Abstract
We describe a patient with severe and progressive encephalitis of unknown etiology. We performed rapid metagenomic sequencing from cerebrospinal fluid and identified Powassan virus, an emerging tick-borne flavivirus that has been increasingly detected in the United States.
Keywords: Powassan virus, encephalitis, metagenomic sequencing
Encephalitis is a challenging clinical syndrome for which current diagnostic testing for infectious, autoimmune, and neoplastic causes often yields no identifiable etiology. Here we present a case of severe, progressive encephalitis that was ultimately shown by serology to be due to Powassan virus, an emerging flavivirus transmitted by Ixodes scapularis ticks. We performed metagenomic sequencing and detected Powassan virus in cerebrospinal fluid 4 weeks before the diagnosis was made by serology, demonstrating the utility of this method for timely pathogen detection in severe central nervous system infection.
CASE REPORT
A 61-year-old man was admitted in December 2016 with bilateral headache, gait instability, lethargy, and confusion. He was initially evaluated in the Emergency Department of a referring hospital, where computed tomography (CT) of his head was normal and he declined lumbar puncture and admission. Because of multiple tick bites in the preceding 2 weeks, he was prescribed doxycycline for presumed Lyme disease. Over the next 48 hours, he developed worsening confusion, weakness, and ataxia. He returned to the referring hospital and was admitted.
The patient’s medical history was notable for Crohn’s disease treated with adalimumab, atrial fibrillation requiring anticoagulation, hypertension, and a prior right frontal embolic stroke. He lived in a heavily wooded area in New Hampshire, had frequent tick exposures, and worked as a construction contractor in basements with uncertain rodent and bat exposures. He had no recent travel.
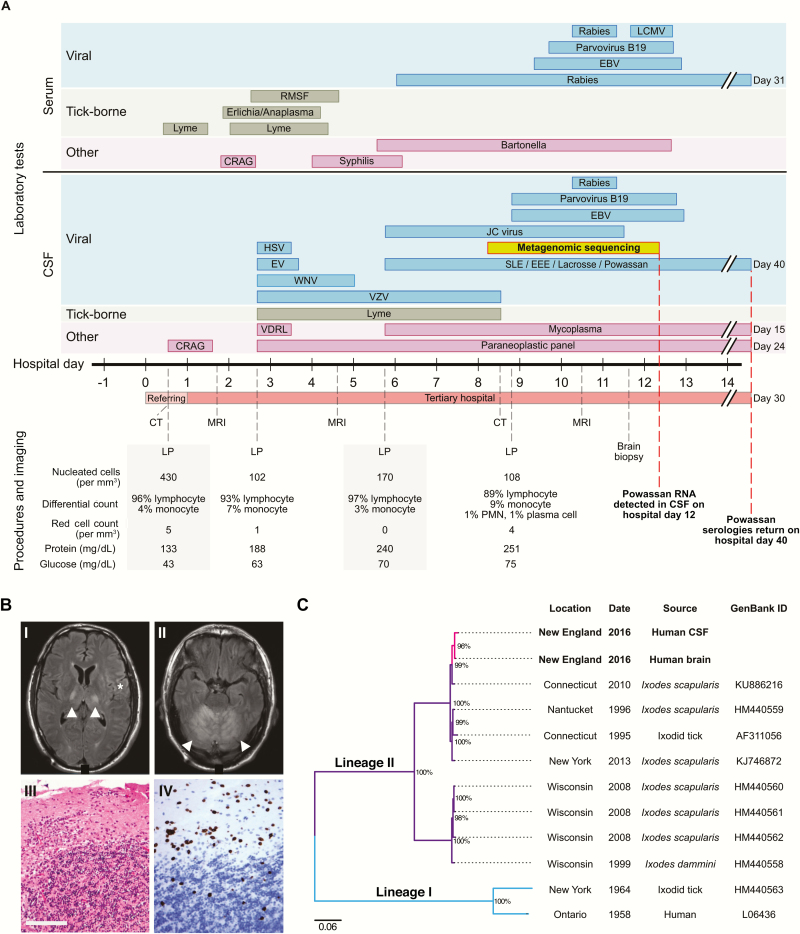
Upon admission to the referring hospital, physical examination was notable for an erythematous, indurated lesion at the site of a tick bite on his medial right thigh. He was drowsy and confused, profoundly ataxic without dysmetria, and had right facial hemiparesis. He underwent lumbar puncture, which demonstrated 430 leukocytes/mm3 (96% lymphocytes), a protein level of 133 mg/dL, and a glucose level of 43 mg/dL (Figure 1A). No opening pressure was recorded. He had no other laboratory abnormalities. He was empirically treated for meningitis but within 12 hours of admission required endotracheal intubation for airway protection, at which time he was transferred to our hospital (hospital day 1).
Figure 1.
Clinical data and genome sequencing data. A, Timeline of diagnostic testing, imaging, and procedures. For diagnostic tests, each bar indicates the time between specimen collection and receipt of results. B, Brain imaging and brain biopsy findings. Axial brain MRI images from hospital day 1 show focal T2-weighted/fluid-attenuated inversion recovery hyperintensities of the bilateral thalamus (panel I, arrowheads) and cortex (panel I, asterisk) and confluent hyperintensities of the cerebellum (panel II, arrowheads). Hematoxylin and eosin staining of a right posterior fossa biopsy performed on hospital day 11 shows extensive lymphocytic infiltration and thickening of dura and diffuse inflammation of the cerebellum (panel III). CD3 staining of the cerebellum reveals a predominantly T-cell lymphocytic infiltrate in the granule cell and molecular layers (panel IV). Scale bar = 100 um. C, Phylogenetic tree of Powassan virus genomes. The partial Powassan virus genomes identified in this study are labeled in pink. These sequences cluster together and with other Powassan virus lineage II genomes isolated from Ixodes ticks in the Northeast United States. A separate cluster of Powassan virus lineage II genomes isolates from Ixodes ticks in the Midwest United States is also demonstrated, and all lineage II branches are labeled in purple. Powassan virus lineage I genomes are included for reference (labeled in blue). Branches are labeled with the location, date, source, and GenBank ID of each Powassan virus genome. Nodes with at least 80% bootstrap support are labeled with percentage of bootstrap support. Ixodes dammini has been renamed Ixodes scapularis. Abbreviations: CRAG, cryptococcal antigen; CSF, cerebrospinal fluid; EBV, Epstein-Barr virus; EEE, Eastern equine encephalitis virus; HSV, herpes simplex virus; IVIG, intravenous immunoglobulin; LCMV, lymphocytic choriomeningitis virus; LP, lumbar puncture; MRI, magnetic resonance imaging; PMN, polymorphonuclear leukocyte; RMSF, Rocky Mountain spotted fever; SLE, Saint Louis encephalitis virus; CT, computed tomography; VDRL, Venereal Disease Research Laboratory; VZV, varicella-zoster virus; WNV, West Nile virus.
On examination, the patient did not open his eyes to stimulation or follow commands despite minimal sedation. His left leg exhibited triple flexion to noxious stimulation. Magnetic resonance imaging (MRI) of the brain showed T2-weighted hyperintensity of the bilateral thalami and cerebral cortex and diffuse T2-weighted hyperintensity of the cerebellum, with no ischemia or hemorrhage (Figure 1B). On hospital day 4, repeat brain MRI showed increased symmetric hyperintensities of the cortex and cerebellum with associated leptomeningeal enhancement, new involvement of the midbrain, and partial effacement of the fourth ventricle without hydrocephalus, suggestive of disease progression. An electroencephalogram showed continuous diffuse irregular delta waves, slowing of the background, and occasional right frontal broad sharp wave discharges, without seizures.
Lumbar punctures performed at our hospital showed mild improvement in the lymphocytic pleocytosis (Figure 1A). Numerous tests for infectious, autoimmune, and neoplastic causes of encephalitis were negative (Figure 1A and Supplementary Table 1). On hospital day 11, the patient underwent a right suboccipital craniotomy with biopsy of the dura and underlying cerebellum (Supplementary Table 2). Histopathology and immunohistochemical studies, which returned on day 18, revealed diffuse lymphoid infiltrates (Figure 1B) and no evidence of vasculitis, infarction, or infection by spirochetes and common viral pathogens.
The patient was treated with empiric broad-spectrum antimicrobials (Supplementary Table 3). Given concern for arboviral infection, intravenous immunoglobulin was administered on hospital days 6–10, with minimal improvement in the patient’s clinical status.
The patient enrolled in a research study, and metagenomic sequencing from cerebrospinal fluid (CSF) was performed on hospital day 8, which identifed Powassan virus within 96 hours, as described below. The diagnosis was confirmed 40 days after admission by serologic testing performed at the Centers for Disease Control and Prevention (CDC) from a CSF sample sent on hospital day 2. Histopathologic evaluation of brain biopsy specimens for Powassan virus at the CDC were equivocal.
The patient had minimal neurological recovery and was discharged to an acute care facility on hospital day 30. Seven months after discharge, he was reportedly able to nod his head to questions and slightly move his upper extremities and toes.
METHODS
Metagenomic sequencing was performed using published methods [1] on CSF from a clinically indicated lumbar puncture performed on hospital day 5, plasma and whole blood from hospital day 4, and brain tissue from the biopsy performed on hospital day 11. Libraries were multiplexed and sequenced using an Illumina platform, along with water and/or CSF from an uninfected clinical sample as negative controls. Details are in the Supplementary Appendix.
RESULTS
Powassan virus was detected in CSF from the initial rapid sequencing run completed on hospital day 11. Among 2.4 million total sequencing reads, 10 reads belonged to Powassan virus. No other plausible pathogens were detected by metagenomic analysis or dedicated searches for specific bacteria and viruses. Powassan virus was not detected in 2 negative control samples from the same sequencing run (uninfected CSF and water), in any previous controls in our laboratory, in the patient’s plasma (7.3 million reads), or the patient’s whole blood (9.3 million reads); it was also not isolated by inoculation of patient CSF or whole blood into weanling balb/c mice, and these mice remained seronegative.
Deeper massively parallel sequencing using hybrid capture technology (see Supplementary Appendix), performed using 2 independent libraries from CSF, confirmed the finding of Powassan virus and enabled assembly of a partial Powassan virus genome (19%). Metagenomic sequencing and hybrid capture from the brain tissue sample also detected Powassan virus and allowed assembly of another partial genome (48%). These partial genomes were highly similar and belonged to Powassan lineage II (Figure 1C and Supplementary Figures 1 and 2). Detection of Powassan virus in this subject was confirmed by quantitative reverse transcription polymerase chain reaction (qRT-PCR) (Supplementary Table 4).
DISCUSSION
We describe a patient with severe encephalitis secondary to Powassan virus, in whom the etiologic agent was identified by rapid metagenomic sequencing 4 weeks earlier than by standard serologic testing. Our case underscores the utility of metagenomic sequencing to detect pathogens in CSF in a clinically meaningful timeframe; this has been reported previously in a patient with neuroleptospirosis [2], but is not yet routine. Our results emphasize the extremely high sensitivity of metagenomic sequencing because we detected virus in CSF despite negative mouse inoculation studies and in brain tissue despite inconclusive immunohistochemistry. Finally, we demonstrate that metagenomic sequencing enables genomic characterization of pathogens, which is critical for surveillance of emerging and understudied pathogens.
Powassan virus, an emerging flavivirus transmitted by Ixodes ticks, has been increasingly detected in New England, where most human infections are presumed to be Powassan lineage II, also known as deer tick cirus [3–9]. Similar to other cases, our patient had altered sensorium and no peripheral laboratory abnormalities; CSF profile had normal glucose, elevated protein, and pleocytosis; and brain MRI showed T2-weighted/ fluid-attenuated inversion recovery hyperintensities within the basal ganglia, thalamus, and cerebellum. He was afebrile but was receiving immunomodulatory therapy, which could suppress fever. As illustrated by this patient, Powassan virus encephalitis confers high morbidity and mortality.
Powassan virus may be responsible for a large number of unrecognized infections in humans, including nonneuroinvasive disease. There is a substantial need for increased testing, including the development of rapid diagnostic tests. Currently, the gold standard for diagnosis is a screening antibody test followed by a confirmatory plaque reduction neutralization test at the CDC [10]. In our case, results took 5 weeks, which was too slow to affect patient management.
Metagenomic sequencing has been successfully used to identify a range of pathogens in research studies and holds promise as a powerful clinical diagnostic strategy. To obtain clinically relevant results using metagenomic sequencing, 2 factors are important to consider. First, the extremely high sensitivity necessitates appropriate controls to reduce the possibility of false-positive results arising from environmental contamination (Supplementary Figure 3). In our case, although few Powassan virus reads were detected, they were found only in this patient’s CSF sample and not in 2 negative control samples sequenced in parallel. We further confirmed our results with 2 independent sequencing libraries and qRT-PCR. Second, because metagenomic sequencing is relatively new, with still-evolving test characteristics, results must be interpreted in the context of clinical information, highlighting the need for close communication between clinical and research teams.
Although no specific antiviral therapy exists for Powassan virus, our results helped relieve uncertainty for the patient’s family and care team. Should specific therapeutic interventions have been available, our results may have facilitated their use earlier during the course of illness. More broadly, implementation of metagenomic sequencing can avoid unnecessary testing and guide decisions regarding the use of antimicrobial and immunomodulatory treatments. Rapid pathogen detection also has implications for public health, enabling timely interventions in vector surveillance and eradication efforts.
Our identification of Powassan virus encephalitis by rapid metagenomic sequencing highlights the utility of this unbiased approach in identifying pathogens in a clinically meaningful timeframe and supports its development as a diagnostic methodology. This case also underscores the emergence of Powassan virus as an important cause of severe encephalitis and emphasizes the need for further study of its epidemiology and pathogenesis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We would like to thank Asim Ahmed, MD, and Daniel Park, PhD, for valuable comments on the manuscript, and Cormac Kinsella, Simon Ye, Katherine Siddle, PhD, and Andreas Gnirke, PhD, for expert technical advice.
Financial support. This work was supported by the National Institutes of Health (KL2 TR001100 to A. P., T32 AI007061 to S. K., and T32 AG000222 to S. S. M.), a Massachusetts General Hospital Department of Neurology SPARK Award to S. S. M. and T. C., and a generous Broadnext10 gift from the Broad Institute.
Potential conflicts of interest. S. T. reports consultation on tick-borne disease diagnostics with Oxford Immunotec, Fuller Laboratories, and Meridian Biosciences. E. R. reports consultation with T2 Biosystems and TBS Technologies. M. F. reports a sponsored research grant from Biogen. All other authors declare no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Matranga CB, Andersen KG, Winnicki S et al. Enhanced methods for unbiased deep sequencing of Lassa and Ebola RNA viruses from clinical and biological samples. Genome Biol 2014; 15:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson MR, Naccache SN, Samayoa E et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med 2014; 370:2408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piantadosi A, Rubin DB, McQuillen DP et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Telford SR 3rd, Armstrong PM, Katavolos P et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 1997; 3:165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebel GD, Spielman A, Telford SR 3rd. Phylogeny of North American Powassan virus. J Gen Virol 2001; 82:1657–65. [DOI] [PubMed] [Google Scholar]
- 6. Tavakoli NP, Wang H, Dupuis M et al. Fatal case of deer tick virus encephalitis. N Engl J Med 2009; 360:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Khoury MY, Camargo JF, White JL et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease–endemic areas of New York, U.S.A. Emerg Infect Dis 2013; 19:1926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El Khoury MY, Hull RC, Bryant PW et al. Diagnosis of acute deer tick virus encephalitis. Clin Infect Dis 2013; 56:e40–7. [DOI] [PubMed] [Google Scholar]
- 9. Tutolo JW, Staples JE, Sosa L, Bennett N. Notes from the field: Powassan virus disease in an infant — Connecticut, 2016. MMWR Morb Mortal Wkly Rep 2017; 66:408–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Infection. CDC Arboviral Disease Case Definition 2015. https://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/. Accessed 2 May 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.