Abstract
African tick bite fever is the most commonly encountered travel-associated rickettsiosis, occurring in as many as 5% of travelers returning from rural subequatorial Africa. This case report illustrates that rifampin represents an effective alternative to doxycycline for treatment of African tick bite fever in some selective situations.
Keywords: Rickettsia africae, African tick bite fever, rifampin
Rickettsiae are obligate intracellular bacteria that cause a wide range of zoonotic diseases worldwide. Rickettsia africae, the causative agent of African tick bite fever (ATBF), typically causes a self-limited, acute febrile illness, but can occasionally lead to severe complications including subacute neuropathy [1], myocarditis [2], acute neuropsychiatric symptoms [3], reactive arthritis, and prolonged fevers [4]. Doxycycline is the treatment of choice for all rickettsial diseases and effectively reduces symptom duration and complications; nonetheless, a small percentage of patients cannot receive tetracycline-class antibiotics because of intolerance to these drugs [5–7]. Here we present a case of a patient with ATBF and intolerance to doxycycline who had a rapid and complete response following therapy with rifampin.
CASE PRESENTATION
A 53-year-old immunocompetent white male with a history of doxycycline intolerance presented with 4-day history of a new skin lesion, myalgia, and generalized malaise. These symptoms started about 9 days after being on a safari in the Hluhluwe region of KwaZulu-Natal, South Africa. During this time, he was compliant with antimalarial prophylaxis (atovaquone/proguanil), and used insect repellant. The patient did not recall any tick bites. Prior to his trip, he had visited a travel clinic and was current on all of his vaccines. His doxycycline intolerance consisted of blistering and ulcerated oral lesions in the corner of his mouth as well as between digits of his hands within hours of exposure of a 100-mg oral dose of this drug administered for the treatment of rosacea. This reaction also occurred when a lower dosage (40 mg daily) was administered.
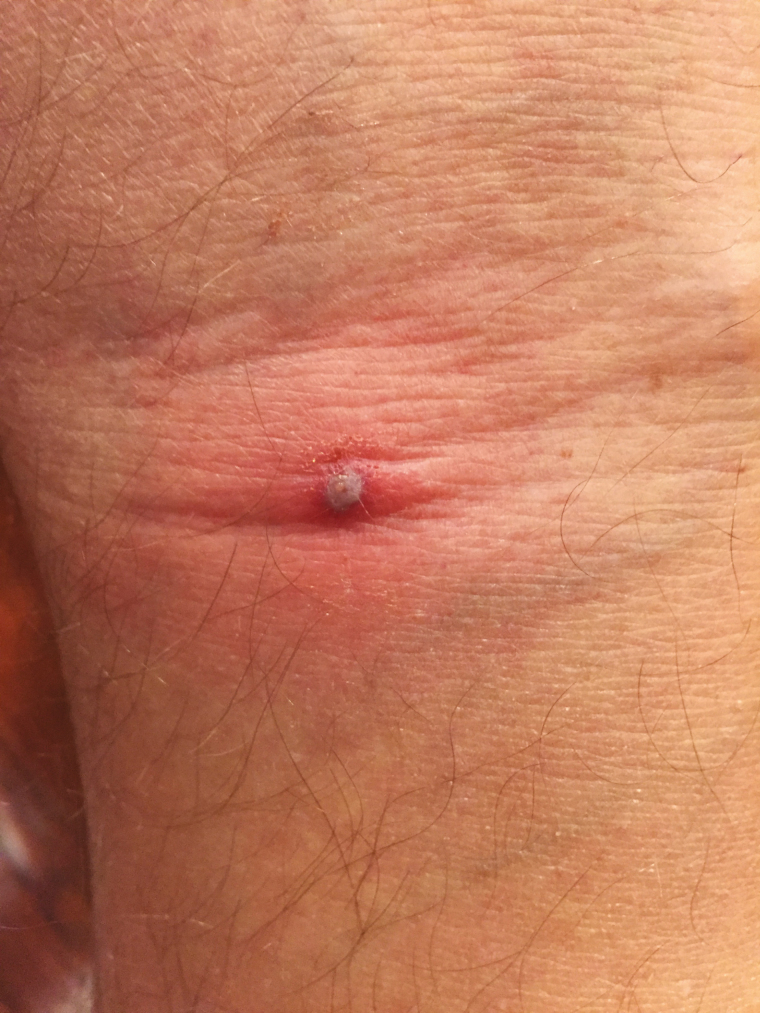
When seen in the clinic he appeared to be generally well, with stable vital signs, and was afebrile. Physical examination was remarkable for tender firm lymph nodes in the inguinal and right popliteal region and a 1-cm pustular tender nodule with surrounding erythema and induration in his right popliteal fossa (Figure 1), but no rash was present. A presumptive diagnosis of ATBF was made. Because of the patient’s history of doxycycline intolerance and overall clinical stability, no initial treatment was prescribed. A punch biopsy was performed on the nodule, which was sent to the Centers for Disease Control and Prevention for diagnostic testing. However, 36 hours later the patient developed fever to 38.3°C and headache. A real-time polymerase chain reaction (PCR) assay targeting a segment of the 23S ribosomal RNA gene of Rickettsia species [8] was performed on DNA extracted from a portion of the skin biopsy specimen and confirmed a rickettsial infection. The patient was started on rifampin 450 mg twice a day. Within 12 hours of starting therapy, his temperature returned to normal and his condition improved considerably with resolution of his headache and myalgia. The patient completed a 10-day course of rifampin with complete resolution of symptoms. Rickettsia africae was subsequently isolated in Vero E6 cells after 10 days. A nested PCR assay amplified a 532-bp segment of the sca0 (ompA) gene [9, 10], and sequencing of this amplicon revealed 100% identity with the corresponding sequence of R. africae. A convalescent serum sample collected approximately 5 weeks after recovery revealed a reciprocal immunoglobulin antibody titer to R. africae of 2048.
Figure 1.
Pustular lesion with surrounding erythema and induration in the right popliteal fossa.
DISCUSSION
Standard therapy for adult patients with ATBF is doxycycline 100 mg twice daily [11]. Alternatives to doxycycline for the treatment of ATBF are limited. Chloramphenicol is identified as an alternative drug, but oral formulations are not currently available in the United States [12, 13]. Ciprofloxacin [4, 5, 12] and some macrolides, including azithromycin and erythromycin [14, 15], have been used alone or in combination with other antibiotics as treatment of suspected ATBF, although the efficacy of these antibiotics against R. africae is inconclusive. Josamycin, another macrolide, has been recommended for treatment of ATBF in pregnant women based on in vitro sensitivity [12, 16], but clinical data are lacking.
In the current case report, we document a patient with culture-confirmed ATBF who was successfully treated with rifampin. Although rifampin is not considered a first-line treatment for any rickettsial infection, it has been used successfully as monotherapy to treat pregnant women and children infected with Anaplasma phagocytophilum [17–19]. We opted to treat this patient with rifampin because of his inability to take doxycycline, and because most Rickettsia species, including R. africae, are highly susceptible to rifampin in vitro [16, 20]. A few case reports describe the use of rifampin in combination with erythromycin or azithromycin to successfully treat Mediterranean spotted fever [21] and ATBF [22], but there are no reports of its successful use as monotherapy for ATBF. Our patient’s prompt response to rifampin suggests that its use may be considered in specific clinical situations when doxycycline is contraindicated. Some other pathogenic Rickettsia species endemic to sub-Saharan Africa, including Rickettsia conorii and Rickettsia sibirica mongolotimonae [23], are also susceptible to rifampin in vitro [16].
Rifampin resistance occurs in several bacteria, including Staphylococcus aureus [24], Neisseria meningitidis [25], Mycobacterium tuberculosis [26], Escherichia coli [27, 28], and Streptococcus pneumoniae [29]. In addition, a few pathogenic species of spotted fever group Rickettsia, including Rickettsia massiliae and Rickettsia aeschlimannii, are resistant to rifampin in vitro [16, 30], and therapeutic failures have been reported when rifampin is used as primary therapy for rickettsial infections in regions where R. massiliae is prevalent [31, 32]. Finally, because R. massiliae and R. aeschlimannii cause disease manifestations similar to R. africae, and occur sympatrically with R. africae in some areas of Africa [23], rifampin should not be considered as first-line therapy for all patients returning from Africa with fever and an eschar, but reserved only for specialized situations such as PCR-confirmed infections with rifampin-susceptible rickettsial pathogens as described in this report.
CONCLUSIONS
ATBF is the most commonly encountered travel-associated rickettsiosis, occurring in as many as 5% of travelers returning from rural subequatorial Africa [4, 33]. Physicians should consider this infection in patients who present with compatible signs and symptoms and a travel history to a region where R. africae is endemic. Treatment is generally empiric because accurate diagnostic assays are largely retrospective and of limited availability. This case report illustrates that rifampin represents an effective alternative to doxycycline for treatment of ATBF in some selective situations.
Notes
Disclaimer. The findings and conclusions are those of the authors and do not necessarily reflect the views of the US Department of Health and Human Services.
Financial support. V. G. F. was supported by the National Institutes of Health (NIH; K24-AI093969).
Potential conflicts of interest. V. G. F. served as Chair of the V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Cubist/Merck, Karius, Contrafect, and Genentech; has NIH Small Business Innovation Research grants pending (Affinergy, Locus, Medical Surface, Inc); has been a paid consultant for Achaogen, Astellas, Arsanis, Affinergy, Basilea, Bayer, Cerexa, Contrafect, Cubist, Debiopharm, Durata, Grifols, Genentech, MedImmune, Merck, Medicines Co, Pfizer, Novartis, Novadigm, Theravance, and xBiotech; has received honoraria from Theravance, Green Cross; and has a patent pending in sepsis diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jensenius M, Fournier PE, Fladby T et al. Sub-acute neuropathy in patients with African tick bite fever. Scand J Infect Dis 2006; 38:114–8. [DOI] [PubMed] [Google Scholar]
- 2. Bellini C, Monti M, Potin M, Dalle Ave A, Bille J, Greub G. Cardiac involvement in a patient with clinical and serological evidence of African tick-bite fever. BMC Infect Dis 2005; 5:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson Y, Chappuis F, Loutan L. African tick-bite fever: four cases among Swiss travelers returning from South Africa. J Travel Med 2004; 11:225–8. [DOI] [PubMed] [Google Scholar]
- 4. Jensenius M, Fournier PE, Vene S et al. ; Norwegian African Tick Bite Fever Study Group African tick bite fever in travelers to rural sub-equatorial Africa. Clin Infect Dis 2003; 36:1411–7. [DOI] [PubMed] [Google Scholar]
- 5. Rashid R, Pasqualotto AC, Denning DW. A case of spotted fever group rickettsiosis imported into the United Kingdom and treated with ciprofloxacin: a case report. J Med Case Rep 2008; 2:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shapiro LE, Knowles SR, Shear NH. Comparative safety of tetracycline, minocycline, and doxycycline. Arch Dermatol 1997; 133:1224–30. [PubMed] [Google Scholar]
- 7. Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther 2005; 27:1329–42. [DOI] [PubMed] [Google Scholar]
- 8. Kato CY, Chung IH, Robinson LK, Austin AL, Dasch GA, Massung RF. Assessment of real-time PCR assay for detection of Rickettsia spp. and Rickettsia rickettsii in banked clinical samples. J Clin Microbiol 2013; 51:314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173:1576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roux V, Fournier PE, Raoult D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J Clin Microbiol 1996; 34:2058–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensenius M, Fournier PE, Kelly P, Myrvang B, Raoult D. African tick bite fever. Lancet Infect Dis 2003; 3:557–64. [DOI] [PubMed] [Google Scholar]
- 12. Raoult D, Drancourt M. Antimicrobial therapy of rickettsial diseases. Antimicrob Agents Chemother 1991; 35:2457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biggs HM, Behravesh CB, Bradley KK et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep 2016; 65:1–44. [DOI] [PubMed] [Google Scholar]
- 14. Miller GB, Gear JH. Treatment of tick-bite fever with erythromycin. S Afr Med J 1984; 66:694–7. [PubMed] [Google Scholar]
- 15. Caruso G, Zasio C, Guzzo F et al. Outbreak of African tick-bite fever in six Italian tourists returning from South Africa. Eur J Clin Microbiol Infect Dis 2002; 21:133–6. [DOI] [PubMed] [Google Scholar]
- 16. Rolain JM, Maurin M, Vestris G, Raoult D. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother 1998; 42:1537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buitrago MI, Ijdo JW, Rinaudo P et al. Human granulocytic ehrlichiosis during pregnancy treated successfully with rifampin. Clin Infect Dis 1998; 27:213–5. [DOI] [PubMed] [Google Scholar]
- 18. Shields K, Cumming M, Rios J et al. Transfusion-associated Anaplasma phagocytophilum infection in a pregnant patient with thalassemia trait: a case report. Transfusion 2015; 55:719–25. [DOI] [PubMed] [Google Scholar]
- 19. Krause PJ, Corrow CL, Bakken JS. Successful treatment of human granulocytic ehrlichiosis in children using rifampin. Pediatrics 2003; 112:e252–3. [DOI] [PubMed] [Google Scholar]
- 20. Raoult D, Roussellier P, Vestris G, Tamalet J. In vitro antibiotic susceptibility of Rickettsia rickettsii and Rickettsia conorii: plaque assay and microplaque colorimetric assay. J Infect Dis 1987; 155:1059–62. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J, Lasri Y, Landau Z. Mediterranean spotted fever in pregnancy. Scand J Infect Dis 1999; 31:202–3. [DOI] [PubMed] [Google Scholar]
- 22. Haemel AK, Bearden A, Longley BJ, Crnich C. Black spots in the returning traveler. Dermatol Online J 2013; 19:20393. [PubMed] [Google Scholar]
- 23. Parola P, Paddock CD, Socolovschi C et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev 2013; 26:657–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raad I, Hanna H, Jiang Y et al. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob Agents Chemother 2007; 51:1656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berkey P, Rolston K, Zukiwski A, Gooch G, Bodey GP. Rifampin-resistant meningococcal infection in a patient given rifampin chemoprophylaxis. Am J Infect Control 1988; 16:250–2. [DOI] [PubMed] [Google Scholar]
- 26. Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CRJr. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med 1993; 328:527–32. [DOI] [PubMed] [Google Scholar]
- 27. Goldstein BP. Resistance to rifampicin: a review. J Antibiot (Tokyo) 2014; 67:625–30. [DOI] [PubMed] [Google Scholar]
- 28. Hartmann G, Honikel KO, Knüsel F, Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta 1967; 145:843–4. [DOI] [PubMed] [Google Scholar]
- 29. van Tilburg PM, Bogaert D, Sluijter M, Jansz AR, de Groot R, Hermans PW. Emergence of rifampin-resistant Streptococcus pneumoniae as a result of antimicrobial therapy for penicillin-resistant strains. Clin Infect Dis 2001; 33:e93–6. [DOI] [PubMed] [Google Scholar]
- 30. Drancourt M, Raoult D. Characterization of mutations in the rpoB gene in naturally rifampin-resistant Rickettsia species. Antimicrob Agents Chemother 1999; 43:2400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bella F, Espejo E, Uriz S, Serrano JA, Alegre MD, Tort J. Randomized trial of 5-day rifampin versus 1-day doxycycline therapy for Mediterranean spotted fever. J Infect Dis 1991; 164:433–4. [DOI] [PubMed] [Google Scholar]
- 32. Cardeñosa N, Segura F, Raoult D. Serosurvey among Mediterranean spotted fever patients of a new spotted fever group rickettsial strain (Bar29). Eur J Epidemiol 2003; 18:351–6. [DOI] [PubMed] [Google Scholar]
- 33. Ericsson CD, Jensenius M, Fournier P-E, Raoult D. Rickettsioses and the international traveler. Clin Infect Dis 2004; 39:1493–9. [DOI] [PubMed] [Google Scholar]