Persistent double-stranded DNA viremia episodes temporally correlate with virus-specific end-organ disease and are associated with increased overall mortality after hematopoietic cell transplantation. First positive viral load >2 log10 copies/mL (range, 2.04–3.06 per virus) predicts persistent episodes and provides an early metric for intervention.
Keywords: transplant, virus, kinetics, threshold, mortality
Abstract
Background
Improved understanding of double-stranded DNA (dsDNA) virus kinetics after hematopoietic cell transplantation (HCT) would facilitate development of therapeutic strategies.
Methods
We tested weekly plasma samples from 404 patients through day 100 after allogeneic HCT for cytomegalovirus (CMV), human herpesvirus (HHV) 6A and 6B, BK polyomavirus (BKV), adenovirus (AdV), and Epstein-Barr virus (EBV) using quantitative polymerase chain reaction. Episodes lasting ≤1 week were defined as blips and >1 week as persistent. We described virus-specific kinetics, analyzed the association of virus area under the curve (AUC) with overall mortality, and identified risk factors for persistent episodes.
Results
We identified 428 episodes of CMV, 292 of BKV, 224 of HHV-6B, 46 of AdV, and 53 of EBV. CMV and BKV had the highest proportions of persistent episodes (68% and 80%, respectively). Detection and kinetics varied by virus. HHV-6B episodes reached maximum levels fastest and had the shortest intervals between detection and end-organ disease. End-organ disease occurred within 14 days of viremia in 68% of cases, generally during persistent episodes. For all viruses, higher viral load AUC increased risk for overall mortality through day 365, persistent episodes had higher viral load than blips, and higher first positive viral load significantly increased risk for persistent episodes. First viral load >2 log10 copies/mL (range, 2.04–3.06 per virus) had high specificity for persistent episodes.
Conclusions
Persistent high viral load dsDNA viremia episodes after allogeneic HCT predict mortality. Virus-specific kinetics can guide timing and thresholds for early intervention in studies of novel agents.
Reactivation of or infection with double-stranded DNA (dsDNA) viruses, including human herpesviruses (HHVs), adenovirus (AdV), and BK polyomavirus (BKV), remain important causes of morbidity and mortality after allogeneic hematopoietic cell transplant (HCT) [1–5]. There is a dose-response relationship between the frequency and magnitude of virus detection and subsequent complications [6]. Prophylactic and preemptive treatment strategies mitigate the impact of herpes simplex virus (HSV) types 1 and 2, varicella zoster virus, and cytomegalovirus (CMV) [7], but other dsDNA viruses continue to contribute to poor outcomes post-HCT [1–5].
Studies of viral dynamics informed the development and implementation of new treatment strategies for infections such as human immunodeficiency virus, hepatitis C virus, influenza, and HSV-2 [8–10]. Studies of CMV viral load kinetics guided screening and treatment approaches to prevent CMV disease in immunocompromised hosts [7, 11, 12], but other dsDNA virus kinetics following HCT are poorly characterized overall. Given the broader availability of diagnostic testing (eg, polymerase chain reaction [PCR] assays) [13], studies demonstrating direct and indirect complications associated with dsDNA viremia [2, 4, 14], and the development of novel antiviral therapeutics with broad activity [15–17], a better understanding of the kinetics and implications of dsDNA virus detection after allogeneic HCT is important.
We previously demonstrated frequent plasma detection of CMV, BKV, HHV-6B, Epstein-Barr virus (EBV), and AdV in a cohort of allogeneic HCT recipients [6]. The cumulative exposure to these viruses was associated with overall mortality. Here, we investigate the individual kinetics of these dsDNA viruses after allogeneic HCT, predictors of prolonged viremia, and the association of individual virus detection with overall mortality.
METHODS
Patients
We identified 404 patients of any age who received a first allogeneic HCT at our center during 2007–2014. We prespecified a target cohort consisting of human leukocyte antigen (HLA)–matched, HLA-mismatched, and cord blood HCT recipients with 125–150 patients per group. In each group, we included consecutive patients for whom we had ≥60% of weekly plasma samples while alive between day 0 and day 100 post-HCT. Specimens were left over from clinical CMV testing and preserved when available in an unbiased fashion. Two HCTs involving recipients or donors with possible inherited chromosomally integrated (ci) HHV-6 were excluded; ciHHV-6 was defined as persistent levels ≥100 copies per/mL in ≥80% of plasma samples [18]. We abstracted demographic and clinical information from medical records and databases. Nine patients receiving a second HCT before day 100, at a median of 58 days, were censored for all analyses at the last sampling time preceding the second HCT.
Laboratory Methods
Samples were retrospectively tested for BKV, HHV-6B, HHV-6A, AdV, and EBV. CMV results were obtained from clinical testing. Real-time quantitative PCR was performed for each virus as described in the Supplementary Data, and detection of 1 copy of virus DNA/reaction (50 copies/mL of plasma) was considered positive. CMV results were converted from international units (IU) to copies per milliliter to allow for comparisons on the same scale. Our conversion factor to the World Health Organization standard is 4 copies = 1 IU.
Definitions
Patient characteristics are defined in Table 1. Antiviral treatment strategies and end-organ disease definitions are detailed in the Supplementary Data. Overall mortality was defined as mortality occurring for any reason.
Table 1.
Demographic and Clinical Characteristics of the Study Cohort Compared to Excluded Hematopoietic Cell Transplantation Recipients During the Study Period
| Characteristic | All HCT Recipients During Study Period (n = 1926) | Study Cohort (n = 404) | Excluded Patients (n = 1522a) |
|---|---|---|---|
| Age >21 y | 1591 (83) | 358 (89) | 1233 (81) |
| Female sex | 809 (42) | 179 (44) | 630 (41) |
| White | 1430 (74) | 277 (69) | 1153 (76) |
| CMV donor negative, recipient negative | 563 (29) | 57 (14) | 506 (33) |
| HCT comorbidity indexb | |||
| Low (0) | 223 (18) | 70 (17) | 153 (18) |
| Intermediate (1–2) | 391 (31) | 133 (33) | 258 (30) |
| High (≥3) | 658 (52) | 201 (50) | 457 (53) |
| Underlying disease | |||
| Acute leukemia | 928 (48) | 195 (48) | 733 (48) |
| Chronic leukemia | 186 (10) | 58 (14) | 128 (8) |
| Lymphoma | 232 (12) | 41 (10) | 191 (13) |
| Nonmalignant | 158 (8) | 24 (6) | 134 (9) |
| Other | 422 (22) | 86 (21) | 336 (22) |
| Higher-risk diseasec | 394 (20) | 75 (19) | 319 (21) |
| Myeloablative conditioning regimend | 683 (35) | 155 (38) | 528 (35) |
| HCT typee | |||
| HLA-matched | 1392 (72) | 154 (38) | 1238 (81) |
| HLA-mismatched | 302 (16) | 125 (31) | 177 (12) |
| Cord blood | 232 (12) | 125 (31) | 107 (7) |
| Donor related | 703 (37) | 131 (32) | 572 (38) |
| Acute GVHD, grade 3-4f | 246 (13) | 46 (11) | 200 (13) |
| Steroids ≥ 1 mg/kgg | NA | 252 (62) | NA |
Data are presented as No. (%).
Abbreviations: CMV, cytomegalovirus; GVHD, graft-vs-host-disease; HCT, hematopoietic cell transplantation; HLA, human leukocyte antigen; NA, not available.
aUnknown data for patients in the following categories: race, n = 89; CMV serostatus, n = 54; HCT comorbidity index, n = 654.
bBased on the HCT comorbidity index [27].
cHigher-risk disease refers to diagnoses other than acute myeloid leukemia, acute lymphoblastic leukemia, or lymphoma in first remission, chronic myeloid leukemia in chronic phase, and refractory anemia without excess blasts.
dMyeloablative regimens included any regimen containing ≥800 cGY TBI, any regimen containing carmustine/etoposide/cytarabine/melphalan, or any regimen containing busulfan/cyclophosphamide with or without antithymocyte globulin.
eCategories include related and unrelated donors. HLA-matched HCTs were defined as 10/10 allele or antigen match and included 76 related and 78 unrelated donors. HLA-mismatched HCTs included 55 related donors and 70 unrelated donors; this group consisted of 47 haploidentical, 76 with 9/10 matched, and 2 with 8/10 matched donors. All cord blood HCTs were mismatched and 12 were single unit.
fAcute graft-vs-host disease grades were categorized as previously described [28].
gMaximum steroid dose (prednisone equivalent) administered during first 100 days post-HCT.
Episode Analyses
Virus detection episodes were defined based on periods of consecutive positive samples with a single negative test before and after. Multiple episodes were possible per patient. Episode duration was calculated assuming episodes started at the midpoint between the first positive and preceding negative sample and ended at the midpoint between the last positive sample and the proceeding negative sample, up to a maximum of 3.5 days. To account for imprecision due to variable sampling times, duration was categorized on a scale of weeks. We classified all episodes lasting >4 weeks in a single category. We further dichotomized episodes as blips (ie, 1 positive sample was assigned a duration of 1 week) or persistent episodes. Censoring is described in the Supplementary Data.
The cumulative distribution of episode duration was estimated using the Kaplan-Meier estimate. Duration trajectories were estimated with uncensored episodes using loess splines. We used linear mixed models to evaluate the relationship between episode duration (predictor) and mean viral load per virus episode (outcome). Receiver operating characteristic (ROC) curves were generated (pROC package in the R programming language) to evaluate the sensitivity and specificity of first positive viral load thresholds (log10 scale) as predictors for blip vs persistent episodes. Area under the ROC curve was estimated using a nonparametric method [19].
We used regression with generalized estimating equations (GEEs) to identify risk factors at the time of first virus detection that predicted progression to a persistent episode. Risk factors for inclusion in the model were selected a priori and included baseline variables of age, HLA match, stem cell source, underlying disease risk, and conditioning regimen, as well as time-dependent variables of acute graft-vs-host disease (GVHD), neutrophil engraftment date, absolute lymphocyte count <200 cells/μL, maximum prednisone-equivalent steroid dose within the preceding 14 days, viral load, number of concurrent dsDNA viruses detected, and any antiviral use (ganciclovir, foscarnet, or cidofovir). GEE models were estimated using the geepack package in the R programming language assuming a log link to estimate relative risk with a robust variance estimator [20, 21].
Cord blood HCT recipients had twice weekly CMV testing, but we only included weekly results in the primary analyses to maintain balanced testing across HCT types and viruses. A sensitivity analysis including all CMV results is presented in the Supplementary Data.
Overall Mortality Analysis
We used Cox proportional hazards regression models to evaluate the association between virus detection in the first 100 days post-HCT with overall mortality through day 365. The cumulative burden of virus exposure was calculated as the average area under the curve (AUC) using log10 copies/mL for each virus by summing quantitative PCR results and dividing by the number of days followed. Virus detection was incorporated as a time-dependent variable during the testing period through day 100 and as a time-invariant variable between days 101 and 365, using the AUC at the last recorded value through day 100. Demographic and clinical characteristics from Table 1 were considered for adjusted analyses. Covariates with P values ≤.2 in univariable analyses were retained in final adjusted models if P values were <.1. Statistical significance was defined as 2-sided P < .05. We used SAS software version 9.4 TS1M3 (SAS Institute, Cary, North Carolina) for these analyses.
RESULTS
Patient demographics and clinical characteristics of the study cohort and excluded patients are shown in Table 1. The distribution of characteristics was similar between selected and excluded patients aside from HCT type (as prespecified) and CMV serostatus. We retrospectively tested 4990 plasma samples obtained within 100 days post-HCT with a median of 13 samples per patient (interquartile range [IQR], 12–14) and 7 days between samples (IQR, 7–7). Ganciclovir, foscarnet, or cidofovir was administered to 247 patients (61%) within the first 100 days in 128 (32%), 108 (27%), and 20 (5%) patients at any time, respectively (not mutually exclusive).
A High Proportion of Viremic Episodes Persisted for >1 Month
Virus detection, persistence, and expansion differed by virus. CMV had the greatest number of episodes (428), followed by BKV (292), HHV-6B (224), AdV (46), and EBV (53) (Table 2). Most viruses had a median of 1 episode per patient except for CMV (median of 2 episodes). Virus detection occurred throughout the 14-week observation period; HHV-6B was detected the earliest (median 3 weeks) and AdV and EBV the latest (median 6 weeks).
Table 2.
Characteristics of Post–Hematopoietic Cell Transplantation Virus Detection per Patient
| Virus | Patients, No.a | Episode No., Total (Blip vs Persistent)b | Est. % Blipsc | Est. % Persistent >4 wkc | Episodes per Patient (IQR)d | First Detect, wk (IQR)d | Max Detect, wk (IQR)d | First Viral Load (IQR)e | Max Viral Load (IQR)f |
|---|---|---|---|---|---|---|---|---|---|
| CMV | 252 | 428 (135 vs 255) | 32 | 35 | 2 (1–2) | 5 (3–7) | 7 (5–9) | 2.2 (2.0–2.5) | 2.8 (2.3–3.3) |
| BKV | 218 | 292 (58 vs 208) | 20 | 63 | 1 (1–2) | 4 (2–6) | 10 (6–12) | 3.3 (2.7–3.8) | 4.1 (3.3–4.8) |
| HHV-6Bg | 188 | 224 (98 vs 91) | 44 | 23 | 1 (1–1) | 3 (3–4) | 4 (3–5) | 2.9 (2.5–3.4) | 3.2 (2.6–4.0) |
| AdV | 42 | 46 (23 vs 17) | 50 | 39 | 1 (1–1) | 6 (3–10) | 9 (5–12) | 2 (1.8–3.0) | 2 (1.8–4.2) |
| EBV | 37 | 53 (21 vs 24) | 41 | 30 | 1 (1–2) | 6 (4–7) | 9 (5–11) | 2.1 (1.9–2.4) | 2.2 (2.0–3.0) |
Data are presented as median (IQR) unless otherwise indicated.
Abbreviations: AdV, adenovirus; BKV, BK polyomavirus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; est., estimated; HHV-6B, human herpesvirus 6B; IQR, interquartile range; wk, post-HCT week.
aNumber of patients with at least 1 episode per virus.
bCensored episodes with duration ≤1 week are included in the total count but could not be classified as a blip or persistent episode.
cPercentage of blip and persistent episodes >4 weeks were estimated using KM estimator to account for censored episodes (see Figure 1).
dThe numbers presented are the median for the given category.
eMedian first detected viral load per patient (log10 copies/mL).
fMedian maximum viral load per patient (log10 copies/mL).
gHHV-6A was only detected in 1 patient and included in the HHV-6B category.
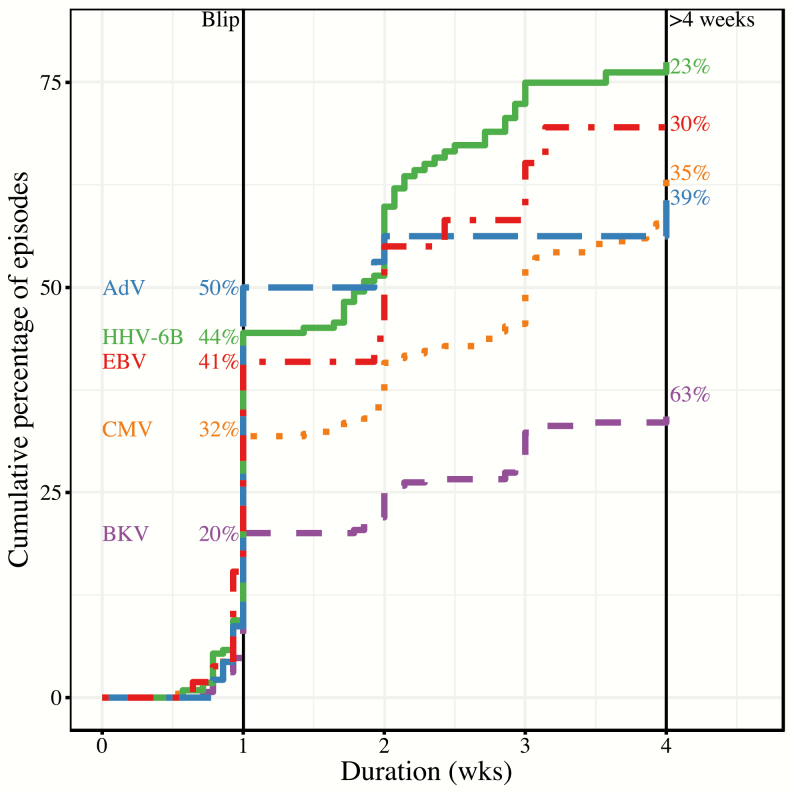
For all viruses, the majority of episodes were either blips or persistent episodes detected for >4 weeks (Figure 1). AdV had the highest proportion of blips (50%) and BKV had the lowest (20%) (Table 2). BKV episodes were the longest with a median duration >4 weeks (Figure 1).
Figure 1.
Kaplan-Meier estimated cumulative distribution of episode duration for each virus. Blip episodes had a duration ≤1 week. The vertical line at 1 week denotes the cutoff for blips; the percentage of blips is listed to the left. Estimated median episode duration occurs where the curves cross 50%. All episodes >4 weeks (vertical line at 4 weeks) were categorized together; the percentage is listed to the right. Curves are color coded by virus type. Using cytomegalovirus as an example, 32% of episodes were blips (therefore, 68% were persistent), the estimated median episode duration was 3 weeks, and 35% of episodes lasted >4 weeks. Abbreviations: AdV, adenovirus; BKV, BK polyomavirus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6B, human herpesvirus 6B.
Viral Loads Were Higher During Persistent Episodes Relative to Blips
We demonstrated shared and unique kinetic features of specific virus episodes. For all viruses, viral loads of blip episodes tended to be lower than the first positive, mean, maximum, and last positive viral loads of persistent episodes (Supplementary Figure 1A). Generally, mean viral load increased as episode duration increased (Supplementary Figure 1B), and persistent episodes had significantly higher mean viral loads than blips (Supplementary Figure 2). Spline estimated viral load trajectories based on episode duration had relatively conserved patterns among viruses but some differences depending on episode duration (Supplementary Figure 1C). CMV expansion dynamics were the slowest, and peak viral load increased with longer duration. For BKV, longer episodes (≥3 weeks) showed an early peak viral load followed by plateau. HHV-6B had the most consistent kinetics for all episodes lasting <4 weeks, which were characterized by rapid progression to similar peak viral loads followed by rapid decay. HHV-6B episodes lasting >4 weeks peaked early, decayed slightly, and remained at a plateau.
End-Organ Disease Is Temporally Related to Onset of Persistent Viremia
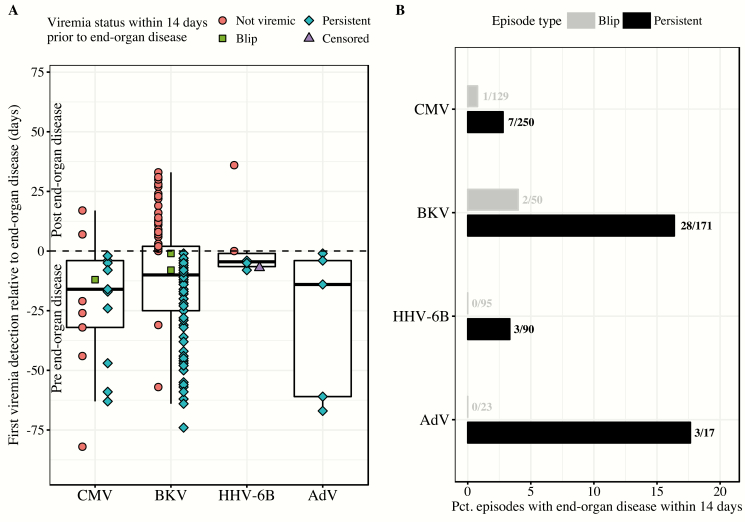
There were 36 cases of end-organ disease (excluding BKV) in 34 patients, comprising 24 cases of CMV (20 gastrointestinal, 4 pulmonary), 6 cases of HHV-6B (central nervous system), 5 cases of AdV (2 gastrointestinal, 2 pulmonary, 1 gastrointestinal and pulmonary), and 1 case of EBV (central nervous system); there were no cases of EBV-associated posttransplant lymphoproliferative disease. There were 101 cases of BKV-associated cystitis. Most patients with end-organ disease had plasma detection of the associated virus at some time post-HCT. The proportion of patients who developed virus-specific end-organ disease in the absence of any viremia was as follows: CMV, 3/24 (2 gastrointestinal and 1 pulmonary); BKV, 7/101; HHV-6B, 0/6; AdV, 0/5; EBV, 1/1. Figure 2A exhibits the temporal relationship of end-organ disease with virus detection and episode type. Among all patients with end-organ disease and viremia, first plasma detection occurred a median of 10 days before end-organ disease diagnosis (median day by virus: CMV, 16; BKV, 10; HHV-6B, 5; AdV, 14). Viremia was present within a 14-day window prior to diagnosis in 68% of cases (by virus: CMV, 62%; BKV, 68%; HHV-6B, 67%; AdV, 100%), almost always in the context of persistent episodes. End-organ disease diagnosis within 14 days or 28 days of persistent episode onset occurred relatively frequently for BKV (16% and 27%, respectively) and AdV (18% and 24%) but infrequently for CMV (3% and 5%) and HHV-6B (3% and 3%) (Figure 2B).
Figure 2.
The temporal relationship of end-organ disease with virus detection and episode type among viremic patients. A, The distribution of first plasma virus detection and episode type relative to end-organ disease diagnosis among patients with viremia and end-organ disease during the study period. Boxes represent the interquartile range (IQR), and whiskers extend to within 1.5 times the IQR of the first and third quartiles (Tukey box plot). Shapes depict episode type and whether the episode occurred within a 14-day window preceding end-organ disease diagnosis. Circles depict start date of episodes in which virus detection occurred outside a 14-day window prior to end-organ disease diagnosis. Most end-organ disease diagnoses had episodes (square and diamond shapes) within a 14-day window prior to disease: cytomegalovirus (CMV), 62%; BK polyomavirus (BKV), 68%; human herpesvirus 6B (HHV-6B), 67%; adenovirus (AdV), 100%. B, The percentage of blip or persistent viremia episodes preceding end-organ disease cases within 14 days. Fractions indicate the number of end-organ disease cases occurring within 14 days after episode onset (numerator) over the total number of episodes (denominator). For 1 HHV-6B case, first plasma detection and end-organ disease diagnosis date were the same; we classified this episode as a nonpreceding episode. In the single case of Epstein-Barr virus (EBV) end-organ disease, the patient never had EBV detected in plasma.
Higher Viral Load Area Under the Curve Is Associated With Overall Mortality
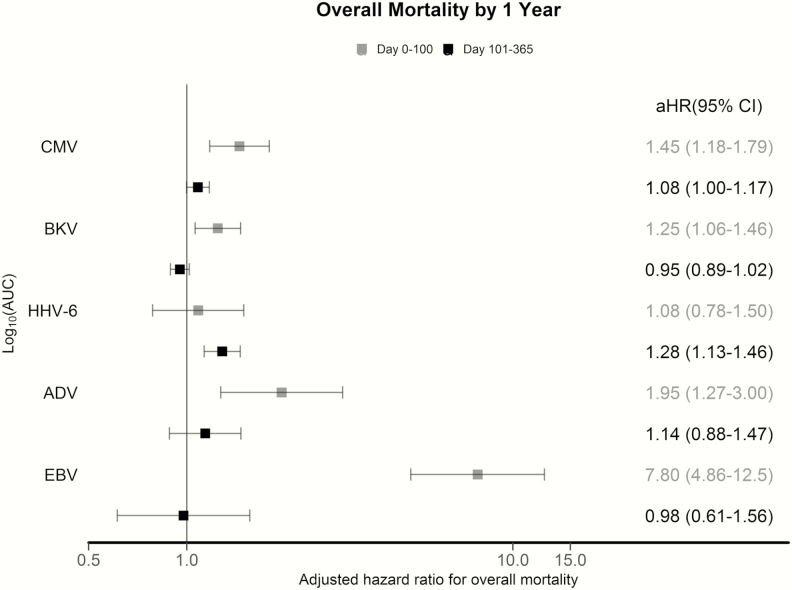
There were 125 deaths (32%) by day 365 post-HCT; 36 patients (9%) died in days 0–100 and 89 patients (23%) died in days 101–365. We used Cox regression models to evaluate the association of cumulative viral load AUC per virus through day 100 with overall mortality by day 100 and between days 101 and 365. Higher cumulative viral load AUC for each virus except HHV-6B was independently associated with overall mortality by day 100 post-HCT; CMV and HHV-6B were associated with overall mortality between days 101 and 365 (Figure 3). Models were adjusted for age, HCT comorbidity index, underlying disease risk, conditioning regimen, acute GVHD, and cumulative steroid dose AUC during the first 100 days post-HCT.
Figure 3.
Forest plot of the association of cumulative viral load area under the curve (AUC) with overall mortality through day 365 post–hematopoietic cell transplantation (HCT). We calculated the cumulative viral load AUC on a log10 copies/mL scale for each virus per patient by summating quantitative polymerase chain reaction results and dividing by the number of days followed. Cumulative viral load AUC was incorporated as a time-dependent variable through day 100 (grey) and a time-invariant variable through day 365 (because testing ended at day 100 [black]). All models were adjusted for age, HCT comorbidity index score, underlying disease risk, type of conditioning regimen (myeloablative vs not), time-dependent acute graft-vs-host disease grade 3–4, and time-dependent cumulative steroid dose AUC during the first 100 days post-HCT (steroid use was categorized as 0, <1 mg/kg, 1 to <2 mg/kg, and ≥2 mg/kg prednisone equivalent per day). Abbreviations: AdV, adenovirus; aHR, adjusted hazard ratio; AUC, area under the curve; BKV, BK polyomavirus; CI, confidence interval; CMV, cytomegalovirus; EBV, Epstein-Barr virus; HHV-6 human herpesvirus 6.
Viral and Host Risk Factors for Persistent Episodes
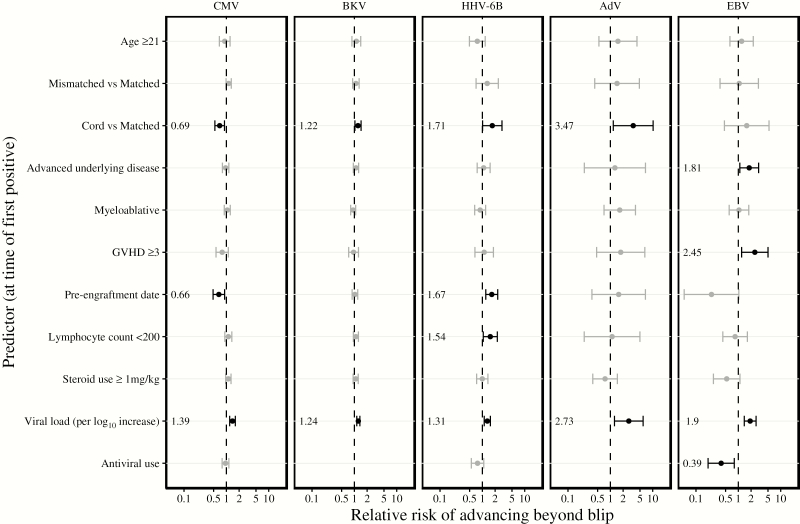
We next analyzed predictors of persistent episodes. We constructed ROC curves to evaluate the sensitivity and specificity of first positive viral load as a predictor of having a persistent episode (Supplementary Figure 3). First positive viral load roughly discriminated between blips and persistent episodes but performance, based on area under the ROC curve, varied by virus (Supplementary Table 2). Viral load thresholds >2 log10 copies/mL (range, 2.04–3.06) had only 50% sensitivity but preserved low false-positive rates for all viruses (range, 0–32%). These thresholds had positive likelihood ratios ≥2 (ratios > 1 increase probability of the event) for BKV, HHV-6B, AdV, and EBV. Slightly lower viral load thresholds (range, 1.75–2.36 log10 copies/mL) yielded 75% sensitivity with higher false-positive rates (range, 19%–59%). These thresholds maintained positive likelihood ratios >2 for AdV and EBV.
First positive viral load was associated with risk of persistent episodes for all viruses in an adjusted regression model: each log10 copies/mL increase was associated with a 1.2–2.7 relative risk of progressing to a persistent episode (Figure 4). Additional risk factors for persistent episodes at the time of first virus detection included cord blood HCT (BKV, HHV-6B, AdV), advanced underlying disease (EBV), acute GVHD grades 3–4 (EBV), pre–neutrophil engraftment (HHV-6B), absolute lymphocyte count <200 cells/μL (CMV, HHV-6B), and steroid dose ≥1 mg/kg within the preceding 14 days (CMV). Patients already receiving antivirals for other indications were less likely to progress to persistent episodes for EBV.
Figure 4.
Characteristics associated with advancing from a blip to a persistent episode at the time of first positive test result. We used generalized estimating equations to estimate the association of each variable with risk of developing a persistent episode at the time of the first positive test. All covariates were included in a multivariable model. Acute graft-versus-host disease grade, neutrophil engraftment date, absolute lymphocyte count <200 cells/μL, maximum prednisone-equivalent steroid dose within the preceding 14 days, viral load, number of concurrent double-stranded DNA viruses detected, and antiviral use (ganciclovir, foscarnet, or cidofovir) were time-dependent variables based on the date of the first positive test. Most antiviral use (ie, ganciclovir and foscarnet) in this cohort did not target BK polyomavirus or adenovirus, so antiviral use was not incorporated into analyses for these viruses. Concurrent detection of other viruses did not increase risk for developing persistent episodes for any of the viruses and was excluded from the final model. Dots represent the relative risk and error bars represent the 95% confidence interval. Bolded bars were statistically significant (P < .05). Abbreviations: AdV, adenovirus; BKV, BK polyomavirus; CMV, cytomegalovirus; EBV, Epstein-Barr virus; GVHD, graft-vs-host disease; HHV-6B human herpesvirus 6B.
DISCUSSION
In this diverse cohort of allogeneic HCT recipients with substantial exposure to antivirals active against dsDNA viruses, we detected CMV, BKV, and HHV-6B in plasma from the majority of patients, with less frequent detection of AdV and EBV. We demonstrated that higher viral load at first detection was partially predictive of persistent episodes for all viruses after controlling for factors affecting immune reconstitution. Identifying patients at risk for persistent episodes is important given that persistent episodes had higher overall mean viral load and were more likely to progress to end-organ disease compared to blips. Additionally, higher viral load AUC was associated with overall mortality within 100 days after HCT for each virus except HHV-6B, and between days 101 and 365 for CMV and HHV-6B.
Our study identified that dsDNA virus detection and kinetics in immunocompromised patients have a temporal relationship with outcomes and could be incorporated into treatment strategies. While viremia was common and occurred frequently in the absence of end-organ disease, most end-organ disease occurred in the context of persistent episodes. Among patients with end-organ disease, diagnosis occurred within 14 days of viremia in approximately 60%–70% of CMV, BKV, and HHV-6 cases, and in all cases of AdV. End-organ disease absent plasma detection was uncommon but occurred most frequently for CMV (13%) and BKV (7%). These data, along with the finding that the cumulative viral load AUC was associated with early and sometimes late overall mortality, support the potential benefits of prophylactic instead of preemptive treatment. Future study designs should consider endpoints evaluating a reduction in persistent viremia.
Optimal treatment approaches may vary by virus. For example, HHV-6B episodes had the highest initial viral load, reached maximum levels at or within a week of onset, and had very short duration between plasma detection and end-organ disease diagnosis (median, 5 days) in contrast to other viruses (median, >7 days). This suggests that a prophylactic approach to end-organ disease prevention may be more efficacious for HHV-6B, which is supported by a study of preemptive HHV-6B treatment that did not significantly mitigate HHV-6B reactivation and end-organ disease [22]. Alternatively, more frequent HHV-6B testing might be beneficial. A preemptive approach may be sufficient for other viruses, and our data suggest that first viral loads >2–3 log10 copies/mL may provide an early metric for identifying safe but clinically significant opportunities to intervene. Such an approach is supported by studies in CMV [11] and AdV [23], which underscore the importance of early interventions to mitigate duration and extent of viral replication. However, despite the success of preemptive therapy for reducing CMV end-organ disease, CMV viremia remains a risk factor for poor outcomes [1], emphasizing the need for better therapeutic options. CMV prophylaxis with novel small molecules or vaccines has shown promise in early studies [24, 25].
The natural history of virus reactivation was likely impacted by antiviral use, primarily with ganciclovir and foscarnet targeting CMV but with potential effects on HHV-6B and EBV. An anti-CMV effect is suggested by relatively low CMV viral loads and expansion kinetics compared to other viruses (Supplementary Figure 1). First positive CMV viral loads were also the least predictive of progression to persistent episodes by ROC curve analysis, likely due to preemptive therapy (Supplementary Table 2). Additionally, lower risk for CMV progression to persistent episodes in cord blood vs HLA-matched HCT recipients (Figure 4) was likely due to more aggressive preemptive treatment among cord blood HCT recipients. Our data also demonstrated a prophylactic effect of antivirals for EBV (Figure 4).
Strengths of this study include a diverse cohort of HCT recipients with regular testing through day 100 post-HCT. Identification of risk factors at the time of first virus detection is a novel approach to patient risk stratification, and improved understanding of viral kinetics may inform the potential success of viral prevention strategies. The use of viral load AUC may also serve as an informative endpoint in interventional trials. A limitation of our study was the lack of more frequent sampling. Given the high frequency of episodes with only 1 positive test, these data may underestimate the true burden of dsDNA virus detection. A supplementary analysis incorporating twice-weekly testing data for CMV in cord blood HCT recipients demonstrated that more frequent sampling identified additional episodes, although these were generally blips also characterized by lower viral load than persistent episodes (Supplementary Data). In addition to improving the quality of episode classification, increased sampling may refine our understanding of viral expansion and decay kinetics as demonstrated for HSV-2 [8, 26], as well as strengthen temporal associations between viremia and end-organ disease diagnosis to improve preemptive approaches. The lack of international standardized PCR assays during this study period, except for CMV, limits generalizability of viral load thresholds. The majority of patients in this study were adults, which may explain the lower incidence of AdV reactivation compared with other studies [23], and 19% of patients ≤21 years old had adenoviremia compared with 9% of patients >21 years old. Patients receiving a second HCT before day 100 may be at particularly high risk for viral infections, and censoring at this time may have led to an underestimate of episode number and duration, as well as mortality.
In conclusion, we identified that longer episodes of viremia after allogeneic HCT resulted in higher viral loads and a greater burden of virus exposure for each virus, which was in turn associated with increased overall mortality. Findings from this study underscore the limitations of preemptive antiviral treatment strategies. A one-size-fits-all approach for the prevention of dsDNA viruses may not be the most effective strategy. First positive viral load thresholds can be used in the design of interventional trials to study the efficacy of novel strategies to mitigate persistent episodes and improve outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Author contributions. J. A. H., B. T. M., J. T. S., M. B., D. M. Z., and W. L. were responsible for the design of the study and interpretation of the data. B. T. M., J. A. H., and H. X. analyzed the data and created the figures. Data were collected by J. A. H., F. M., and C. S. D. Laboratory work was carried out or supervised by M.-L. H., T. S.-A., and K. R. J. All authors contributed to the writing and revision of the manuscript and approved the final version.
Acknowledgments. We thank E. Lisa Chung, Laurel Joncas-Schronce, Laura Sissons-Ross, and Zach Stednick for help with data collection and management; and Heather Andrew, Lizanne Ngo, Jo Tono, Jessica Yi, Tracy Santo Hayes, and Susan McArdle for preparing and testing samples. Samples were obtained from the Infectious Disease Sciences Biorepository at the Fred Hutchinson Cancer Research Center.
Financial support. This work was supported in part by an investigator-initiated grant from Chimerix, Inc. This work was also supported by grants from the National Institutes of Health (5K23AI119133-02 to J. A. H. and K24HL093294 to M. B.). Additional resources were provided by the National Institutes of Health (HL088021, CA78902, CA18029, and HL122173).
Potential conflicts of interest. J. A. H. has served as a consultant for Chimerix, Inc and Nohla Therapeutics, Inc and has received research support from Chimerix, Inc and Shire. G. N. is an employee of, and owns stock in, Chimerix, Inc. D. M. Z. has received research support from Chimerix, Inc. M. B. has received grants and personal fees from Merck and Co, Astellas, Shire, Roche/Genentech, Gilead, and Chimerix, and personal fees from Clinigen and Microbiotix. J. T. S. has received research support from Chimerix, Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Green ML, Leisenring W, Xie H et al. . Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 2016; 3:e119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zerr DM, Boeckh M, Delaney C et al. . HHV-6 reactivation and associated sequelae after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18:1700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Erard V, Huang ML, Ferrenberg J et al. . Quantitative real-time polymerase chain reaction for detection of adenovirus after T cell-replete hematopoietic cell transplantation: viral load as a marker for invasive disease. Clin Infect Dis 2007; 45:958–65. [DOI] [PubMed] [Google Scholar]
- 4. Erard V, Kim HW, Corey L et al. . BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005; 106:1130–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner HJ, Wessel M, Jabs W et al. . Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation 2001; 72:1012–9. [DOI] [PubMed] [Google Scholar]
- 6. Hill JA, Mayer BT, Xie H et al. . The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood 2017; 129:2316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomblyn M, Chiller T, Einsele H et al. ; Center for International Blood and Marrow Research; National Marrow Donor Program; European Blood and MarrowTransplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Disease Canada; Centers for Disease Control and Prevention Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant 2009; 15:1143–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schiffer JT, Wald A, Selke S, Corey L, Magaret A. The kinetics of mucosal herpes simplex virus-2 infection in humans: evidence for rapid viral-host interactions. J Infect Dis 2011; 204:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perelson AS, Ribeiro RM. Modeling the within-host dynamics of HIV infection. BMC Biol 2013; 11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canini L, Perelson AS. Viral kinetic modeling: state of the art. J Pharmacokinet Pharmacodyn 2014; 41:431–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet 2000; 355:2032–6. [DOI] [PubMed] [Google Scholar]
- 12. Åsberg A, Humar A, Rollag H et al. . Lessons learned from a randomized study of oral valganciclovir versus parenteral ganciclovir treatment of cytomegalovirus disease in solid organ transplant recipients: the VICTOR Trial. Clin Infect Dis 2016; 62:1154–60. [DOI] [PubMed] [Google Scholar]
- 13. Inazawa N, Hori T, Hatakeyama N et al. . Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol 2015; 87:1427–35. [DOI] [PubMed] [Google Scholar]
- 14. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 2002; 185:273–82. [DOI] [PubMed] [Google Scholar]
- 15. Prichard MN, Williams JD, Komazin-Meredith G et al. . Synthesis and antiviral activities of methylenecyclopropane analogs with 6-alkoxy and 6-alkylthio substitutions that exhibit broad-spectrum antiviral activity against human herpesviruses. Antimicrob Agents Chemother 2013; 57:3518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Florescu DF, Keck MA. Development of CMX001 (brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther 2014; 12:1171–8. [DOI] [PubMed] [Google Scholar]
- 17. Papadopoulou A, Gerdemann U, Katari UL et al. . Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014; 6:242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pellett PE, Ablashi DV, Ambros PF et al. . Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol 2012; 22:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–45. [PubMed] [Google Scholar]
- 20. Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol 2011; 174:984–92. [DOI] [PubMed] [Google Scholar]
- 21. Halekoh U, Højsgaard S, Yan J. The R Package geepack for generalized estimating equations. J Stat Softw 2006; 15:1–11. [Google Scholar]
- 22. Ishiyama K, Katagiri T, Hoshino T, Yoshida T, Yamaguchi M, Nakao S. Preemptive therapy of human herpesvirus-6 encephalitis with foscarnet sodium for high-risk patients after hematopoietic SCT. Bone Marrow Transplant 2011; 46:863–9. [DOI] [PubMed] [Google Scholar]
- 23. Grimley MS, Chemaly RF, Englund JA et al. . Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant 2017; 23:512–21. [DOI] [PubMed] [Google Scholar]
- 24. Marty F, Ljungman P, Chemaly RF et al. . A phase III randomized, double-blind, placebo-controlled trial of letermovir (LET) for prevention of cytomegalovirus (CMV) infection in adult CMV-seropositive recipients of allogeneic hematopoietic cell transplantation (HCT) (abstract). In: BMT Tandem Meeting 2017. [Google Scholar]
- 25. Nakamura R, Rosa C La, Longmate J et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol. 2016; 3:e87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schiffer JT, Swan D, Al Sallaq R et al. . Rapid localized spread and immunologic containment define herpes simplex virus-2 reactivation in the human genital tract. Elife 2013; 2:e00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sorror ML, Maris MB, Storb R et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106:2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leisenring WM, Martin PJ, Petersdorf EW et al. . An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood 2006; 108:749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.