% CI, 28%–69%) had a grade 2 or higher AE while on treatment or up to 28 days after treatment discontinuation (Table 4). No SAEs, according to ICH criteria, were reported and no treatment discontinuations due to an AE were reported. Four participants experienced a grade 2 sign or symptom; however, only 2 were considered “probably related” to the study regimen (insomnia and fatigue; Supplemental Table 2A). The single grade 3 sign or symptom was left toe pain and was not felt to be related to the study regimen. Five participants had a grade 2 laboratory abnormality. Two events were considered “probably or possibly related” to study treatment (elevated total bilirubin and low serum phosphate). Four participants experienced grade 2 and 3 laboratory AEs 4 weeks or more after discontinuing study treatment; all of these were elevations in liver enzymes and all occurred in participants with confirmed virologic relapse (Supplemental Table 2B).
Table 4.
Adverse Events and Discontinuations
| Event a |
Sofosbuvir-Ribavirin for 12 Weeks
(N = 17) |
| ≧Grade 2 AEs, n (%) | 8 (47) |
| Grade 3‒4 AE, n (%) | 1 (6) |
| Serious AE, n (%) | 0 |
| Treatment discontinuation due to AE, n (%) | 0 |
| Death, n (%) | 0 |
| Grade 3‒4 laboratory abnormality, n (%) | 0 |
Abbreviation: AE, adverse event.
aGrade 2 AE, insomnia, herpes lesion, fever with congestion, fatigue. Grade 3 AE, left big toe pain (not related). Grade 2 laboratory AE, elevated total bilirubin ×2, elevated fasting blood glucose, low phosphate, elevated fasting total cholesterol.
The mean change in CD4+ cell count from baseline to on-treatment week 12 was –50 cells/mm3 (90% CI, –93 to –7 cells/mm3), to 12 weeks post-treatment was +11 cells/mm3 (90% CI, –38 to +59 cells/mm3), and to 24 weeks post-treatment was +62 cells/mm3 (90% CI, +25 to +98 cells/mm3). All participants had HIV-1 RNA values <50 copies/mL during study treatment.
Adherence
Self-reported adherence was collected for study drugs. Four participants did not complete all adherence reporting. One participant reported a single missed dose of sofosbuvir and ribavirin and 2 participants reported missed doses of ribavirin twice in the past 4 days, overall suggesting excellent self-reported adherence.
DISCUSSION
Few published studies have investigated treatment approaches to acute HCV infection using DAA regimens in HIV-infected individuals. The first wave HCV NS3/4A protease inhibitors have been studied in combination with pegylated-interferon and ribavirin, achieving SVR12 rates of 84%–86% [15, 16]. The oral combination of sofosbuvir-ribavirin was the first approved interferon-free therapy for the treatment of chronic genotype 2 and 3 HCV infection, and a recent report of the DARE-C II trial suggested that a 6-week duration of sofosbuvir-ribavirin is insufficient for the treatment of acute HCV infection [10, 17]. The sofosbuvir-containing regimens without interferon for treatment of acute HCV in HIV-1 infected individuals (SWIFT-C) trial explored a 12-week duration of sofosbuvir-ribavirin in participants acutely infected with HCV, regardless of genotype, and chronically infected with HIV-1. Although all participants suppressed HCV RNA on therapy, 7 suffered relapse. While 12 weeks of sofosbuvir-ribavirin had a lower relapse rate than that reported in the DARE-C II trial, with approximately 25% genotype 3 infection, the response rate is still insufficient.
The overall SVR12 of 59% did not achieve noninferiority compared to the study-defined historical SVR rate of 60%. Relapse was the reason for treatment failure in all 7 participants who failed therapy. Reinfection, which has been reported to occur more frequently in high-risk individuals, is a less likely explanation for treatment failure based on the HCV sequence analysis, although reinfection from the same viral strain cannot be excluded [18, 19]. Viral kinetics were similar to chronic infection studies; 70% of participants had achieved an undetectable HCV RNA by on-treatment week 4 and all participants had an undetectable HCV RNA by on-treatment week 8. The majority of participants (80%) who had the slowest viral kinetics (first undetectable HCV RNA at week 8) achieved SVR12. Neither baseline HCV RNA nor IL28B genotype were associated with treatment outcome; however, this phase 1 study was underpowered for exploring predictors of relapse.
The only clinical or demographic factor that differed between participants who achieved SVR vs those who relapsed was ribavirin concentration at week 12 of sofosbuvir-ribavirin treatment. Ribavirin concentrations were 52% higher in those who achieved SVR. The cause of this discrepancy in ribavirin exposures is unclear but could relate to pharmacokinetic variability, a chance imbalance between groups, or differences in adherence despite the high levels of self-reported adherence. All participants had high self-reported adherence to sofosbuvir-ribavirin, but self-report has been shown to overestimate medication adherence [20–22]. Ribavirin has a long plasma half-life (7–12 days) [23–25], thus concentrations reflect cumulative drug dosing. There are no data on expected ribavirin exposures in individuals with perfect adherence, but mean (standard deviation) ribavirin concentrations in the 7 SWIFT-C participants who relapsed were lower than the median (IQR) ribavirin steady-state concentrations in 388 HCV-monoinfected individuals with genotype 3 disease in the phase 3 trials of sofosbuvir-ribavirin (1745 [908, 2131] vs 2750 [2229, 3316] ng/mL) [26] and the 20 participants who received weight-based ribavirin plus sofosbuvir in the NIAID SPARE trial (1745 [908, 2131] vs 2357 [1883, 3085] ng/mL) [27]. The DARE-C II trial did report a lower numerical ribavirin concentration in relapsed participants, but this did not meet statistical significance. This difference may be due to the shorter course of therapy and thus less drop-off in adherence.
Natural clearance of HCV infection can bias results of small single-arm trials in acute HCV infection toward greater perceived benefit of the intervention. However, overall the prevalence of the favorable IL28B CC allele was low (24%) and the median entry HCV RNA was high (6.36 log10 IU/mL), suggesting a low influence of these potential confounders. The median time from first laboratory evidence of acute infection to study entry and treatment dosing was 140 days (approximately 20 weeks) with the range of 91 to 172 days (13 to 24 weeks), confirming enrollment in the 12- to 24-week acute infection window. While there is potential that treatment earlier (first 12 weeks) in the course of acute HCV infection could improve treatment response with this regimen, this design approach also risks treating patients more likely to spontaneously clear the infection.
Overall the sofosbuvir-ribavirin regimen was well tolerated, with no SAEs and no treatment discontinuations. No participant required RBV dose reduction. Significant (≥ grade 2) AEs deemed related to the study drugs were uncommon. As has been previously reported on ribavirin, absolute CD4 count decreased by approximately 50 cells/mm3 (90% CI, –93 to –7 cells/mm3), but by 24 weeks post-treatment participants had gained an additional 62 cells/mm3 (90% CI, 25 to 98 cells/mm3) from baseline. No participant experienced HIV viral breakthrough during the study.
The primary limitations of this study are the result of the phase 1 design. The study was only powered to show noninferiority of the primary outcome, SVR12, to the study-defined historical SVR rate assuming a high underlying true response rate. Thus all secondary objectives were exploratory and underpowered, limiting our ability to determine risk factors for relapse; although no obvious trends emerged with the exception of ribavirin exposures. In addition, the majority of participants had genotype 1 infection, for which this regimen is not approved.
In conclusion, the sofosbuvir-ribavirin regimen for 12 weeks for the treatment of acute HCV infection in HIV-1–infected persons achieved on-treatment undetectable HCV RNA in all participants but suffered a high (41%) relapse rate. In line with the DARE-C II trial, more effective therapies are needed for the treatment of acute HCV infection in HIV-infected individuals. Due to the rapid advancement in the field, a protocol amendment was completed for A5327 to proceed with cohort 2 using 8 weeks of the DAA combination regimen of ledipasvir, a NS5A inhibitor, and sofosbuvir, which is FDA approved as a fixed dose combination tablet regimen for 12 weeks for the treatment of chronic HCV genotype 1, 4, 5, and 6 infections. Emerging experience with this regimen for 6 weeks has been reported in acute HCV infection in HIV-infected [28] and HIV-uninfected [29] cohorts. However, to date, all-oral DAA studies in acute HCV are heterogeneous and exploratory in nature. Overall, most suggest that the previously recognized immunologic benefit of therapy in the acute infection period may have less impact with interferon-free therapies. Further studies, including cohort 2 of SWIFT-C, which is ongoing and evaluating 8 weeks of daily ledipasvir-sofosbuvir (90 mg/400 mg) in this population, will inform our approach to the treatment of acute HCV infection.
Supplementary Material
Notes
Acknowledgments. The study team thanks all participants for taking part in this study, the AIDS Clinical Trials Group, Study Data and Monitoring Committee, participating Clinical Research Sites (CRSs), and the specialty laboratories. We also thank Gilead Sciences for providing sofosbuvir and ribavirin and funding Hepatitis C Virus RNA testing.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the (NIH; UM1 AI068634, UM1 AI068636, and UM1 AI106701). The research was also been supported, in part, by a grant(s) funded by the Center for AIDS Research and Gilead Sciences. J. K. was supported by the National Institutes of Health [R01 DA040499].
Potential conflicts of interest. S. N. reports grants from Abbvie, Bristol Meyers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck, Tacere, and Vertex Pharmaceuticals; nonfinancial support from Eviral Hep, IAS-USA, Platform Q Health Inc., and Practice Point Communications; and personal fees from the Infectious Diseases Society of America, outside the submitted work. D. S. F. owns stock in Gilead. R. C. reports grants from Gilead Sciences, Mass Biologics, Merck, Abbvie, BMS, and Boehringer Ingelheim, outside the submitted work. All other authors have no reported conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144:705–14. [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention, C.f.D.C.a.P., Statistics and Surveillance. 2015. [Google Scholar]
- 3. Onofrey S, Aneja J, Haney GA, et al. Underascertainment of acute hepatitis C virus infections in the U.S. surveillance system: a case series and chart review. Ann Intern Med 2015; 163:254–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zibbell JE, Iqbal K, Patel RC, et al. ; Centers for Disease Control and Prevention Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. Morb Mortal Wkly Rep 2015; 64:453–8. [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention, C.f.D.C.a.P., Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men—New York City, 2005–2010. Morb Mortal Wkly Rep 2011; 60:945–50. [PubMed] [Google Scholar]
- 6. Jaeckel E, Cornberg M, Wedemeyer H, et al. ; German Acute Hepatitis C Therapy Group Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med 2001; 345:1452–7. [DOI] [PubMed] [Google Scholar]
- 7. Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat 2010; 17:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews GV, Hellard M, Haber P, et al. ; Australian Trial in Acute Hepatitis C Study Group Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis 2009; 48:650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boesecke C, Stellbrink HJ, Mauss S, et al. Does baseline HCV genotype have an impact upon treatment outcome of acute HCV infection in HIV co-infected individuals. 18th Conference on Retroviruses and Opportunistic Infections February 27-March 2, 2011; Boston, MA., 2011. Abstract 113. [Google Scholar]
- 10. Sofosbuvir, U.S. prescribing information. Foster City, CA: Gilead Sciences, 2015. [Google Scholar]
- 11. European, A.T.N.A.H.C.I.C.P., Acute hepatitis C in HIV-infected individuals: recommendations from the European AIDS Treatment Network (NEAT) consensus conference. AIDS 2011. 25: 399–409. [DOI] [PubMed] [Google Scholar]
- 12. Deterding K, Grüner N, Buggisch P, et al. ; Hep-Net Acute HCV-III Study Group Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis 2013; 13:497–506. [DOI] [PubMed] [Google Scholar]
- 13. COPEGUS (RBV, U.T., US Prescribing Information. Roche Laboratories Inc., Nutley, NJ: p. Revised: February 2013. [Google Scholar]
- 14. US Department of Health and Human Services, N.I.o.H., National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 2.0. [November 2014]. Available from: http://rsc.tech-res.com/Document/safetyandpharmacovigilance/DAIDS_AE_GRADING_TABLE_v2_NOV2014.pdf. [Google Scholar]
- 15. Fierer DS, Dieterich DT, Mullen MP, et al. ; New York Acute Hepatitis C Surveillance Network Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men. Clin Infect Dis 2014; 58:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hullegie SJ, Claassen MA, van den Berk GE, et al. Boceprevir, peginterferon and ribavirin for acute hepatitis C in HIV infected patients. J Hepatol 2016; 64:807–12. [DOI] [PubMed] [Google Scholar]
- 17. Martinello M, Gane E, Hellard M, et al. Sofosbuvir and ribavirin for 6 weeks is not effective among people with recent hepatitis C virus infection: the DARE-C II study. Hepatology 2016; 64:1911–21. [DOI] [PubMed] [Google Scholar]
- 18. Grady BP, Schinkel J, Thomas XV, Dalgard O. Hepatitis C virus reinfection following treatment among people who use drugs. Clin Infect Dis 2013; 57Suppl 2:S105–10. [DOI] [PubMed] [Google Scholar]
- 19. Ingiliz P, Krznaric I, Stellbrink HJ, et al. Multiple hepatitis C virus (HCV) reinfections in HIV-positive men who have sex with men: no influence of HCV genotype switch or interleukin-28B genotype on spontaneous clearance. HIV Med 2014; 15:355–61. [DOI] [PubMed] [Google Scholar]
- 20. Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. J Infect Dis 2002; 185Suppl 2:S143–51. [DOI] [PubMed] [Google Scholar]
- 21. Miller LG, Hays RD. Measuring adherence to antiretroviral medications in clinical trials. HIV Clin Trials 2000; 1:36–46. [DOI] [PubMed] [Google Scholar]
- 22. Paterson DL, Potoski B, Capitano B. Measurement of adherence to antiretroviral medications. J Acquir Immune Defic Syndr 2002; 31Suppl 3:S103–6. [DOI] [PubMed] [Google Scholar]
- 23. Wu LS, Rower JE, Burton JR, Jr, et al. Population pharmacokinetic modeling of plasma and intracellular ribavirin concentrations in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother 2015; 59:2179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rower JE, Meissner EG, Jimmerson LC, et al. Serum and cellular ribavirin pharmacokinetic and concentration-effect analysis in HCV patients receiving sofosbuvir plus ribavirin. J Antimicrob Chemother 2015; 70:2322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khakoo S, Glue P, Grellier L, et al. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol 1998; 46:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shih YC, Bekele NB, Xu Y. Use of Bayesian net benefit regression model to examine the impact of generic drug entry on the cost effectiveness of selective serotonin reuptake inhibitors in elderly depressed patients. Pharmacoeconomics 2007; 25:843–62. [DOI] [PubMed] [Google Scholar]
- 27. Hatu G, Bailly F, Pourcelot E, et al. Lower ribavirin biodisponibility in patients with HIV-HCV coinfection in comparison with HCV monoinfected patients. BMC Infect Dis 2014; 14:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rockstroh J, Bhagani S, Hyland RH, et al. Ledipasvir/sofosbuvir for 6 weeks in HIV-infected patients with acute HCV infection. Conference on Retroviruses and Opportunistic Infections (CROI) February 22–25, 2016: p. Boston. Abstract 154LB. [Google Scholar]
- 29. Deterding K, Spinner CD, Schott E, et al. Ledipasvir plus sofosbuvir fixed-dose combination for six weeks in patients with acute hepatitis C virus genotype 1 monoinfection (HepNet Acute HCV I): an open-label, single-arm, phase 2 study. Lancet Infect Dis 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.