Summary
We find no evidence to support a difference in the risk of death among pregnant women with suspected or confirmed Ebola virus disease (EVD) as compared to nonpregnant women. Limited data suggest poor fetal and neonatal outcomes in EVD-affected pregnancies.
Keywords: Ebola virus disease, pregnant, Liberia, Sierra Leone, maternal mortality.
Abstract
Background.
Reliable data are lacking on pregnancy outcomes during Ebola virus disease (EVD) epidemics. We aimed to characterize symptoms and outcomes among pregnant women admitted to Ebola treatment units (ETUs) with suspected and confirmed EVD to better inform obstetric management.
Methods.
We analyzed a retrospective cohort of reproductive-aged women presenting to 5 West African ETUs from September 2014 to September 2015. We compared clinical symptoms, risk of EVD diagnosis, and mortality between pregnant and nonpregnant women.
Results.
Of 729 reproductive-aged women admitted to study ETUs, 44 (6%) reported pregnancy. Thirteen of 44 pregnant women (30%) tested EVD positive; 6 of 13 (46%) died. Pregnant women were less likely than nonpregnant women to report anorexia, asthenia, diarrhea, fever, myalgias/arthralgias, nausea, or vomiting (P < .05) at admission. Pregnant women with suspected EVD had the same risk, however, of laboratory-confirmed EVD (30% vs 24%, P = .38). While pregnant women with confirmed EVD had similar Ebola viral loads on presentation to nonpregnant women, as measured by initial cycle threshold (26.4 vs 23.2, P = .16), they were less likely to have myalgias/arthralgias (P< .001) and vomiting (P = .02). Both all-cause mortality (14% vs 19%, P = .39) and EVD-specific mortality (46% vs 54%, P = .60) were not significantly different between pregnant and nonpregnant women. Two neonates born live in the ETU died within 8 days.
Conclusions.
We find no evidence to support a difference in the risk of death between pregnant women with suspected or confirmed EVD compared to nonpregnant women. Limited data suggest poor fetal and neonatal outcomes in EVD-affected pregnancies.
From 2014–2016, West Africa faced the largest recorded Ebola virus disease (EVD) epidemic in history, with 28616 suspected and confirmed EVD cases and at least 11310 fatalities [1]. Historically, pregnant women with EVD were thought to be at increased risk of severe illness and death due to altered immune function and placental infection [2, 3]. To date, a total of 111 cases of pregnant patients with concern for EVD have been reported [2–12], with an aggregate maternal mortality rate of 86%. The majority of these cases come from the first recognized Ebola outbreak in Zaire in 1976, where data retrospectively collected primarily from family member interviews suggested 82 pregnant women had probable EVD, among whom 73 (89%) died. This was similar to the overall outbreak mortality of 88%, though most patients included in this analysis never had definitive testing for EVD. In addition, almost all cases in the 1976 Zaire outbreak had received injections in the setting of poor infection control at a single hospital or one of its prenatal or outpatient clinics or had close contact with a case that did; there were no survivors among all of those with parenteral injection as their mode of contact [2, 3].
In contrast, the limited outcomes data published to date from the 2014–2016 West Africa outbreak suggest that EVD mortality in pregnancy may be lower than previously reported, with only 5 EVD-infected pregnant women dying of 12 reported cases [4–12]. To date, fetal outcomes have been reported for 59 confirmed or suspected EVD cases in pregnancy, which resulted in 47 (80%) stillbirths or miscarriages and 12 (20%) live births, all of whom died within 19 days of life [2–12]. Suspected fatal EVD cases in prior outbreaks were less often laboratory confirmed, potentially biasing mortality estimates.
Obstetric care providers face considerable challenges and ethical dilemmas managing pregnancy during EVD epidemics, exacerbated by overwhelmed health systems [13] and a scarcity of data on EVD in pregnancy. During an outbreak, pregnant women with vaginal bleeding or other symptoms may be classified as suspected for EVD based on clinical case definitions, even though other etiologies such as placenta previa, placental abruption, or even normal labor may be more likely etiologies of their symptoms. As a result, many EVD-negative pregnant women are evaluated and treated in Ebola treatment units (ETUs), placing them at risk for nosocomial EVD [14]. Once admitted to the ETU, obstetric care guidelines often recommend that healthcare workers do not intervene during delivery to avoid exposure to Ebola-infected body fluids, based on limited historical data suggesting poor maternal and fetal survival [15]. More data are needed on presenting signs and symptoms of EVD in pregnancy, and how EVD outcomes compare to nonpregnant patients, to better inform management principles. Though devastating, the 2014–2016 Ebola outbreak did provide a rare opportunity to learn about EVD and improve the evidence base for guidelines, particularly in subpopulations such as pregnant women. Here, we aim to better characterize symptoms and outcomes among pregnant women and nonpregnant reproductive-aged women admitted to ETUs with suspected EVD to better inform outbreak triage and management strategies.
METHODS
Study Design and Ethical Review
This retrospective cohort includes patient data collected at 5 ETUs operated by International Medical Corps in Liberia and Sierra Leone between 15 September 2014 and 15 September 2015 as part of its comprehensive response to the EVD epidemic [16]. Ethical approval for this study and informed consent exemption was provided by Sierra Leone Ethics and Scientific Review Committee and the institutional review boards of the University of Liberia–Pacific Institute for Research and Evaluation, Lifespan (Rhode Island Hospital), and Partners Healthcare.
Participants and Setting
All women of reproductive age admitted to ETUs run by International Medical Corps between 15 September 2014 and 15 September 2015 were included for analysis. On arrival to the ETU, all patients were triaged by trained clinical staff, who evaluated each patient to be admitted for either a positive EVD test prior to arrival or classification as a suspected case based on national and World Health Organization EVD clinical case definition (Supplementary Appendix). Patients not meeting the case definition were referred to other healthcare facilities. Pregnancy was defined by patient self-report or documentation of positive urine pregnancy test in the patient’s chart. All female patients were asked routinely about pregnancy status at admission to ETUs in Sierra Leone, but this information was collected in Liberia based on provider discretion. Gestational age (GA) was calculated using patient report of last normal menstrual period.
Laboratory Testing
All patients admitted to the ETU were tested within 24 hours for Ebola virus using quantitative Ebola Zaire real-time reverse-transcription polymerase chain reaction (RT-PCR) of blood samples. Patients initially testing negative remained at the ETU and were retested 48 hours later, due to poor sensitivity of RT-PCR early in the disease course. A cycle threshold (Ct) value >40 was considered negative for Ebola virus according to laboratory protocols described in detail elsewhere [16].
Clinical Management
Protocol-based treatment was standardized at all 5 ETUs and included empiric antimalarial therapy, broad-spectrum antibiotics, oral rehydration solution (ORS), gastrointestinal prophylaxis, and nutritional supplementation. Treatment for fever, pain, nausea, and delirium was given as needed. Patients with moderate to severe dehydration or inability to hydrate with ORS received bolus or maintenance intravenous crystalloid fluids (Supplementary Appendix).
Data Collection
Patient demographics including age, sex, and country were collected on admission and whether the patient had contact with anyone ill. Temperature was recorded as febrile (>38.0°C) or afebrile (≤38.0°C). Clinical variables of interest included day of symptom onset, and symptoms on presentation included abdominal pain, anorexia, asthenia, bleeding, diarrhea, dyspnea, fever, headache, hiccups, jaundice, myalgias/arthralgias, nausea, throat pain, and vomiting. Clinical data were recorded at triage and at least daily from admission to discharge. Outcome variables collected included results of confirmatory EVD testing, length of stay, and vital status on discharge.
All data were collected on standardized paper forms by trained clinical staff for routine care and epidemiologic surveillance. Data from each ETU were entered into a separate electronic database by local data officers and later aggregated for all ETUs. Full details on methods for data collection and aggregation have been published elsewhere [16].
Data Analysis
Analysis was limited to women of reproductive age (15–49 years), with vital status data on ETU discharge being our primary outcome of interest. Vital status was defined as death, discharged alive after repeat-negative EVD testing, or transferred for further management. Results of confirmatory EVD testing and ETU length of stay were secondary outcomes of interest. Descriptive statistics were used to characterize the cohort. Comparisons between baseline characteristics and outcomes were made between pregnant and nonpregnant reproductive-aged women, with a secondary analysis restricted to patients with confirmed EVD. The χ2 or Fisher exact test was used to compare categorical variables; t test was used for continuous normal variables; and Wilcoxon rank-sum test was used for nonparametric variables. Logistic regression analysis was performed to determine the independent association of pregnancy status and each outcome, controlling for potential confounders including age and country of origin. For all analyses, P < .05 was considered statistically significant. Analyses were conducted in R version 3.2.1 and Stata 12 software (StataCorp, College Station, Texas).
RESULTS
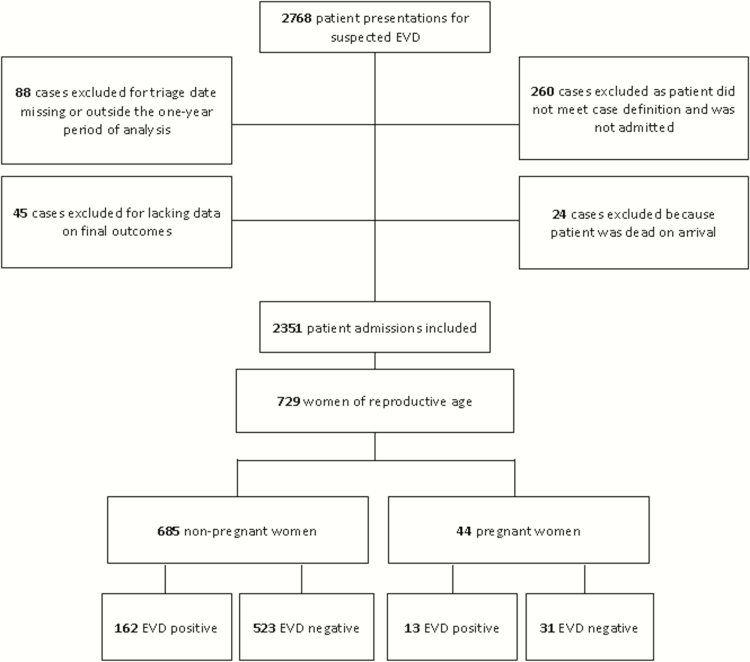
Among 2351 admitted patients with outcome data, 729 reproductive-aged women were included in this analysis (Figure 1). Forty-four women were pregnant, with a median gestational age (GA) of 26 weeks (interquartile range [IQR], 13–30 weeks). Thirteen of 44 (30%) pregnant women tested EVD positive.
Figure 1.
Study flow diagram. Abbreviation: EVD, Ebola virus disease.
Baseline Characteristics and Outcomes
Of 729 reproductive-aged women, median age was 30 years (IQR, 23–38 years). Approximately 67% were from Sierra Leone, with the remainder from Liberia. Overall, 175 of 729 (24%) had laboratory-confirmed EVD, and 135 of 729 (19%) died prior to ETU discharge. Mortality was significantly higher in women with laboratory-confirmed EVD (93/175 [53%]) compared with EVD-negative women (42/553 [8%]) (P < .001).
Baseline characteristics on arrival to the ETU and final outcomes by pregnancy status among all women of reproductive age are displayed in (Table 1). Pregnant women were younger than nonpregnant women and less likely to have fever, myalgias/arthralgias, nausea, vomiting, diarrhea, anorexia, and asthenia on arrival to the ETU. There were no significant differences in likelihood of EVD diagnosis (30% vs 24%, P = .38), ETU length of stay (3 days vs 3 days, P = .86), or all-cause mortality (14% vs 19%, P = .39) between pregnant and nonpregnant women (Table 1). After controlling for age and country of origin, there were no significant differences in odds of EVD diagnosis (odds ratio [OR], 1.6; 95% confidence interval [CI], .8–3.1) or all-cause mortality (OR, 0.8; 95% CI, .3–1.9) by pregnancy status. In an analysis restricted to EVD-negative women, similar findings were observed (Table 2).
Table 1.
Comparison of Baseline Data and Final Outcomes for All Nonpregnant and Pregnant Women Admitted to Study Ebola Treatment Units Between 15 September 2014 and 15 September 2015
| Characteristic | Not Pregnant | Pregnant | P Valuea |
|---|---|---|---|
| All patients | 94 (685) | 6 (44) | … |
| Demographic characteristics | |||
| Age, y, median (IQR) | 30 (23–40) | 25 (20–31) | .00 |
| Country | … | … | .01 |
| Liberia | 37 (252) | 18 (8) | … |
| Sierra Leone | 63 (433) | 82 (36) | … |
| Clinical symptoms at triage | |||
| Days of symptoms, median (IQR) | 3 (2–6) | 2 (1–5) | .28 |
| Abdominal pain | 55 (375) | 64 (28) | .25 |
| Anorexia | 67 (462) | 45 (20) | .00 |
| Asthenia | 74 (508) | 59 (26) | .03 |
| Bleeding | 11 (73) | 18 (8) | .12 |
| Diarrhea | 40 (256) | 15 (6) | .00 |
| Dyspnea | 35 (238) | 23 (10) | .10 |
| Fever | 78 (535) | 57 (25) | .00 |
| Headache | 64 (435) | 50 (22) | .07 |
| Hiccups | 11 (75) | 9 (4) | .70 |
| Jaundice | 5 (37) | 2 (1) | .37 |
| Myalgias or arthralgias | 62 (424) | 34 (15) | .00 |
| Nausea | 43 (185) | 22 (8) | .02 |
| Throat pain | 27 (183) | 14 (6) | .06 |
| Vomiting | 46 (313) | 23 (10) | .00 |
| Epidemiological characteristics | |||
| Contact with someone ill | 32 (196) | 37 (16) | .44 |
| Laboratory | |||
| EVD positive | 24 (162) | 30 (13) | .38 |
| Outcome | |||
| Length of stay, d, median (IQR) | 3 (2–4) | 3 (2–3) | .86 |
| Mortality | 19 (129) | 14 (6) | .39 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: EVD, Ebola virus disease; IQR, interquartile range.
aBased on χ2 test or t test as appropriate.
Table 2.
Comparison of Baseline Data and Final Outcomes for Ebola Virus Disease (EVD)–Negative Nonpregnant Women and EVD-Negative Pregnant Women Admitted to Study Ebola Treatment Units Between 15 September 2014 and 15 September 2015
| Characteristic | EVD Negative, Not Pregnant | EVD Negative, Pregnant | P Valuea |
|---|---|---|---|
| All patients | 94 (523) | 6 (31) | … |
| Demographic characteristics | |||
| Age, y, median (IQR) | 30 (23–38) | 25 (20–28) | .01 |
| Country | … | … | .02 |
| Liberia | 36 (188) | 16 (5) | … |
| Sierra Leone | 64 (335) | 84 (26) | … |
| Clinical symptoms at triage | |||
| Days of symptoms, median (IQR) | 3 (1–6) | 3 (2–6) | .22 |
| Abdominal pain | 56 (293) | 68 (21) | .20 |
| Anorexia | 68 (353) | 42 (13) | .00 |
| Asthenia | 74 (386) | 52 (16) | .01 |
| Bleeding | 11 (60) | 19 (6) | .19 |
| Diarrhea | 34 (167) | 10 (3) | .01 |
| Dyspnea | 37 (193) | 23 (7) | .11 |
| Fever | 80 (416) | 55 (17) | .00 |
| Headache | 63 (331) | 48 (15) | .10 |
| Hiccups | 10 (54) | 6 (2) | .49 |
| Jaundice | 7 (34) | 0 (0) | .14 |
| Myalgias or arthralgias | 60 (314) | 39 (12) | .02 |
| Nausea | 43 (143) | 23 (6) | .05 |
| Throat pain | 27 (141) | 16 (5) | .18 |
| Vomiting | 44 (228) | 26 (8) | .05 |
| Epidemiological characteristics | |||
| Contact with someone ill | 18 (84) | 26 (8) | .25 |
| Outcome | |||
| Length of stay, d, median (IQR) | 3 (3–3) | 3 (2–3) | .98 |
| Mortality | 8 (42) | 0 (0) | .10 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: EVD, Ebola virus disease; IQR, interquartile range.
aBased on Fisher exact and Wilcoxon rank-sum tests.
Thirteen of 175 (7%) reproductive-aged women with confirmed EVD were pregnant. Baseline characteristics and outcomes among EVD-positive women of reproductive age are presented in (Table 3) by pregnancy status. Pregnant women with EVD were younger than nonpregnant women with EVD, and were significantly less likely to present with myalgias/arthralgias and vomiting on arrival to the ETU. Pregnant women with EVD had similar initial Ct values to nonpregnant women (26 vs 23, P = .17), similar length of stay (10 vs 7 days, P = .69) and overall mortality (46% vs 54%, P = .60) (Table 3). After controlling for age and country of origin in a multivariable logistic regression model predicting mortality among EVD-positive women, pregnancy was not associated with increased odds of death (adjusted OR, 0.9; 95% CI, .3–3.0).
Table 3.
Comparison of Baseline Data and Final Outcomes for Ebola Virus Disease (EVD)–Positive Nonpregnant and EVD-Positive Pregnant Women Admitted to Study Ebola Treatment Units Between 15 September 2014 and 15 September 2015
| Characteristic | EVD Positive, Not Pregnant | EVD Positive, Pregnant | P Valuea |
|---|---|---|---|
| All patients | 93 (162) | 7 (13) | … |
| Demographic characteristics | |||
| Age, y, median (IQR) | 33 (24–42) | 24 (20–32) | .02 |
| Country | … | … | .24 |
| Liberia | 40 (64) | 23 (3) | … |
| Sierra Leone | 60 (98) | 77 (10) | … |
| Clinical symptoms at triage | |||
| Days of symptoms, median (IQR) | 3 (2–6) | 3 (2–3) | .36 |
| Abdominal pain | 51 (82) | 54 (7) | .82 |
| Anorexia | 67 (109) | 54 (7) | .37 |
| Asthenia | 75 (122) | 77 (10) | 1.00 |
| Bleeding | 8 (13) | 15 (2) | .31 |
| Diarrhea | 57 (89) | 27 (3) | .06 |
| Dyspnea | 28 (45) | 23 (3) | 1.00 |
| Fever | 73 (119) | 62 (8) | .35 |
| Headache | 64 (104) | 54 (7) | .55 |
| Hiccups | 13 (21) | 15 (2) | .68 |
| Jaundice | 2 (3) | 8 (1) | .27 |
| Myalgias or arthralgias | 68 (110) | 23 (3) | .00 |
| Nausea | 43 (42) | 20 (2) | .20 |
| Throat pain | 26 (42) | 8 (1) | .19 |
| Vomiting | 52 (85) | 15 (2) | .02 |
| Epidemiological characteristics | |||
| Contact with someone ill | 79 (112) | 67 (8) | .33 |
| Laboratory | |||
| Initial Ct value, median (IQR) | 23 (20–27) | 26 (19–35) | .17 |
| Outcome | |||
| Length of stay, d, median (IQR) | 7 (4–14) | 10 (2–17) | .69 |
| Mortality | 54 (87) | 46 (6) | .60 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: Ct, cycle threshold; EVD, Ebola virus disease; IQR, interquartile range.
aBased on Fisher exact and Wilcoxon rank-sum tests as appropriate.
Clinical Course of EVD in Pregnancy
Of pregnant women with laboratory-confirmed EVD, 6 of 13 (46%) died, 6 of 13 (46%) survived to discharge, and 1 of 13 (8%) was transferred to an ETU specializing in pregnancy care and survived to discharge. Pregnant patients with EVD were significantly more likely to have recent contact with someone ill (67% vs 26%, P = .03), longer length of stay (10 days vs 3 days, P = .02), and higher mortality than pregnant patients without EVD (46% vs 0%, P < .01).
Figure 2 displays the proportion of clinical signs and symptoms reported during the entire ETU admission course among pregnant (n = 13) and nonpregnant (n = 154) reproductive-aged EVD-positive patients. The most common symptoms among pregnant EVD positive patients were abdominal pain (85%) and nausea/vomiting (69%). Hiccups (8%) and nonhemorrhagic rash (8%) were the least frequent symptoms.
Figure 2.
Proportion of patients reporting symptoms at any time during the course of their illness among Ebola virus disease (EVD)–positive pregnant and EVD-positive nonpregnant women admitted to study Ebola treatment units (ETUs) between 15 September 2014 and 15 September 2015.
Pregnancy Outcomes
Pregnancy outcomes of women with EVD are presented in (Table 4). Two of 13 patients (15%) had preterm vaginal deliveries of live neonates in the ETU. One neonate born at 25 weeks GA (by last menstrual period and bedside ultrasound dating at 24 weeks) died on ETU day 2 with maternal survival. The second born at 35 weeks GA was EVD-negative on ETU day 1 but tested EVD-positive on ETU day 5 and died on ETU day 9; this case also resulted in maternal death. No disease-specific signs were noted in these neonates. Two other pregnant EVD-positive patients (2/13 [15%]) had spontaneous abortions in the ETU and subsequent maternal death. Three additional patients (3/13 [23%]) died in the ETU while pregnant. One patient (1/13 [8%]) initially had normal fetal activity, followed by ultrasound-documented fetal demise after repeat maternal blood testing became negative for EVD. Induction and delivery of a stillborn fetus was completed prior to ETU discharge. One patient (1/13 [8%]) had a termination of pregnancy after recovery from EVD. Four patients (4/13 [31%]) recovered from EVD and were discharged pregnant; their pregnancy outcomes are unknown. Of note, there were no deaths among EVD-negative pregnant women (Table 2).
Table 4.
Pregnancy-Related Outcomes for Ebola Virus Disease–Positive Pregnant Women Admitted to Study Ebola Treatment Units Between 15 September 2014 and 15 September 2015
| Outcome | Gestational Age, Weeks | Maternal Outcome | Fetal Outcome |
|---|---|---|---|
| Live birth | |||
| Case 1 | 25 | Discharged on ETU day 15 | Death on ETU day 2a |
| Case 2 | 35 | Death on ETU day 3 | Death on ETU day 9a |
| Spontaneous abortion | |||
| Case 1 | 13 | Death on ETU day 10 | SAB on ETU day 3b |
| Case 2 | 14 | Death on ETU day 2 | SAB on ETU day 1b |
| Termination of pregnancy | |||
| Case 1 | 4 | Discharged on ETU day 21c | TOP on ETU day 21b |
| Fetal demise and induced delivery | |||
| Case 1 | 30 | Discharged on ETU day 32 | FDID |
| Maternal death while pregnant | |||
| Case 1 | 28 | Death on ETU day 6 | … |
| Case 2 | 30 | Death on ETU day 1 | … |
| Case 3 | 35 | Death on ETU day 1 | … |
| Unknown | |||
| Case 1 | 13 | Discharged on ETU day 18 | NA |
| Case 2 | NA | Discharged on ETU day 12 | NA |
| Case 3 | NA | Discharged on ETU day 11 | NA |
| Case 4 | NA | Discharged on ETU day 27 | NA |
Abbreviations: ETU, Ebola treatment unit; FDID, fetal demise and induced delivery; NA = not available; SAB, spontaneous abortion; TOP, termination of pregnancy.
aIndicates ETU day of the baby.
bIndicates ETU day of the mother.
cPatient was transferred to outside facility on ETU day 18 and discharged 3 days later.
DISCUSSION
Our study is among the largest reports to date of pregnancy outcomes during the 2014–2016 West Africa EVD outbreak. Mortality among pregnant women with EVD was lower in our study (46%) compared to prior epidemics (86%) [2, 3]. In addition, we found no evidence of higher mortality in EVD-positive pregnant women compared with EVD-positive nonpregnant women of reproductive age, and no evidence of more severe disease, as measured by initial Ct value. Despite prior data suggesting high mortality in pregnant patients with EVD, our data do not support the idea that pregnant women are at higher risk for death compared with nonpregnant patients with EVD [17, 18].
Large outcome studies from the 2014–2016 West African EVD outbreak have reported overall case fatality ratios of 37%–75% [19–23], also lower than in prior epidemics [1, 19]. Despite small sample sizes, reports from the recent epidemic suggest that EVD mortality rates in pregnancy may be lower than previously reported, with 5 deaths of 12 (42%) women with known outcomes, similar to our finding of 46% mortality. Lower mortality overall during the 2014–2016 West Africa EVD outbreak may reflect improvements in EVD care compared with historical outbreaks, improved detection of less severe cases, or changes in the virulence of the disease.
With only 24% of women admitted to ETUs in our study testing positive for EVD, our findings suggest that most women screened for EVD in an ETU may have an alternative diagnosis. EVD was the diagnosis for a minority (30%) of symptomatic pregnant patients admitted, and the low probability of eventual EVD diagnosis should be considered when making decisions about life-saving invasive treatment while awaiting results of EVD testing [24]. Although no EVD-negative pregnant patients died in our cohort, it is important to acknowledge that ETU admission comes with the risk of nosocomial infection with EVD. In addition, pregnant women admitted to ETUs often lack access to surgical and neonatal care that could be life-saving. Thus, the decision of whether to admit a pregnant woman suspected of EVD to an ETU or triage her elsewhere is critical. Preliminary studies suggest that rapid point-of-care testing could help serve as a screening test for EVD in future epidemics, improving management decisions for pregnant patients [25–27]. Supplemental clinical criteria may also be required to more effectively screen for EVD among pregnant women.
Currently, supportive care is the mainstay of Ebola management until effective Ebola-specific vaccines and therapeutics are available [11], and expectant management of labor seems to be an appropriate strategy [15, 17]. Obstetric interventions such as fetal monitoring, assisted delivery, episiotomy, cesarean delivery, induction of labor, or pregnancy interruption were considered in the 2014–2016 West Africa EVD outbreak on a case-by-case basis [28]. Though the decision to intervene must be individualized and based on the physiological state of the mother, GA of the fetus, available resources, trained personnel, and protective equipment, more aggressive interventions should not be withheld based on the assumption that maternal outcomes are generally poor. Fetal outcomes to date are, however, universally poor and should be considered in maternal management plans [2–12].
In addition to careful deliberation about obstetric intervention, appropriate anticipatory guidance should be provided to all pregnant women with EVD. Toward this end, urine pregnancy testing should be considered as part of routine ETU testing in order to appropriately counsel pregnant women with EVD on potential pregnancy complications and management options. Those with EVD in pregnancy near to term should be managed in an ETU through delivery to ensure appropriate birth precautions are taken. If an EVD survivor is discharged while pregnant, referral should be made to specialized centers to develop plans for labor, delivery, and access to emergency obstetric and newborn care, as Ebola virus persistence has been documented in amniotic fluid, cord blood, and placental samples in cases of negative RT-PCR testing for Ebola virus in maternal blood [7, 11]. As this presents the risk for infection to family or the community in the case of a home delivery, contact should be maintained between these women and their local EVD management teams. Women should be educated in anticipation of a potential precipitous delivery outside an ETU, and provided with an appropriate clean delivery kit, barrier protection, and decontamination supplies to protect delivery attendants and household members.
We report on one of the largest cohorts of pregnant patients managed in an ETU setting to date, but the small overall number of cases still limits the power of our study to detect significant differences between pregnant and nonpregnant patients. However, our results were robust when controlling for age and country of origin in multivariable logistic regression analysis, with no increased odds of all-cause or EVD mortality associated with pregnancy. In addition, we report that initial Ct values were similar between pregnant and nonpregnant EVD-positive patients, an important marker of illness severity.
The results we present here are based on data collected for clinical and epidemiological purposes by clinicians in extremely difficult conditions with limited resources [16]. Another limitation is that pregnancy status and gestational age were often based on patient self-report, as urine pregnancy testing or confirmatory ultrasound were not routinely available in all ETUs. In Sierra Leone, female patients were routinely asked about pregnancy status at admission, whereas in Liberia it was provider-dependent. This difference in practice likely contributes to the higher pregnancy rates documented in Sierra Leone, and may have led patients with early pregnancies to be misclassified as nonpregnant, particularly in Liberia. This limitation of pregnancy self-reporting is, however, common to prior reports of EVD in pregnancy. In addition, EVD outcomes are likely worse in late pregnancy due to physiologic changes. Potential misclassification of early pregnancy as nonpregnant EVD cases is unlikely to significantly impact results. Similarly, lack of diagnostic testing to detect important comorbidities, such as HIV status, diabetes, maternal hypertension, and other medical conditions, limits inferences about the relative importance of these contributors to survival in both pregnant and nonpregnant patients [4, 8, 11, 12]. The ability to implement rapid testing for EVD, pregnancy, and other comorbidities would greatly aid in triage and management decisions in this population. We have no follow-up data for patients after ETU discharge. Though all EVD patients must have negative EVD testing and be clinically recovered prior to ETU discharge, post-ETU complications cannot be excluded. Last, the fetal outcomes for the 4 pregnant EVD survivors discharged from the ETUs are unknown.
CONCLUSIONS
We found no direct or indirect evidence to support an increased risk of death from EVD in pregnant women, compared with nonpregnant reproductive-aged women. Fetal survival was poor, however, based on the limited outcome data available. Based on our report of higher overall survival rates in pregnant EVD patients as compared with historical data from prior outbreaks, corroboration with other outcomes data is warranted to guide optimal management strategies in future epidemics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the governments of Liberia, Sierra Leone, and Guinea for contributing to International Medical Corps’ humanitarian response; all of our generous institutional, corporate, foundation, and individual donors who placed their confidence and trust in International Medical Corps and made our work during the Ebola epidemic possible; the United States Naval Medical Research Center, Public Health England, the European Union Mobile Laboratory, and the Nigerian Laboratory for providing laboratory data to our ETUs (Ebola Treatment Unit); all members of our research review committee and other technical teams that contributed to this research, including Rabih Torbay, Ann Canavan, Dennis Walto, Dan Rodman, Yoav Rappaport, Samuel Grindley, Syed Hassan, Erin Shedd, Ryan Burbach, Saikrishna Madhireddy, Benedict Adjogah, Melody Xie, Nadezda Sekularac, Inka Weissbecker, Sean Casey, Farrah Zughni, Natalie Sarles, and August Felix; medical directors Vanessa Wolfman and Kassahun Gebrehiwot and the monitoring and evaluation staff including Annie Abbate, Razia Laghari, Allison Stewart, Alex Tran, Matthew Siakor, David Mansaray, Lamin Bangura, Sorie Sesay, and Joseph Fangawa; and all other data collection officers at our ETUs.
Financial support. International Medical Corps provided all funding for this research study. L. M. B. reports salary support from the National Institutes of Health (research training grant number R25 TW009337 funded by the Fogarty International Center and the National Institute of Mental Health and the T32 Ruth L. Kirschstein National Research Service Award grant number 5T32AI007433-22).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. 2014 Ebola outbreak in West Africa: case counts Available at: http://www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/case-counts.html. Accessed 17 October 2016.
- 2. Mupapa K, Mukundu W, Bwaka MA, et al. Ebola hemorrhagic fever and pregnancy. J Infect Dis 1999; 179:S11–2. [DOI] [PubMed] [Google Scholar]
- 3. Report of an International Commission Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ 1978; 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 4. Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med 2014; 371:2054–7. [DOI] [PubMed] [Google Scholar]
- 5. Oduyebo T, Pineda D, Lamin M, Leung A, Corbett C, Jamieson DJ. A pregnant patient with Ebola virus disease. Obstet Gynecol 2015; 126:1273–5. [DOI] [PubMed] [Google Scholar]
- 6. Maganga GD, Kapetshi J, Berthet N, et al. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med 2014; 371:2083–91. [DOI] [PubMed] [Google Scholar]
- 7. Caluwaerts S, Fautsch T, Lagrou D, et al. Dilemmas in managing pregnant women with Ebola: 2 case reports. Clin Infect Dis 2016; 62:903–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baize S, Pannetier D, Oestereich L, et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371:1418–25. [DOI] [PubMed] [Google Scholar]
- 9. Bower H, Grass JE, Veltus E, et al. Delivery of an Ebola virus-positive stillborn infant in a rural community health center, Sierra Leone, 2015. Am J Trop Med Hyg 2016; 94:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akerlund E, Prescott J, Tampellini L. Shedding of Ebola virus in an asymptomatic pregnant woman. N Engl J Med 2015; 372:2467–9. [DOI] [PubMed] [Google Scholar]
- 11. Baggi F, Taybi A, Kurth A, et al. Management of pregnant women infected with Ebola virus in a treatment centre in Guinea, June 2014. Euro Surveill 2014; 19:2–5. [DOI] [PubMed] [Google Scholar]
- 12. Schieffelin JS, Shaffer JG, Goba A, et al. ; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lori JR, Rominski SD, Perosky JE, et al. A case series study on the effect of Ebola on facility-based deliveries in rural Liberia. BMC Pregnancy Childbirth 2015; 15:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden EC. Maternal health: Ebola’s lasting legacy. Nature 2015; 519:24–6. [DOI] [PubMed] [Google Scholar]
- 15. Médecins Sans Frontières. Guidance paper: Ebola treatment centre (ETC): pregnant & lactating women. Geneva, Switzerland: MSF, 2014. [Google Scholar]
- 16. Roshania R, Mallow M, Dunbar N, et al. Successful implementation of a multicountry clinical surveillance and data collection system for Ebola virus disease in West Africa: findings and lessons learned. Glob Health Sci Pract 2016; 4:394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Guidance for screening and caring for pregnant women with Ebola virus disease for healthcare providers in U.S. hospitals Available at: http://www.cdc.gov/vhf/ebola/healthcare-us/hospitals/pregnant-women.html Accessed 17 October 2016.
- 18. Jamieson DJ, Uyeki TM, Callaghan WM, Meaney-Delman D, Rasmussen SA. What obstetrician-gynecologists should know about Ebola: a perspective from the Centers for Disease Control and Prevention. Obstet Gynecol 2014; 124:1005–10. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014; 371:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qin E, Bi J, Zhao M, et al. Clinical features of patients with Ebola virus disease in Sierra Leone. Clin Infect Dis 2015; 61:491–5. [DOI] [PubMed] [Google Scholar]
- 21. Yan T, Mu J, Qin E, et al. Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak. Eur J Clin Microbiol Infect Dis 2015; 34:2089–95. [DOI] [PubMed] [Google Scholar]
- 22. Wong JY, Zhang W, Kargbo D, et al. Assessment of the severity of Ebola virus disease in Sierra Leone in 2014–2015. Epidemiol Infect 2016; 144:1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agua-Agum J, Ariyarajah A, Blake IM, et al. ; WHO Ebola Response Team Ebola virus disease among male and female persons in West Africa. N Engl J Med 2016; 374:96–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deaver JE, Cohen WR. Ebola virus screening during pregnancy in West Africa: unintended consequences. J Perinat Med 2015; 43:649–55. [DOI] [PubMed] [Google Scholar]
- 25. Walker NF, Brown CS, Youkee D, et al. Evaluation of a point-of-care blood test for identification of Ebola virus disease at Ebola holding units, Western Area, Sierra Leone, January to February 2015. Euro Surveill 2015; 20. pii:21073. [DOI] [PubMed] [Google Scholar]
- 26. Broadhurst MJ, Kelly JD, Miller A, et al. ReEBOV antigen rapid test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study. Lancet 2015; 386:867–74. [DOI] [PubMed] [Google Scholar]
- 27. Phan JC, Pettitt J, George JS, et al. Lateral flow immunoassays for Ebola virus disease detection in Liberia. J Infect Dis 2016; 214:222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bebell LM, Riley LE. Ebola virus disease and Marburg disease in pregnancy: a review and management considerations for filovirus infection. Obstet Gynecol 2015; 125:1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.