Age, historical plasma viral load, and prior ART regimen changes were independently associated with increased hazard of CD4 decline and death while off ART. ART reengagement interventions need further development and may consider prioritizing individuals with these poorer prognostic factors.
Keywords: HIV/AIDS, antiretroviral therapy, treatment retention, CD4, disease progression
Abstract
Background
Suboptimal retention is among the biggest challenges to realize the full benefits of combination antiretroviral therapy (ART). We aimed to describe ART interruption patterns and identify determinants of disease progression while off ART in British Columbia, Canada.
Methods
With population-level data on ART utilization and laboratory testing in British Columbia (1996–2015), we described the timing, frequency, and duration of ART interruptions (a gap of ≥90 days in ART dispensation records). A 4-state continuous-time Markov model was implemented to identify determinants of disease progression during individuals’ first ART interruption episode. Disease progression was measured according to CD4-based state transitions (cells/μL: ≥500 to 200–499; 200–499 to <200; ≥500 to death; 200–499 to death; and <200 to death).
Results
Among individuals initiating ART, 3129 (38.6%) interrupted ART over a median 8-year follow-up (interquartile range [IQR], 4.3–13.5 years). Those interrupting ART had a median of 1 interruption (IQR, 1.0–3.0), with the first interruption occurring 12.8 (IQR, 4.0–36.1) months after ART initiation, lasting for 7.5 (IQR, 4.1–20.3) months. The proportion of individuals interrupting ART within the first year of ART initiation decreased over time; however, the absolute number of individuals interrupting ART remained high. In a multivariable analysis, age, historical plasma viral load, and ART regimen changes prior to interruption were associated with increased hazard of CD4 decline and death.
Conclusions
Our results demonstrate that ART interruptions are common even in a high-resource setting with universal free access to human immunodeficiency virus care. Further efforts are needed to promote ART reengagement and may consider prioritizing individuals with poorer prognostic factors.
The use of combination antiretroviral therapy (ART) has successfully transformed human immunodeficiency virus (HIV) disease from an acute fatal condition into a chronic condition [1]. Along with early diagnosis and treatment initiation [2], continuous ART retention is essential to achieving sustained HIV suppression [3], decreased emergence of drug resistance [4], and improved survival and quality of life [5–7], as well as reduced HIV transmission [8]. Ensuring ART retention is critical to HIV treatment-as-prevention strategies and ultimately reaching the goal of an AIDS-free generation [9].
Poor adherence and continuity of care are among the biggest challenges to realize the full benefits of ART in both high-income and resource-limited settings [9, 10]. The Joint United Nations Programme on HIV/AIDS (UNAIDS) has established its 90-90-90 target to address gaps in the cascade of HIV care [11]—that is, by 2020, 90% of people living with HIV (PLHIV) will be diagnosed, 90% of those diagnosed will receive ART, and 90% of those on ART will have suppressed plasma viral loads (pVLs) [11]. In British Columbia (BC), Canada, progress has been made in improving the HIV care continuum over time [12], but gaps exist at each stage of cascade including achieving suppressed pVL [13]. Similar gaps have been reported globally [14], underscoring the pressing need to optimize ART continuation.
Existing evidence supports benefits of several interventions in improving adherence and viral suppression, including text messages, cognitive-behavioral therapy, and peer navigators, although their effects appear to be modest and wane over time [15]. Previous studies have also identified risk factors associated with ART nonadherence/interruption, including younger age [16–19], injection drug use [16, 18, 19], poor patient–provider relationship [20], and higher levels of regimen complexity [21]. Knowledge is limited, however, regarding population-level patterns of ART interruption and determinants of disease progression while off ART. This is partly due to the paucity of population-level drug dispensation data [22].
Utilizing the population-level data in BC, Canada, between 1996 and 2015, we aimed to first describe ART interruption patterns and then summarize the determinants of disease progression following the first ART interruption.
MATERIALS AND METHODS
Study Population
We considered all individuals who were antiretroviral naive at age ≥19 years and initiated combination ART between 1 August 1996 and 30 September 2015, as observed in the BC Centre for Excellence in HIV/AIDS (BC-CfE) Drug Treatment Program data. The study cohort is followed in a unique environment characterized by universal medical care, including free in- and outpatient care, laboratory monitoring, and antiretroviral drugs. Antiretroviral drugs are centrally distributed by the BC-CfE according to provincial treatment guidelines [23], which remain consistent with those put forward by the International AIDS Society (IAS) [24]. The initial fill of HIV medications is usually a 1-month supply, and refills for stable patients are usually a 2- to 3-month supply [25]. Both CD4 and pVL are also monitored according to these guidelines [23].
Our data capture complete antiretroviral drug dispensation, drug resistance, and pVL test data, and an estimate of 80% CD4 test data in the province [26]. The study sample is comprised of individuals infected mostly with subtype B virus [27]. The study was approved by the University of British Columbia and Providence Health Care research ethics board.
Measures
ART interruption was defined as a minimum 90-day gap between the prescription refill date and the date when previously dispensed medications were expected to be finished. We chose ≥90 days given our clinical experience on an ongoing province-wide “reengagement and engagement in treatment for antiretroviral interrupted and naive populations” intervention, where alerts were sent to providers whose patients have a ≥60-day gap in antiretroviral drug dispensing records. We found that many of these individuals were still taking their medication (albeit with low adherence), indicating the need for a longer time period to capture true ART interruption.
Disease progression while off ART was the primary endpoint in the analysis to address the second study objective, operationalized by a matrix with 5 possible transitions (CD4 count [cells/μL] ≥500 to 200–499; 200–499 to <200; ≥500 to death; 200–499 to death; and <200 to death). We applied an “ad hoc smoothing” technique [28], whereby transitions between CD4 strata were only allowed when 2 consecutive CD4 measurements were observed, to address intraindividual variation in time between CD4 measurements. All-cause death was ascertained through a linkage to provincial vital statistics data. Off-ART deaths were classified as such if they occurred during an off-ART period, or within 90 days of reinitiating ART; the latter aimed to capture individuals who reinitiated ART as a result of advanced disease.
We considered a list of variables to examine their relationships with disease progression, including (1) fixed covariates: male (yes/no), aboriginal ethnicity (yes/no/unknown), people who inject drugs (PWID) (yes/no/unknown), hepatitis C virus positive (HCV+) at ART initiation (yes/no/unknown), and (2) time-dependent covariates: age, year, time from ART initiation to interruption (in months), HIV drug resistance (yes/no), nadir CD4 count and historical average pVL before interruption, regimen change (yes/no), and receiving a modern regimen (yes/no). Drug resistance was considered as nonreversible, and determined as detecting mutations based on a modification of the 2014 IAS-USA mutation list [29]. Historical average pVL was measured as area under the pVL curve, as sustained high pVL periods are associated with CD4 declines. We coded missing values as a third category in several categorical covariates to avoid the exclusion of individuals due to missing covariates.
Statistical Analysis
Descriptive statistics and univariate analysis were employed to characterize ART interruption patterns among all individuals initiating ART. First, we evaluated the proportion of individuals interrupting ART during follow-up and compared their characteristics to those remaining on ART continuously. Second, we examined the number of first and subsequent interruption episodes by calendar year and, among those with a minimum 1-year follow-up, the proportions of individuals interrupting ART within the first year. Third, we investigated the proportion of individuals who received at least 1 CD4/pVL test while off ART and compared the time intervals between CD4/pVL measurements pre– and post–ART interruption among individuals having at least 2 measurements to examine their retention in HIV care while off ART.
A parametric continuous-time, multistate Markov (MSM) model [30] was implemented to identify determinants of disease progression following the first ART interruption and before ART resumption, among individuals with at least 2 observed disease status measurements (either CD4 or death) during the first off-ART period. Individuals were censored at the point of the last CD4 test during the first interruption episode. MSM models have previously been applied to model HIV disease progression by CD4 cell count strata [28, 31, 32]. These models efficiently handle heavily censored data, such as when the exact time of disease onset is unknown or when a subject is observed over a portion of his/her disease history [32]. The assumption of noninformative sampling times was satisfied in the current study as CD4 measurements typically occur at regular intervals as part of routine care [30]. A covariate was assumed to affect each baseline transition intensity by a proportional factor.
At first, we described the frequency of observed disease state transitions, and estimated the average annual transition probabilities from the unadjusted MSM model. We then examined the association between each covariate and disease transitions using bivariate models. At last, we constructed multivariable models to summarize factors associated with disease progression. As the covariates PWID and HCV+ were highly positively correlated, we evaluated the nested models (with both covariates vs with either of them) using likelihood ratio tests, and a P value ≤.05 was considered as favoring the more complex model. Covariate effects on 2 transitions (CD4 count ≥500 to death; and 200–499 to death) were not estimated due to small numbers of transitions [30].
To check the robustness of results, we repeated multivariable analyses among first and subsequent ART interruptions, additionally adjusting for numbers of interruption episodes, as well as among a remaining subset of individuals (n = 1657) after excluding those with any missing covariates.
All statistical analyses were executed in SAS version 9.4 and R version 3.2.5 software. The R msm package version 1.6.4 was used for MSM modeling [30].
RESULTS
A total of 8110 ART-naive individuals initiated combination ART between 1996 and 2015 in BC. Among them, 3129 (38.6%) interrupted ART for ≥90 days over a median 8-year follow-up (interquartile range [IQR], 4.3–13.5 years), resulting in 6549 off-ART episodes. Individuals interrupting ART were younger at ART initiation (38 vs 42 years) and were more likely to be aboriginal (19.7% vs 8.1%), PWID (51.0% vs 21.2%), and HCV+ (55.1% vs 25.3%), compared with individuals remaining on ART (Table 1). Those interrupting ART had a median of 1 interruption (IQR, 1.0–3.0), with the first interruption occurring 12.8 (IQR, 4.0–36.1) months after ART initiation, lasting for 7.5 (IQR, 4.1–20.3) months (Table 1).
Table 1.
Characteristics at Antiretroviral Therapy (ART) Initiation of 8110 ART-Naive Individuals Who Initiated ART in British Columbia, by ART Interruption (≥90 Days), 1996–2015
| Characteristic | Total | Ever Interrupted ART | Never Interrupted ART | P Valuea |
|---|---|---|---|---|
| (N = 8110) | (n = 3129) | (n = 4981) | ||
| Age, y, median (IQR) | 40.7 (33.6–48.1) | 38.2 (32.2–44.8) | 42.4 (34.9–49.7) | <.001 |
| Male sex | 6636 (81.8) | 2342 (74.8) | 4294 (86.2) | <.001 |
| White race | ||||
| Yes | 4913 (60.6) | 1841 (58.8) | 3072 (61.7) | <.001 |
| No | 1849 (22.8) | 792 (25.3) | 1057 (21.2) | |
| Unknown | 1348 (16.6) | 496 (15.9) | 852 (17.1) | |
| Aboriginal ethnicity | ||||
| Yes | 1022 (12.6) | 617 (19.7) | 405 (8.1) | <.001 |
| No | 5740 (70.8) | 2016 (64.4) | 3724 (74.8) | |
| Unknown | 1348 (16.6) | 496 (15.9) | 852 (17.1) | |
| People who inject drugs | ||||
| Yes | 2651 (32.7) | 1595 (51.0) | 1056 (21.2) | <.001 |
| No | 3629 (44.7) | 1034 (33.0) | 2595 (52.1) | |
| Unknown | 1830 (22.6) | 500 (16.0) | 1330 (26.7) | |
| CD4 count <200 cells/μLb | ||||
| Yes | 2938 (36.2) | 1162 (37.1) | 1776 (35.7) | <.001 |
| No | 4513 (55.6) | 1675 (53.5) | 2838 (57.0) | |
| Unknown | 659 (8.1) | 292 (9.3) | 367 (7.4) | |
| HIV drug resistancec | 705 (8.7) | 277 (8.9) | 428 (8.6) | .69 |
| Hepatitis C positive | ||||
| Yes | 2984 (36.8) | 1724 (55.1) | 1260 (25.3) | <.001 |
| No | 4476 (55.2) | 1200 (38.4) | 3276 (65.8) | |
| Unknown | 650 (8.0) | 205 (6.6) | 445 (8.9) | |
| Modern regimend | 5700 (70.3) | 1611 (51.5) | 4089 (82.1) | <.001 |
| Calendar year | ||||
| 1996–2003 | 2562 (31.6) | 1678 (53.6) | 884 (17.7) | <.001 |
| 2004–2007 | 1622 (20.0) | 607 (19.4) | 1015 (20.4) | |
| 2008–2011 | 2182 (26.9) | 598 (19.1) | 1584 (31.8) | |
| 2012–2015 | 1744 (21.5) | 246 (7.9) | 1498 (30.1) | |
| Follow-up, y, median (IQR) | 5.6 (2.4–10.0) | 8.0 (4.3–13.5) | 4.4 (1.5–7.9) | <.001 |
| Interruption episodes, median (IQR) | … | 1.0 (1.0–3.0) | … | |
| Time from ART initiation to first interruption, mo, median (IQR) | … | 12.8 (4.0–36.1) | … | |
| Duration of first interruption episode, mo, median (IQR) | … | 7.5 (4.1–20.3) | … | |
| Duration of each interruption episode, mo, median (IQR) | 6.5 (4.0–14.9) | |||
| Total months off ART, median (IQR) | … | 18.9 (7.0–41.3) | … | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range.
aPearson χ2 and Mann-Whitney tests were used to compare categorical and continuous variables, respectively.
bBaseline CD4 count was determined based on the CD4 test performed within 3 months of ART initiation.
cHIV drug resistance was considered as nonreversible, and determined as detecting mutations based on a modification of the 2014 International AIDS Society–USA mutation list.
dModern regimens included those based on lamivudine plus tenofovir or emtricitabine plus tenfovir combined with efavirenz, rilpivirine, or etravirine or a boosted protease inhibitor or integrase inhibitor regimen; small numbers of individuals could have received tipranavir, maraviroc, or enfuvirtide.
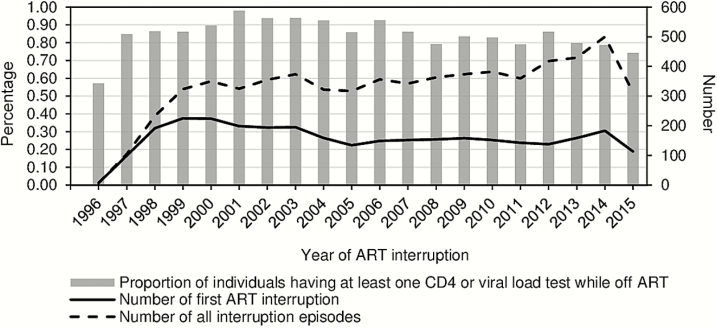
The proportion of individuals interrupting ART within the first year decreased from 31.7% to 18.7%, 13.5%, and 11.0%, respectively, among those initiating ART in 1996–2003, 2004–2007, 2008–2011, and 2012–2014. The decrease was statistically significant when comparing against the previous period, except for 2012–2014 (P = .077). However, absolute numbers of individuals interrupting ART the first time every year remained high (around 150), with a gradual increase from 138 in 2012 to 183 in 2014; similarly, total numbers of interruptions increased from 360 in 2011 to 500 in 2014 (Figure 1). The numbers observed in 1996 and 2015 represented partial years.
Figure 1.
Longitudinal trends of antiretroviral therapy (ART) interruption and individuals’ retention to human immunodeficiency virus (HIV) care (n = 3129). The study sample initiated ART between August 1996 and September of 2015 and was followed up till the end of 2015. Gray bars indicate proportion of individuals participating in HIV monitoring at least once after interrupting ART; solid line indicates total number of individuals interrupting ART the first time; dashed line indicates the total number of interruption episodes.
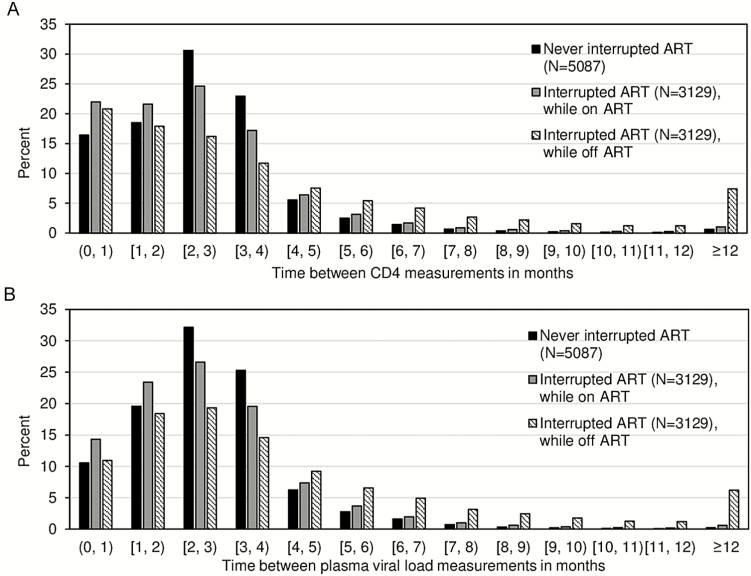
We observed a large proportion of individuals having at least 1 CD4 or pVL test during the first interruption (Figure 1), with the proportion being higher in the early 2000s. The time intervals between CD4/pVL tests were comparable among individuals interrupting and not interrupting ART during on-ART periods (Figure 2A and 2B), with 95.0% and 96.6% of observed pVL measurement pairs being less than 6 months apart, respectively. The intervals were longer for pVL measurements during off-ART periods, yet the majority (79.0%) were within 6 months apart. Similar patterns were observed over time (Supplementary Figure 1).
Figure 2.
Distributions of time between CD4 measurements (A) and time between plasma viral load measurements (B) before and after all interruption episodes (1996–2015). Time between CD4 and plasma viral load tests was measured among those having at least 2 tests. Abbreviation: ART, antiretroviral therapy.
Individuals (2212/3129 [70.7%]) with at least 2 observed disease status measurements during the first ART interruption were included in analyses of disease progression. The median time from ART interruption to the last CD4 measurement or death was 8.3 (IQR, 3.9–21.8) months. Model estimated average probability of all-cause mortality after 1 year of ART interruption was 2%, 4%, or a remarkable 19% for those with a CD4 count of ≥500, 200–499, and <200 cells/μL, respectively, at ART dropout (Table 2).
Table 2.
Frequency of the Observed Disease State Transitions and the Estimated Average Annual Transition Probabilities During the First Antiretroviral Therapy Interruption Episode (N = 2212)a
| CD4 Strata at time t, cells/μL | CD4 Strata at Time t+1, Cells/μL | |||
|---|---|---|---|---|
| ≥500 | 200–499 | <200 | Deathb | |
| Observed CD4 Transitions | ||||
| ≥500 | 2073 | 446 | 19 | 13 |
| 200–499 | 0 | 5097 | 484 | 42 |
| <200 | 0 | 0 | 2133 | 108 |
| Model-Estimated Annual Transition Probabilities | ||||
| ≥500 | 0.49 | 0.41 | 0.08 | 0.02 |
| 200–499 | 0 | 0.70 | 0.26 | 0.04 |
| <200 | 0 | 0 | 0.81 | 0.19 |
aTransition probabilities were estimated using a 4-state continuous-time Markov model among individuals who had at least 2 observed disease status measurements (either CD4 measurements or death) during the first episode of antiretroviral therapy (ART) interruption.
bOff-ART deaths, classified as such if they occurred during off-ART period, or within 90 days of reinitiating ART.
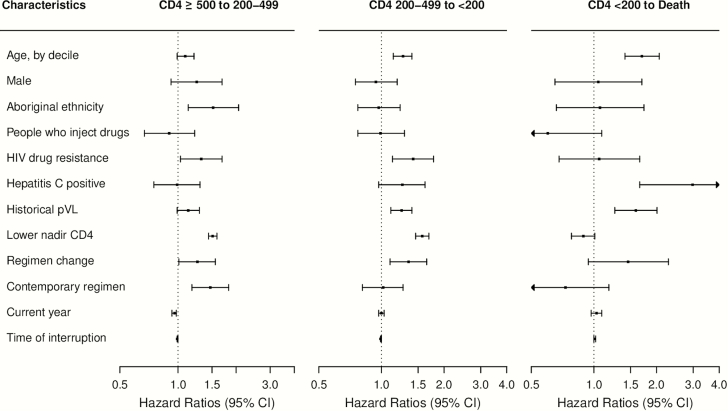
Independent determinants of CD4 decline or death during first-ART interruption are shown in Figure 3 and Supplementary Table 1. In the multivariable analysis, we found that increased age, elevated historical pVL, and ART regimen changes prior to interruption appeared to be associated with increased hazard of CD4 decline and death. For example, the adjusted hazard ratios (aHRs) for transitions from CD4 count (cells/μL) 200–499 to <200 were 1.27 (95% confidence interval [CI], 1.14–1.40) per 10-year increase in age; 1.25 (95% CI, 1.11–1.40) per log10-unit increase in historical pVL and 1.35 (95% CI, 1.10–1.65) with prior ART regimen changes. Similarly, every 10-year increase in age, and every log10-unit increase in historical pVL were associated with 70% (95% CI, 41%–106%) and 59% (95% CI, 26%–101%) increase in the hazard of progression from CD4 count <200 cells/μL to death, respectively.
Figure 3.
Adjusted hazard ratios associated with disease state transition intensities during the first antiretroviral therapy (ART) interruption episode among 2212 individuals. Four-state Markov models estimated the baseline transition intensities for each of the 5 possible transitions (CD4 count [cells/μL] ≥500 to 200–499; 200–499 to <200; ≥500 to death; 200–499 to death; and <200 to death); covariate effects of the 2 transitions (≥500 to death; and 200–499 to death) was not estimated due to the small observed transition number; results of the missing categories of the covariates were not shown. HIV drug resistance was considered as nonreversible, and determined as detecting mutations based on a modification of the 2014 International AIDS Society–USA mutation list. Historical plasma viral load (pVL) was measured as area under the pVL curve prior to ART interruption. Modern (contemporary) regimens included those based on lamivudine + tenofovir or emtricitabine + tenfovir combined with efavirenz, rilpivirine, or etravirine or a boosted protease inhibitor or integrase inhibitor regimen; small numbers of individuals could have received tipranavir, maraviroc, or enfuvirtide. Time of interruption was measured by time from ART initiation to interruption in months. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; lower nadir CD4, lowest CD4 cell count prior to antiretroviral therapy interruption, per 100 cells/μL decline; pVL, plasma viral load.
In addition, drug resistance and lower nadir CD4 counts (per 100 cells/μL decline) were associated with increased hazard of CD4 decline from ≥500 to 200–499 (aHRs, 1.32 [95% CI, 1.03–1.69] and 1.51 [95% CI, 1.44–1.59]), and from 200–499 to <200 cells/μL (aHRs, 1.42 [95% CI, 1.13–1.78] and 1.57 [95% CI, 1.46–1.69]), respectively. In contrast, being HCV+ was strongly associated with increased hazard of death (2.98 [95% CI, 1.66–5.32]).
Results of the sensitivity analyses among first and subsequent ART interruptions as well as among a subset of individuals following exclusion of those with missing covariates were consistent with main findings (Supplementary Table 2).
DISCUSSION
This study examined disease progression and associated factors while off ART in a population-based setting. Our results demonstrate that ART interruptions are common even among individuals on ART in a high-resource setting with universal free access to HIV care, and that these interruptions are associated with remarkable disease progression.
We identified that older age, higher historical pVL, and prior ART regimen changes were associated with increased hazard of CD4 decline and death. Lower nadir CD4 was associated with CD4 decline, but not with death. Potential explanations might include that individuals with lower nadir CD4 were more likely to reinitiate ART [33]. These findings were supported by another study among PLHIV in 82 centers across Europe, which reported positive associations between age, pVL, CD4 ≤200 cells/μL, and progression to AIDS or death among individuals interrupting ART. The authors did not distinguish outcomes occurring during ART interruption or after ART resumption, and current pVL/CD4 count was examined in the European study compared to historical pVL/CD4 count in our analysis [17]. Moreover, both studies did not observe an independent association between injection drug use and disease progression, suggesting that poorer ART treatment outcomes among PWID on ART observed by previous studies may be mediated by factors such as adherence [34]. However, about 12% of our analyzed sample had an unknown PWID status, and unknown PWID status was associated with increased hazard of progression to death. If PWID were more likely to be classified as missing, our estimates on PWID and death might be biased downward toward null.
The interpretation of factors associated with partial transitions may be limited and require evaluation in future studies. We found that receiving a modern regimen was associated with faster CD4 declines from ≥500 to 200–499 cells/μL after interruption. This might be partly explained by residual confounding associated with individuals’ clinical disease status, as we observed that individuals interrupting a modern regimen had lower CD4 at ART initiation, indicating they might have more advanced disease compared to those interrupting a nonmodern regimen. We also observed a strong association between HCV+ and progression from CD4 <200 cells/μL to death; although HCV+ was highly positively correlated with PWID, the observed association remained large whether adjusting or not adjusting for PWID (aHR, 2.98 [95% CI, 1.66–5.32] vs 1.92 [95% CI, 1.15–3.19]). As our study considered all-cause mortality instead of AIDS-related death, the association may be partly attributable to HCV-related death. Nevertheless, a previous study found a lack of association between time-dependent HCV status and AIDS or death among individuals interrupting ART [17].
Our findings suggest a need to improve and maintain ART retention among an increasing number of PLHIV. Prior studies reported pervasive ART interruptions in both high- and low-resource settings [16, 18, 19, 35]. More than 38% of our study sample had interrupted ART by end of 2015. However, it was encouraging that the proportion of individuals interrupting ART within the first year of initiation decreased over time. The same trend was observed among PLHIV participating in a Canadian multisite cohort in BC, Quebec, and Ontario [19]. Moreover, 1 study among the same BC population reported a decline in durations of interruption, where interruption was defined as a gap of ≥30 days [16]. Despite improvements in individuals’ retention to ART, we found the absolute number ART interruptions remained high, with an increase since 2012, corresponding to changes in ART initiation guidelines put forward in 2012, offering ART to all PLHIV regardless of CD4 count [24].
In the context of relatively sparse evidence on effective and cost-effective interventions to improve ART persistence [9, 15, 18, 36, 37], evidence to inform development, optimization, and prioritization of interventions should be carefully examined. The prognostic factors identified in our study should be considered in parallel with risk factors for ART interruption/nonadherence to inform ART retention and reengagement interventions. For instance, strategies to prevent ART interruptions might focus on individuals with younger age and PWID, who had poorer ART adherence [16–19, 35]; however, interventions to reengage individuals who already interrupted ART might prioritize those with increased risk of disease progression, including older individuals, and those with prior experience in switching ART regimens, higher historical pVL level, lower nadir CD4 count, and drug resistance.
The current study also reveals that individuals interrupting ART continue participation in CD4/pVL tests, suggesting that there may be barriers specific to ART retention, but not to HIV monitoring care. These barriers may include avoiding medication side effects and being suspicious of treatment effectiveness, which were identified in a systematic review [38] as well as among a selected cohort of PLHIV in BC [35]. Future studies on different barriers to ART retention and routine clinical care may provide more insights on improving ART continuation.
Finally, our model-based disease transition probability estimates while off ART provide important parameter inputs for mathematical models used to estimate HIV transmission dynamics and make resource allocation decisions. These estimates were lacking in the literature, and disease progression among ART-discontinuing PLHIV was often assumed to be identical to ART-naive untreated PLHIV [39, 40], which ignored potential effect of prior regimens.
Our study has several limitations. First, structured treatment interruptions existed before 2006 [7, 24] but could not be distinguished from patient-initiated interruptions, which should be considered when interpreting the trends of ART interruption and HIV care receipt patterns over time. Second, the determination of ART interruption relied on medication dispensation data along with a 90-day threshold and were thus measured with some unavoidable degree of misclassification. Regardless, our definition aimed to balance the trade-off between sensitivity and specificity, and would capture interruptions and/or very low adherence, which warrant attention. Third, our analysis on disease progression did not include individuals who were lost to follow-up or did not participate in CD4 tests while off ART, who might have different disease progression. We explicitly compared their characteristics and our study sample in Supplementary Table 3. Fourth, covariate effects could not be estimated for all transitions due to small numbers of certain observed transitions. Fifth, although we summarized associations between a number of covariates and disease progression, there is always potential for unmeasured confounding in observational studies. However, the residual confounding might be less critical in the case when identification of subgroups of high risk rather than causal inference was of particular interest. Finally, the study was conducted in a setting with universal free HIV care, so certain findings may not be applicable elsewhere.
To conclude, our findings provide population-level estimates of ART interruption patterns and prognostic factors of disease progression while off ART. These factors should be communicated with health providers and PLHIV, and be considered together with risk factors for interruption to inform ART retention strategies. Despite observed improvement over time, further efforts are needed to promote ART reengagement and may consider prioritizing individuals with older age, higher levels of historical pVL, and prior ART regimen change experience.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We acknowledge all BC Ministry of Health (BCMoH) and Vancouver Coastal Health (VCH) decision support staff involved in data access and procurement, including Miranda Compton (VCH), Theodora Consolacion (BC Centre for Disease Control [BC-CDC]), Monika Lindegger (BC-CDC), Al Cassidy (BCMoH), and Joleen Wright and Karen Luers (VCH). We also acknowledge Ciro Panessa, Nancy South, and Mark Gilbert for their contributions to the STOP HIV/AIDS study group. Finally, we acknowledge Kathy Lepik (BC Centre for Excellence in HIV/AIDS) for providing her clinical insights on the HIV antiretroviral drug dispensing practice in BC.
Financial support. This work was supported by the BC Ministry of Health–funded “Seek and Treat for Optimal Prevention of HIV & AIDS” pilot project; a grant from the National Institutes of Health (NIH)/National Institute on Drug Abuse (grant number R01-DA-041747); and by Genome Canada (142HIV), the Canadian Institutes of Health Research, Genome British Columbia, and Genome Quebec funding to P. R. H. and J. S. G. M. In addition, B. N. is supported by a Michael Smith Foundation for Health Research Scholar award.
Potential conflicts of interest. J. S. G. M. is supported with grants paid to his institution by the British Columbia Ministry of Health and by the NIH (R01-DA-036307). He has also received limited unrestricted funding, paid to his institution, from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck, and ViiV Healthcare. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Hogg R, Lima V, Sterne JAC et al. . Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372: 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anglemyer A, Rutherford GW, Easterbrook PJ et al. . Early initiation of antiretroviral therapy in HIV-infected adults and adolescents: a systematic review. AIDS 2014; 28(suppl 2):S105–18. [DOI] [PubMed] [Google Scholar]
- 3. Paterson DL, Swindells S, Mohr J et al. . Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 4. Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003; 37:1112–8. [DOI] [PubMed] [Google Scholar]
- 5. Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 x 10(9) cells/L. Ann Intern Med 2003; 139:810–6. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira e Silva AC, Reis RK, Nogueira JA, Gir E. Quality of life, clinical characteristics and treatment adherence of people living with HIV/AIDS. Rev Lat Am Enfermagem 2014; 22:994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Sadr WM, Lundgren JD, Neaton JD et al. . CD4+count-guided interruption of antiretroviral treatment. New Engl J Med 2006; 355: 2283–96. [DOI] [PubMed] [Google Scholar]
- 8. Cohen MS, Chen YQ, McCauley M et al. ; HPTN 052 Study Team Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, Mills EJ. Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clin Infect Dis 2014; 59(suppl 1):S21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mills EJ, Nachega JB, Buchan I et al. . Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA 2006; 296:679–90. [DOI] [PubMed] [Google Scholar]
- 11. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the HIV epidemic. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 12. Nosyk B, Montaner JSG, Colley G et al. . The cascade of HIV care in British Columbia, Canada, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis 2014; 14: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. BC Centre for Excellence in HIV/AIDS. HIV monitoring quarterly report for British Columbia, second quarter 2016. Vancouver: BC Centre for Excellence in HIV/AIDS, 2016. [Google Scholar]
- 14. Joint United Nations Programme on HIV/AIDS. How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response: fact sheet. Geneva, Switzerland: UNAIDS, 2015. [Google Scholar]
- 15. Kanters S, Park JJ, Chan K et al. . Interventions to improve adherence to antiretroviral therapy: a systematic review and network meta-analysis. Lancet HIV 2017; 4:e31–40. [DOI] [PubMed] [Google Scholar]
- 16. Nosyk B, Lourenco L, Min JE, Shopin D, Lima VD, Montaner JS. Characterizing retention in HAART as a recurrent event process: insights into ‘cascade churn.’ AIDS 2015; 29: 1681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holkmann Olsen C, Mocroft A, Kirk O et al. ; EuroSIDA Study Group Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med 2007; 8:96–104. [DOI] [PubMed] [Google Scholar]
- 18. Kranzer K, Ford N. Unstructured treatment interruption of antiretroviral therapy in clinical practice: a systematic review. Trop Med Int Health 2011; 16:1297–313. [DOI] [PubMed] [Google Scholar]
- 19. Samji H, Taha TE, Moore D et al. ; Canadian Observational Cohort (CANOC) Collaboration Predictors of unstructured antiretroviral treatment interruption and resumption among HIV-positive individuals in Canada. HIV Med 2015; 16:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med 2004; 19:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nachega JB, Parienti JJ, Uthman OA et al. . Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58:1297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Medland NA, McMahon JH, Chow EP, Elliott JH, Hoy JF, Fairley CK. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc 2015; 18:20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. BC Centre for Excellence in HIV/AIDS. Therapeutic guidelines for antiretroviral (ARV) treatment of adult HIV infection. Vancouver: BC Centre for Excellence in HIV/AIDS, 2015. [Google Scholar]
- 24. Günthard HF, Aberg JA, Eron JJ et al. ; International Antiviral Society–USA Panel Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society–USA panel. JAMA 2014; 312:410–25. [DOI] [PubMed] [Google Scholar]
- 25. BC Centre for Excellence in HIV/AIDS. How to obtain HIV medication through the drug treatment program: HIV medication dispensing-dispensed quantity of HIV medications. Available at: http://www.cfenet.ubc.ca/drug-treatment-program/how-obtain-hiv-medication#dispensing. Accessed 9 May 2017. [Google Scholar]
- 26. BC Centre for Disease Control, BC Centre for Excellence in HIV/AIDS. HIV monitoring quarterly report: technical report. 2015. [Google Scholar]
- 27. Alexander CS, Montessori V, Wynhoven B et al. . Prevalence and response to antiretroviral therapy of non-B subtypes of HIV in antiretroviral-naive individuals in British Columbia. Antivir Ther 2002; 7:31–5. [PubMed] [Google Scholar]
- 28. Sypsa V, Touloumi G, Kenward M, Karafoulidou A, Hatzakis A. Comparison of smoothing techniques for CD4 data in a Markov model with states defined by CD4: an example on the estimation of the HIV incubation time distribution. Stat Med 2001; 20:3667–76. [DOI] [PubMed] [Google Scholar]
- 29. Wensing AM, Calvez V, Günthard HF et al. . 2014 update of the drug resistance mutations in HIV-1. Top Antivir Med 2014; 22:642–50. [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson C. Multi-state modelling with R: the msm package. Cambridge, UK. 2016. Available at: http://cran.stat.nus.edu.sg/web/packages/msm/vignettes/msm-manual.pdf. [Google Scholar]
- 31. Nosyk B, Min J, Lima VD et al. . HIV-1 disease progression during highly active antiretroviral therapy: an application using population-level data in British Columbia: 1996–2011. J Acquir Immune Defic Syndr 2013; 63: 653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathieu E, Loup P, Dellamonica P, Daures JP. Markov modelling of immunological and virological states in HIV-1 infected patients. Biom J 2005; 47:834–46. [DOI] [PubMed] [Google Scholar]
- 33. Toulson AR, Harrigan R, Heath K et al. . Treatment interruption of highly active antiretroviral therapy in patients with nadir CD4 cell counts >200 cells/mm3. J Infect Dis 2005; 192:1787–93. [DOI] [PubMed] [Google Scholar]
- 34. Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis 2005; 41:1027–34. [DOI] [PubMed] [Google Scholar]
- 35. Samji H, Chen Y, Salters K, Montaner JS, Hogg RS. Correlates of unstructured antiretroviral treatment interruption in a cohort of HIV-positive individuals in British Columbia. AIDS Behav 2014; 18:2240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walensky RP, Borre ED, Bekker LG et al. . The anticipated clinical and economic effects of 90-90-90 in South Africa. Ann Intern Med 2016; 165: 325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nosyk B, Min JE, Krebs E et al. ; on behalf of the STOP HIV/AIDS Study Group. The cost-effectiveness of HIV testing and treatment engagement initiatives in British Columbia, Canada: 2011–2013, Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mills EJ, Nachega JB, Bangsberg DR et al. . Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med 2006; 3:e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nosyk B, Min JE, Lima VD, Hogg RS, Montaner JS; STOP HIV/AIDS study Group Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study. Lancet HIV 2015; 2:e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mauskopf J, Kitahata M, Kauf T, Richter A, Tolson J. HIV antiretroviral treatment: early versus later. J Acquir Immune Defic Syndr 2005; 39:562–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.