HIV-infected pregnant women on protease inhibitor (PI)–based combination antiretroviral therapy (cART) had elevated estradiol levels compared with HIV-uninfected women. Estradiol levels in the fetal compartment (cord blood) were inversely correlated with birth weight centile in the HIV-infected, PI-cART–exposed women.
Keywords: HIV, pregnancy, combination antiretroviral therapy, estradiol, growth restriction
Abstract
Background
Human immunodeficiency virus (HIV)–infected pregnant women on protease inhibitor (PI)–based combination antiretroviral therapy (cART) have a greater risk for adverse birth outcomes, and an association with steroid hormone levels has been implicated. The objective of this study was to investigate the association between PI-cART and estradiol levels in pregnancy.
Methods
Fifty-five HIV-infected and 49 HIV-uninfected Canadian pregnant women were followed prospectively throughout gestation. All HIV-infected women were on a PI-based cART regimen. Maternal plasma samples were collected at 12–18 weeks, 24–28 weeks, 34–38 weeks, at delivery, and from the cord. Birth outcomes were recorded. Levels of estradiol, dehydroepiandrosterone sulfate (DHEAS), sex hormone–binding globulin (SHBG), cortisol, and adrenocorticotropic hormone (ACTH) were quantified by enzyme-linked immunosorbent assay.
Results
HIV-infected women exposed to PI-cART had higher estradiol levels in maternal and cord plasma compared to HIV-uninfected women (median [interquartile range] for cord estradiol: 23.9 ng/mL [16.4–36.4] for HIV-infected exposed to PI-cART and 15.7 ng/mL [12.2–21.2] for HIV-negative; P = .0025). HIV-infected women had higher DHEAS levels in cord plasma that correlated with cord and maternal delivery estradiol levels. Cortisol and ACTH levels did not differ between groups. In the HIV-infected women, cord estradiol levels correlated negatively with birth weight centile (r = –0.47, P = .0016).
Conclusions
Our data suggest that PI-cART exposure in pregnancy is associated with elevated levels of estradiol, likely driven by higher fetal DHEAS production. Cord estradiol levels were inversely correlated with birth weight centile in infants born to PI-cART–exposed women, suggesting that fetal exposure to high estradiol levels may be contributing to cART-associated fetal growth restriction.
(See the Major Article by McDonald et al on pages 428–36.)
Approximately 1.5 million HIV-infected (HIV+) women become pregnant yearly, and more than two-thirds of these women are now able to access combination antiretroviral therapy (cART) during pregnancy [1]. The benefits of cART in practically eliminating vertical HIV transmission from mother to child far outweigh the possible risks of exposing the fetus to highly potent drugs during gestation. However, as the number of HIV+ women on cART increases, there are growing concerns about the safety and toxicity of cART on pregnancy outcomes and on the developing fetus [2–8]. Studies originating from North America, Europe, and Africa suggest that HIV+ pregnant women on cART have higher rates of preterm birth (PTB), small for gestational age (SGA), and low birth weight (LBW) infants, which may be more pronounced with protease inhibitor (PI)–based regimens (PI-cART) [5–9]. The mechanisms underlying cART-associated adverse birth outcomes have not been well defined. We have previously reported an association between fetal growth restriction in HIV+ women on PI-cART and lower progesterone levels during pregnancy due to a dysregulation in the metabolism of progesterone [10, 11].
Steroid hormones are chemical messengers that control and regulate a number of physiological and cellular activities. Successful implantation and pregnancy require a well-coordinated interplay between maternal, placental, and fetal physiology, which is facilitated by steroid hormones [12, 13]. Any alterations in steroid hormone homeostasis may significantly affect pregnancy outcomes, including fetal growth and time of parturition.
The steroid hormone estrogen is produced at every stage of pregnancy and regulates an array of fetal developmental processes. During pregnancy, the placenta functions as the primary site of estrogen production but requires precursors from the maternal and fetal circulation. This is essential because the placenta lacks the capacity to produce the C19 steroid precursors of estrogen, dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) [13]. The levels of estradiol (E2), the most biologically potent estrogen, rise throughout pregnancy to reach 100-fold prepregnancy levels by term. This excess E2 is found primarily in the maternal circulation but also crosses to the fetal compartment where it regulates developmental activities such as the promotion of fetal organogenesis, regulation of the fetal neuroendocrine system, and parturition [14].
Endocrine dysfunction is common among HIV+ individuals, and altered levels of E2 have been reported in patients on cART [15]. However, only a handful of studies have investigated the effects of cART on the levels of E2, and no data are available in the context of pregnancy, although high levels of the E2 precursor DHEAS have been reported in neonates exposed to PI-cART in utero [15–17]. The objectives of this study were to investigate E2 levels at different gestational time-points in HIV+ women on PI-cART and to examine the association between E2 levels and adverse birth outcomes.
METHODS
Ethics Statement
This study was approved by the Research Ethics Boards at St Michael’s Hospital, Mount Sinai Hospital, University Health Network, and Women’s College Hospital, Toronto, Canada. Written informed consent was collected from all participants.
Study Population
Plasma samples were obtained from HIV+ (n = 55) and HIV-uninfected (HIV–) (n = 49) pregnant women who consented to participate in a biobank program supporting research relevant to HIV and pregnancy. Women included in this study were recruited from 4 different sites in Toronto, Canada, from September 2010 to December 2015 and were prospectively followed throughout their pregnancy. The inclusion and exclusion criteria and detailed recruitment procedures for the cohort have been previously published [10]. In brief, women were included if they were ≥18 years old, with confirmed singleton pregnancy, and confirmed HIV status (HIV+ or HIV–). Women were excluded if they had preexisting hypertension (blood pressure >140/90 mm Hg), type I or II diabetes, renal disease, autoimmune disease, collagen vascular disease, documented opportunistic infection (in HIV+ group), current illicit drug use, or current tobacco use. Matching between HIV+ and HIV– women was performed on the basis of race, maternal age (±5 years), parity (0, 1, or >1), and body mass index (BMI) (<25 or >25 kg/m2). All HIV+ women received cART (Table 1); 32 (58.2%) initiated cART prior to conception and 23 (41.8%) initiated cART during pregnancy (6 in first, 14 in second, and 3 in third trimester).
Table 1.
Participant Characteristics and Birth Outcomes
| Characteristic | HIV-Infected (n = 55) |
HIV-Uninfected (n = 49) |
P Value |
|---|---|---|---|
| Maternal age, y, median (IQR) | 33 (30–37) | 32 (29–35) | .23 |
| Prepregnancy BMI, median (IQR) | 23.5 (21.9–27.6) | 24.3 (21.5–28.6) | .83 |
| Race | |||
| Black | 41 (74.6) | 33 (67.4) | .88 |
| White | 11 (20.0) | 13 (26.5) | |
| Hispanic | 1 (1.8) | 1 (2.0) | |
| Asian | 2 (3.6) | 2 (4.1) | |
| ARV regimen | |||
| PI | 55 (87.3) | NA | |
| Lopinavir/ritonavir | 32 (50.7) | ||
| Atazanavir/ritonavir | 20 (31.8) | ||
| Darunavir/ritonavir | 3 (4.8) | ||
| HIV viral load near delivery, copies/mL, median (IQR) | 40 (40–66) | NA | |
| Undetectable HIV viral load | 41 (74.5) | NA | |
| CD4+ T-cell count near delivery, cells/μL, median (IQR) | 527 (400–670) | NA | |
| Parity | |||
| 0 | 23 (41.8) | 16 (32.6) | .63 |
| 1 | 20 (36.4) | 21 (42.9) | |
| ≥2 | 12 (21.8) | 12 (24.5) | |
| Infant sex | |||
| Male | 31 (56.4) | 21 (42.9) | .20 |
| Female | 24 (43.6) | 27 (55.1) | |
| Missing | 0 (0) | 1 (2.0) | |
| Delivery | |||
| Vaginal | 30 (54.6) | 31 (63.3) | .08 |
| Cesarean, no labor | 13 (23.6) | 14 (28.6) | |
| Cesarean, labor | 12 (21.8) | 3 (6.1) | |
| Missing | 0 (0) | 1 (2.0) | |
| Preterm birth | 9 (16.4) | 0 (0) | .003 |
| Gestational age at birth, wk, median (IQR) | 38.6 (37.7–40.0) | 40.1 (39.1–40.9) | <.0001 |
| Small for gestational age | 15 (27.3) | 0 (0) | <.0001 |
| Low birth weight | 13 (23.6) | 0 (0) | <.0001 |
| Birth weight, g, median (IQR) | 3010 (2595–3430) | 3571 (3302–3722) | <.0001 |
| Birth weight, percentile, median (IQR) | 24.3 (8.9–57.0) | 53.5 (30.8–70.8) | .001 |
Data are presented as No. (%) of subjects unless otherwise indicated. Race, parity, small for gestational age, preterm delivery, and infant sex comparisons were assessed by χ2 analysis; comparisons of median values were assessed by the Mann-Whitney test.
Abbreviations: ARV, antiretroviral; BMI, body mass index; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; PI, protease inhibitor.
Gestational age was determined based on reporting of last menstrual period and confirmed by ultrasonography. PTB was defined as spontaneous delivery before 37 weeks of gestation. SGA was defined as birth weight <10th percentile for gestational age. Birth weight centiles were calculated accounting for gestational age, infant sex, and mother’s world region of birth according to Ray et al [18]. None of the infants in this study acquired HIV.
Maternal plasma samples (heparinized) were collected at 3 different gestational time-points defined as early (12–18 weeks), mid (24–28 weeks), and late (34–38 weeks), between 9:30 am and 12:30 pm, and at delivery. Samples were not available for all participants at all time-points. Cord plasma samples were collected at delivery. All blood samples were processed within 1 hour of collection by spinning at 1000g for 15 minutes. Plasma was collected and frozen at –80°C until analysis.
Hormone Assays
E2, sex hormone–binding globulin (SHBG), DHEAS, cortisol, and adrenocorticotropic hormone (ACTH) were measured in the maternal compartment (maternal plasma) and in the fetal compartment (cord plasma) by competitive enzyme-linked immunosorbent assay (ELISA) performed according to the manufacturer’s instruction. All ELISA kits were obtained from DRG International (Springfield, New Jersey). Plasma samples were diluted 1:50 (E2), 1:300 (SHBG), 1:2 (cortisol), and assayed undiluted for DHEAS and ACTH. All samples were assayed in duplicate. Bioavailable estradiol was calculated using a simplified 1-ligand/2-protein version of the method published by Mazer [19]. The model included measured E2 (ligand) and SHBG (protein), and assumed a default value of 4.3 g/dL for albumin (protein). Testosterone, which also binds to SHBG, was not included in the model.
Statistical Methods
Differences in demographic and categorical data were computed using χ2 or Fisher exact test. For continuous variables, means/medians were compared using Student t test or Mann-Whitney U test as appropriate. For time course analyses, analyte levels were loge transformed and 2-way analysis of variance was used to determine main effects of gestational period and treatment, and the interactions between them. Correlation was assessed using Spearman rank correlation coefficient (r). Linear and logistic regression analyses were used to examine relationships between analytes and birth outcomes. A 2-sided P value <.05 was used as the cutoff for statistical significance. All statistical analyses were performed using Stata (version 12.0) and GraphPad Prism (version 5.0) software.
RESULTS
Birth Outcomes
Fifty-five HIV+ women and 49 HIV– women were included in this study. The characteristics of the women and description of the regimens are presented in Table 1. In the HIV+ group, the rate of spontaneous PTB was 16.4% (9 of 55), the rate of SGA births was 27.3% (15 of 55), and the rate of LBW was 23.6% (13 of 55). None of the HIV– women experienced PTB, SGA, or LBW. The median birth weight (P < .0001), birth weight centile (P = .001), and gestational age at birth (P < .0001) were all significantly lower in the HIV+ compared with the HIV– group (Table 1).
Estradiol Concentrations Are Elevated in HIV+ Women on Protease Inhibitor-Combination Antiretroviral Therapy
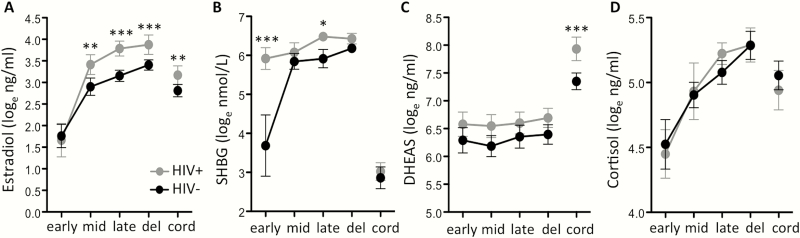
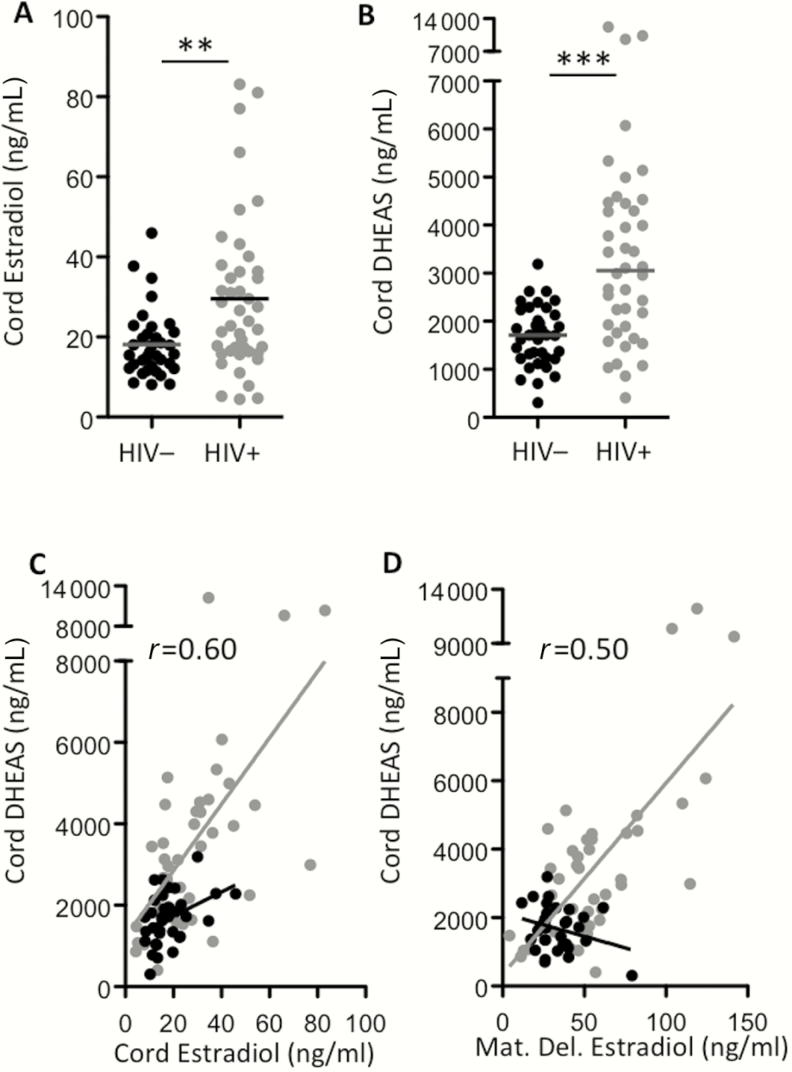
Maternal E2 levels were significantly higher in the HIV+ group compared to the HIV– group at mid and late gestation and at delivery (Figure 1). From the midpoint to delivery, E2 levels in the HIV+ group were approximately 2-fold higher than in the HIV– group (Table 2) and previously reported E2 levels for uncomplicated pregnancy [20]. Cord E2 levels (used to define the fetal compartment) were also 50% higher in the HIV+ compared with the HIV– group (P = .0025) (Table 2 and Figure 2A). Estradiol levels did not differ between the 3 different PIs at any time-point.
Figure 1.
Hormone levels in maternal and cord plasma of human immunodeficiency virus (HIV)–infected (HIV+) and HIV-uninfected (HIV–) women. Loge-transformed (A) estradiol, (B) sex hormone binding globulin (SHBG), (C) dehydroepiandrosterone sulphate (DHEAS), and (D) cortisol in HIV– and HIV+ protease inhibitor combination antiretroviral therapy–exposed women. Hormone levels measured by enzyme-linked immunosorbent assay in maternal and cord plasma. Gestational week of sample collection as follows: early, 12–18 weeks; mid, 24–28 weeks; late, 34–38 weeks. Maternal delivery (del) and cord blood (cord) samples were also collected. Data are mean with 95% confidence interval. Statistical comparisons in maternal levels by 2-way analysis of variance with Bonferroni posttest, and in cord levels by t test. Asterisks indicate significant differences between HIV+ and HIV– groups. * indicates P < .05, ** indicates P < .01 and *** indicates P < .0001. N for HIV– and HIV+, respectively: early: 20, 24; mid: 42, 36; late: 42, 41; delivery: 41, 46; cord: 35, 43.
Table 2.
Total Measured Estradiol and Bioavailable Estradiol in Maternal Plasma Across Gestation and Cord Plasma at Delivery
| Gestation | Estradiola | No. | HIV-Uninfected | No. | HIV-Infected, PI-cART Exposed | P Value |
|---|---|---|---|---|---|---|
| Early | E2 | 20 | 5572 (4186–7143) | 26 | 5400 (3787–8585) | .96 |
| bE2 | 3292 (1529–5292) | 656.9 (481.1–1162) | <.0001 | |||
| Mid | E2 | 42 | 16854 (10938–33683) | 37 | 38116 (17890–54400) | .0011 |
| bE2 | 3353 (1459–5689) | 3837 (2429–6548) | .39 | |||
| Late | E2 | 42 | 21593 (17110–33476) | 44 | 44448 (28262–70434) | <.0001 |
| bE2 | 3564 (2327–4877) | 5072 (2802–6863) | .049 | |||
| Delivery | E2 | 40 | 27265 (25705–39144) | 43 | 53024 (37638–77306) | <.0001 |
| bE2 | 4659 (2946–5466) | 5720 (3602–12187) | .024 | |||
| Cord | E2 | 35 | 15684 (12194–21208) | 43 | 23928 (16359–36403) | .0025 |
| bE2 | 13700 (10255–17719) | 20668 (13681–30516) | .0036 |
Data are presented as median with interquartile range. All values are in picogram per milliliter (pg/mL). P values of < .05 are shown in bold.
Abbreviations: bE2, calculated bioavailable estradiol; cART, combination antiretroviral therapy; E2, estradiol; HIV, human immunodeficiency virus; PI, protease inhibitor.
aTotal estradiol measured by enzyme-linked immunosorbent assay. Bioavailable estradiol calculated using a simplified version of the Mazer model [19, 21]. Comparisons by Mann-Whitney test.
Figure 2.
Estradiol and dehydroepiandrosterone sulfate (DHEAS) are elevated in cord plasma from human immunodeficiency virus–infected (HIV+) protease inhibitor combination antiretroviral therapy (PI-cART)–exposed women. Estradiol (A) and DHEAS (B) measured by enzyme-linked immunosorbent assay in cord plasma of HIV-uninfected (HIV–) (black, n = 35), HIV+ PI-cART–exposed (gray, n = 43) women. The line indicates the median. Statistical comparisons by Mann-Whitney test, P= .0025 for (A) and P < .0001 for (B). Correlation between cord DHEAS and cord estradiol (C) and cord DHEAS and maternal delivery (Mat. Del.) estradiol (D) assessed using Spearman rank test. C, r= 0.60 (95% confidence interval [CI], .35–.77), P < .0001 for HIV+; r= 0.40 (95% CI, .06–.65), P= .019 for HIV–. D, r= 0.50 (95% CI, .21–.71), P= .0013 for HIV+. Asterisks indicate significant differences between HIV+ and HIV– groups. * indicates P < .05 and *** indicates P < .0001. Sensitivity analysis was performed excluding the 3 high data points in cord DHEAS in the HIV+ group. All relationships remained significant.
Sex Hormone–Binding Globulin Levels Are High in Early Pregnancy in HIV+ Women
SHBG is the high-affinity binding protein for both E2 and testosterone. In the HIV+ group, maternal SHBG levels were 10-fold higher compared to the HIV– group in the early time-point (P < .0001), and increased only marginally throughout gestation (Figure 1B). As expected, cord plasma SHBG levels were significantly lower than SHBG levels in the maternal plasma; and were similar between groups (Figure 1B).
We computed bioavailable E2 (bE2) levels from the measured levels of total E2 and SHBG using a simplified version of the Mazer method [19, 21]. At the early time-point, bE2 was significantly lower in the HIV+ women compared with HIV– women (P < .0001; Table 2), due to the high levels of SHBG. By the midpoint, bE2 levels were similar between groups. By the late time-point and at delivery, bE2 levels were approximately 20% and 40% higher respectively in the HIV+ group compared with the HIV– group (Table 2).
In cord plasma, bE2 levels were approximately 50% higher in the HIV+ group compared with the HIV– group, suggesting that fetuses in the HIV+ group were exposed to higher than normal levels of bE2 (Table 2). In women who initiated PI-cART during pregnancy, we observed an inverse correlation between cord bE2 and gestational week of PI-cART initiation (r = –0.66, P = .0024) (Supplementary Figure 1).
Cord Dehydroepiandrosterone Sulfate Levels Are Elevated in HIV+ Women on Protease Inhibitor-Combination Antiretroviral Therapy
During pregnancy, the principal C19 precursor for E2 synthesis is DHEAS, supplied in equal parts by the fetal and maternal adrenal glands [22]. Maternal plasma DHEAS levels were similar between groups (Figure 1C). However, cord levels of DHEAS were significantly higher in the HIV+ group compared with the HIV– group (median [interquartile range]): HIV+: 2993 (1759–4455) ng/mL vs HIV–: 1710 (1229–2183) ng/mL (P < .0001; Figure 2B).
We observed a positive correlation between cord E2 and cord DHEAS levels in both the HIV+ (r = 0.60 [95% confidence interval {CI}, .35–.77], P < .0001), and HIV– groups (r = 0.40 [95% CI, .06–.65], P = .019) (Figure 2C). Cord DHEAS levels in the HIV+ group also correlated with maternal E2 levels at delivery (r = 0.50 [95% CI, .21–.71], P = .0013) (Figure 2D).
Combination Antiretroviral Therapy Has No Effect on Cord Levels of Adrenocorticotropic Hormone and Cortisol
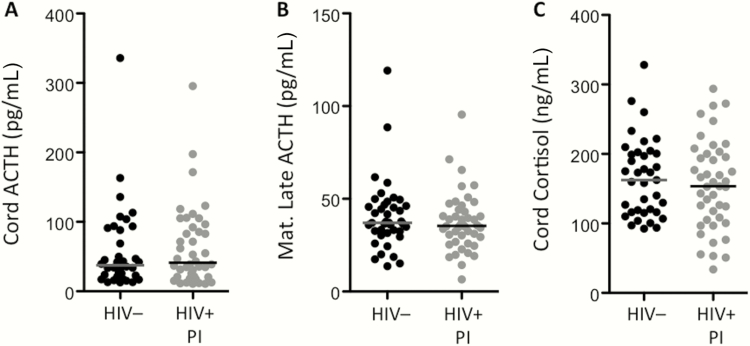
Higher levels of DHEAS in the cord blood may indicate altered homeostasis of the fetal hypothalamic-pituitary-adrenal (HPA) axis [23]. To probe this further, we compared the levels of cortisol and ACTH in cord and maternal blood of HIV+ women on PI-cART and HIV– women. ACTH regulates the production of both cortisol and DHEAS. Unlike DHEAS, cord and maternal levels of cortisol did not differ between groups (Figure 1D and Figure 3C). ACTH levels were also similar between groups (Figure 3A and 3B).
Figure 3.
Adrenocorticotropic hormone (ACTH) and cortisol levels do not differ between human immunodeficiency virus (HIV)–infected (HIV+) and HIV-uninfected (HIV–) pregnant women. Cord ACTH (A), maternal delivery ACTH (B), and cord cortisol (C) levels assessed in plasma by enzyme-linked immunosorbent assay. Data are shown for HIV– (black) and HIV+ protease inhibitor (PI) combination antiretroviral therapy–exposed (gray) women. The line indicates the median. Statistical comparisons by Mann-Whitney test. No significant differences were observed.
Cord E2 Levels Correlate With Birth Weight Centile and Increased Odds of Small for Gestational Age in HIV+ Women on Protease Inhibitor-Combination Antiretroviral Therapy
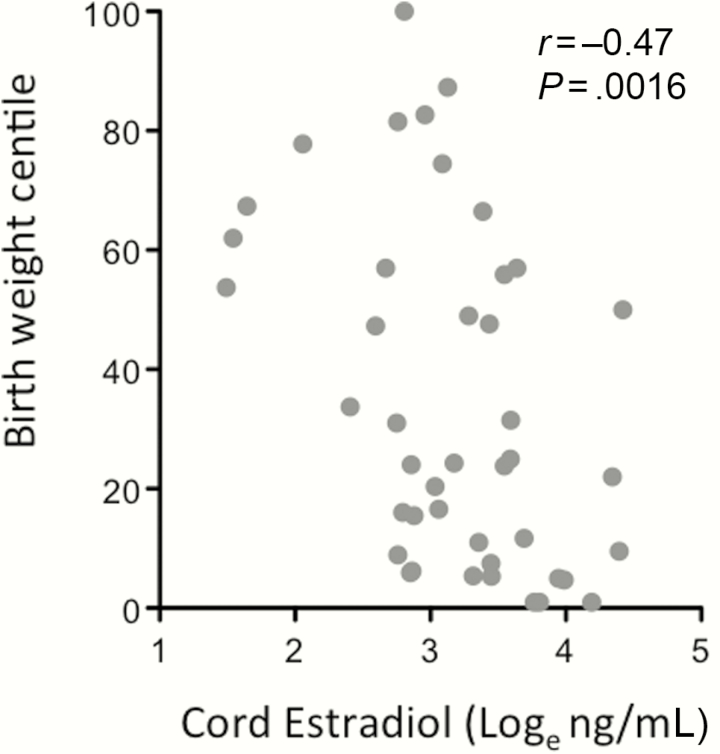
We next examined whether E2 levels correlated with adverse birth outcomes. E2 levels in the cord were inversely correlated to birth weight centile in the HIV+ group (r = –0.47 [95% CI, –.68 to –.18], P = .0016; Figure 4), but not in the HIV– group (r = –0.2 [95% CI, –.51 to .15], P = 0.25). A similar correlation was observed between birth weight centile and cord bE2 (r = –0.45 [95% CI, –.67 to –.16], P = .0026) in the HIV+ group but not in the HIV– group. We have previously reported that maternal progesterone levels at the mid time-point were lower in HIV+ PI-cART–exposed compared with HIV– pregnant women, and that progesterone levels at this time-point correlated with birth weight centile in the HIV+ group [10]. We assessed whether the relation between cord E2 levels and birth weight centile would be maintained after adjusting for maternal progesterone levels (at the mid time-point) in the subgroup of HIV+ women for whom both cord E2 and maternal progesterone levels were available (n = 15), using linear regression analysis. Cord E2 levels remained a significant predictor of birth weight centile in HIV+ women after adjusting for maternal progesterone levels (unadjusted coefficient for loge E2: –29.7 [standard error, 8.6], P = .004; adjusted coefficient for loge E2: –28.8 [standard deviation, 12.3], P = .038).
Figure 4.
Cord estradiol levels correlate with birth weight centile in human immunodeficiency virus (HIV)–infected (HIV+) protease inhibitor combination antiretroviral therapy (PI-cART)–exposed women. Loge-transformed cord estradiol levels are plotted against birth weight centile for HIV+ PI-cART–exposed women, n = 43. Correlation assessed using Spearman rank test: r= –0.47 (95% confidence interval, –.68 to –.18), P= .0016.
We performed logistic regression to assess the relation between E2 levels and SGA, LBW, and PTB. In the HIV+ group, we observed a significant association between cord E2 and SGA, but not PTB or LBW. The odds ratio of SGA associated with a 1 loge increase in cord E2 was 4.36 (95% CI, 1.17–16.23), P = .028. Maternal levels of E2 across gestation did not correlate with birth outcomes. We did not observe any correlation between E2 levels or SGA and viral load, CD4 count, maternal age, parity, or prepregnancy BMI.
DISCUSSION
Our data show that HIV+ pregnant women on PI-cART regimens had elevated E2 levels in mid and late pregnancy, at delivery, and in the cord blood compared with HIV– women. We present evidence that higher maternal and cord E2 levels in HIV+ PI-cART–exposed women may be driven by an increase in the levels of DHEAS (the E2 precursor) in the fetal compartment. We further report an inverse correlation between cord E2 levels and birth weight centile, and an association between high cord E2 and increased odds of an SGA birth in HIV+ women exposed to PI-cART.
E2 can exist in the circulation as free, bound to albumin, or bound to SHBG [24]. Free and albumin-bound E2 are considered bioavailable to downstream cellular functions. SHBG levels were dysregulated in the HIV+ group, in the maternal but not in the cord blood. In normal pregnancies, SHBG levels rise in concert with E2 [25]; however, this concurrent increase was not observed in the HIV+ women. HIV+ women had 10-fold higher levels of SHBG at the early time-point, and while HIV– women showed an increase in SHBG mirroring that of E2, SHBG levels rose only minimally throughout pregnancy in the HIV+ women. High SHBG levels have been reported in several studies of male and female HIV+ nonpregnant adults in the pre- and post-cART era [26]. It is possible that both HIV infection and/or antiretrovirals may have contributed to the SHBG profile of our HIV+ women.
To put our E2 and SHBG data into context, we calculated bioavailable E2 according to the Mazer method [19, 21]. These data should be interpreted with caution as we did not directly measure albumin and did not include testosterone in the model (which competes with E2 for SHBG binding). The 10-fold higher levels of SHBG in the HIV+ group led to significantly lower bioavailable E2 in early pregnancy. The high SHBG levels observed in the maternal compartment of HIV+ women may help protect the mother from the effects of high circulating E2. While maternal total E2 levels in mid to late pregnancy were 2-fold higher in the HIV+ compared with HIV– women, bioavailable E2 was only 20% higher. However, SHBG levels were lower in the fetal compartment in both groups, and our data suggest that fetuses in the HIV+ group were exposed to significantly higher levels of both total and bioavailable E2 compared with the HIV– group. In addition, we observed a negative correlation between cord bioavailable E2 and gestational week of PI-cART start, suggesting that first trimester/early exposure to PI-cART may lead to greater E2 dysregulation, although these data should be viewed with caution given the small sample size.
In studies of assisted reproduction, a fetal exposure to a high E2 environment has been associated with increased risk of LBW and SGA births [27, 28]. Several mechanisms have been proposed including impaired endometrial receptivity and restraint spiral artery remodeling leading to poor placenta perfusion, and metabolic abnormalities including alterations in the insulin-like growth factor axis and polyunsaturated fatty acid metabolism [27, 29]. Concurring with observations of fetal high E2 exposure leading to growth restriction, we observed an inverse correlation between cord E2 and birth weight centile, and an association between high cord E2 and increased odds of an SGA birth in the PI-cART group. Low birth weight, poor growth trajectories, and metabolic abnormalities have been reported in HIV-exposed, uninfected children [30]. Whether high in utero E2 exposure could contribute to these is worth exploring further.
Maintenance of physiological E2 levels during pregnancy requires coordinated positive and negative feedback mechanisms between the mother and fetus that are regulated by the interplay between ACTH, cortisol, and DHEAS through the HPA axis [14, 31]. In the fetal adrenal cortex, the fetal zone expresses CYP17 early in pregnancy and produces DHEAS, acting as the primary source of E2 precursors during pregnancy [13]. Although there were no appreciable differences in the concentrations of maternal DHEAS among the groups, the HIV+ women had higher levels of cord DHEAS, which correlated positively with E2 in the cord, and E2 in maternal plasma at delivery. These data are in agreement with studies that showed a correlation between DHEAS and E2 concentration in pregnancy [32], and suggest a fetal origin of excess DHEAS fueling E2 production by the placenta in HIV+ PI-cART–exposed women. High DHEAS levels have been previously reported in neonates exposed to PI-cART in utero [17]. We did not observe differences in the levels of cortisol or ACTH between groups, which suggests the absence of a generalized disturbance in the fetal HPA axis.
The high E2 levels observed in our study may be driven by additional mechanisms. It has been reported that a downregulation of estrogen receptor (ER) in the fetal zone of the adrenal gland disrupts the negative feedback control of estrogen synthesis, which leads to an increase in estrogen production [14]. Thus, one putative mechanism leading to the excess E2 production in HIV+ women exposed to PI-cART may be the loss of responsiveness to high E2 due to the downregulation of ER or disruption of ER signaling. This proposed mechanism seems plausible because PIs have been shown to downregulate ER [33]. This hypothesis needs to be further investigated. Additionally, PIs, and ritonavir in particular, are known inhibitors of cytochrome P (CYP) 450 enzymes involved in the metabolism of E2 and DHEAS, including CYP34A [34]. Inhibition of CYP3A could lead to higher E2 levels.
Our study has several strengths including its prospective design with multiple sampling points throughout pregnancy, the inclusion of HIV– women with similar age, BMI, and parity as the HIV+ women, and the exclusion of confounders such as preexisting hypertension, diabetes, drug use, and smoking.
Our study has several limitations. Our sample size is relatively small, and the HIV+ women were on a variety of PI regimens. The absence of adverse birth outcomes in the HIV– group may amplify the differences in hormonal levels between groups. Our reported associations between E2 levels and birth outcomes are correlative and do not prove causation. With our current study design, it is impossible to delineate the effect of HIV from cART on E2 levels, although it would be unethical to include an HIV+ treatment-naive cohort given the current treatment guidelines. Additionally, because all the HIV+ women included in this study received a PI-based regimen, it is not possible to determine whether the effects on E2 levels were PI dependent or not. However, in a separate study using samples from 322 Ugandan women randomized to receive either lopinavir/ritonavir (PI)–based or efavirenz (non-PI)–based cART, we have been able to demonstrate that E2 levels were higher in the women receiving PI-based cART compared with those receiving non-PI-based cART [35].
In conclusion, we report higher than normal levels of E2 in maternal and cord blood of HIV+ women pregnant women on PI-cART, likely driven by increased production of fetal DHEAS. A correlation between fetal exposure to high E2 and lower birth weight centile was observed. The etiology of the adverse birth outcomes experienced by HIV+ cART-exposed women is undoubtedly multifactorial. Taken together with previous observations of lower progesterone levels [10, 11], our data support a significant impact of PI-based cART on sex steroid homeostasis in pregnancy. Our study provides needed information toward a better understanding of the mechanisms that underlie SGA outcomes in the context of cART use, and adds to our knowledge of the safety of antiretroviral use in pregnancy. Given the pleotropic effects of E2 on fetal development, future studies assessing the impact of high in utero E2 exposure on factors beyond growth parameters, such as genitourinal, metabolic, and cognitive outcomes, in HIV-exposed, uninfected children are merited.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the women who participated in the study; the study coordinators and labor and delivery staff at the recruiting sites; and Dr T. Brown for critical review of the manuscript.
Financial support. This work was supported by the Canadian Institutes of Health Research (CIHR) (MOP-130398 and IHD-123784 to L. S.); CIHR Emerging Team Grant in Maternal Health (to M. L., L. S., M. H. Y.); Ontario HIV Treatment Network (G655 to L. S.); Canadian Foundation for AIDS Research (to L. S.); and CIHR Canadian HIV Trials Network (to K. A. B.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Joint United Nations Programme on HIV/AIDS. Fact sheet Available at: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf. Accessed 11 November 2016.
- 2. Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth?J Infect Dis 2006; 193:1195–201. [DOI] [PubMed] [Google Scholar]
- 3. Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21:607–15. [DOI] [PubMed] [Google Scholar]
- 4. Grosch-Woerner I, Puch K, Maier RF et al. Multicenter Interdisciplinary Study Group Germany/Austria Increased rate of prematurity associated with antenatal antiretroviral therapy in a German/Austrian cohort of HIV-1-infected women. HIV Med 2008; 9:6–13. [DOI] [PubMed] [Google Scholar]
- 5. Patel K, Shapiro DE, Brogly SB et al. P1025 Team of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis 2010; 201:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Townsend C, Schulte J, Thorne C et al. Pediatric Spectrum of HIV Disease Consortium, the European Collaborative Study and the National Study of HIV in Pregnancy and Childhood Antiretroviral therapy and preterm delivery-a pooled analysis of data from the United States and Europe. BJOG 2010; 117:1399–410. [DOI] [PubMed] [Google Scholar]
- 7. Sibiude J, Warszawski J, Tubiana R et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost?Clin Infect Dis 2012; 54:1348–60. [DOI] [PubMed] [Google Scholar]
- 8. Shapiro RL, Hughes MD, Ogwu A et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010; 362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fowler MG, Qin M, Fiscus SA et al. IMPAACT 1077BF/1077FF PROMISE Study Team Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016; 375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papp E, Mohammadi H, Loutfy MR et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis 2015; 211:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papp E, Balogun K, Banko N et al. Low prolactin and high 20-α-hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV-infected pregnant women exposed to protease inhibitor-based combination antiretroviral therapy. J Infect Dis 2016; 213:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guller S, Gravanis A, Gurpide E. Steroid metabolizing enzymes associated with the microvillar membrane of human placenta. J Steroid Biochem 1986; 24:935–44. [DOI] [PubMed] [Google Scholar]
- 13. Mesiano S, Jaffe RB. Developmental and functional biology of the primate fetal adrenal cortex. Endocr Rev 1997; 18:378–403. [DOI] [PubMed] [Google Scholar]
- 14. Kaludjerovic J, Ward WE. The interplay between estrogen and fetal adrenal cortex. J Nutr Metab 2012; 2012:837901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collazos J, Martínez E, Mayo J, Ibarra S. Sexual dysfunction in HIV-infected patients treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 31:322–6. [DOI] [PubMed] [Google Scholar]
- 16. Lamba H, Goldmeier D, Mackie NE, Scullard G. Antiretroviral therapy is associated with sexual dysfunction and with increased serum oestradiol levels in men. Int J STD AIDS 2004; 15:234–7. [DOI] [PubMed] [Google Scholar]
- 17. Simon A, Warszawski J, Kariyawasam D et al. ANRS French Perinatal Cohort Study Group Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 2011; 306:70–8. [DOI] [PubMed] [Google Scholar]
- 18. Ray JG, Sgro M, Mamdani MM et al. Birth weight curves tailored to maternal world region. J Obstet Gynaecol Can 2012; 34:159–71. [DOI] [PubMed] [Google Scholar]
- 19. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids 2009; 74:512–9. [DOI] [PubMed] [Google Scholar]
- 20. Kuijper EA, Ket JC, Caanen MR, Lambalk CB. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online 2013; 27:33–63. [DOI] [PubMed] [Google Scholar]
- 21. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999; 84:3666–72. [DOI] [PubMed] [Google Scholar]
- 22. Siiteri PK, MacDonald PC. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab 1966; 26:751–61. [DOI] [PubMed] [Google Scholar]
- 23. Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev 2011; 32:317–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunn JF, Nisula BC, Rodbard D. Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma. J Clin Endocrinol Metab 1981; 53:58–68. [DOI] [PubMed] [Google Scholar]
- 25. Hammond GL. Plasma steroid-binding proteins: primary gatekeepers of steroid hormone action. J Endocrinol 2016; 230:R13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin ME, Benassayag C, Amiel C, Canton P, Nunez EA. Alterations in the concentrations and binding properties of sex steroid binding protein and corticosteroid-binding globulin in HIV+ patients. J Endocrinol Invest 1992; 15:597–603. [DOI] [PubMed] [Google Scholar]
- 27. Hu XL, Feng C, Lin XH et al. High maternal serum estradiol environment in the first trimester is associated with the increased risk of small-for-gestational-age birth. J Clin Endocrinol Metab 2014; 99:2217–24. [DOI] [PubMed] [Google Scholar]
- 28. Imudia AN, Awonuga AO, Doyle JO et al. Peak serum estradiol level during controlled ovarian hyperstimulation is associated with increased risk of small for gestational age and preeclampsia in singleton pregnancies after in vitro fertilization. Fertil Steril 2012; 97:1374–9. [DOI] [PubMed] [Google Scholar]
- 29. Jin M, Lv PP, Yu TT, Shen JM, Feng C, Huang HF. IGFBP1 involved in the decreased birth weight due to fetal high estrogen exposure in mice. Biol Reprod 2016; 95:96. [DOI] [PubMed] [Google Scholar]
- 30. Desmonde S, Goetghebuer T, Thorne C, Leroy V. Health and survival of HIV perinatally exposed but uninfected children born to HIV-infected mothers. Curr Opin HIV AIDS 2016; 11:465–76. [DOI] [PubMed] [Google Scholar]
- 31. Albrecht ED, Henson MC, Walker ML, Pepe GJ. Modulation of adrenocorticotropin-stimulated baboon fetal adrenal dehydroepiandrosterone formation in vitro by estrogen at mid- and late gestation. Endocrinology 1990; 126: 3083–8. [DOI] [PubMed] [Google Scholar]
- 32. Albrecht ED, Pepe GJ. The placenta remains functional following fetectomy in baboons. Endocrinology 1985; 116: 843–5. [DOI] [PubMed] [Google Scholar]
- 33. Xiang J, Wang Y, Su K et al. Ritonavir binds to and downregulates estrogen receptors: molecular mechanism of promoting early atherosclerosis. Exp Cell Res 2014; 327:318–30. [DOI] [PubMed] [Google Scholar]
- 34. Jiménez-Nácher I, Alvarez E, Morello J, Rodriguez-Nóvoa S, de Andrés S, Soriano V. Approaches for understanding and predicting drug interactions in human immunodeficiency virus-infected patients. Expert Opin Drug Metab Toxicol 2011; 7:457–77. [DOI] [PubMed] [Google Scholar]
- 35. McDonald C, Conroy AL, Gamble JL,. et al. Estradiol levels are altered in humna immunodeficiency virus-infected pregnant women randomized to efavirenz- versus lopinavir/ritonavir-based antiretroviral therapy. Clin Infect Dis 2017; doi:10.1093/cid/cix772 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.