Summary
Cytomegalovirus urinary shedding detected at the time of labor and delivery in HIV-infected pregnant women not on antiretrovirals was associated with infant HIV and CMV transmission.
Keywords: HIV, pregnancy, congenital CMV, CMV viruria, HIV perinatal transmission.
Abstract
Background.
Cytomegalovirus (CMV) urinary shedding in pregnant women infected with human immunodeficiency virus (HIV) was evaluated to determine whether it poses an increased risk for congenital CMV infection (cCMV).
Methods.
A subset of mother-infant pairs enrolled in the perinatal NICHD HPTN 040 study (distinguished by no antiretroviral use before labor) was evaluated. Maternal and infant urines were tested by qualitative real-time polymerase chain reaction (RT-PCR) for CMV DNA with quantitative RT-PCR performed on positive specimens.
Results.
Urine specimens were available for 260 women with 85.4% from the Americas and 14.6% from South Africa. Twenty-four women (9.2%) had detectable CMV viruria by qualitative PCR. Maternal CMV viruria was not associated with mean CD4 cell counts or HIV viral load but was associated with younger maternal age (P = .02). Overall, 10 of 260 infants (3.8%) had cCMV. Women with detectable peripartum CMV viruria were more likely to have infants with cCMV than those without: 20.8% (5/24) versus 2.1% (5/236), (P = .0001). Women with CMV viruria had significantly higher rates of HIV perinatal transmission (29.2% vs. 8.1%, P = .002). They were 5 times (adjusted odds ratio [aOR] = 5.6, 95% confidence interval [CI] 1.9–16.8) and nearly 30 times (aOR, 29.7; 95% CI, 5.4–164.2) more likely to transmit HIV and CMV to their infants, respectively. Maternal gonorrhea (aOR, 19.5; 95% CI, 2.5–151.3) and higher maternal HIV log10 viral load (OR, 2.8; 95% CI, 1.3–6.3) were also significant risk factors for cCMV.
Conclusion.
In this cohort of HIV-infected pregnant women not on antiretrovirals, urinary CMV shedding was a significant risk factor for CMV and HIV transmission to infants.
Clinical Trials Registration Number.
Cytomegalovirus (CMV) is an important yet neglected cause of congenital infection, which may lead to sensorineural hearing loss and developmental delay [1, 2]. Congenital CMV infection (cCMV) may result from maternal primary infection, reactivation, or reinfection during pregnancy with CMV [3]. The prevalence of cCMV has been suggested to be ≤1% in industrialized nations but may be higher in resource-limited countries, where CMV seropositivity is higher [1, 4–6]. Other studies have suggested that cCMV rates may be higher among infants born to mothers infected with human immunodeficiency virus (HIV) in the pre-antiretroviral era compared to infants born to HIV-uninfected women [7–12]. HIV-infected infants may be more likely to demonstrate symptomatic cCMV than HIV-uninfected infants, and CMV infection may lead to more rapid progression of infant HIV infection [7–12].
CMV may be shed in bodily fluids such as saliva, breast milk, urine, and cervical secretions [3]. Studies evaluating CMV viruria in pregnant women have suggested intermittent detection of the virus in urine specimens [13–16]. Although detection of CMV in bodily fluids may be a marker of viral replication [17], the majority of prior studies in healthy pregnant women have not shown that maternal CMV shedding in urine and/or cervical secretions is associated with an increased risk of cCMV in infants [14, 16, 18–22].
However, less is known about the relevance of CMV shedding in urine and cervical specimens, among women with impaired cell-mediated immunity such as HIV-infected pregnant women [23]. This is of particular importance because impaired immunity may lead to reactivation or persistence of CMV infection [24].
In order to address the lack of published research evaluating the role of maternal CMV shedding and the development of congenital CMV in high-risk populations such as HIV-infected pregnant women, the present substudy of the NICHD HPTN 040 cohort was developed. The primary objective of this analysis was to evaluate rates of CMV viruria among HIV-infected pregnant women and to determine potential associations between maternal CMV viruria and cCMV. Additional secondary objectives include determination of risk factors for maternal CMV viruria, cCMV, and HIV perinatal transmission.
METHODS
The study population was a subset of mother-infant pairs enrolled in the National Institute of Child Health and Human Development (NICHD) HIV Prevention Trials Network (HPTN) 040 conducted from April 2004 to June 2011, for whom maternal and infant urines were available. One mL aliquots of maternal urine were tested with qualitative CMV DNA real-time polymerase chain reaction (RT-PCR), and positive specimens were tested by quantitative CMV DNA RT-PCR. Infant urines were similarly tested, with mother-infant pair results correlated.
Study Design
NICHD/HPTN 040 was a phase 3, triple-arm, randomized, open-label, multicenter study that evaluated the efficacy, safety, and tolerance of 3 different infant antiretroviral prophylaxis regimens for the prevention of intrapartum HIV transmission to infants born to HIV-infected pregnant women who had not received antiretroviral drugs during pregnancy [25].
HPTN 040 enrolled 1,684 HIV-infected pregnant women diagnosed with HIV infection at the time of labor and delivery from multiple sites in Brazil, South Africa, Argentina, and the United States. All women provided written informed consent prior to study enrollment. Infants <32 weeks of gestational age were excluded from study participation. Although the primary endpoint of the parent study was infant HIV infection status at 3 months of age, infants were followed until 6 months of age for safety and toxicity monitoring in the parent study.
At the time of labor and delivery, maternal plasma HIV RNA levels and CD4+ T-lymphocyte subsets were obtained. Serologic testing for syphilis was also performed at the time of labor and delivery per standard of care. Testing for Neisseria gonorrhoeae and Chlamydia trachomatis was performed using the Xpert ® CT/NG assay (Cepheid, Sunnyvale, CA) on stored maternal urines.
HIV Diagnosis
Infant blood specimens were used to perform HIV DNA polymerase chain reaction (PCR) within 48 hours of birth and at 10–14 days, 4–6 weeks, 3 months, and 6 months of age. Positive infant HIV results were confirmed by repeat testing. Diagnosis of infant HIV infection required 2 positive HIV DNA PCR tests (Roche Molecular Systems Inc., Basel, Switzerland) collected on different days. In utero HIV infection was defined as a positive HIV DNA PCR test result at birth with positive results on repeat testing. Intrapartum HIV infection was defined as a negative HIV DNA PCR result at birth with a confirmed positive HIV DNA PCR result on subsequent testing. All HIV-exposed infants enrolled in the study were exclusively formula fed.
Specimen Collection and CMV Testing
Stored maternal urine samples, 1 per patient, were collected at the time of labor and delivery or within 48 hours after birth. Maternal urines were frozen at –80o C and stored at study sites. Thawed 1 mL aliquots of maternal urine were then tested by qualitative RT-PCR for CMV DNA (FOCUS Diagnostics CMV Analyte Specific Reagent). Maternal urines with positive qualitative results were then tested by quantitative CMV DNA RT-PCR. Infant urines also collected within 48 hours after birth were similarly tested with mother-infant pair results correlated. Infants with detectable CMV in urine in the first 48 hours of life were diagnosed with presumed cCMV. Testing of maternal and infant samples was performed after the conclusion of the parent study, and thus, results were not available to inform clinical management. Further detailed analysis of cCMV as a risk factor for HIV perinatal transmission as well as HIV perinatal transmission as a risk factor for cCMV are delineated in separate HPTN 040 substudies [26].
Statistical Analysis
The χ2 (or Fisher exact test) was used to test the difference in proportions for categorical variables, and the 2-sample t-test (or Kruskal-Wallis) test was used to test the mean or median differences for continuous variables as appropriate. The univariate and multivariable logistic models were used to examine the associations of outcomes (infant HIV status, cCMV, and maternal CMV viruria) with potential risk factors, respectively. The covariates with an overall Type III P < .15 from the univariate models were included in the initial full multivariable model for model selection. All computations were performed using SAS software v9.4 (Cary, NC, USA).
Human Subjects
The study was approved by the institutional review boards and national ethics committees at each participating site.
RESULTS
Maternal Profile
Urine specimens were available for 260 women. The majority of women (85.4%) were from the Americas (Brazil, Argentina, US); 14.6% were from South Africa. The mean maternal age was 27.1 years with the majority less than 30 years of age. High rates of bacterial sexually transmitted infections (Chlamydia trachomatis, Neisseria gonorrhoeae, and Treponema pallidum), illegal substance use, alcohol use, and lack of prenatal care were observed (Table 1). The mean maternal CD4 count was 477 (SD 301) cells/mm3, and the median maternal HIV viral load was 12 953 (range of 0–2 700 000) copies/mL.
Table 1.
Maternal Characteristics Summary and Risk Factors for Maternal CMV Viruria
| Total (N = 260) | CMV Viruria Detected (N = 24) | CMV Viruria Not Detected (N = 236) | Unadjusted | ||
|---|---|---|---|---|---|
| n (col %) | n (row %) | n (row %) | OR (95% CI) | P-value | |
| Study arm | |||||
| ZDV | 83 (31.9) | 8 (9.6) | 75 (90.4) | 1.00 | |
| ZDV+NVP | 87 (33.5) | 7 (8.0) | 80 (92.0) | 0.82 (0.28– 2.37) | .71 |
| ZDV+3TC+NFV | 90 (34.6) | 9 (10.0) | 81 (90.0) | 1.04 (0.38 – 2.84) | .94 |
| Maternal age (years) | |||||
| 13–24 | 99 (38.1) | 13 (13.1) | 86 (86.9) | 6.20 (1.36 – 28.31) | .02 |
| 25–29 | 77 (29.6) | 9 (11.7) | 68 (88.3) | 5.43 (1.13 – 25.97) | .03 |
| 30 and older | 84 (32.3) | 2 (2.4) | 82 (97.6) | 1.00 | |
| Mean maternal HIV viral load (SD) (copies/mL) Maternal HIV viral load, categorical (copies/mL) | 73 108 (249 313) | 70 907 (253 893) | 94 664 (202 185) | … | .91 |
| ≤400 | 15 (5.8) | 1 (6.7) | 14 (93.3) | 1.00 | |
| 401 to ≤ 10,000 | 101 (38.8) | 9 (8.9) | 92 (91.1) | 1.37 (0.16 – 11.65) | .77 |
| 10,001 to 100,000 | 109 (41.9) | 10 (9.2) | 99 (90.8) | 1.41 (0.17 – 11.91) | .75 |
| Log 10 of maternal HIV viral load | 259 (99.6) | 24 (9.3) | 235 (90.7) | 1.13 (0.70 – 1.83) | .62 |
| Mean maternal CD4 count (SD) (cells/mm 3) | 477 (301) | 442 (179) | 481 (311) | … | .92 |
| Maternal CD4 count (cells/ mm 3 )/100 | 256 (98.5) | 24 (9.4) | 232 (90.6) | 0.95 (0.82 – 1.11) | .53 |
| Region | |||||
| Americas | 222 (85.4) | 23 (10.4) | 199 (89.6) | 1.00 | |
| South Africa | 38 (14.6) | 1 (2.6) | 37 (97.4) | 0.23 (0.03 – 1.79) | .16 |
| Syphilis | |||||
| Yes | 20 (7.7) | 1 (5.0) | 19 (95.0) | 0.49 (0.06 – 3.86) | .50 |
| No | 239 (92.3) | 23 (9.6) | 216 (90.4) | 1.00 | |
| CT or NG | |||||
| No | 209 (82.9) | 21 (10.0) | 188 (90.0) | 1.00 | |
| Yes | 43 (17.1) | 2 (4.7) | 41 (95.3) | 0.44 (0.10–1.94) | .28 |
| CT | |||||
| No | 212 (84.1) | 21 (9.9) | 191 (90.1) | 1.00 | |
| Yes | 40 (15.9) | 2 (5.0) | 38 (95.0) | 0.48 (0.11–2.13) | .33 |
| NG | |||||
| No | 239 (94.8) | 23 (9.6) | 216 (90.4) | 1.00 | |
| Yes | 13 (5.2) | 0 (0.0) | 13 (100) | 0.54 (0–2.60) | .56 |
| Any of these STIs | |||||
| Yes | 59 (22.8) | 3 (5.1) | 56 (94.9) | 0.46 (0.13–1.59) | .22 |
| No | 200 (77.2) | 21 (10.5) | 179 (89.5) | 1.00 | |
| Prenatal care | |||||
| No | 99 (38.1) | 8 (8.1) | 91 (91.9) | 1.00 | |
| Yes | 159 (61.2) | 16 (10.1) | 143 (89.9) | 1.27 (0.52–3.09) | .59 |
| Alcohol use during pregnancy | |||||
| ≥1/week | 25 (9.7) | 2 (8.0) | 23 (92.0) | 1.18 (0.25–5.66) | .84 |
| >1/month, <1/week | 14 (5.4) | 2 (14.3) | 12 (85.7) | 2.26 (0.45–11.38) | .32 |
| ≤1/month | 60 (23.2) | 9 (15.0) | 51 (85.0) | 2.39 (0.94– 6.10) | .07 |
| Never | 160 (61.8) | 11 (6.9) | 149 (93.1) | 1.00 | |
| Illegal substance use | |||||
| Yes | 38 (14.7) | 3 (7.9) | 35 (92.1) | 0.82 (0.23–2.88) | .75 |
| No | 221 (85.3) | 21 (9.5) | 200 (90.5) | 1.00 | |
Abbreviations: 3TC = lamivudine; CI, confidence interval; CMV, cytomegalovirus; CT = Chlamydia trachomatis; HIV, human immunodeficiency virus; NFV = nelfinavir; NG = Neisseria gonorrhoeae; NVP = nevirapine; OR, odds ratio; SD, standard deviation; STI = sexually transmitted infection; ZDV = zidovudine.
Twenty-four women (9.2%) had detectable CMV viruria by qualitative PCR. The majority of women with viruria (91.7%) had low levels of detectable CMV (<200 copies/mL), whereas 2 women had higher levels at 236 and 53 524 CMV copies/mL, respectively. Of potential risk factors for maternal CMV viruria (infant antiretroviral regimen parent study arm, study region, prenatal care, sexually transmitted infections [STIs], alcohol use, tobacco use, maternal CD4 count, and maternal HIV viral load), only maternal age was a predictor for maternal CMV viruria in univariate analysis (P = .02). Mean age among women with CMV viruria was significantly lower (24.3 years, SD 4.8) compared to those without viruria (27.4 years, SD 6.5), P = .006. Younger women (ages 13–24 years and 25–29 years) were significantly more likely to have CMV viruria at the time of delivery (odds ratio [OR], 6.2; 95% confidence interval [CI], 1.4–28.3) and (OR, 5.4; 95% CI, 1.1–26), respectively. Mean HIV viral load and CD4 cell count were not significantly associated with CMV viruria (P = .91 and P = .92, respectively) (Table 1).
Maternal CMV Viruria and Other Risk Factors for Congenital CMV Infection
Overall, 10 (3.8%) infants had cCMV, with CMV detected from urine at the time of birth (range of 367–592 274 copies/mL). cCMV rates varied by detection of maternal CMV viruria. Among mothers with detectable urinary CMV at the time of delivery, 20.8% had infants with cCMV. In contrast, only 2.1% of infants born to mothers with undetectable urinary CMV had cCMV. The 2 women with the highest levels of CMV viruria had infants with cCMV. Women with CMV viruria were 12 times (OR, 12.2; 95% CI, 3.2–45.7) more likely to have an infant with cCMV. The relationship between maternal CMV viruria and cCMV was even more pronounced when the analysis was adjusted for mode of delivery, maternal gonococcal infection, and maternal HIV log10 viral load (aOR, 29.7; 95% CI, 5.4–164.2) (Table 2).
Table 2.
Unadjusted and Adjusted Risk Factors for Congenital CMV (cCMV) and Perinatal HIV Transmission
| CMV Positive (N = 10) | CMV Negative (N = 250) | Unadjusted | Adjusted | P-value | HIV Positive (N = 26) | HIV Negative (N =234) | Unadjusted | P-value | Adjusted | P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (row %) | n (row %) | OR (95% CI) | P-value | OR (95% CI) | n (row %) | n (row%) | OR (95% CI) | OR (95% CI) | ||||
| Maternal CMV | ||||||||||||
| Detected | 5 (20.8) | 19 (79.2) | 12.16 (3.23 - 45.74) | .0002 | 29.72 (5.38–164.15) | .0001 | 7 (29.2) | 17 (70.8) | 4.70 (1.73–12.75) | .002 | 5.57 (1.85–16.78) | .002 |
| Not detected | 5 (2.1) | 231 (97.9) | 1.00 | 1.00 | 19 (8.1) | 217 (91.9) | 1.00 | 1.00 | ||||
| Study arm | ||||||||||||
| ZDV | 2 (2.4) | 81 (97.6) | 1.00 | 14 (16.9) | 69 (83.1) | 1.00 | 1.00 | |||||
| ZDV+NVP | 3 (3.4) | 84 (96.6) | 1.45 (0.24–8.88) | .69 | 10 (11.5) | 77 (88.5) | 0.64 (0.27–1.53) | .32 | 0.65 (0.26–1.63) | .36 | ||
| ZDV+3TC+NFV | 5 (5.6) | 85 (94.4) | 2.38 (0.45 – 12.63) | .31 | 2 (2.2) | 88 (97.8) | 0.11 (0.02–0.51) | .01 | 0.10 (0.02–0.46) | .003 | ||
| Log 10 of HIV maternal viral load | 10 (3.9) | 249 (96.1) | 2.82 (1.27 – 6.26) | .01 | 26 (10.0) | 233 (90.0) | 1.66 (1.03–2.69) | .04 | 1.72 (1.02–2.90) | .04 | ||
| NG | ||||||||||||
| No | 7 (2.9) | 232 (97.1) | 1.00 | 1.00 | 25 (10.5) | 214 (89.5) | 1.00 | |||||
| Yes | 2 (15.4) | 11 (84.6) | 6.03 (1.12 – 32.46) | .04 | 19.45 (2.50 – 151.34) | .005 | 1 (7.7) | 12 (92.3) | 0.71 (0.09–5.72) | .75 | ||
| Duration of rupture of membranes | ||||||||||||
| Unknown | 3 (14.3) | 18 (85.7) | 2.57 (0.64–10.40) | .18 | ||||||||
| >24 hours | 2 (40.0) | 3 (60.0) | 10.30 (1.52–69.67) | .02 | ||||||||
| 12–24 hours | 0 (0.0) | 9 (100) | <0.01 (<0.01 to >999) | .98 | ||||||||
| 6 to <12 hours | 3 (14.3) | 18 (85.7) | 2.57 (0.64–10.40) | .18 | ||||||||
| 5 to <6 hours | 9 (16.1) | 47 (83.9) | 2.96 (1.11–7.89) | .03 | ||||||||
| <0.5 hours | 9 (6.1) | 139 (93.9) | 1.00 | |||||||||
Log10 of HIV maternal viral load indicates that for each 1 unit log10 viral load increase, the odds of having an infant with cCMV or HIV increase 2.8 times or 1.7 times, respectively.
Abbreviations: 3TC, lamivudine; CI, confidence interval; CMV, cytomegalovirus; HIV, human immunodeficiency virus; NFV, nelfinavir; NG, Neisseria gonorrhoeae; NVP, nevirapine; OR, odds ratio; ZDV, zidovudine.
Apart from maternal CMV viruria, maternal gonococcal infection and maternal HIV viral load were also associated with cCMV. Women with gonococcal infection were six times more likely to have an infant with cCMV (OR, 6; 95% CI, 1.1–32.5), a difference that was even more pronounced in the adjusted analysis (aOR, 19.5; 95% CI, 2.5–151.3), when controlled for maternal urine CMV, mode of delivery, and log10 HIV viral load. Higher maternal HIV log10 viral load were also associated with nearly a 3-fold increased risk of cCMV (OR, 2.8; 95% CI, 1.3–6.3). (Table 2).
Maternal Peripartum CMV Viruria and HIV Perinatal Transmission and Infant Mortality
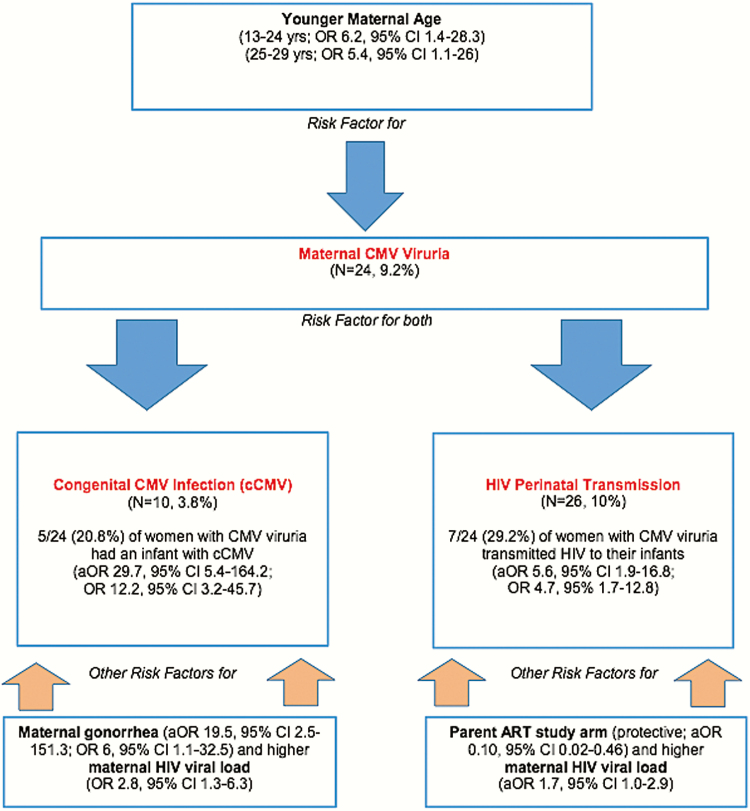
Women with CMV viruria also had significantly higher rates of HIV transmission to their infants (29.2% vs. 8.1%, P = .002) and were nearly 5 times more likely to transmit HIV to their infants (OR, 4.7; 95% CI, 1.7–12.8) on univariate analysis, with similar findings on multivariate analysis adjusted for infant study arm, maternal log10 viral load, and duration of ruptured membranes (aOR, 5.6; 95% CI, 1.9–16.8). (Table 2). Among the 7 HIV-infected infants born to women with CMV viruria, 5 were infected with HIV in utero and 2 were infected intrapartum. The one infant with both cCMV and HIV infection was infected with HIV in utero. Although there was an association between maternal CMV viuria and infant CMV acquisition (P = .0001, Table 2) and infant HIV acquisition (P = .002, Table 2), we did not observe an association between infant HIV and congenital CMV (cCMV) infection (P = .66, Table 3). Significant observed risk factors for maternal CMV viruria, cCMV, and HIV perinatal transmission in this study are summarized in Figure 1.
Table 3.
HIV Perinatal Transmission and Congenital CMV (cCMV)
| Total (N = 260) | Infant Congenital CMV (cCMV) | P-value | ||
|---|---|---|---|---|
| CMV Negative (N = 250) | CMV Positive (N = 10) | |||
| n (%) | n (%) | n (%) | ||
| Infant HIV | ||||
| HIV negative | 234 (90.0) | 225 (90.0) | 9 (90.0) | .66 |
| HIV infected in utero | 14 (5.4) | 13 (5.2) | 1 (10.0) | |
| HIV infected intrapartum | 12 (4.6) | 12 (4.8) | 0 (0.0) | |
Abbreviations: CMV, cytomegalovirus; HIV, human immunodeficiency virus.
Figure 1.
Summary of observed study risk factors for maternal CMV viruria, congenital CMV infection (cCMV), and HIV perinatal transmission.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; CMV, cytomegalovirus; HIV, human immunodeficiency virus; OR, odds ratio.
DISCUSSION
We evaluated the association between maternal CMV viruria and both cCMV and perinatal HIV transmission in a cohort of HIV-infected pregnant women, who were not receiving antenatal antiretroviral drugs, and found that infants born to women with CMV viruria at the time of labor and delivery were significantly more likely to have cCMV as well as HIV infection.
The profile of women and infant pairs in this smaller substudy was similar to that of our other published NICHD HPTN 040 analyses [27–29]. Among the population of late-presenting HIV-infected pregnant women included in this subanalysis, the majority of women were from the Americas (primarily Brazil) and had high rates of illegal substance usage, alcohol usage, lack of prenatal care, and other sexually transmitted infections (chlamydia, gonorrhea, and syphilis) [27–29].
The frequency of maternal CMV viruria (9.2%) detected at the time of labor and delivery corresponds to rates reported in prior studies of healthy pregnant and nonpregnant women without HIV, which have ranged from 1.4–13% [14, 17, 20, 22, 30]. A few isolated published studies have reported on the detection of CMV cervical shedding in HIV-infected pregnant women, among whom CMV shedding rates were extremely high (66%) [31]. Only 1 study has evaluated rates of CMV urinary shedding in HIV-infected pregnant or nonpregnant women, reporting a prevalence of 7%, similar to our results [23]. Our study is distinguished from the earlier one from the late 1990s of HIV-infected pregnant women because it employed sensitive diagnostic methods to detect CMV viruria (using CMV PCR as opposed to culture) [32, 33] and its inclusion of high-risk HIV-infected pregnant women from countries outside the United States (primarily Brazil and South Africa), where seroprevalence of CMV and rates of cCMV are believed to be higher [23].
Nearly 21% of our HIV-infected pregnant women with CMV viruria at the time of labor and delivery had an infant with cCMV. These women had a nearly 30-fold (adjusted OR, 29.7) increased risk of having an infant with cCMV as compared to women without CMV viruria. These findings contrast to the majority of other published studies, which have not found an association between maternal CMV urinary or cervical shedding and an increased risk of in utero CMV transmission [16, 18–22, 34, 35]. We are aware of only 1 other study that has documented high rates of cCMV among infants (10%) born to women with CMV viruria, which reported that high titers of maternal urinary CMV were significantly associated with cCMV [14]. Interestingly, that study, which took place more than 2 decades ago, only evaluated pregnant women without HIV infection. It is possible that the HIV infection status of pregnant women in our study, compared to the majority of prior studies, could account for the differing results.
Another interesting finding from our study was that maternal Neisseria gonorrhoeae was a risk factor for cCMV. Women with this STI were 6 times more likely (OR, 6; adjusted OR, 19.5) to have an infant with cCMV, and our findings correspond to results of other studies in the literature [3, 36–43].
Over 29% of women with CMV viruria transmitted HIV to their infants, and women with CMV viruria at the time of labor and delivery were 5 times (adjusted OR, 5.6) more likely to have an infant infected with HIV. These results contrast with the 1 other study evaluating maternal CMV viruria in HIV-infected pregnant women and its relationship to HIV perinatal transmission, which did not show a significant association of CMV viruria and transmission [23]. Our study also differed because it evaluated only HIV-infected pregnant women not receiving any antiretroviral drugs (such as zidovudine) during pregnancy.
Our findings that CMV viruria may be a marker for both in utero transmission of CMV and HIV underscores the complexities of these relationships [11, 12, 44]. One hypothesis for our findings is that immunosuppression inherent in pregnancy, particularly in the third trimester, along with that from HIV infection, may lead to more sustained local CMV reactivation and/or reinfection of the genital/urinary tract as evidenced by increased CMV viral shedding (i.e., viruria) [11, 12, 44]. In addition, CMV may also itself induce a pro-inflammatory state in the genital tract that may contribute to increased HIV-shedding [45]. These factors, along with decreased maternal antibodies and placental antibody transfer associated with untreated maternal HIV infection, may lead to an increased likelihood of CMV placental infection, inflammation, and ultimately CMV in utero transmission among infants as well as an increased risk of HIV perinatal infection, particularly in utero HIV transmission [11, 31].
One limitation of our study was that the sample size was restricted to women with available urine samples from our primary HPTN 040 study [25]. Thus, the lack of association between HIV perinatal transmission and cCMV was likely a reflection of our sample size. Furthermore, the magnitude of maternal CMV viruria detected in our cohort was low. The limited number of women with higher levels of CMV viruria (i.e., >200 copies/mL) in our study precluded determination of whether the degree of maternal viruria had any impact on the risk of CMV transmission to infants. Cervical specimens were also not collected in the parent study, which precluded evaluation of CMV cervical shedding rates. In addition, given the high seroprevalence of CMV among HIV-infected women in Brazil based on published studies by members of our team, maternal CMV serology was not done. We believe that the majority of maternal CMV infections in our cohort were likely due to CMV recurrence or reinfection [5, 6]. However, definitive information on maternal CMV seropositivity was not available for these women to make any conclusive statements in this regard. Women in our study were not on antiretroviral drugs during pregnancy; therefore, our findings should not be extrapolated to women who are adequately treated for HIV.
CONCLUSION
Urinary CMV shedding at the time of delivery in HIV-infected pregnant women not on antiretroviral drugs was relatively common in this high-risk cohort. Our findings suggest that maternal CMV viruria at the time of birth is a significant risk factor for both CMV and HIV transmission to infants born to women who did not receive antiretroviral treatment during pregnancy. In particular, our results appear to underscore the necessity of controlling maternal HIV infection during pregnancy through use of antiretrovirals to prevent both HIV and CMV transmission to neonates. Additional studies are needed to evaluate the role of both CMV urinary and genital shedding during pregnancy and the potential role this may play in the risk of in utero transmission of both HIV and CMV.
NICHD HPTN 040 STUDY TEAM
In addition to the authors, members of the NICHD/HPTN 040/PACTG 1043 protocol team include the following: Argentina, Buenos Aires- Foundation for Maternal and Infant Health (FUNDASAMIN): Mariana Ceriotto, Edgardo Szyld, Silvia Marzo. Brazil, Belo Horizonte- Federal University of Minas Gerais: Flavia Faleiro Ferreira, Fabiana Kakehasi. Porto Alegre-Hospital Nossa Senhora da Conceicao: Rita Lira. Porto Alegre-Hospital Femina: Carla Franceschini de Fraga, Rita Lira. Porto Alegre-Irmandade da Santa Casa de Misericordia de Porto Alegre: Debora Fernandes Coelho, Alberto Sanseverino, Luis Carlos Ribeiro. Rio de Janeiro-Hospital dos Servidores do Estado: M. Leticia Santos Cruz, Ezequias Martins, Jacqueline Anita de Menezes, Luisa Andrea Torres Salgado. Rio de Janeiro- Hospital Geral de Nova Iguaçu: Ana Valeria Cordovil, Andréa Gouveia, Priscila Mazzucanti, Jorge Eurico Ribeiro. Ribeirao Preto -Universidade de Sao Paulo: Geraldo Duarte, Adriana Aparecida Tiraboschi Barbaro, Carolina Sales Vieira. Sao Paulo-Universidade Federal de Sao Paulo: Daisy Maria Machado, Regina Succi. South Africa, Capetown-Stellenbosch University and Tygerberg Hospital: Mark Cotton, Jeanne Louw, Elke Maritz. Johannesburg-Perinatal HIV Research Unit, University of Witwatersrand and Chris Hani Baragwanath Hospital: Sarita Lalsab, Shini Legoete, James Alasdair McIntyre, Mandisa Nyati. United States, Baltimore-Johns Hopkins University: Allison Agwu, Jean Anderson, Joan Bess, Jonathan Ellen, Todd Noletto, Nancy Hutton. Gainesville-Shands Hospital: Carol Delany, Robert M. Lawrence. Jacksonville-University of Florida: Chas Griggs, Mobeen Rathore, Kathleen Thoma, Michelle Tucker. Long Beach- Miller Childrens Hospital: Audra Deveikis, Susan Marks. Newark-University Medical and Dental School of NJ: Linda Bettica, James M. Oleske. San Juan City-San Juan City Hospital: Midnela Acevedo Flores, Elvia Pérez. Oswaldo Cruz Foundation, Rio de Janeiro (FIOCRUZ): Francisco I Bastos, Beatriz Grinsztejn, Ronaldo I. Moreira, Marilia Santini de Oliveira, Monica Derrico, Valéria Ribeiro, Thiago Torres e FIOTEC (Fundação para o Desenvolvimento Científico e Tecnológico). University of California-Davis: Ruth Dickover. Boston University: Mark Mirochnick. Westat, Inc.: Margaret Camarca, James Bethel, Emmanuel Aluko, Yolanda Bertucci, Jennifer Bryant, Patty Chen, Barbara Driver, Ruby Duston, Adriana Ferreira, Priya Guyadeen, Sarah Howell, Marsha Johnson, Linda Kaufman, Naomi Leshabane, Lilya Meyerson, Rita Patel, Lubima Petrova, Georgine Price, Susan Raitt, Scott Watson, Yiling Xu, Eunice Yu. Other protocol team members included, George Siberry and Jennifer Read from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), Elizabeth Smith and Sheryl Zwerski from the National Institute of Allergy and Infectious Diseases (NIAID).
Notes
Author’s contribution. K. A. drafted the initial substudy design and data analysis, drafted the initial manuscript, revised, and approved the final manuscript as submitted.
J. X. designed the data collection instruments, organized data entry for the initial study, and provided all data collected for this study. J. X. also assisted in providing methods for data analysis, confirmed and finalized primary data analysis study results presented in this paper.
B. A. assisted with preparation and coordination of urine samples from study sites and provided laboratory support in the United States.
E. J., J. H. P., B. S., R. F., R. K., J. P., and M. M. M.-P., G. G. and G. T. were responsible for initial study design, patient recruitment, and patient care enrollment in this study at sites in Brazil and in South Africa. They also reviewed and revised the manuscript and approved the final manuscript as submitted.
M. M. provided laboratory support in Brazil for study conduct, specimen storage, transfer of specimens to the United States, and participated in data analysis.
J. D. K. provided additional oversight for the current substudy design, data analysis, reviewed, revised, and approved final manuscript as submitted.
D. H. W., L. M., J. M., Y. B., V. V., and K. N.-S. supervised the original protocol development, design of the data collection instruments, supervised data collection at all sites, critically reviewed the manuscript, and approved of the final manuscript as submitted. K. N.-S. was the principal investigator of the parent study as well as this current substudy.
Acknowledgment. We thank the patients and their families who enrolled in this trial. We also thank Marita McDonough and Lauren Petrella from Boehringer Ingelheim Pharmaceuticals and Helen Watson from GlaxoSmithKline (on behalf of ViiV Healthcare) for assistance with the donation of study drugs from their respective companies for the conduct of the parent study. We also acknowledge laboratory personnel who conducted all of the urine specimen preparation and shipment, Mary Ann Hausner and Jessica Liu.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the US Department of Health and Human Services, or the US Department of State. Affiliated universities, programs or companies of the authors.
Financial support. The NICHD HPTN 040 study was supported by NICHD contract no. HHSN267200800001C (NICHD Control no. N01-HD-8-0001) and U01 AI047986 (Brazilian AIDS Prevention Trials International Network), NIAID/ NIH. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The original parent study was supported in part by Boehringer Ingelheim Pharmaceuticals Inc. (BIPI), and GlaxoSmithKline, on behalf of ViiV Healthcare. Support was also provided by the UCLA Children’s Discovery and Innovation Institute (CDI) through the Harry Winston Fellowship Award, the UCLA AIDS Institute, the UCLA Center for AIDS Research (CFAR) NIH/ NIAID AI02869 and AI28697, and the UCLA Pediatric AIDS Coalition.
Potential conflicts of interest. The authors certify no potential conflicts of interest. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
for the NICHD HPTN 040 Study Team:
Mariana Ceriotto, Edgardo Szyld, Silvia Marzo, Flavia Faleiro Ferreira, Fabiana Kakehasi, Rita Lira, Carla Franceschini de Fraga, Rita Lira, Debora Fernandes Coelho, Alberto Sanseverino, Luis Carlos Ribeiro, M. Leticia Santos Cruz, Ezequias Martins, Jacqueline Anita de Menezes, Luisa Andrea Torres Salgado, Ana Valeria Cordovil, Andréa Gouveia, Priscila Mazzucanti, Jorge Eurico Ribeiro, Geraldo Duarte, Adriana Aparecida Tiraboschi Barbaro, Carolina Sales Vieira, Daisy Maria Machado, Regina Succi, Mark Cotton, Jeanne Louw, Elke Maritz, Sarita Lalsab, Shini Legoete, James Alasdair McIntyre, Mandisa Nyati, Allison Agwu, Jean Anderson, Joan Bess, Jonathan Ellen, Todd Noletto, Nancy Hutton, Carol Delany, Robert M. Lawrence, Chas Griggs, Mobeen Rathore, Kathleen Thoma, Michelle Tucker, Audra Deveikis, Susan Marks, Linda Bettica, James M. Oleske, Midnela Acevedo Flores, Elvia Pérez, Francisco I Bastos, Beatriz Grinsztejn, Ronaldo I. Moreira, Marilia Santini de Oliveira, Monica Derrico, Valéria Ribeiro, Thiago Torres e FIOTEC, Ruth Dickover, Margaret Camarca, James Bethel, Emmanuel Aluko, Yolanda Bertucci, Jennifer Bryant, Patty Chen, Barbara Driver, Ruby Duston, Adriana Ferreira, Priya Guyadeen, Sarah Howell, Marsha Johnson, Linda Kaufman, Naomi Leshabane, Lilya Meyerson, Rita Patel, Lubima Petrova, Georgine Price, Susan Raitt, Scott Watson, Yiling Xu, Eunice Yu, George Siberry, Jennifer Read, Elizabeth Smith, and Sheryl Zwerski
References
- 1. Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26:86–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbi M, Binda S, Caroppo S, Primache V. Neonatal screening for congenital cytomegalovirus infection and hearing loss. J Clin Virol 2006; 35:206–9. [DOI] [PubMed] [Google Scholar]
- 3. Cannon MJ, Hyde TB, Schmid DS. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 2011; 21:240–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Sande MA, Kaye S, Miles DJ et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One 2007; 2:e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis 2009; 49:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto AY, Mussi-Pinhata MM, Cristina P, Pinto G, Moraes Figueiredo LT, Jorge SM. Congenital cytomegalovirus infection in preterm and full-term newborn infants from a population with a high seroprevalence rate. Pediatr Infect Dis J 2001; 20:188–92. [DOI] [PubMed] [Google Scholar]
- 7. Guibert G, Warszawski J, Le Chenadec J et al. ; French Perinatal Cohort. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis 2009; 48:1516–25. [DOI] [PubMed] [Google Scholar]
- 8. Kovacs A, Schluchter M, Easley K et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med 1999; 341:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J 1996; 15:1102–6. [DOI] [PubMed] [Google Scholar]
- 10. Chandwani S, Kaul A, Bebenroth D et al. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J 1996; 15:310–4. [DOI] [PubMed] [Google Scholar]
- 11. Mwaanza N, Chilukutu L, Tembo J et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-Saharan Africa. Clin Infect Dis 2014; 58:728–35. [DOI] [PubMed] [Google Scholar]
- 12. King CC, Ellington SR, Kourtis AP. The role of co-infections in mother-to-child transmission of HIV. Curr HIV Res 2013; 11:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khare M, Sharland M, Manyonda I, Rice P, Bland JM, Griffiths P. Use of serial maternal urine cytomegalovirus PCR to detect primary CMV infection in seronegative pregnant women. J Virol Methods 2004; 119:31–5. [DOI] [PubMed] [Google Scholar]
- 14. Nankervis GA, Kumar ML, Cox FE, Gold E. A prospective study of maternal cytomegalovirus infection and its effect on the fetus. Am J Obstet Gynecol 1984; 149:435–40. [DOI] [PubMed] [Google Scholar]
- 15. Peckham CS, Johnson C, Ades A, Pearl K, Chin KS. Early acquisition of cytomegalovirus infection. Arch Dis Child 1987; 62:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montgomery R, Youngblood L, Medearis DN Jr. Recovery of cytomegalovirus from the cervix in pregnancy. Pediatrics 1972; 49:524–31. [PubMed] [Google Scholar]
- 17. Şahiner F, Honca M, Çekmez Y et al. The role of maternal screening in diagnosing congenital cytomegalovirus infections in highly immune populations. Ir J Med Sci 2015; 184:475–81. [DOI] [PubMed] [Google Scholar]
- 18. Bello C, Whittle H. Cytomegalovirus infection in Gambian mothers and their babies. J Clin Pathol 1991; 44:366–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. New advances in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2008; 41:192–7. [DOI] [PubMed] [Google Scholar]
- 20. Shen CY, Chang SF, Yen MS, Ng HT, Huang ES, Wu CW. Cytomegalovirus excretion in pregnant and nonpregnant women. J Clin Microbiol 1993; 31:1635–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pass RF, Stagno S, Dworsky ME, Smith RJ, Alford CA. Excretion of cytomegalovirus in mothers: observations after delivery of congenitally infected and normal infants. J Infect Dis 1982; 146:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Reynolds DW, Stagno S, Hosty TS, Tiller M, Alford CA Jr. Maternal cytomegalovirus excretion and perinatal infection. N Engl J Med 1973; 289:1–5. [DOI] [PubMed] [Google Scholar]
- 23. Pitt J, Schluchter M, Jenson H et al. Maternal and perinatal factors related to maternal-infant transmission of HIV-1 in the P2C2 HIV study: the role of EBV shedding. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV-1 Infection (P2C2 HIV) Study Group. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 19:462–70. [DOI] [PubMed] [Google Scholar]
- 24. Rola-Pleszczynski M, Frenkel LD, Fuccillo DA et al. Specific impairment of cell-mediated immunity in mothers of infants with congenital infection due to cytomegalovirus. J Infect Dis 1977; 135:386–91. [DOI] [PubMed] [Google Scholar]
- 25. Nielsen-Saines K, Watts DH, Veloso VG et al. ; NICHD HPTN 040/PACTG 1043 Protocol Team. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med 2012; 366:2368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nielsen-Saines K, for NICHD HPTN 040 Protocol Team Increased CMV co-infection with in utero-acquired HIV-1. Oral abstract no. 2695.8. In: Pediatric Academic Society Meetings; Washington, DC, United States, May 4–7, 2013. [Google Scholar]
- 27. Adachi K, Klausner JD, Bristow CC et al. ; NICHD HPTN 040 Study Team. Chlamydia and gonorrhea in HIV-infected pregnant women and infant HIV transmission. Sex Transm Dis 2015; 42:554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adachi K, Klausner JD, Xu J et al. Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-infected pregnant women and adverse infant outcomes. Pediatr Infect Dis J 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yeganeh N, Watts HD, Camarca M et al. ; NICHD HPTN 040P1043 Study Team. Syphilis in HIV-infected mothers and infants: results from the NICHD/HPTN 040 study. Pediatr Infect Dis J 2015; 34:e52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stagno S, Pass RF, Dworsky ME, Alford CA Jr. Maternal cytomegalovirus infection and perinatal transmission. Clin Obstet Gynecol 1982; 25:563–76. [DOI] [PubMed] [Google Scholar]
- 31. Slyker J, Farquhar C, Atkinson C et al. Compartmentalized cytomegalovirus replication and transmission in the setting of maternal HIV-1 infection. Clin Infect Dis 2014; 58:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ross SA, Ahmed A, Palmer AL et al. ; National Institute on Deafness and Other Communication Disorders CHIMES Study. Detection of congenital cytomegalovirus infection by real-time polymerase chain reaction analysis of saliva or urine specimens. J Infect Dis 2014; 210:1415–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Vries JJ, van der Eijk AA, Wolthers KC et al. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. J Clin Virol 2012; 53:167–70. [DOI] [PubMed] [Google Scholar]
- 34. Numazaki Y, Yano N, Morizuka T, Takai S, Ishida N. Primary infection with human cytomegalovirus: virus isolation from healthy infants and pregnant women. Am J Epidemiol 1970; 91:410–7. [DOI] [PubMed] [Google Scholar]
- 35. Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011; 17:1285–93. [DOI] [PubMed] [Google Scholar]
- 36. Fowler KB, Pass RF. Risk factors for congenital cytomegalovirus infection in the offspring of young women: exposure to young children and recent onset of sexual activity. Pediatrics 2006; 118:e286–92. [DOI] [PubMed] [Google Scholar]
- 37. Pereira LH, Embil JA, Haase DA, Manley KM. Cytomegalovirus infection among women attending a sexually transmitted disease clinic: association with clinical symptoms and other sexually transmitted diseases. Am J Epidemiol 1990; 131:683–92. [DOI] [PubMed] [Google Scholar]
- 38. Ross SA, Novak Z, Ashrith G et al. Association between genital tract cytomegalovirus infection and bacterial vaginosis. J Infect Dis 2005; 192:1727–30. [DOI] [PubMed] [Google Scholar]
- 39. Embil JA, Garner JB, Pereira LH, White FM, Manuel FR. Association of cytomegalovirus and herpes simplex virus infections of the cervix in four clinic populations. Sex Transm Dis 1985; 12:224–8. [DOI] [PubMed] [Google Scholar]
- 40. Chandler SH, Holmes KK, Wentworth BB et al. The epidemiology of cytomegaloviral infection in women attending a sexually transmitted disease clinic. J Infect Dis 1985; 152:597–605. [DOI] [PubMed] [Google Scholar]
- 41. Jordan MC, Rousseau WE, Noble GR, Steward JA, Chin TD. Association of cervical cytomegaloviruses with venereal disease. N Engl J Med 1973; 288:932–4. [DOI] [PubMed] [Google Scholar]
- 42. Clarke LM, Duerr A, Feldman J, Sierra MF, Daidone BJ, Landesman SH. Factors associated with cytomegalovirus infection among human immunodeficiency virus type 1-seronegative and -seropositive women from an urban minority community. J Infect Dis 1996; 173:77–82. [DOI] [PubMed] [Google Scholar]
- 43. Mostad SB, Kreiss JK, Ryncarz AJ et al. Cervical shedding of cytomegalovirus in human immunodeficiency virus type 1–infected women. J Med Virol 1999; 59:469–73. [PubMed] [Google Scholar]
- 44. Khamduang W, Jourdain G, Sirirungsi W et al. ; Program for HIV Prevention and Treatment (PHPT) Study Group. The interrelated transmission of HIV-1 and cytomegalovirus during gestation and delivery in the offspring of HIV-infected mothers. J Acquir Immune Defic Syndr 2011; 58:188–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lurain NS, Robert ES, Xu J et al. HIV type 1 and cytomegalovirus coinfection in the female genital tract. J Infect Dis 2004; 190:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]