Pregnancy malaria induces a proinflammatory immune response. We find that malaria-related systemic inflammatory responses such as CXCL9 are associated with risks of pregnancy loss and preterm delivery.
Keywords: placental malaria, CXCL9, pregnancy loss, preterm delivery
Abstract
Background
Pregnancy malaria (PM) is associated with a proinflammatory immune response characterized by increased levels of cytokines and chemokines such as tumor necrosis factor–α, interferon-γ, interleukin 10 (IL-10), and CXCL9. These changes are associated with poor outcomes including low birthweight delivery and maternal anemia. However, it is unknown if inflammatory pathways during malaria are related to pregnancy loss and preterm delivery (PTD).
Methods
Cytokine and chemokine levels were measured in maternal peripheral blood at enrollment, gestational week 30–32, and delivery, and in placental blood, of 638 women during a longitudinal cohort study in Ouelessebougou, Mali. Plasmodium falciparum infection was assessed by blood smear microscopy at all visits.
Results
PM was associated with increased levels of cytokines and chemokines including IL-10 and CXCL9. In a competing risks model adjusted for known covariates, high CXCL9 levels measured in the peripheral blood during pregnancy were associated with increased risk of pregnancy loss and PTD. At delivery, high IL-10 levels in maternal blood were associated with an increase in pregnancy loss, and increased IL-1β levels in placental blood were associated with pregnancy loss and PTD.
Conclusions
PM is associated with increased proinflammatory cytokine and chemokine levels in placental and maternal peripheral blood. Systemic inflammatory responses to malaria during pregnancy predict increased risk of pregnancy loss and PTD.
Clinical Trials Registration
NCT01168271.
Pregnant women are more susceptible to Plasmodium falciparum infection compared with their nonpregnant counterparts [1]. Susceptibility diminishes with successive pregnancies, distinguishing malaria from other infections that afflict pregnant women [1]. Pregnancy malaria (PM) is associated with poor pregnancy outcomes; severe maternal anemia and low birth weight deliveries are common sequelae in Africa [2]. In areas of low malaria transmission where pregnant women have little or no immunity to malaria, PM causes severe acute syndromes in mothers as well as fetal mortality [2]. In high transmission zones, PM is not associated with severe acute syndromes in the mother, but is associated with prematurity and stillbirth [3, 4]. In an analysis of hospital records between 1948 and 2002, the perinatal mortality rate was significantly higher in malaria-endemic than nonmalarious areas [5]. Meta-analysis of studies reporting placental malaria infection status similarly showed increased risk of stillbirth in women with placental malaria (odds ratio, 2.19; 95% confidence interval [CI], 1.49–3.22) [5].
During PM, parasite sequestration in the placenta is followed by the accumulation of an inflammatory infiltrate consisting mainly of macrophages. In areas of high malaria transmission, placental inflammatory infiltrates are more common in primigravidae, as they lack specific immunity to placental parasites. The placental infiltrate has been associated with poor pregnancy outcomes such as low birthweight (LBW) delivery and preterm delivery (PTD) [6, 7]. Cytokines and chemokines are secreted by sequestered immune cells as well as placental villi [8]. Levels of tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), interleukin (IL) 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1, macrophage migration inhibitory factor (MIF), CXCL8, CXCL9, CXCL-10, and CXCL13 are higher in infected vs uninfected women [8–16]. High levels of TNF-α, IFN-γ, the chemokine CXCL9 that is regulated by IFN-γ, and MIF have been associated with an increase in LBW deliveries, particularly among primigravid women [9, 13, 16–18]. High placental MIF levels were also associated with stillbirths [16].Currently, the relationship between placental or peripheral inflammation and PTD or early pregnancy loss in the context of malaria remains underexplored. In this study, we measured levels of cytokines and chemokines (IL-1β, IL-6, IL-10, TNF-α, IL-8, and CXCL9) at several time points during pregnancy, and related these to PTD as well as pregnancy loss (defined as miscarriage, stillbirth, and early neonatal death).
METHODS
Human Subjects and Clinical Procedures
Women participating in this study were enrolled between November 2010 and October 2013 into a longitudinal cohort conducted by the Immunoepidemiology Project in Ouelessebougou, Mali. Pregnant women aged 15–45 years presenting at the antenatal clinic were asked to participate and gave informed consent after receiving a study explanation form and an oral explanation from the study clinicians in their native language. The protocol and study procedures were approved by the institutional review board of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (ClinicalTrials.gov identifier NCT01168271), and the Ethics Committee of the Faculty of Medicine, Pharmacy and Dentistry at the University of Bamako, Mali.
After enrollment, women underwent a clinical evaluation including obstetrical examination and a thorough medical history review of the current and previous pregnancies. Follow-up included scheduled monthly clinical examination until 1 month postdelivery and at any time participants felt sick. Malaria infections were treated with quinine or with artemether-lumefantrine. Women received 0–3 doses of intermittent presumptive treatment with sulfadoxine-pyrimethamine (IPTp-SP), depending on gestational age at enrollment, number of doses already taken prior to enrollment, and use of other drugs to treat malaria episodes during follow-up visits. Gestational age was determined by ultrasound examination with a Siui CTS-7700+ ultrasound scanner. Gestational age was estimated by crown–rump length prior to gestational week 14 and by biparietal diameter and femur length afterward. PM was defined as the presence of any parasite in peripheral blood or placental blood smear. Blood smear was concluded to be negative after examination of at least 100 high-power fields in the thick smear. Pregnancy loss includes cases of miscarriage, stillbirth, and early neonatal death. Miscarriage was defined as pregnancy ending before gestational week 28, and stillbirth as delivery of a nonviable baby at a gestational age of ≥28 weeks. Early neonatal death was defined as death within the first 7 days. PTD was defined as birth prior to 37 weeks’ gestational age.
Cytokine and Chemokine Assays
Some blood samples were not available at time of assay. These analyses included samples from 638 women: 603, 433, and 591 peripheral blood samples collected at enrollment, gestational week 30–32, and delivery, respectively, and 589 placental blood samples.
Plasma samples were analyzed using a multiplex bead-based platform (Bioplex, Bio-Rad, Irvine, California). The multiplex assay was developed in-house as previously described [18, 19]. The detection limits for the different analytes were as follows: IL-1β, 2.3 pg/mL; IL-6, 2.5 pg/mL; IL-8, 3.1 pg/mL; IL-10, 2.4 pg/mL; TNF-α, 1.9 pg/mL; CXCL9, 149.7 pg/mL.
Statistical Analysis
Only singleton pregnancies were included in these analyses.
Differences in soluble mediator levels between groups at each time point were analyzed by Mann-Whitney test, using JMP version 12 software (SAS Institute, Cary, North Carolina). P values were corrected for multiple comparisons by Holm method, using R function p.adjust. Adjusted P values <.05 were considered significant. Adjusted P values are reported.
To estimate if cytokine and chemokine levels at enrollment and at gestational week 30–32 predict increased risk of pregnancy loss and PTD, the Lunn-McNeil competing risks model (LMCRM) [20] was fitted, including all 6 soluble mediators (log-transformed) in 1 model. The model was adjusted for several covariates that are associated with pregnancy outcomes. Time-dependent variables were used for the covariates cumulative IPTp use, malaria infection during follow-up visits, and malaria at the time of antibody measurement. In addition, the model was adjusted for the baseline covariates gravidity and insecticide-treated net (ITN) use. The analysis was conducted in R with packages survival and data.table.
Multinomial logistic regressions were used for cross-sectional analyses at delivery (maternal peripheral blood and placental blood) to examine the relationships between soluble mediators and pregnancy outcomes. The 3 levels of the categorical outcome were defined as pregnancy loss, PTD, and term delivery. Term delivery served as the baseline-category reference group. The predictors for the delivery include cytokine levels (log-transformed), adjusted for gravidity, malaria infection during follow-up visits, malaria at the time of antibody measurement, IPTp, and ITN. These analyses were carried out in R (version 3.3.2 using the function multinom from the nnet package). For these cross-sectional analyses, a P value <.05 was considered significant.
RESULTS
Study Population
The number of samples and gestational ages at visit, stratified by gravidity and pregnancy outcomes, are shown in Table 1. Gestational age at enrollment did not differ significantly between the 3 gravid groups among women with term delivery, pregnancy loss, or PTD. At delivery, the mean gestational age of term newborns was significantly higher in multigravid than primigravid and secundigravid women (mean [standard deviation] gestational age, 39.2 [1.3] years in primigravidae; 39.2 [1.1] years in secundigravidae; and 39.6 [1.2] years in multigravidae; P = .007).
Table 1.
Study Population Stratified By Maternal Gravidity and Malaria Infection Status at Each Visit
| Visit | Group | PM Status | No. | Gestational Age, wka, Mean (SD) | P Valueb |
|---|---|---|---|---|---|
| Enrollment | Primigravid | PM- | 94 | 21.7 (7.1) | .8 |
| PM+ | 63 | 22.0 (4.7) | |||
| Secundigravid | PM– | 89 | 20.5 (6.5) | .1 | |
| PM+ | 29 | 22.5 (5.5) | |||
| Multigravid | PM– | 278 | 21.1 (6.5) | .6 | |
| PM+ | 50 | 21.8 (6.5) | |||
| GW 30–32 | Primigravid | PM– | 99 | ||
| PM+ | 10 | ||||
| Secundigravid | PM– | 78 | |||
| PM+ | 3 | ||||
| Multigravid | PM– | 230 | |||
| PM+ | 13 | ||||
| Delivery | Primigravid | PM– | 128 | 38.7 (1.9) | .0009 |
| PM+ | 24 | 36.9 (2.3) | |||
| Secundigravid | PM– | 102 | 38.8 (1.5) | .4 | |
| PM+ | 12 | 38.6 (1.4) | |||
| Multigravid | PM– | 294 | 39.4 (1.6) | .9 | |
| PM+ | 31 | 39.5 (1.4) | |||
| Pregnancy outcome | Primigravid | Pregnancy loss | 17 | ||
| PTD | 25 | ||||
| Secundigravid | Pregnancy loss | 8 | |||
| PTD | 14 | ||||
| Multigravid | Pregnancy loss | 17 | |||
| PTD | 8 |
Pregnancy loss includes miscarriages, stillbirths, and early neonatal death.
Abbreviations: GW, gestational week; PM, pregnancy malaria; PTD, preterm delivery; SD, standard deviation.
aGestational age at delivery includes viable singleton births.
bPM– and PM+ were compared by Mann-Whitney test adjusted for multiple comparison by Holm method.
Malaria infection was most common at enrollment: 40.4%, 24.6%, and 15.2% of primigravid, secundigravid, and multigravid women, respectively, were infected at enrollment, whereas 15.8%, 10.5%, and 9.5% of primigravid, secundigravid, and multigravid women, respectively were infected at delivery. Infection prevalence was higher among primigravid than multigravid women at enrollment and at delivery (P < .0001 and P = .06, respectively). Among primigravidae, secundigravidae, and multigravidae, 67.1%, 73.7%, and 76.2%, respectively, received ≥2 doses of IPTp-SP. Positive blood smears were treated per Malian Ministry of Health guidelines, and 55%, 43%, and 33% of primigravidae, secundigravidae, and multigravidae, respectively, received treatment for malaria during study follow-up.
Infection Modifies Cytokine and Chemokine Levels
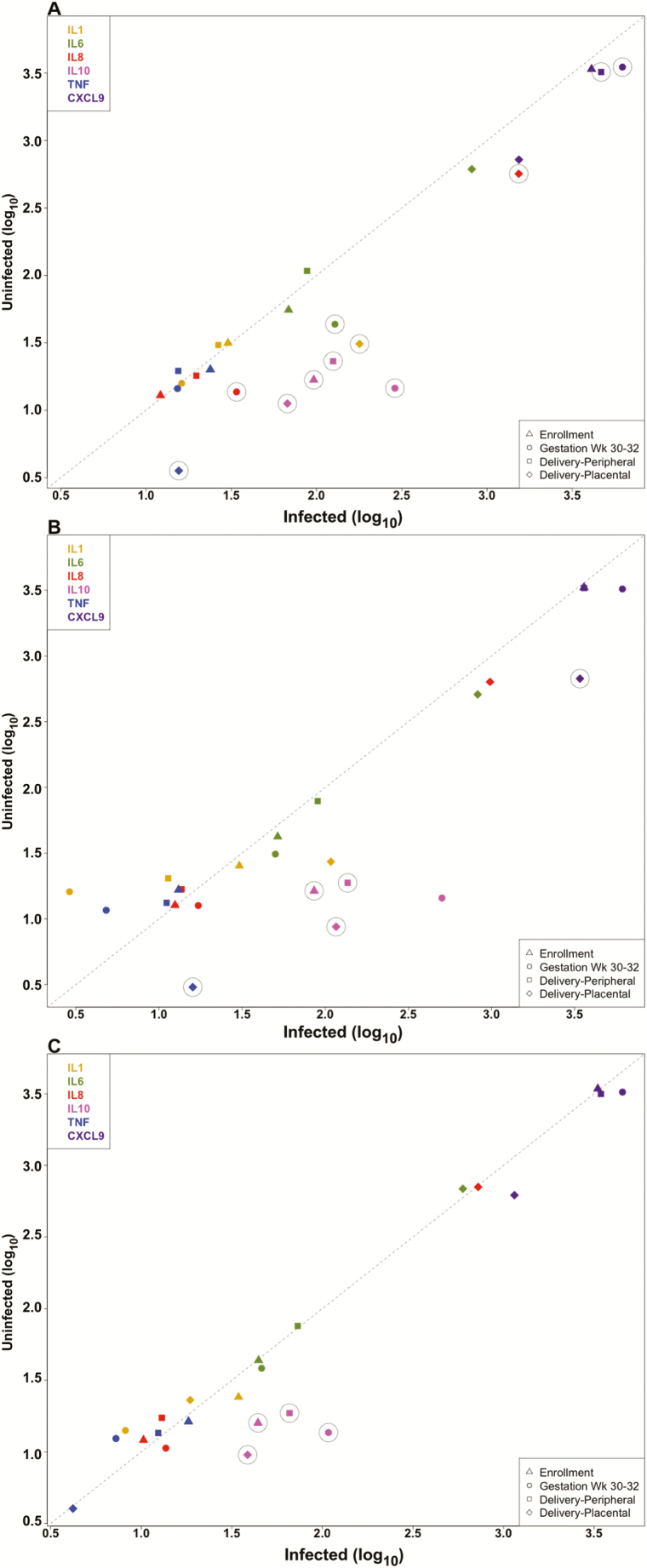
Cytokines and chemokines examined here include those previously associated with low birthweight delivery in malaria-infected primigravidae (TNF-α and CXCL9), a marker of inflammatory placental malaria (IL-10), and those involved in term delivery (IL-1β, IL-6, and IL-8) [9, 18, 21, 22]. Levels were analyzed in peripheral blood samples collected during pregnancy and at delivery, and in placental blood (Figure 1 and Supplementary Table 1). Peripheral blood IL-10 levels were significantly higher in infected than uninfected primigravidae and multigravidae at all time-points and in secundigravidae at enrollment and at delivery (P < .05). At gestational week 30–32, levels of IL-6, IL-8, and CXCL9 were higher in infected than uninfected primigravidae; at delivery, CXCL9 levels were higher in infected than uninfected primigravidae (P < .05). At enrollment, peripheral IL-10 levels were higher in infected than uninfected women for all gravidity groups (Supplementary Table 1), but levels were significantly lower in infected multigravidae vs infected primigravidae and secundigravidae (mean level, 78.4 in primigravidae, 73.1 in secundigravidae, and 43.9 in in multigravidae; P < .0001).
Figure 1.
Soluble mediator levels during pregnancy in malaria-infected and uninfected women. Mean cytokine and chemokine levels in primigravidae (A), secundigravidae (B), and multigravidae (C) are shown. Circles around data points indicate that differences between uninfected and infected women were statistically significant for that endpoint. Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
PM was associated with higher placental levels of IL-1β, IL-8, IL-10, and TNF-α in primigravidae, of IL-10, TNF-α, and CXCL9 in secundigravidae, and of IL-10 in multigravidae (Figure 1 and Supplementary Table 1).
Levels of Soluble Mediators Predict Risk of Pregnancy Loss and Preterm Delivery
We examined whether cytokine and chemokine levels during pregnancy predict pregnancy loss (defined as miscarriage, stillbirth, and early neonatal death) and PTD. Separately, we examined the associations between cytokine and chemokine levels measured at delivery in maternal peripheral blood and in the placenta with these 2 outcomes. Table 2 shows the results from the LMCRM for associations between cytokine and chemokine levels during pregnancy and pregnancy outcomes adjusted for multiple covariates described above. To examine the independent contribution of each cytokine and chemokine measured, all were included in the same model.
Table 2.
Competing Risk Analysis on the Effect of Cytokine and Chemokine Levels During Pregnancy on Pregnancy Loss and Preterm Delivery
| Soluble Mediator | Pregnancy Loss | PTD | Pregnancy Loss and PTD |
|---|---|---|---|
| IL-1β | 1.00 (.96–1.04) | 0.99 (.96–1.02) | 0.99 (.97–1.02) |
| IL-6 | 0.93 (.87–.99) | 0.97 (.91–1.03) | 0.95 (.91–.99) |
| IL-10 | 1.00 (.91–1.1) | 1.01 (.93–1.1) | 1.00 (.93–1.07) |
| TNF-α | 1.04 (.98–1.11) | 1.02 (.97–1.08) | 1.03 (.99–1.08) |
| IL-8 | 1.02 (.94 –1.11) | 0.94 (.88–1.00) | 0.98 (.93–1.03) |
| CXCL9 | 1.36 (1.08–1.72) | 1.44 (1.15–1.81) | 1.41 (1.20–1.66) |
Data are presented as hazard ratio (95% confidence interval). The Lunn and McNeil competing risks model was used to assess the effect of cytokine and chemokine levels during pregnancy on outcomes adjusted for factors that might influence pregnancy outcomes (gravidity, malaria infection, insecticide-treated net use, number of intermittent presumptive treatment doses).
Abbreviations: IL, interleukin; PTD, preterm delivery; TNF, tumor necrosis factor.
Increased CXCL9 levels predicted increased risk of pregnancy loss (hazard ratio [HR], 1.365; P = .009) and PTD (HR, 1.446; P = .001), while IL-6 was associated with reduced risk of pregnancy loss. Because an increase in CXCL9 levels predicted both increased risk of pregnancy loss and PTD, we further evaluated the differences between these 2 competing risks. The coefficients corresponding to the differences between these 2 competing risks using LMCRM were not statistically significant and the 2 outcomes were combined: CXCL9 levels predicted an increase of the combined risks of pregnancy loss and PTD (HR, 1.41; P < .0001), and IL-6 reduced the risk (HR, 0.95 [95% CI, .91–.99]; P = .02).
At delivery, increased IL-10 levels in maternal peripheral blood were associated with increased risk of pregnancy loss (relative risk, 1.1; P = .02) (Table 3). High placental IL-1β levels were associated with PTD, and with stillbirths or early neonatal deaths (Table 3).
Table 3.
Multinomial Regression Analysis of Cytokines and Chemokines at Delivery on Pregnancy Loss and Preterm Delivery
| Blood Sample | Outcome | Soluble Mediator | RR (95% CI) |
|---|---|---|---|
| Delivery maternal peripheral | Pregnancy loss | IL-1β | 0.99 (.96–1.00) |
| IL-6 | 1.11 (.97–1.30) | ||
| IL-10 | 1.1 (1.00–1.23) | ||
| TNF-α | 0.99 (.92–1.10) | ||
| IL-8 | 0.93 (.85–1.00) | ||
| CXCL9 | 1.21 (.96–1.50) | ||
| PTD | IL-1β | 0.97 (.94–1.00) | |
| IL-6 | 0.98 (.90–1.10) | ||
| IL-10 | 1.00 (.89–1.10) | ||
| TNF-α | 1.01 (.95–1.10) | ||
| IL-8 | 0.95 (.87–1.00) | ||
| CXCL9 | 1.15 (.91–1.50) | ||
| Placental blood | Pregnancy lossa | IL-1β | 1.1 (1.00–1.2) |
| IL-6 | 1.05 (.97–1.10) | ||
| IL-10 | 1.04 (.96–1.10) | ||
| TNF-α | 1.02 (.96–1.10) | ||
| IL-8 | 1.01 (.92–1.10) | ||
| CXCL9 | 0.94 (.89–1.00) | ||
| PTD | IL-1β | 1.10 (1.00–1.10) | |
| IL-6 | .096 (.88–1.10) | ||
| IL-10 | 0.96 (.90–1.00) | ||
| TNF-α | 0.99 (.93–1.00) | ||
| IL-8 | 0.99 (.91–1.10) | ||
| CXCL9 | 1.00 (.95–1.10) |
Models were adjusted for factors that might influence pregnancy outcomes (gravidity, malaria infection, insecticide-treated net use, number of intermittent presumptive treatment doses).
Abbreviations: CI, confidence interval; IL, interleukin; RR, relative risk; PTD, preterm delivery; TNF, tumor necrosis factor.
aPregnancy loss includes stillbirths and early neonatal death cases.
Among the covariates included in the analysis, gravidity was significantly associated with pregnancy outcomes. Primigravidae and secundigravidae had higher risk of pregnancy loss and PTD (primigravidae: HR, 4.03 [95% CI, 2.12–7.67], P < .0001; secundigravidae: HR, 2.88 [95% CI, 1.41–5.88], P = .004), compared to multigravidae.
DISCUSSION
Malaria during pregnancy is associated with poor outcomes for both mothers and their newborn children. PM is associated with maternal anemia, LBW delivery, fetal loss, and PTD. In this study, we found that maternal cytokines and chemokines were elevated during PM. Further, peripheral levels during pregnancy predicted PTD and pregnancy loss. In competing risks analysis, high CXCL9 levels in maternal blood predicted pregnancy loss or PTD. The findings suggest that the systemic inflammatory response is contributing to these malaria sequelae. The association between systemic proinflammatory responses and pregnancy loss and PTD is consistent with previous animal model studies in which TNF-α, IFN-γ, or IL-2 administered to pregnant mice induced abortion [23].
Histologically, placental malaria in susceptible women, mainly primigravidae, has unique features that differ from other placental pathologies. Placental malaria is characterized by an increased number of macrophages, cytotoxic T cells, and B cells in placental intervillous spaces, resulting in increased proinflammatory cytokines as described here and in previous studies [8, 9, 13, 14, 24]. High placental IFN-γ and CXCL9 levels are associated with a reduction in birthweight [9, 13, 18]. In the current study, systemic levels of CXCL9 during pregnancy predict fetal loss and PTD. CXCL9 levels in peripheral blood were higher than in placental blood, suggesting that peripheral production of CXCL9 is independent of placental CXCL9. During pregnancy, syncytiotrophoblast microvesicles are released into maternal peripheral blood and have been proposed to play a role in regulating maternal immune responses [25, 26]. The number and composition of microparticles and exosomes change in complicated pregnancy [27], and placental microvesicles from preeclamptic vs normal term women induced higher secretion of proinflammatory cytokines by peripheral mononuclear cells [28]. Malaria infection is associated with increased microparticles, and their levels correlate with disease severity [29]. One study reported that placental malaria is not associated with a change in microparticle counts, but instead levels of microRNA (miRNA) type miRNAL-517c carried by exosomes was higher in women with placental malaria [30]. We speculate that increased exosome numbers or changes in microparticle composition in women with pregnancy loss or PTD activate peripheral mononuclear cells, resulting in proinflammatory immune responses associated with these 2 poor outcomes. Differences in the composition of placental microparticles and exosomes circulating in maternal peripheral blood between women with and without placental malaria remain to be defined.
IL-10 was previously described as a marker of inflammatory placental infection, with elevations in both placental and maternal peripheral blood [15, 31]. Among primigravidae, peripheral IL-10 levels are higher among infected women with histological evidence of inflammation vs those without [31]. High IL-10 levels during placental malaria may counterbalance proinflammatory responses as a mechanism to reduce damage [32]. In this study, high IL-10 levels at delivery were associated with increased risk of pregnancy loss, which suggests that chronic placental inflammation is associated with pregnancy loss. Suguitan et al [33] found that higher parasitemia, higher IL-10 and TNF-α levels, and lower TNF-α/IL-10 ratio in placental blood was associated with PTD, but these relationships were not significant in a multivariate logistic regression model.
Here, increased levels of placental IL-1β were associated with increased relative risk of pregnancy loss and PTD, consistent with previous animal and human studies describing a significant role for IL-1β in PTD (reviewed in [34]). Also, in a comparative transcriptome analysis of placenta tissue from malaria-infected women with inflammation vs noninfected women, multiple cytokines and chemokines including IL-1β were upregulated in inflammatory placental malaria [14], supporting the role of inflammatory cytokines in poor pregnancy outcomes.
The novel observation in the current study is that the systemic maternal inflammatory response to PM, as exemplified by CXCL9, is associated with pregnancy loss and PTD. High peripheral CXCL9 levels during pregnancy are associated with increased risk of pregnancy loss and PTD. Although CXCL9 levels were higher in both the placental and maternal peripheral blood of malaria-infected primigravidae, only CXCL9 levels in maternal peripheral blood were associated with poor pregnancy outcomes. These results emphasize the role of the maternal compartment in influencing pregnancy outcomes in the context of malaria, and suggest that assays to predict risk of pregnancy complications based on maternal peripheral blood are a tractable goal.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Acknowledgments. We thank the women in Ouelessebougou, Mali, for participation in the study. Rathy Mohan managed the clinical database, and staff at the community health centers supported follow-up of study participants.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McGregor IA. Epidemiology, malaria and pregnancy. Am J Trop Med Hyg 1984; 33:517–25. [DOI] [PubMed] [Google Scholar]
- 2. Desai M, ter Kuile FO, Nosten F et al. . Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 3. Braun V, Rempis E, Schnack A et al. . Lack of effect of intermittent preventive treatment for malaria in pregnancy and intense drug resistance in western Uganda. Malar J 2015; 14:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watson-Jones D, Weiss HA, Changalucha JM et al. . Adverse birth outcomes in United Republic of Tanzania—impact and prevention of maternal risk factors. Bull World Health Organ 2007; 85:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Geertruyden JP, Thomas F, Erhart A, D’Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg 2004; 71:35–40. [PubMed] [Google Scholar]
- 6. Muehlenbachs A, Fried M, McGready R et al. . A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. J Infect Dis 2010; 202:1608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ordi J, Ismail MR, Ventura PJ et al. . Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol 1998; 22:1006–11. [DOI] [PubMed] [Google Scholar]
- 8. Suguitan AL Jr, Leke RG, Fouda G et al. . Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. J Infect Dis 2003; 188:1074–82. [DOI] [PubMed] [Google Scholar]
- 9. Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-gamma and TNF-alpha associated with pregnancy outcomes. J Immunol 1998; 160:2523–30. [PubMed] [Google Scholar]
- 10. Moormann AM, Sullivan AD, Rochford RA et al. . Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. J Infect Dis 1999; 180:1987–93. [DOI] [PubMed] [Google Scholar]
- 11. Abrams ET, Brown H, Chensue SW et al. . Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. J Immunol 2003; 170:2759–64. [DOI] [PubMed] [Google Scholar]
- 12. Chaisavaneeyakorn S, Moore JM, Mirel L et al. . Levels of macrophage inflammatory protein 1 alpha (MIP-1 alpha) and MIP-1 beta in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clin Diagn Lab Immunol 2003; 10:631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kabyemela ER, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Fetal responses during placental malaria modify the risk of low birth weight. Infect Immun 2008; 76:1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy PE. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J Immunol 2007; 179:557–65. [DOI] [PubMed] [Google Scholar]
- 15. Chêne A, Briand V, Ibitokou S et al. . Placental cytokine and chemokine profiles reflect pregnancy outcomes in women exposed to Plasmodium falciparum infection. Infect Immun 2014; 82:3783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh PP, Lucchi NW, Blackstock A, Udhayakumar V, Singh N. Intervillous macrophage migration inhibitory factor is associated with adverse birth outcomes in a study population in central India. PLoS One 2012; 7:e51678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogerson SJ, Brown HC, Pollina E et al. . Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infect Immun 2003; 71:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong S, Kurtis JD, Pond-Tor S, Kabyemela E, Duffy PE, Fried M. CXC ligand 9 response to malaria during pregnancy is associated with low-birth-weight deliveries. Infect Immun 2012; 80:3034–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coutinho HM, McGarvey ST, Acosta LP et al. . Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. J Infect Dis 2005; 192:528–36. [DOI] [PubMed] [Google Scholar]
- 20. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995; 51:524–32. [PubMed] [Google Scholar]
- 21. Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013; 69:212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hebisch G, Grauaug AA, Neumaier-Wagner PM, Stallmach T, Huch A, Huch R. The relationship between cervical dilatation, interleukin-6 and interleukin-8 during term labor. Acta Obstet Gynecol Scand 2001; 80:840–8. [DOI] [PubMed] [Google Scholar]
- 23. Chaouat G, Menu E, Clark DA, Dy M, Minkowski M, Wegmann TG. Control of fetal survival in CBA x DBA/2 mice by lymphokine therapy. J Reprod Fertil 1990; 89:447–58. [DOI] [PubMed] [Google Scholar]
- 24. Ordi J, Menendez C, Ismail MR et al. . Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. J Infect Dis 2001; 183:1100–7. [DOI] [PubMed] [Google Scholar]
- 25. Southcombe J, Tannetta D, Redman C, Sargent I. The immunomodulatory role of syncytiotrophoblast microvesicles. PLoS One 2011; 6:e20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol 2017; 119:98–106. [DOI] [PubMed] [Google Scholar]
- 27. Mitchell MD, Peiris HN, Kobayashi M et al. . Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol 2015; 213:S173–81. [DOI] [PubMed] [Google Scholar]
- 28. Holder BS, Tower CL, Jones CJ, Aplin JD, Abrahams VM. Heightened pro-inflammatory effect of preeclamptic placental microvesicles on peripheral blood immune cells in humans. Biol Reprod 2012; 86:103. [DOI] [PubMed] [Google Scholar]
- 29. Mantel PY, Marti M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol 2014; 16:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moro L, Bardají A, Macete E et al. . Placental microparticles and microRNAs in pregnant women with Plasmodium falciparum or HIV infection. PLoS One 2016; 11:e0146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malar J 2008; 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yao Y, Simard AR, Shi FD, Hao J. IL-10-producing lymphocytes in inflammatory disease. Int Rev Immunol 2013; 32:324–36. [DOI] [PubMed] [Google Scholar]
- 33. Suguitan AL Jr, Cadigan TJ, Nguyen TA et al. . Malaria-associated cytokine changes in the placenta of women with pre-term deliveries in Yaounde, Cameroon. Am J Trop Med Hyg 2003; 69:574–81. [PubMed] [Google Scholar]
- 34. Nadeau-Vallée M, Obari D, Quiniou C et al. . A critical role of interleukin-1 in preterm labor. Cytokine Growth Factor Rev 2016; 28:37–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.