Although statins and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers demonstrated modest negative neurocognitive effects on single cognitive domains, these effects were overwhelmed by lack of effect on the primary outcome of global neurocognitive function and by the well-established cardiovascular benefits.
Keywords: Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Antagonists, Hydroxymethylglutaryl-CoA Reductase Inhibitors (statins), neurocognitive disorders, HIV
Abstract
Background
Although statins, angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) are generally well tolerated, the impact of these therapies individually or in combination on the change in neurocognitive function in persons with human immunodeficiency virus infection is unknown.
Methods
The study included participants in the AIDS Clinical Trials Group Longitudinal Linked Randomized Trials cohort participants not receiving a statin or ACEI/ARB within 30 days of first neurologic assessment (baseline), with assessments by NPZ-3 (z score of averaged Trailmaking A and B tests and digit symbol test [DST]) from ≥2 measurements. Marginal structural models estimated the causal effect of statin or ACEI/ARB initiation on neurocognitive function; initial constant slope was assumed during the first year of treatment and a second constant slope thereafter.
Results
Of 3949 eligible participants, 16% started therapy with a statin, 11% with an ACEI/ARB, and 5% with both. Statin therapy had no significant effect on the composite NPZ-3 (primary outcome), Trailmaking B test, or DST. A small, nonsignificant positive effect on the Trailmaking A test was seen during year 1 (estimate, 0.088; 95% confidence interval, −.010 to .187; P = .08) and a small but significant negative effect (−0.033; −.058 to −.009; P = .007) in each subsequent year. ACEI/ARB therapy had a significant negative effect on the DST (−0.117; 95% confidence interval, −.217 to .016; P = .02) during year 1 but minimal effect in subsequent years or on other neurocognitive domains.
Conclusions
In summary, although modest declines in neurocognitive performance were seen in single domains with statin or ACEI/ARB therapy, we did not find consistent evidence that statins or ACEI/ARB have an effect on global neurocognitive function. Future studies should focus on long-term neurocognitive effects.
Despite significant advances in the treatment of human immunodeficiency virus (HIV) infection, neurocognitive impairment (NCI) is observed among persons receiving antiretroviral therapy (ART). Moreover, the incidence of asymptomatic or mild cognitive impairment has remained stable despite early and effective ART, with some cohorts reporting an overall increase in the prevalence owing to an increasing number of individuals living with and aging with HIV/AIDS [1]. Associations between vascular disease and NCI are well recognized, and increasing age and the presence of vascular disease also seem to influence the occurrence and progression of HIV-related NCI [2, 3]. Furthermore, albuminuria was associated with an increased risk of NCI in the general population [4–8], and proteinuria (defined as a urine protein to creatinine ratio [uPCR] >200 mg/g) was associated with prevalent and incident NCI in HIV-infected persons enrolled in the AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) cohort [9]. It is possible that vascular changes in the central nervous system and kidneys, leading to cognitive impairment or proteinuria, respectively, imply common pathogenic mechanisms underlying both cardiovascular and neurocognitive disorders.
Statins (3-hydroxymethyl, 3-methylglutaryl coenzyme A reductase inhibitors) are the most widely prescribed cholesterol-reducing medications, and they reduce cardiovascular events by 25%–40% [10]. Equipoise exists regarding the effect of statins on cognitive function. Several small case series and case reports described statin-associated worsening cognitive function [11–15], which prompted a Food and Drug Administration warning for statins that included a risk of adverse neurocognitive events [16]. However, numerous larger observational studies and meta-analyses, including a Cochrane review, have since found no consistent association of statins with neurocognitive decline, and some have suggested a beneficial effect of statins on cognition [17–24]. Little is known about the effects of statins on neurocognitive function in persons with HIV; in a subset of statin users from the CHARTER HIV study (n = 63), statins were not associated with improved neuropsychological performance [25].
Renin angiotensin blockade with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) are effective in lowering albuminuria, in addition to the expected blood pressure lowering effects. In an analysis of 2 large clinical trials, telmisartan, an ARB with anti-inflammatory properties, was associated with significantly reduced odds of neurocognitive decline among subjects with baseline macroalbuminuria [26].
Although statins and ACEI/ARB are generally safe, well tolerated, and often coprescribed, the impact of these therapies individually, or in combination, on the change in neurocognitive function of HIV-infected persons is unknown. The ACTG ALLRT study provides an ideal cohort to evaluate the effects of statin therapy and/or ACEI/ARB treatment on neurocognitive function over time, in HIV-infected persons who recently started ART. Using a novel method of marginal structural modeling, the impact of these therapies can be estimated from the observational ACTG ALLRT cohort by mimicking a randomized clinical trial.
METHODS
The ACTG ALLRT cohort was a longitudinal cohort study of HIV-infected participants who received randomly assigned ART as part of prospective clinical trials of ART treatment within the ACTG network [27]. Between 2000 and 2007, a total of 5972 participants were enrolled; follow-up ended in 2013. For this analysis, baseline was defined as the time of the first neurologic assessment after enrollment in ALLRT, after ART initiation. Participants included in the current analysis were not taking a statin or an ACEI/ARB within 30 days of the first neurologic assessment (baseline visit), had neurologic function testing available at least once within 30 months after baseline, and had no known neurocognitive dysfunction, current/prior central nervous system opportunistic infections, psychotic disease, active major depressive disorder, and/or use of antipsychotic medication at or before baseline.
Outcomes
Neurologic function was determined by the Hopkins Verbal Learning Test–Revised (HVLT-R) and the average z scores of 3 combined tests of neurocognitive performance (NPZ-3): Trailmaking A and B tests (TrA, TrB) and the Wechsler Adult Intelligence Scale–Revised digit symbol test (DST) every 48 weeks. Raw scores on HVLT-R and NPZ-3 components were standardized and adjusted for demographic factors (sex, age, race, and educational status) using normative data. Standardized t scores were converted to z scores for the primary outcome [28]. HVLT-R was introduced in the ALLRT study in 2007 [29]. For all standardized scores, positive scores indicate better neurologic function, and negative scores indicate worse neurologic function.
Statins were categorized as (1) lipophilic (atorvastatin, simvastatin, lovastatin, fluvastatin, cerivastatin, and pitavastatin) or (2) hydrophilic (pravastatin and rosuvastatin). Urine samples (random spot) were collected every 48 weeks; the uPCR was calculated (<0.2 is equivalent to 0.2 g of protein per day and considered normal). Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Statistical Analyses
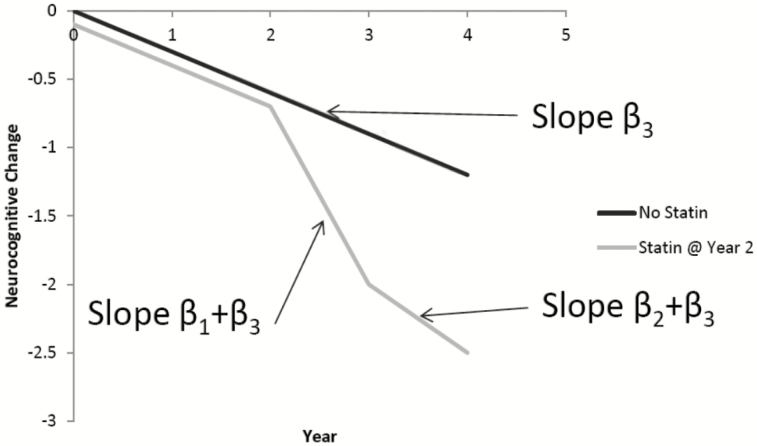
Time-varying confounders may predict statin and/or ACEI/ARB use and may also be associated with cognitive function, leading to time-dependent confounding by indication. This analysis used marginal structural models to estimate the causal effect of statin and/or ACEI/ARB therapy within year 1 (ie, early effects of therapy) and during each subsequent year of therapy (ie, prolonged effects of therapy) on neurocognitive outcomes. As illustrated in Figure 1, we expect neurocognitive function to follow a certain trajectory (β3) without treatment. We hypothesized that the trajectory of neurocognitive function in the first year of statin or ACEI/ARB therapy reflects the baseline slope in addition to the drug effect (β1 + β3), but this trajectory may differ from the longer-term effect after year 1 (β2 + β3), in regard to adverse effects and potential mechanisms.
Figure 1.
This model assumes the rate of neurocognitive change is the same (β3) until statin or ACEI/ARB therapy is initiated, after which we hypothesize that the rate of change in year 1 of therapy (β1 + β3) may differ from the effect for each subsequent year (β2 + β3). Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
In observational studies, therapy is initiated due to underlying risk factors. The control population is thus different than the treatment population. Marginal structural models attempt to mimic a randomized controlled trial by weighting patients to account for factors that may have influenced the initial treatment decision (to start a statin or ACEI/ARB). We used stabilized inverse probability of treatment weights to adjust for measured time-dependent confounders and stabilized inverse probability of censoring weights to adjust for potential selection bias due to censoring [30]. The final weighted general estimating equation (GEE) model used an exchangeable working correlation structure.
The following covariates were included as confounders potentially associated with statin initiation and neurologic outcomes: baseline age (<40, 40–60, or >60 years), sex, white race, Spanish-speaking status, and education level. Additional variables used both baseline and time-updated status: smoking status; diagnoses of cardiovascular or cerebrovascular disease; diagnosis of diabetes mellitus; CD4+ T-cell count (<200, 200–500, or >500 cells/μL; low-density lipoprotein (LDL) cholesterol level (<100, 100–160, or >160 mg/dL); systolic blood pressure >160 mm Hg, diastolic blood pressure >100 mm Hg, or use of antihypertensives, including ACEI/ARB; and virologic suppression (plasma HIV-1 RNA values <400 vs ≥400 copies/mL).
Covariates included in the ACEI/ARB models were similar except that time-updated body mass index (<18.5, 18.5–30, or >30 kg/m2) and estimated glomerular filtration rate (continuous; milliliters per minute per 1.73 square meters) were included; lipid criteria included LDL values or use of lipid-lowering drugs other than statins (bile acid sequestrants, fibrates, fish oils, ezetimibe, or niacin); and ACEI/ARB use was replaced with statin use. Participants who went 30 months without a neurologic examination or discontinued ALLRT for reasons other than completion of protocol, death, or site closure were censored. Covariates that were considered confounders potentially associated with censoring and neurologic outcomes included baseline age; sex, white race, Spanish-speaking status, current or prior intravenous drug use at baseline, and time-updated HIV-1 RNA level.
NPZ-3 (primary outcome) and individual components of NPZ-3 and HVLT-R (secondary) outcomes were examined separately. Sensitivity analyses were performed to investigate the effect of statins, censoring at ACEI/ARB initiation, and, for ACEI/ARB therapy, censoring at statin initiation. Exploratory analyses assessed factors associated with neurologic scores after statin initiation and compared hydrophilic versus lipophilic effects using unweighted GEE models, starting follow-up at statin initiation. The third exploratory analysis assessed the effect of ACEI/ARB initiation on uPCR using a piece-wise unweighted GEE model with a knot at the time of treatment initiation. This analysis included all available outcomes for the subset of participants starting ACEI/ARB therapy.
All analyses considered initiation of statin or ACEI/ARB treatment as intention to treat. Differences were considered statistically significant at P <.05; no adjustment was made for multiple comparisons. Analyses were performed using SAS software, version 9.4 (SAS Institute).
RESULTS
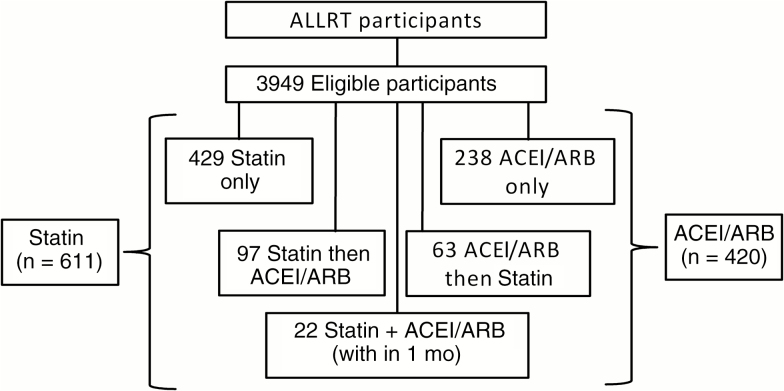
Of 5972 ALLRT participants, 3949 met inclusion criteria. Of these participants, 611 (15.5%) started therapy with a statin, 420 (10.6%) with an ACEI/ARB, and 182 (4.6%) with both during the follow-up period (Figure 2). At baseline, 53% of participants were <40 years old, and a majority were male (82%), white (56%), and nonsmokers (62%), with CD4+ T-cell counts >200/µL (79%) and an HIV-1 RNA levels <400 copies/mL (73%), Table 1. Participants in the statin and ACEI/ARB analyses received a median of 16 weeks of ART before initial neurocognitive testing (interquartile range [IQR], 8–60 and 8–57 weeks for statin and ACEI/ARB analysis, respectively). Specific statin and ACEI/ARB receipt are shown in Table 2. A median of 133 weeks (IQR, 55–245 weeks) lapsed between the first NPZ-3 assessment and statin initiation, and a median of 180 weeks (76–321 weeks) between the first NPZ-3 assessment and ACEI/ARB initiation.
Figure 2.
Of AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT) participants, the number of participants receiving statin and/or ACEI/ARB therapy. Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Table 1.
Study Characteristics at Baseline (Initial Neurocognitive Assessment)
| Characteristic | Patients, No. (%)a | ||
|---|---|---|---|
| Overall (N = 3949) | Statin Initiated (n = 611) | ACEI/ARB Initiated (n = 377) | |
| Age group, y | |||
| >60 | 85 (2) | 22 (4)b | 11 (3) |
| 40–60 | 1787 (45) | 392 (64) | 247 (66) |
| <40 | 2077 (53) | 197 (32) | 119 (32) |
| Female sex | 700 (18) | 94 (15) | 74 (20) |
| White race | 2221 (56) | 387 (63)b | 213 (56) |
| Spanish speaking | 558 (14) | 82 (13) | 35 (9)b |
| Educational levels | |||
| Less than high school | 551 (14) | 73 (12) | 46 (12) |
| High school or some college | 2279 (58) | 353 (58) | 217 (58) |
| College or above | 1119 (28) | 185 (30) | 114 (30) |
| Current or prior IVDU | 302 (8) | 31 (5)b | 27 (7) |
| Current smoker | 1495 (38) | 226 (37) | 126 (33) |
| CVD or DM | 143 (4) | 54 (9)b | 6 (2)b |
| Hypertension | 763 (19) | 93 (15)b | 90 (24)b |
| Dyslipidemia | 334 (8) | 135 (22)b | 55 (15)b |
| LDL level, mg/dL | |||
| >160 | 314 (8) | 159 (26)b | 44 (12)b |
| 100–160 | 1748 (44) | 330 (54) | 197 (52) |
| <100 | 1887 (48) | 122 (20) | 136 (36) |
| Body mass index, kg/m2 | |||
| >30 | 627 (16) | 108 (18)b | 95 (25)b |
| 25–30 | 1389 (35) | 239 (39) | 144 (38) |
| <25 | 1933 (49) | 264 (43) | 138 (37) |
| Taking ART | 3871 (98) | 601 (98) | 377 (98) |
| PI + NRTI backbonec | 1733 (44) | 226 (37) | 168 (45) |
| INSTI + NRTI backbone | 351 (9) | 18 (3) | 20 (5) |
| NNRTI + NRTI backbone | 1114 (28) | 200 (33) | 115 (31) |
| Other | 673 (17) | 157 (26) | 66 (18) |
| Duration of ART before baseline, median (IQR), wk | … | 16 (8–60) | 16 (8–57) |
| CD4+ T-cell count, cells/µL | |||
| <200 | 831 (21) | 118 (19) | 96 (25) |
| 200–500 | 2030 (51) | 315 (52) | 181 (48) |
| >500 | 1088 (28) | 178 (29) | 100 (27) |
| HIV-1 RNA <400 copies/mL | 2885 (73) | 437 (72) | 264 (70) |
| eGFR, mean (SD), mL/min/1.73 m2 | 104.2 (19.3) | 98.8 (18.6)b | 98.4 (19.6)b |
| Time between baseline and drug initiation, median (IQR), wk | … | 133 (55–245) | 180 (76–321) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ART, antiretroviral therapy; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HIV-1, human immunodeficiency virus type; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; IVDU, intravenous drug use; LDL, low-density lipoprotein cholesterol; NNRTI, nonnucleotide reverse-transcriptase inhibitor; NRTI, nucleotide/nucleoside reverse-transcriptase inhibitor; PI, boosted protease inhibitor; SD, standard deviation.
aData represent No. (%) of patients unless otherwise specified.
bSignificant difference between participants starting or not starting therapy (statin or ACEI/ARB) (P < .05 by Kruskal-Wallis, Fisher exact, or Wilcoxon test).
cAn NRTI backbone is defined as 2 NRTI drugs.
Table 2.
Specific Statin and ACEI/ARB Frequency
| Statin or ACEI/ARB | Patients, No. (%) |
|---|---|
| Statin (n = 611) | |
| Atorvastatin | 301 (49) |
| Pravastatin | 222 (36) |
| Simvastatin | 47 (8) |
| Rosuvastatin | 27 (4) |
| Othera | 15 (2) |
| ACEI/ARB (n = 377) | |
| ACEI | |
| Lisinoprilb | 238 (63) |
| Enaloprilb | 39 (10) |
| Benazapril | 23 (6) |
| Quinapril | 16 (4) |
| Ramipril | 12 (3) |
| Fosinopril | 8 (2) |
| Captopril | 3 (<1) |
| ARB | |
| Losartanb | 18 (5) |
| Valsartan | 13 (3) |
| Otherc | 7 (2) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
aLess than 1% of patients were taking lovastatin, fluvastatin, or cervistatin.
bIncludes combination tablets with hydrochlorothiazide.
cLess than 1% of patients were taking irbesartan, telmisartan, olmesartan, or cadesartan.
Statin Effects
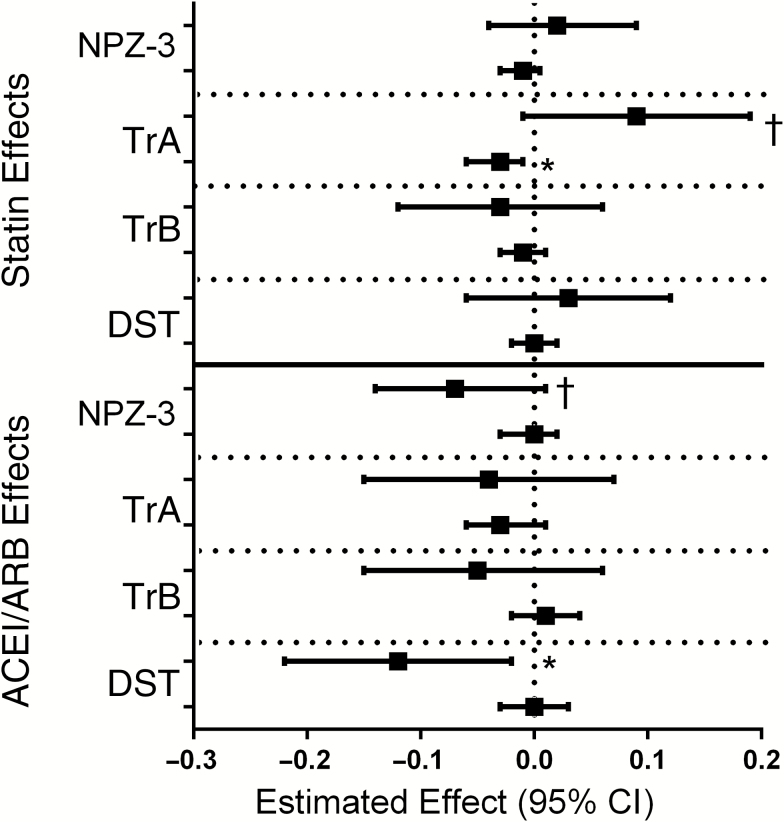
In the adjusted statin model, neither the first nor subsequent years of statins had a statistically significant impact on the composite NPZ-3 scores (Table 3 and Figure 3); similar effects were seen in sensitivity models, which censored at ACEI/ARB initiation (Table 3). On individual NPZ-3 components (secondary outcomes), the first year of statin therapy was associated with a trend toward improved TrA score (estimate, 0.088; 95% confidence interval [CI], −.010 to .187; P = .08), and subsequent years of statin therapy were associated with small but significantly lower TrA scores (−0.033; −.058 to −.009; P = .007). No significant effects were seen with TrB, DST (Figure 3), or HVLT-R (Table 4) scores.
Table 3.
Statin and ACEI/ARB Effects on Composite NPZ-3 Scores in Marginal Structural Modelsa
| Drug Effect on NPZ-3 Score | Estimate (95% CI) | P Value |
|---|---|---|
| Statin causal effect | ||
| Year 1 | 0.025 (−0.043 to .093) | .47 |
| After year 1 (per year) | −0.012 (−0.029 to .005) | .16 |
| Years since baseline | 0.061 (.057–.065) | <.001 |
| Statin causal effect, censored at ACEI/ARB initiation | ||
| Year 1 | −0.029 (−.102 to .045) | .45 |
| After year 1 (per year) | −0.008 (−.27 to .011) | .38 |
| Years since baseline | 0.063 (.059–.068) | <.001 |
| ACEI/ARB causal effect | ||
| Year 1 | −0.068 (−.14 to .008) | .08 |
| After year 1 (per year) | −0.005 (−.027 to .017) | .65 |
| Years since baseline | 0.064 (0.060–.068) | <.001 |
| ACEI/ARB causal effect, censored at statin initiation | ||
| Year 1 | −0.111 (−.211 to −.012) | .03 |
| After year 1 (per year) | 0.007 (−.029 to .043) | .71 |
| Years since baseline | 0.064 (0.059–.068) | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval NPZ-3, z score of averaged Trailmaking (Tr) A and B and digit symbol test (DST).
aModels adjusted for baseline age, sex, race, language, education level, smoking status, dyslipidemia, cardiovascular disease or diabetes, CD4+ T-cell count, human immunodeficiency virus type 1 RNA level <400 copies/mL, body mass index, estimated glomerular filtration rate, and hypertension.
Figure 3.
Estimated effect of statins (top) or ACEI/angiotensin receptor blocker (ARB) (bottom) therapy within the first year (β1 + β3 shown on the top line) or for each subsequent year after year 1 (β2 + β3 shown on the bottom line)
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; NPZ-3, z score of averaged Trailmaking (Tr) A and B and digit symbol test (DST). *P < .05; †P > .05 but < .10.
Table 4.
Statin and ACEI/ARB Effects on HVLT-R in Marginal Structural Modelsa
| Drug Effect on HVLT-R Score | Estimate (95% CI) | P Value |
|---|---|---|
| Statin causal effect | ||
| Year 1 | −0.123 (−.271 to .026) | .11 |
| After year 1 (per year) | 0.028 (−.040 to .096) | .42 |
| Years since baseline | 0.053 (0.043–.064) | <.001 |
| Statin causal effect, censored at ACEI/ARB initiation | ||
| Year 1 | −0.150 (−.296 to −.005) | .04 |
| After year 1 (per year) | 0.036 (−.032 to .105) | .30 |
| Years since baseline | 0.050 (0.039–.060) | <.001 |
| ACEI/ARB causal effect | ||
| Year 1 | 0.000 (−.155 to .155) | >.99 |
| After year 1 (per year) | 0.045 (−.037 to .127) | .28 |
| Years since baseline | 0.051 (0.041–.061) | <.001 |
| ACEI/ARB causal effect, censored at statin initiation | ||
| Year 1 | −0.033 (−.200 to .133) | .69 |
| After year 1 (per year) | 0.079 (−.026 to .183) | .14 |
| Years since baseline | 0.051 (0.040–.062) | <.001 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; HVLT-R, Hopkins Verbal Learning Test–Revised.
aModels adjusted for baseline age, sex, race, language, education level, smoking status, dyslipidemia, cardiovascular disease or diabetes, CD4+ T-cell count, human immunodeficiency virus type 1 RNA level <400 copies/mL, body mass index, estimated glomerular filtration rate, and hypertension.
When the effect of statin initiation was limited to participants (17%) with baseline NPZ-3 scores less than −1, there was no significant effect on NPZ-3 scores in year 1 (NPZ-3 change, 0.031; 95% CI, −.155 to .216; P = .75). In contrast, a significant negative effect was seen with subsequent years of statin therapy (−0.059; 95% CI, −.11 to −.004; P = .04). As shown in Table 5, these changes were driven by a negative effect on TrA score (−0.080; 95% CI, −.134 to −.027; P = .003).
Table 5.
Statin and ACEI/ARB Effects in Marginal Structural Models, Restricted to Participants with Baseline NPZ-3 Score Less Than −1
| Drug Effect | Estimate (95% CI) | P Value |
|---|---|---|
| Statin causal effect on TrA score | ||
| Year 1 | 0.031 (−.178 to .239) | .77 |
| After year 1 (per year) | −0.080 (−.134 to −.027) | .003 |
| Statin causal effect on TrB score | ||
| Year 1 | 0.032 (−.189 to .253) | .78 |
| After year 1 (per year) | −0.037 (−.101 to .028) | .26 |
| Statin causal effect on DST | ||
| Year 1 | 0.060 (−.173 to .292) | .62 |
| After year 1 (per year) | −0.057 (−.130 to .016) | .12 |
| ACEI/ARB causal effect on TrA score | ||
| Year 1 | 0.106 (−.192 to .405) | .49 |
| After year 1 (per year) | −0.146 (−.221 to −.071) | <.001 |
| ACEI/ARB causal effect on TrB score | ||
| Year 1 | −0.155 (−.444 to .133) | .29 |
| After year 1 (per year) | −0.035 (−.099 to .028) | .28 |
| ACEI/ARB causal effect on DST | ||
| Year 1 | −0.220 (−.433 to −.008) | .04 |
| After year 1 (per year) | 0.003 (−.073 to .078) | .95 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CI, confidence interval; NPZ-3, z score of averaged Trailmaking (Tr) A and B and digit symbol test (DST).
Exploratory multivariable analyses identified covariates associated with neurocognitive scores after statin initiation. Greater time in the study (and hence, greater time receiving ART) was associated with improved NPZ-3 scores; older age at statin initiation and suppressed HIV-1 RNA (<400 copies/mL) at the time of statin initiation were associated with lower NPZ-3 scores. In contrast, HVLT-R scores were higher among participants with lower CD4+ T-cell counts at statin initiation and with longer duration of statin therapy.
The effect of hydrophilic vs lipophilic statin on neurocognitive scores was also explored. For the NPZ-3 outcomes, 358 patients started therapy with a lipophilic and 253 with a hydrophilic statin; for the HVLT-R outcomes, 131 started therapy with a lipophilic and 138 with a hydrophilic statin. No significant difference between statin type was detected for NPZ-3 scores during year 1 (lipophilic statin, −0.055 [95% CI, −.151 to .041]; hydrophilic statin, 0.015 [−.129 to .098]; P = .59) or subsequent years (lipophilic, −0.042 [−.072 to −.012]; hydrophilic, −0.034 [−.063 to −.006]; P = .44). In contrast, lipophilic statins were associated with significantly lower HVLT-R scores (−0.060; 95% CI, −.349 to .229) than hydrophilic statins (0.343; .070 to .615; P = .04) in the first year of therapy but not in subsequent years (lipophilic, −0.019 [−.126 to .088]; hydrophilic, 0.029 [−.084 to .141]; P = .44).
Angiotensin-Converting Enzyme Inhibitor/Angiotensin Receptor Blocker Effects
In the adjusted ACEI/ARB model, the first year of therapy was associated with a trend toward poorer performance on NPZ-3 scores (−0.068; 95% CI, −.14 to .008; P = .08). The effect was more pronounced in sensitivity analyses that censored participants at the time of statin initiation (−0.111; 95% CI, −.211 to −.012; P = .03; Table 3). Significantly lower performance on the DST was observed during year 1 of ACEI/ARB therapy (−0.117; 95% CI, −.217 to .016; P = .02), with minimal effect in each subsequent year (0.00; −.029 to .032, P = .94). There was no significant effect of ACEI/ARB on the other NPZ-3 components (Figure 3) or the HVLT-R score (Table 4).
When the effect of ACEI/ARB initiation was limited to participants (17%) with NPZ-3 scores less than −1 at baseline, there was no significant effect on NPZ-3 scores in year 1 (NPZ-3 change, −0.093; 95% CI, −.197 to .143; P = .33), but there was a trend toward significant reduction in subsequent years of ACEI/ARB therapy (estimate, −0.057; −.115 to −.001; P = .053). Similar to the statin effect, these changes were driven by a negative effect on TrA score (Table 5).
In exploratory analyses of the subset of participants starting ACEI/ARB therapy, the rate of change in uPCR before ACEI/ARB initiation (−0.014; 95% CI, −.034 to .005]) did not significantly differ from the rate of change after initiation (−0.012; −.043 to .019); P = .85). The difference remained nonsignificant when analysis was restricted to those with a uPCR >0.2 (−0.036; 95% CI, −.136 to .064; P = .48).
DISCUSSION
In this cohort of ART-treated HIV-infected persons, the addition of statin or ACEI/ARB therapy had no negative effect on neurocognitive function, as shown by the standardized and validated NPZ-3 score, but modest declines were seen on individual components of the NPZ-3 or HVLT-R with both statins and ACEI/ARB. Among HIV-infected participants, heightened inflammation and immune activation, endothelial dysfunction, and atherosclerotic burden may contribute to NCI [31, 32]. Thus, with the known effects of statins on inflammation, endothelial function, and plaque burden, we had expected to see improved NCI with statin therapy, but we did not.
Overall, we failed to identify a statin effect on our primary outcome of global neurocognitive function (NPZ-3 scores), or secondary outcomes of TrB (executive function), DST (speed of processing), or HVLT-R (verbal learning and memory). In contrast, we found a modest net negative effect of statins on TrA, a measure of processing speed and attention, after 4 years of therapy. In exploratory analyses, we also found a negative impact of statin therapy on the primary outcome of global neurocognitive function among participants with baseline impairment, driven primarily by effects on the TrA component, although this was a small percentage of our overall population. Additionally, we found a potentially detrimental effect of lipophilic compared to hydrophilic statins on HVLT-R. The effect of statin type may be the result of unmeasured bias in prescribing, greater central nervous system penetration, or the more potent LDL-lowering effect of lipophilic statins. Theoretically, reduced cholesterol synthesis could impair neurogenesis or lead to neuronal cell death, although the clinical relevance and link to therapeutic statins has not been established [33, 34].
Participants with ACEI/ARB receipt experienced a small decline in NPZ-3 at 1 year, with no additional effect in subsequent years, suggesting limited detrimental effect of ACEI/ARB on neurocognitive performance. Similar to the statin findings, we also found a negative impact of ACEI/ARB therapy on the secondary outcome of TrA among participants with baseline impairment (NPZ-3 score less than −1), and a trend toward a negative effect on the composite NPZ-3 after the first year of therapy. It has been hypothesized that reactive oxygen species (ROS) contribute to non-AIDS comorbid conditions and that reduction in ROS production may reduce the progression of these conditions; indeed, the protective effects of ACEI/ARB therapy seems to be strongly related to their ROS-lowering effects [35, 36]. For example, ACEI/ARB use has been associated with reduced cardiovascular and all-cause mortality rates in patients with diabetes mellitus and in individuals with or at high risk for cardiovascular disease [37]. Moreover, it has been recently shown that ACEI/ARB exposure is associated with a 45% decrease in all-cause mortality rate in HIV-infected ART-suppressed adults (C. W. Wester, C. A. Jenkins, J. R. Koethe, A. Freeman, R. Kalayjian, A. Mendes, A. G. Abraham, J. E. Lake, K. Erlandson, H. Crane, T. R. Sterling, R. D. Moore, and B. E. Shepherd, unpublished data). Thus, the benefits of ACEI/ARB are likely to be mediated at least in part by their robust anti-inflammatory and antioxidant effects, which we expected would have manifest as improved neurocognitive function, but did not.
Albuminuria and proteinuria serve as markers of kidney disease and have been linked to cardiovascular disease, chronic kidney disease, NCI, and increased mortality in HIV-infected adults [38, 39]. Kalayjian et al [9] found a significant association between urinary protein excretion (uPCR) and NCI only in patients having significant proteinuria (uPCR >200 mg/g). We expected that ACEI/ARB initiation would improve neurocognitive function, mediated through a reduction in proteinuria. However, we failed to identify a reduction in proteinuria or improvement in neurocognitive testing after ACEI/ARB initiation. Notably, 53% of the participants were <40 years of age, and only 14% had a uPCR >0.2.
Our findings prompt further investigations into the long-term cognitive effects of statins and ACEI/ARB in HIV infection, particularly among adults with existing mild NCI. The study has several notable limitations. First, our significant findings may be attributable to chance, because a large number of statistical tests were performed. Second, marginal structural models are highly dependent on the included covariates. Inclusion of additional confounding variables might have altered our findings in either direction. Although we attempted to control for factors that influence statin or ACEI/ARB prescription, many unmeasured confounders may contribute, including greater engagement in or knowledge of medical care. Few participants had baseline mild NCI, so conclusions regarding the impact with existing NCI are based on a limited number of participants. Furthermore, we performed only 4 neurocognitive measures in the ALLRT cohort and could have missed changes in other neurocognitive domains. Drug-drug interactions between ART and statins may have altered the statin effect or have led to differing statin dose prescription. We censored participants at the time of death, thus our findings may represent a survivorship bias. In addition, the results of the exploratory analysis may have been influenced by other factors. For example, the association between improved scores over time among participants with either higher viral load or lower CD4+ T-cell count at the time of statin initiation is probably measuring the impact of effective ART on neurocognitive function rather than a statin effect. Those who were the sickest at the time of statin initiation had greater improvements in neurocognitive function than those who were already successfully maintained with ART.
In summary, although we detected modest negative neurocognitive effects for statins and ACEI/ARB therapy on single cognitive domains, these effects are overwhelmed by lack of effect on our primary outcome, the summary NPZ-3 score, and by the well-established cardiovascular benefits of statins and ACEI/ARB therapy. Consistent with findings in the general literature, our study did not find consistent evidence that statins or ACEI/ARB have an effect on global neurocognitive function. Future studies should further investigate the long-term effects of these therapies, particularly among HIV-infected persons with greater cognitive impairment and longer time receiving ART.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institute on Aging (grants K23AG050260 and R01AG054366 to K. M. E.), the National Institute of Allergy and Infectious Diseases (grants AI068636 [Leadership and Operations Center], AI068634 [Statistical and Data Management Center], AI106701 [Laboratory Center], and AI069501, AI036219, AI069432, AI069423, AI069452, and AI067039 [participating clinical trial units]), the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK108438 to E. T. O.), and the Veterans Administration (VISN10 Geriatric Research Educational and Clinical Centers, Louis Stokes Cleveland Veterans Administration Medical Center).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol 2004; 157:3–10. [DOI] [PubMed] [Google Scholar]
- 2. McMurtray A, Nakamoto B, Shikuma C, Valcour V. Small-vessel vascular disease in human immunodeficiency virus infection: the Hawaii aging with HIV cohort study. Cerebrovasc Dis 2007; 24:236–41. [DOI] [PubMed] [Google Scholar]
- 3. McMurtray A, Nakamoto B, Shikuma C, Valcour V. Cortical atrophy and white matter hyperintensities in HIV: the Hawaii aging with HIV cohort study. J Stroke Cerebrovasc Dis 2008; 17:212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barzilay JI, Fitzpatrick AL, Luchsinger J et al. Albuminuria and dementia in the elderly: a community study. Am J Kidney Dis 2008; 52:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol 2010; 171:277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joosten H, Izaks GJ, Slaets JP et al. Association of cognitive function with albuminuria and eGFR in the general population. Clin J Am Soc Nephrol 2011; 6:1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vupputuri S, Shoham DA, Hogan SL, Kshirsagar AV. Microalbuminuria, peripheral artery disease, and cognitive function. Kidney Int 2008; 73:341–6. [DOI] [PubMed] [Google Scholar]
- 8. Weiner DE, Bartolomei K, Scott T et al. Albuminuria, cognitive functioning, and white matter hyperintensities in homebound elders. Am J Kidney Dis 2009; 53:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalayjian RC, Wu K, Evans S et al. Proteinuria is associated with neurocognitive impairment in antiretroviral therapy treated HIV-infected individuals. J Acquir Immune Defic Syndr 2014; 67:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jukema JW, Cannon CP, de Craen AJ, Westendorp RG, Trompet S. The controversies of statin therapy: weighing the evidence. J Am Coll Cardiol 2012; 60:875–81. [DOI] [PubMed] [Google Scholar]
- 11. Padala KP, Padala PR, McNeilly DP, Geske JA, Sullivan DH, Potter JF. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer’s dementia: a prospective withdrawal and rechallenge pilot study. Am J Geriatr Pharmacother 2012; 10:296–302. [DOI] [PubMed] [Google Scholar]
- 12. King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy 2003; 23:1663–7. [DOI] [PubMed] [Google Scholar]
- 13. Orsi A, Sherman O, Woldeselassie Z. Simvastatin-associated memory loss. Pharmacotherapy 2001; 21:767–9. [DOI] [PubMed] [Google Scholar]
- 14. Padala KP, Padala PR, Potter JF. Statins: a case for drug withdrawal in patients with dementia. J Am Geriatr Soc 2010; 58:1214–6. [DOI] [PubMed] [Google Scholar]
- 15. Wagstaff LR, Mitton MW, Arvik BM, Doraiswamy PM. Statin-associated memory loss: analysis of 60 case reports and review of the literature. Pharmacotherapy 2003; 23:871–80. [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs 28 February 2012. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed 4 November 2012.
- 17. Shepardson NE, Shankar GM, Selkoe DJ. Cholesterol level and statin use in Alzheimer disease: II. Review of human trials and recommendations. Arch Neurol 2011; 68:1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ancelin ML, Carrière I, Barberger-Gateau P et al. Lipid lowering agents, cognitive decline, and dementia: the three-city study. J Alzheimers Dis 2012; 30:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sano M, Bell KL, Galasko D et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011; 77:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shepherd J, Blauw GJ, Murphy MB et al. ; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360:1623–30. [DOI] [PubMed] [Google Scholar]
- 21. McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev 2016;1:CD003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Swiger KJ, Martin SS, Tang F et al. Cognitive and physical function by statin exposure in elderly individuals following acute myocardial infarction. Clin Cardiol 2015; 38:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swiger KJ, Manalac RJ, Blumenthal RS, Blaha MJ, Martin SS. Statins and cognition: a systematic review and meta-analysis of short- and long-term cognitive effects. Mayo Clin Proc 2013; 88:1213–21. [DOI] [PubMed] [Google Scholar]
- 24. Ott BR, Daiello LA, Dahabreh IJ et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med 2015; 30:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Letendre SL, Marquie-Beck J, Ellis RJ et al. ; CHARTER Group The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J Neuroimmune Pharmacol 2007; 2:120–7. [DOI] [PubMed] [Google Scholar]
- 26. Barzilay JI, Gao P, O’Donnell M et al. ; ONTARGET and TRANSCEND Investigators Albuminuria and decline in cognitive function: the ONTARGET/TRANSCEND studies. Arch Intern Med 2011; 171:142–50. [DOI] [PubMed] [Google Scholar]
- 27. Smurzynski M, Collier AC, Koletar SL et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials 2008; 9:269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heaton RK, Heaton RK, . Psychological assessment resources I. Revised comprehensive norms for an expanded Halstead-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults, professional manual. Lutz, FL: Psychological Assessment Resources, 2004. [Google Scholar]
- 29. Brandt J, Benedict RHB.. Hopkins Verbal Learning Test–Revised. Administration manual. Lutz, FL: Psychological Assessment Resources, 2001. [Google Scholar]
- 30. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000; 11:550–60. [DOI] [PubMed] [Google Scholar]
- 31. Iantorno M, Schär M, Soleimanifard S et al. Coronary artery endothelial dysfunction is present in HIV-positive individuals without significant coronary artery disease. AIDS 2017; 31:1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edén A, Marcotte TD, Heaton RK et al. Increased intrathecal immune activation in virally suppressed HIV-1 infected patients with neurocognitive impairment. PLoS One 2016; 11:e0157160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cibičková L. Statins and their influence on brain cholesterol. J Clin Lipidol 2011; 5:373–9. [DOI] [PubMed] [Google Scholar]
- 34. Lütjohann D, Stroick M, Bertsch T et al. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids 2004; 69:431–8. [DOI] [PubMed] [Google Scholar]
- 35. Ogawa S, Kobori H, Ohashi N et al. Angiotensin II type 1 receptor blockers reduce urinary angiotensinogen excretion and the levels of urinary markers of oxidative stress and inflammation in patients with type 2 diabetic nephropathy. Biomark Insights 2009; 4:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reynaert NL, Gopal P, Rutten EP, Wouters EF, Schalkwijk CG. Advanced glycation end products and their receptor in age-related, non-communicable chronic inflammatory diseases; overview of clinical evidence and potential contributions to disease. Int J Biochem Cell Biol 2016; 81:403–18. [DOI] [PubMed] [Google Scholar]
- 37. Bangalore S, Fakheri R, Wandel S, Toklu B, Wandel J, Messerli FH. Renin angiotensin system inhibitors for patients with stable coronary artery disease without heart failure: systematic review and meta-analysis of randomized trials. BMJ 2017; 356:j4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gansevoort RT, Matsushita K, van der Velde M et al. ; Chronic Kidney Disease Prognosis Consortium Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes: a collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 2011; 80:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Choi A, Scherzer R, Bacchetti P et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis 2010; 56:872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]