Endothelial dysfunction/reduced nitric oxide bioavailability is associated with worse plasma leakage in dengue patients and occurs early in the course of the disease. Endothelial dysfunction correlates with lower plasma l-arginine and higher arginase-1 levels.
Keywords: dengue, endothelial function, nitric oxide, l-arginine, arginase
Abstract
Background
Dengue can cause increased vascular permeability that may lead to hypovolemic shock. Endothelial dysfunction may underlie this; however, the association of endothelial nitric oxide (NO) pathways with disease severity is unknown.
Methods
We performed a prospective observational study in 2 Vietnamese hospitals, assessing patients presenting early (<72 hours of fever) and patients hospitalized with warning signs or severe dengue. The reactive hyperemic index (RHI), which measures endothelium-dependent vasodilation and is a surrogate marker of endothelial function and NO bioavailability, was evaluated using peripheral artery tonometry (EndoPAT), and plasma levels of l-arginine, arginase-1, and asymmetric dimethylarginine were measured at serial time-points. The main outcome of interest was plasma leakage severity.
Results
Three hundred fourteen patients were enrolled; median age of the participants was 21(interquartile range, 13–30) years. No difference was found in the endothelial parameters between dengue and other febrile illness. Considering dengue patients, the RHI was significantly lower for patients with severe plasma leakage compared to those with no leakage (1.46 vs 2.00; P < .001), over acute time-points, apparent already in the early febrile phase (1.29 vs 1.75; P = .012). RHI correlated negatively with arginase-1 and positively with l-arginine (P = .001).
Conclusions
Endothelial dysfunction/NO bioavailability is associated with worse plasma leakage, occurs early in dengue illness and correlates with hypoargininemia and high arginase-1 levels.
Dengue continues to cause substantial global morbidity, with around 100 million clinically apparent cases estimated to occur every year [1]. Although dengue can present with a broad spectrum of clinical phenotypes, the defining feature of severe disease is altered vascular permeability resulting in a unique plasma leakage syndrome, which can progress to hypovolemic shock, known as dengue shock syndrome (DSS). Typically DSS occurs during a critical phase around day 4–6 of illness as fever resolves [2]. The mechanisms underlying the increased vascular permeability remain to be defined, but endothelial activation/dysfunction is thought to play a key role [3]. Investigating endothelial function clinically is difficult, but novel devices that measure endothelial-dependent vasodilation have been developed over the last decade, which involves physiological stimulation of endothelial release of nitric oxide (NO), reflecting local NO bioavailability [4]. Endothelial dysfunction is a systemic process that results in reduced NO release, causing impaired endothelial-dependent vascular relaxation, which can be quantified peripherally [5].
Alterations in a variety of biochemical precursor molecules and enzymes may result in impaired release of endothelial-derived NO, including deficiency of the substrate l-arginine in the vascular endothelium, inhibition of the enzyme endothelial NO synthase (eNOS), and deficiency of eNOS enzymatic cofactors. Other mechanisms that may alter vascular NO responses include competitive inhibition of NOS by endogenous methylarginines, particularly asymmetric dimethylarginine (ADMA), and increased expression or activity of the enzyme arginase-1 that metabolizes l-arginine [6, 7]. Derangements in these pathways have been found to contribute to endothelial dysfunction associated with the vascular complications observed in sepsis and malaria [8–10].
Although endothelial activation is known to occur in dengue, evidenced by increased release of various adhesion molecules [11, 12], very few data describing dysfunction of the endothelial NO–l-arginine pathway exist, and associations with dengue disease severity remain unknown.
Describing endothelial function across the spectrum of disease and exploring changes during the early febrile phase could identify potentially useful markers for subsequent development of shock, as well as providing insight into the underlying pathogenesis. We therefore set out to investigate associations between endothelial function (reactive hyperemic index [RHI]), plasma levels of l-arginine, ADMA, and arginase-1, in patients with dengue compared with other febrile illness (OFI) at serial time-points during the evolution of the acute illness. The main outcome of interest was plasma leakage severity, with a secondary outcome of mucosal bleeding. We hypothesized (1) that patients with dengue have worse endothelial dysfunction compared to patients with OFI, and (2) that, in the dengue group, endothelial dysfunction is associated with more severe plasma leakage. We also wished to explore associations between key molecules in the NO pathway, endothelial dysfunction, and clinical outcomes.
METHODS
We performed a prospective observational study at the Hospital for Tropical Diseases (HTD), Ho Chi Minh City and the National Hospital for Tropical Diseases (NHTD), Hanoi, Vietnam, between June 2013 and October 2015. Ethical approvals were obtained from the Oxford Tropical Research Ethics Committee and at HTD and NHTD. Written informed consent was obtained from all participants or the parent/guardian of children [13]. Adults and children >3 years of age with a clinical diagnosis of possible dengue were eligible for enrollment into 1 of 2 study arms. In the outpatient arm, participants presenting within 72 hours of fever onset could be enrolled if no obvious alternative cause for the fever was apparent clinically [14]. For the inpatient arm, any individual admitted to NHTD or HTD with suspected dengue with warning signs or with severe dengue was eligible [15]. All patients were reviewed daily until fully recovered or for up to 6 days from enrollment, and also at a follow-up visit 14 days later.
Clinical Endothelial Function Testing
Peripheral artery tonometry (EndoPAT, Itamar Medical, Israel) was used to measure digital vasodilator function. Digital pulse volume changes during reactive hyperemia were recorded in response to a 5-minute arterial occlusion, using a blood pressure (BP) cuff as per standardized methods [5]. A 5-minute baseline recording was performed, after which the BP cuff was rapidly inflated to a predetermined level (50 mm Hg above systolic BP, minimum of 200 mm Hg in adults and 50 mm Hg above systolic BP, no minimum for children) for 5 minutes. A further postocclusion phase was timed for another 5 minutes. The EndoPAT software calculates the RHI, which is the post- to preocclusion amplitude ratio, normalized to values obtained contemporaneously in the control arm. An abnormal ratio is defined by the manufacturer as RHI <1.67 [16]. EndoPAT was performed at 3 time-points; enrollment, defervescence/hospital discharge, and follow-up.
Laboratory Parameters
A full blood count was performed daily, whereas liver and renal function was tested at enrollment and then subsequently if clinically indicated. A research plasma sample was stored every other day. Subsequently, a random selection of patients from each site and enrollment arm who had had EndoPAT tests performed had the following parameters measured at the same time-points specified above on the stored plasma, using commercial enzyme-linked immunosorbent assay (ELISA) kits for l-arginine and ADMA (DLD Diagnostika, Hamburg, Germany) and arginase-1 (Hycult Biotech, Uden, Netherlands).
Dengue Diagnostics
An NS1 test (Platelia ELISA, Bio-Rad) and commercial immunoglobulin M (IgM) and immunoglobulin G (IgG) serology assays (Capture ELISA, Panbio, Australia) were used on acute and convalescent plasma. In addition, reverse-transcription polymerase chain reaction (RT-PCR) was performed on enrollment samples to identify the viral serotype and measure plasma viremia [17]. Patients were defined as having laboratory-confirmed dengue if the RT-PCR or NS1 was positive, and probable dengue if the IgM assay was positive at enrollment or if there was IgM seroconversion between paired specimens. A diagnosis of OFI was assigned to participants with no laboratory evidence of acute or recent flavivirus—that is, if they were negative for RT-PCR, NS1, and IgM/IgG on paired serology. Patients with negative tests at enrollment, but for whom convalescent plasma was not available, were considered unclassifiable.
Clinical Endpoint Definitions
The primary endpoint was plasma leakage severity. Percentage hemoconcentration was defined as the ΔHCT (peak – baseline hematocrit / baseline hematocrit) × 100, and required at least 3 hematocrit recordings during the acute illness. The peak value was the highest measurement during the critical period (illness day 4–6), while the baseline hematocrit was the lowest value (in the following order) from the follow-up sample, a sample obtained within 72 hours of fever onset, or (for hospitalized patients only) the discharge sample, provided no parenteral fluid was administered during the preceding 12 hours. Plasma leakage severity was then graded into 3 categories: 0 indicates no clinically significant leakage (ΔHCT <15% and no signs of clinical fluid accumulation); 1 indicates moderate leakage (ΔHCT 15%–20% and/or any sign of clinical fluid accumulation); 2 indicates severe leakage (ΔHCT >20% and/or shock, or pleural effusion with respiratory compromise). Other outcomes assessed included the occurrence of mucosal bleeding, and overall dengue severity according to the World Health Organization (WHO) 2009 classification.
Statistical Analysis
Data are presented as frequency (percentage) for categorical variables and median (interquartile range [IQR]) for continuous parameters. Comparisons of endothelial parameters were performed between confirmed dengue and OFI patients in the outpatient arm, based on logistic regression models with dengue diagnosis as the outcome of interest, and the endothelial parameters as covariates. Among patients with confirmed dengue, associations with plasma leakage and mucosal bleeding were then explored. These analyses, including trend analysis of endothelial parameters between the 3 plasma leakage grades, were based on linear regression models with endothelial parameters as covariates, and plasma leakage and mucosal bleeding as the outcomes. Overall comparisons (excluding measurements at follow-up) and comparisons in each illness phase were adjusted for age, sex, illness day at enrollment, and illness day of measurement, with additional adjustment for hospitalization in the analyses of clinical outcome. Generalized estimating equations were used to take into account multiple measurements per patient. Associations between endothelial parameters in patients with dengue were assessed by partial correlations controlling for potential confounding variables including age, sex, and illness day of measurement. The significance of partial correlations was assessed based on their Fisher transformation and corresponding bootstrap standard errors. To informally adjust for multiplicity, a significance level of .01 was used for all comparisons. All analyses were performed with the statistical software R version 3.2.2 and the companion package geepack version 1.2-0.
RESULTS
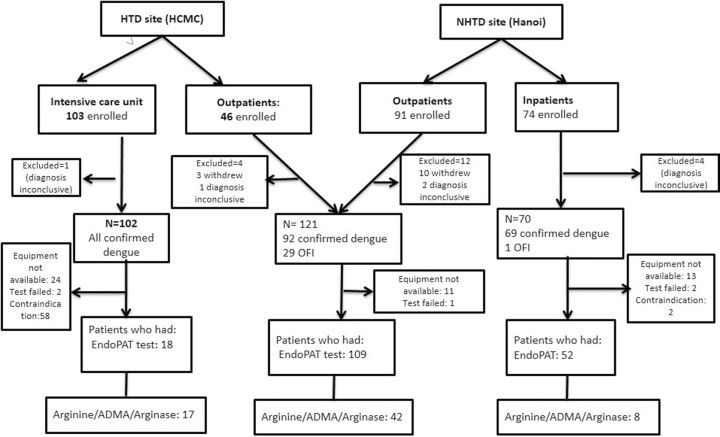
In total, 314 patients were enrolled (Figure 1), of whom 21 withdrew or had inconclusive dengue diagnostics, leaving 293 for the final analysis. Overall, the median age of the participants was 21 (IQR, 13–30) years. The proportion of children (age <15 years) enrolled was 103 of 293 (35%); a higher proportion of children than adults was recruited in the intensive care unit (ICU): 86 of 102 (84%). There was an equal male-to-female ratio. Three patients had type 2 diabetes, 2 patients had controlled hypertension, and 6 patients smoked; the low frequency of these conditions were presumed not to be confounding on endothelial function as measured in this study. Of the 287 patients who had dengue virus (DENV) PCR performed, 201 (70%) were positive, with the following serotypes: 94 (47%) DENV-1; 28 (14%) DENV-2; 37 (18%) DENV-3; 41 (20%) DENV-4; and 1 (1%) mixed infection with DENV-1 and -4 (Table 1).
Figure 1.
Study flow chart. Contraindications included age <10 years at the Hospital for Tropical Diseases site, platelet count <20 × 109/L, or clinically unstable as decided by attending clinician. A random selection of patients who had peripheral artery tonometry testing were chosen to have plasma l-arginine, asymmetric dimethylarginine, and arginase tests. Abbreviations: ADMA, asymmetric dimethylarginine; EndoPAT, peripheral artery tonometry; HCMC, Ho Chi Minh City; HTD, Hospital for Tropical Diseases; NHTD, National Hospital for Tropical Diseases; OFI, other febrile illness.
Table 1.
Enrollment Characteristics for All Patients at Both Sites
| Characteristic | No. | All Patients (N = 293) | No. | Outpatients (n = 121) | No. | Inpatients (n = 70) | No. | ICU (n = 102) |
|---|---|---|---|---|---|---|---|---|
| Age, y | 293 | 21 (13–30) | 121 | 26 (20–32) | 70 | 28 (21–36) | 102 | 11 (8–14) |
| Children (<15 y) | 293 | 103 (35%) | 121 | 12 (10%) | 70 | 5 (7%) | 102 | 86 (84%) |
| Male sex | 293 | 155 (53%) | 121 | 65 (54%) | 70 | 36 (51%) | 102 | 54 (53%) |
| Illness day | 293 | 4 (3–6) | 121 | 3 (2–3) | 70 | 5 (4–6) | 102 | 6 (5–6) |
| Dengue | 293 | 263 (90%) | 121 | 92 (76%) | 70 | 69 (99%) | 102 | 102 (100%) |
| OFI | 293 | 30 (10%) | 121 | 29 (24%) | 70 | 1 (1%) | 102 | 0 (0%) |
| PCR positive | 287 | 201 (70%) | 121 | 85 (70%) | 68 | 49 (72%) | 98 | 67 (68%) |
| Platelets, 109/L | 284 | 86 (30–153) | 118 | 158 (129–198) | 67 | 58 (31–108) | 99 | 29 (19–42) |
| WBC, 109/L | 284 | 4.4 (3.0–6.1) | 118 | 5.0 (3.3–7.2) | 67 | 3.4 (2.5–4.7) | 99 | 4.6 (3.0–5.8) |
| ALB, g/L | 235 | 40.8 (34.2–46.0) | 113 | 46.0 (42.0–48.1) | 24 | 39.0 (36.0–45.0) | 98 | 33.5 (29.2–37.3) |
| AST, U/L | 249 | 60 (32–138) | 117 | 32 (23–45) | 40 | 62 (33–117) | 92 | 164 (105–344) |
| Outcome variables | ||||||||
| Plasma leakage (yes) | 245 | 126 (51%) | 90 | 17 (19%) | 63 | 21 (33%) | 92 | 88 (96%) |
| Mucosal bleed (yes) | 263 | 71 (27%) | 92 | 26 (28%) | 69 | 39 (57%) | 102 | 6 (6%) |
| Hospitalized | 263 | 199 (76%) | 92 | 28 (30%) | 69 | 69 (100%) | 102 | 102 (100%) |
| WHO severity | 263 | 92 | 69 | 102 | ||||
| Dengue | 79 (30%) | 60 (65%) | 19 (28%) | 0 (0%) | ||||
| WS | 96 (37%) | 30 (33%) | 45 (65%) | 21 (21%) | ||||
| Severe | 88 (33%) | 2 (2%) | 5 (7%) | 81 (79%) | ||||
Outcome variables are for dengue-confirmed patients only. Summary statistics are absolute count (%) for categorical variables and median (interquartile range) for continuous data.
Abbreviations: ALB, albumin; AST, aspartate aminotransferase; ICU, intensive care unit; OFI, other febrile illness; PCR, polymerase chain reaction; WBC, white blood cell count; WHO, World Health Organization; WS, warning signs.
In patients with confirmed dengue, 126 of 245 (51%) developed the primary clinical endpoint of plasma leakage (both grades) and 71 of 263 (27%) developed mucosal bleeding (Table 1), while 199 of 263 (71%) were hospitalized at some point during their illness. Considering the WHO classification, there were roughly equal proportions of dengue patients with and without warning signs and with severe dengue (Table 1).
Association of Endothelial Function, l-Arginine, Asymmetric Dimethylarginine, and Arginase-1 Between Dengue and Other Febrile Illness
There was no difference in the RHI or in plasma levels of l-arginine, ADMA, and arginase-1 between patients with dengue and OFI at any of the time-points (Table 2). Considering early illness (days 1–3) compared to follow-up (day >13), dengue patients had lower l-arginine levels (P < .001) (Supplementary Figure 1A) and l-arginine-to-ADMA ratio (P < .001), and higher arginase-1 levels (P = .004) (Supplementary Figure 2A). In the OFI group only, l-arginine was lower during days 1–3 compared to follow-up (49.1 vs 85.9 ng/mL; P < .001).
Table 2.
Endothelial Function and Plasma l-Arginine, Arginase-1, and Asymmetric Dimethylarginine Levels Between Dengue and Other Febrile Illness
| Time-point | OFI (n = 29) | Dengue (n = 92) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| no. | No. | Median (IQR) | no. | No. | Median (IQR) | OR | (95% CI) | P Value | |
| Arginase, ng/mL | 13 | 25 | 101.8 (63.4–203.1) | 29 | 58 | 117.1 (70.8–213.8) | 1.00 | (1.00–1.01) | .344 |
| Days 1–3 | 12 | 12 | 122.6 (85.4–214.7) | 24 | 25 | 183.3(112.0–221.2) | 1.00 | (1.00–1.01) | .318 |
| Days 4–6 | 10 | 10 | 102.4 (55.4–196.5) | 24 | 24 | 98.0 (48.6–170.8) | 1.00 | (1.00–1.01) | .527 |
| Days 7–13 | 3 | 3 | 36.4 (35.4–69.1) | 9 | 9 | 107.7 (92.4–122.1) | 1.04 | (.99–1.08) | .105 |
| Day >13 | 8 | 8 | 78.0 (46.1–99.0) | 16 | 16 | 91.6 (67.5–130.4) | 1.00 | (.99–1.02) | .706 |
| l -arginine, ng/mL | 13 | 25 | 56.2 (43.9–64.0) | 29 | 58 | 50.9 (38.0–67.0) | 1.00 | (.97–1.02) | .825 |
| Days 1–3 | 12 | 12 | 49.1 (42.8–58.9) | 24 | 25 | 43.7 (36.9–62.0) | 0.99 | (.95–1.04) | .698 |
| Days 4–6 | 10 | 10 | 60.1 (55.7–63.2) | 24 | 24 | 51.9 (40.3–66.8) | 0.99 | (.97–1.02) | .549 |
| Days 7–13 | 3 | 3 | 64.7 (53.5–67.1) | 9 | 9 | 62.0 (55.2–69.0) | 1.01 | (.98–1.05) | .456 |
| Day >13 | 8 | 8 | 85.2 (75.8–89.6) | 16 | 16 | 77.0 (64.1–91.9) | 1.01 | (.97–1.04) | .754 |
| RHI | 24 | 46 | 1.75 (1.46–2.44) | 85 | 180 | 1.83 (1.53–2.29) | 0.72 | (.30–1.72) | .461 |
| Days 1–3 | 21 | 21 | 1.63 (1.42–2.47) | 67 | 69 | 1.62 (1.43–2.02) | 0.33 | (.11–1.04) | .058 |
| Days 4–6 | 20 | 21 | 1.74 (1.49–2.34) | 65 | 70 | 1.94 (1.57–2.39) | 1.15 | (.40–3.33) | .794 |
| Days 7–13 | 4 | 4 | 2.16 (1.92–2.27) | 41 | 41 | 2.12 (1.79–2.56) | 1.91 | (.37–9.74) | .436 |
| Day >13 | 17 | 17 | 2.00 (1.80–2.65) | 45 | 45 | 1.86 (1.53–2.16) | 0.35 | (.11–1.14) | .082 |
All analyses were based on logistic regression with generalized estimation equation. Rows in bold represent the overall comparison including measurements from days 1 to 13. The analysis was adjusted for age, sex, illness day at enrollment, and illness day of measurement.
Abbreviations: CI, confidence interval; IQR, interquartile range; no., number of participants, No., number of measurements; OFI, other febrile illness; RHI, reactive hyperemic index.
Associations of Endothelial Function, l-Arginine, Asymmetric Dimethylarginine, and Arginase-1 With Plasma Leakage Severity and Mucosal Bleeding in Dengue Patients
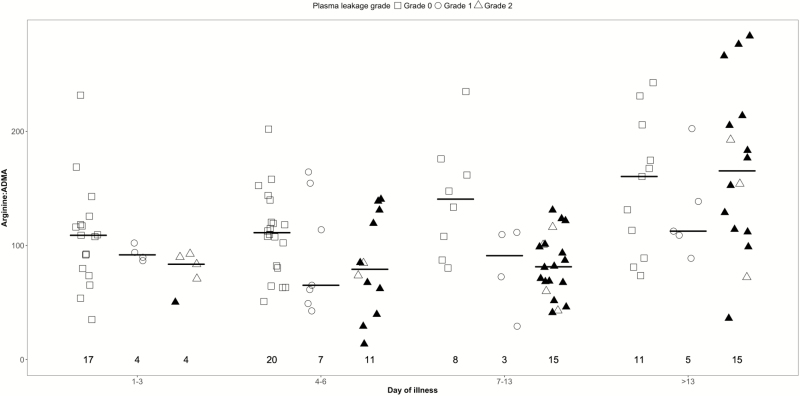
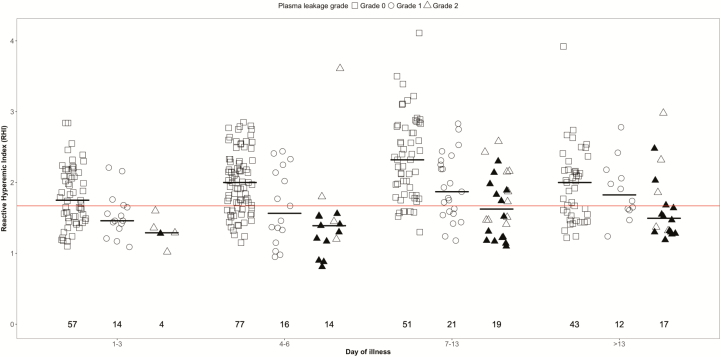
We observed no difference in plasma l-arginine, ADMA, and arginase-1 levels between the plasma leakage severity grades (Table 3 and Supplementary Table1A). There was a lower l-arginine-to-ADMA ratio for both leakage grades 1 and 2 vs no leakage during early convalescence (days 7–13) (Figure 2). The RHI was significantly lower for patients with grade 1 and grade 2 leakage compared to those with no leakage (days 1–13) (Table 3 and Figure 3). The RHI during the critical phase (days 4–6) and early convalescent phase (days 7–13) was significantly lower in those with plasma leakage grade 2 compared to no leakage. Using a linear regression trend test, there was a significant trend in the RHI between the plasma leakage grades overall (P < .001) and for days 1–3 (P = .001), days 4–6 (P = .002), and 7–13 (P < .001). There was no association between endothelial function, ADMA, or arginase-1 levels between patients with and without mucosal bleeding at any of the time points (Supplementary Table 2A).
Table 3.
Association Between Endothelial Function, l-Arginine, Arginase-1, and Plasma Leakage by Illness Phase
| Time-point | no. | No. | No Plasma Leakage (Grade 0) | Plasma Leakage Grade 1 | Plasma Leakage Grade 2 | Grade 1 vs 0 | Grade 2 vs 0 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | no. | No. | Median (IQR) | no. | No. | Median (IQR) | Effect | (95% CI) | P Value | Effect | (95% CI) | P Value | |||
| Arginase, ng/mL | 23 | 45 | 109.7 (66.9–184.4) | 8 | 15 | 117.5 (82.1–248.7) | 19 | 37 | 116.3 (80.8–166.9) | 50.87 | (–62.63, 164.37) | .380 | 24.26 | (–56.38, 104.90) | .556 |
| Days 1–3 | 17 | 17 | 136.3 (103.4–221.2) | 4 | 4 | 158.6 (104.4–224.2) | 4 | 5 | 183.4 (156.6–195.2) | –16.75 | (–116.98, 83.49) | .743 | –16.78 | (–135.68, 102.13) | .782 |
| Days 4–6 | 20 | 20 | 92.0 (54.8–121.2) | 7 | 7 | 116.8 (52.9–237.8) | 11 | 12 | 109.4 (84.9–154.7) | 102.1 | (–67.05, 271.10) | .237 | 105.67 | (–26.61, 237.95) | .117 |
| Days 7–13 | 8 | 8 | 113.0 (85.0–149.7) | 3 | 4 | 121.2 (107.3–213.8) | 15 | 20 | 105.9 (62.4–152.2) | 90.48 | (6.05–174.90) | .036 | 33.05 | (–28.08, 94.19) | .289 |
| Day >13 | 11 | 11 | 90.6 (57.3–115.4) | 5 | 5 | 19.5 (18.0–75.2) | 16 | 17 | 46.7 (36.0–79.3) | 11.42 | (–76.83, 99.67) | .800 | 18.36 | (–27.83, 64.55) | .436 |
| l -arginine, ng/mL | 23 | 45 | 60.0 (43.3–69.0) | 8 | 15 | 47.6 (33.7–76.9) | 19 | 37 | 42.8 (33.5–54.9) | –2.96 | (–21.06, 15.15) | .749 | –15.79 | (–32.22, .65) | .060 |
| Days 1–3 | 17 | 17 | 47.2 (39.9–62.3) | 4 | 4 | 51.7 (46.2–61.4) | 4 | 5 | 33.5 (29.3–35.2) | 11.65 | (–2.28, 25.59) | .101 | –3.87 | (–23.64, 15.90) | .701 |
| Days 4–6 | 20 | 20 | 61.4 (42.3–66.7) | 7 | 7 | 34.3 (28.2–84.0) | 11 | 12 | 42.1 (32.7–48.5) | –4.57 | (–31.00, 21.86) | .735 | –15.22 | (–37.92, 7.48) | .189 |
| Days 7–13 | 8 | 8 | 68.3 (60.2–92.5) | 3 | 4 | 56.0 (31.7–83.2) | 15 | 20 | 51.7 (36.2–66.2) | –9.74 | (–37.58, 18.09) | .493 | –21.07 | (–46.55, 4.42) | .105 |
| Day >13 | 11 | 11 | 83.3 (68.3–93.2) | 5 | 5 | 70.5 (68.1–138.2) | 16 | 17 | 95.9 (74.1–142.1) | 3.58 | (–26.63, 33.79) | .816 | –4.23 | (–33.23, 24.78) | .775 |
| RHI | 101 | 194 | 2.00 (1.62–2.35) | 25 | 54 | 1.66 (1.42–2.18) | 22 | 44 | 1.46 (1.22–1.81) | –0.35 | (–.50, –.20) | <.001 | –0.53 | (–.73, –.33) | <.001 |
| Days 1–3 | 57 | 57 | 1.75 (1.49–2.14) | 14 | 15 | 1.46 (1.38–1.66) | 4 | 5 | 1.29 (1.28–1.36) | –0.24 | –.40, –.08) | .003 | –0.34 | (–.60, –.07) | .012 |
| Days 4–6 | 77 | 82 | 2.00 (1.65–2.30) | 16 | 16 | 1.56 (1.29–2.17) | 14 | 15 | 1.39 (1.18–1.52) | –0.31 | (–.59, –.03) | .032 | –0.49 | (–.84, –.13) | .007 |
| Days 7–13 | 51 | 55 | 2.32 (1.83–2.80) | 21 | 23 | 1.87 (1.58–2.28) | 19 | 24 | 1.62 (1.29–2.02) | –0.46 | (–.70, –.21) | <.001 | –0.54 | (–.77, –.30) | <.001 |
| Day >13 | 43 | 43 | 2.00 (1.54–2.17) | 12 | 12 | 1.82 (1.62–2.09) | 17 | 18 | 1.50 (1.31–1.81) | –0.10 | (–.30, .11) | .371 | –0.25 | (–.52, .02) | .066 |
For each variable, the first row corresponds to the overall comparison, which included all values except values on day >13, and were adjusted for age, sex, and illness day. Other rows correspond to comparison for each illness phase, which included all values during that illness phase and were adjusted for age and sex. Analysis is based on linear regression with generalized estimating equations (variable of interest as outcome and plasma leakage as covariate). Effect (and 95% CI, P value) for grade 1 corresponds to mean difference in variable between grade 1 and 0. Effect (95% CI, P value) for grade 2 corresponds to mean difference in the variable between grade 2 and 0.
Abbreviations: CI, confidence interval; IQR, interquartile range; no. number of participants; No., number of measurements; RHI, reactive hyperemic index.
Figure 2.
Scatterplot of l-arginine-to-asymmetric dimethylarginine (ADMA) ratios for dengue patients by plasma leakage severity and illness phase. Short gray line represents the median value of l-arginine-to-ADMA ratio for each illness phase. The number represents the number of patients that contributed to each group. These graphs are based on 54 patients with at least 1 measurement.
Figure 3.
Scatterplot of endothelial function in dengue patients by plasma leakage severity and illness phase. Short gray line represents the median value of reactive hyperemic index (RHI) during each illness phase. The number represents the number of patients that contributed to each group. Redline represents the 1.67 cutoff, below which is defined as endothelial dysfunction. This graph is based on 109 patients with at least 1 measurement.
Prognostic Potential of Endothelial Function on Days 1–3 for Developing Plasma Leakage in the Critical Phase
Although on illness days 1–3, the levels of arginase-1 tended to be higher and the l-arginine-to-ADMA ratio and RHI tended to be lower in patients who subsequently developed plasma leakage in the critical phase (days 4–6), these parameters were not found to be prognostic using logistic regression and the predefined cutoff of P < .01 (Supplementary Table 3A).
Correlation of Endothelial Function and Plasma l-Arginine, Asymmetric Dimethylarginine, and Arginase-1
There was a negative correlation between RHI and plasma arginase-1 (ρ = –0.21; P = .001) and a positive correlation with l-arginine levels (ρ = 0.27; P = .001). Levels of arginase-1 had a strong negative correlation with l-arginine (ρ = –0.48; P < .001) and borderline correlation with ADMA (ρ = –0.19; P = .010). l-arginine was positively correlated with ADMA (ρ = 0.40; P < .001). We found no correlation between levels of arginase-1 and absolute neutrophil count or monocyte count, nor was there any correlation between enrollment arginase-1 and liver enzyme (aminotransferase) levels. Taken together, these results indicate a strong relationship between endothelial function/NO bioavailability with low levels of serum l-arginine and high levels of arginase.
DISCUSSION
We have shown that endothelium-dependent vasodilation is impaired in dengue and also in OFIs during the acute phase of illness. Contradicting our first hypothesis, we found no difference between endothelial function or l-arginine, arginase-1, and ADMA levels between dengue and OFIs. However, in agreement with our second hypothesis, among patients with confirmed dengue we found that worse endothelial dysfunction was associated with more severe plasma leakage, apparent already in the early febrile phase. Although there was no association with the levels of l-arginine and arginase-1 between the plasma leakage grades, lower l-arginine and higher arginase levels occurred in the early febrile phase of illness and correlated with RHI, suggesting they are involved in the endothelial dysfunction and low NO bioavailability observed in dengue infections. This differs from other endothelial biomarkers such as angiopoietin-2 and VCAM-1 that tend to peak during the critical phase and suggests that hypoargininemia, high arginase, and reduced NO bioavailability are some of the earliest abnormalities in the pathophysiology of dengue [12].
Our results differ from the only other study assessing endothelial function in dengue, performed in Singaporean adults, which demonstrated higher RHI indices in patients with dengue hemorrhagic fever (DHF) compared to uncomplicated dengue [18]. The differences may be due to one or a combination of the following: the Singaporean patients were all older with milder disease, with only 6 DHF patients being hospitalized; the time of enrollment was later in the disease course, when we found a rebound increase in the RHI; and finally the old DHF classification may not be comparable to the severity grades used in our study.
We found that endothelial function was also impaired in the OFI group (which were predominantly other viral illnesses) in the early febrile phase compared with follow-up. The RHI was lower on days 1–3, suggesting that endothelial function and NO bioavailability is reduced early in many infectious diseases, potentially reflecting the influence of nonspecific inflammatory mediators on the vascular system, including proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin 6 [19]. TNF-a can reduce endothelial NO through reduced availability of l-arginine and suppression of eNOS [20]. Further evidence for the role of TNF on endothelial function comes from therapeutics of anti-TNF monoclonal antibodies, which have demonstrated marked transient improvement in endothelial function during treatment [21]. However, although endothelial dysfunction appears to be a common phenomenon in many inflammatory and infectious conditions [22], the degree of impairment also appears to be related to disease severity. One study in adult sepsis demonstrated impaired endothelium-dependent vasodilation that was worse in patients with severe sepsis compared to those with moderate sepsis without organ failure [23]. Similarly, in malaria the degree of endothelial dysfunction has been found to associate with disease severity [24, 25].
A mechanism involving hypoargininemia and increased levels of arginase activity has been proposed in sepsis [26], and it is possible that similar derangements occur in many infectious diseases including malaria and dengue. The source of arginase-1 needs further research; possibilities include release from hepatocytes, but levels did not correlate with liver enzymes. Neutrophil-derived arginase-1 is also a possibility and although we did not demonstrate a correlation between arginase-1 and absolute neutrophil count, a study investigating the early whole blood transcriptional signature of dengue identified arginase-1 and several neutrophil-associated transcripts as being more abundant in dengue patients who progressed to shock [27]. Other possible mechanisms for hypoargininemia in dengue include poor nutritional intake during the acute illness, or leakage out of capillaries. However the levels of l-arginine were lowest in the febrile phase rather than the critical phase, when protein leakage is most marked in dengue. A more likely explanation is that high arginase-1 levels result from early activation and upregulation of neutrophil arginase-1 expression, along with release from neutrophil degranulation; l-arginine is consumed, causing depletion within endothelial cells and resulting in impaired NO bioavailability. We found no changes in levels of ADMA, making competitive inhibition of eNOS as a cause of the endothelial dysfunction less likely; however, the associated inflammatory response, specifically the inhibitory effect of TNF-α on eNOS, may play a role [28].
The timing of the endothelial dysfunction has potentially important implications for therapeutic interventions, particularly those that target the endothelium [29]. A recent trial of lovastatin in adult dengue given within 72 hours of fever did not have an impact on any clinical outcomes [30]. Future trials investigating endothelial modulating therapies should consider either administering the drug earlier (day 1) or targeting patients with evidence of early endothelial dysfunction.
The consequences of impaired endothelial NO release could have several important effects in dengue, not only by impairing vasomotion and altering microcirculatory tissue perfusion, but also may impact vascular permeability directly [12]. Both increased production as well as reduced endothelial NO release can influence microvascular permeability [31]. NO may protect vascular barrier function by inhibiting cellular adhesion. Low NO bioavailability can cause increased platelet and leukocyte adhesion to the vascular endothelial surface [32], and increased albumin leakage has been demonstrated in post capillary venules after NO inhibition [33]. Decreased NO levels also result in increased exocytosis of endothelial Weibel-Palade bodies and release of angiopoietin-2, which has been shown to cause glycocalyx degradation and increased vascular permeability [34, 35]. NO may also protect the microvasculature against mast cell degranulation products and associated permeability alterations [36, 37].
There were several limitations to our study. First, only a small number of patients with severe dengue were studied using EndoPAT, as many of the ICU study participants were excluded due to platelet counts <20 × 109/L, and this may have underestimated the associations between severe dengue and endothelial dysfunction. The numbers of patients enrolled early in the febrile phase who subsequently developed shock were also small, making the prognostic models less robust. Last, plasma levels of arginase-1 may not reflect the activity of the enzyme, and arginase-1 activity assays should be used to confirm that high levels equate to increase enzymatic activity.
CONCLUSIONS
This study has shown that impaired endothelial function is associated with dengue plasma leakage severity. Importantly, we found that endothelial dysfunction occurred early in the course of the disease, with worse function already apparent in the first 72 hours of fever in patients who subsequently developed severe plasma leakage. Endothelial dysfunction as a surrogate for NO bioavailability correlated with lower l-arginine and higher arginase-1 levels, but not ADMA, suggesting that hypoargininemia resulting from high arginase-1 levels plays a role. These findings provide important new information regarding the pathophysiology of progression to severe disease in dengue. Clinical endothelial function tests could be considered not only for screening patients for enrollment in therapeutic intervention trials, but potentially also as a surrogate endpoint for such trials.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank all the medical and nursing staff at the National Hospital for Tropical Diseases in Hanoi and the Hospital for Tropical Diseases in Ho Chi Minh City for patient recruitment and management; Kieu Nguyen Tan Thanh, Su Nguyen Thi Minh, and all the study nurses for their help coordinating the studies; and the Oxford University Clinical Research Unit dengue laboratory staff for sample processing.
Financial support. This work was supported by the Wellcome Trust (grant number 100562/Z/12/Z) and the International Research Consortium on Dengue Risk Assessment, Management and Surveillance (IDAMS) (EU grant number FP7-281803; publication reference number IDAMS 47).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. N Engl J Med 2012; 366:1423–32. [DOI] [PubMed] [Google Scholar]
- 3. Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol 2014; 11:335–45. [DOI] [PubMed] [Google Scholar]
- 4. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation 2007; 115:1285–95. [DOI] [PubMed] [Google Scholar]
- 5. Hamburg NM, Benjamin EJ. Assessment of endothelial function using digital pulse amplitude tonometry. Trends Cardiovasc Med 2009; 19:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J 2001; 15:1264–6. [DOI] [PubMed] [Google Scholar]
- 7. Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med 1993; 329:2002–12. [DOI] [PubMed] [Google Scholar]
- 8. Yeo TW, Lampah DA, Tjitra E et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog 2010; 6:e1000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davis JS, Darcy CJ, Yeo TW et al. Asymmetric dimethylarginine, endothelial nitric oxide bioavailability and mortality in sepsis. PLoS One 2011; 6:e17260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubach MP, Mukemba J, Florence S et al. : Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: association with disease severity. PLoS Pathog 2015, 11:e1004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koraka P, Murgue B, Deparis X et al. Elevation of soluble VCAM-1 plasma levels in children with acute dengue virus infection of varying severity. J Med Virol 2004; 72:445–50. [DOI] [PubMed] [Google Scholar]
- 12. Yacoub S, Lam PK, Vu le HM et al. Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: an observational study. J Infect Dis 2016; 214:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knottnerus A, Tugwell P. STROBE—a checklist to strengthen the reporting of observational studies in epidemiology. J Clin Epidemiol 2008; 61:323. [DOI] [PubMed] [Google Scholar]
- 14. Jaenisch T, Tam DT, Kieu NT et al. Clinical evaluation of dengue and identification of risk factors for severe disease: protocol for a multicentre study in 8 countries. BMC Infect Dis 2016; 16:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization. Dengue: guidelines for treatment, prevention and control. Geneva, Switzerland: WHO, 2009. [PubMed] [Google Scholar]
- 16. Kuvin JT, Patel AR, Sliney KA et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J 2003; 146:168–74. [DOI] [PubMed] [Google Scholar]
- 17. Hue KD, Tuan TV, Thi HT et al. Validation of an internally controlled one-step real-time multiplex RT-PCR assay for the detection and quantitation of dengue virus RNA in plasma. J Virol Methods 2011; 177:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thein TL, Wong J, Leo YS, Ooi EE, Lye D, Yeo TW. Association between increased vascular nitric oxide bioavailability and progression to dengue hemorrhagic fever in adults. J Infect Dis 2015; 212:711–4. [DOI] [PubMed] [Google Scholar]
- 19. Weiner SD, Ahmed HN, Jin Z et al. Systemic inflammation and brachial artery endothelial function in the Multi-Ethnic Study of Atherosclerosis (MESA). Heart 2014; 100:862–6. [DOI] [PubMed] [Google Scholar]
- 20. Goodwin BL, Pendleton LC, Levy MM, Solomonson LP, Eichler DC. Tumor necrosis factor-alpha reduces argininosuccinate synthase expression and nitric oxide production in aortic endothelial cells. Am J Physiol Heart Circ Physiol 2007; 293:H1115–21. [DOI] [PubMed] [Google Scholar]
- 21. Raza K, Carruthers DM, Stevens R, Filer AD, Townend JN, Bacon PA. Infliximab leads to a rapid but transient improvement in endothelial function in patients with primary systemic vasculitis. Ann Rheum Dis 2006; 65:946–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charakida M, Donald AE, Terese M et al. ; ALSPAC (Avon Longitudinal Study of Parents and Children) Study Team Endothelial dysfunction in childhood infection. Circulation 2005; 111:1660–5. [DOI] [PubMed] [Google Scholar]
- 23. Davis JS, Yeo TW, Thomas JH et al. Sepsis-associated microvascular dysfunction measured by peripheral arterial tonometry: an observational study. Crit Care 2009; 13:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yeo TW, Lampah DA, Kenangalem E et al. Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. J Infect Dis 2014; 210:1627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeo TW, Lampah DA, Gitawati R et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 2007; 204:2693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darcy CJ, Woodberry T, Davis JS et al. Increased plasma arginase activity in human sepsis: association with increased circulating neutrophils. Clin Chem Lab Med 2014; 52:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoang LT, Lynn DJ, Henn M et al. The early whole-blood transcriptional signature of dengue virus and features associated with progression to dengue shock syndrome in Vietnamese children and young adults. J Virol 2010; 84:12982–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshizumi M, Perrella MA, Burnett JC Jr, Lee ME. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 1993; 73:205–9. [DOI] [PubMed] [Google Scholar]
- 29. McGown CC, Brookes ZL. Beneficial effects of statins on the microcirculation during sepsis: the role of nitric oxide. Br J Anaesth 2007; 98:163–75. [DOI] [PubMed] [Google Scholar]
- 30. Whitehorn J, Nguyen CVV, Khanh LP et al. Lovastatin for the treatment of adult patients with dengue: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2016; 62:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fukumura D, Gohongi T, Kadambi A et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A 2001; 98:2604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A 1991; 88:4651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurose I, Kubes P, Wolf R et al. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res 1993; 73:164–71. [DOI] [PubMed] [Google Scholar]
- 34. Lukasz A, Hillgruber C, Oberleithner H et al. Endothelial glycocalyx breakdown is mediated by angiopoietin-2. Cardiovasc Res 2017; 113:671–80. [DOI] [PubMed] [Google Scholar]
- 35. Matsushita K, Morrell CN, Cambien B et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003; 115:139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaboury JP, Niu XF, Kubes P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation 1996; 93:318–26. [DOI] [PubMed] [Google Scholar]
- 37. Al-Naemi H, Baldwin AL. Nitric oxide protects venules against histamine-induced leaks. Microcirculation 2000; 7:215–23. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.