Abstract
The aim of the present study was to investigate the potential of a fast gas chromatography (GC) e-nose for freshness discrimination and for prediction of storage time as well as sensory and internal quality changes during storage of hen eggs. All samples were obtained from the same egg production farm and stored at 20 °C for 20 d. Egg sampling was conducted every 0, 3, 6, 9, 12, 16, and 20 d. During each sampling time, 4 egg cartons (each containing 10 eggs) were randomly selected: one carton for Haugh units, one carton for sensory evaluation and 2 cartons for the e-nose experiment. The e-nose study included 2 independent test sets; calibration (35 samples) and validation (28 samples). Every sampling time, 5 replicates were prepared from one egg carton for calibration samples and 4 replicates were prepared from the remaining egg carton for validation samples. Sensors (peaks) were selected prior to multivariate chemometric analysis; qualitative sensors for principal component analysis (PCA) and discriminant factor analysis (DFA) and quantitative sensors for partial least square (PLS) modeling. PCA and DFA confirmed the difference in volatile profiles of egg samples from 7 different storage times accounting for a total variance of 95.7% and 93.71%, respectively. Models for predicting storage time, Haugh units, odor score, and overall acceptability score from e-nose data were developed using calibration samples by PLS regression. The results showed that these quality indices were well predicted from the e- nose signals, with correlation coefficients of R2 = 0.9441, R2 = 0.9511, R2 = 0.9725, and R2 = 0.9530 and with training errors of 0.887, 1.24, 0.626, and 0.629, respectively. As a result of ANOVA, most of the PLS model results were not significantly (P > 0.05) different from the corresponding reference values. These results proved that the fast GC electronic nose has the potential to assess egg freshness and feasibility to predict multiple egg freshness indices during its circulation in the supply chain.
Keywords: egg freshness, fast GC e-nose, chemometric method, quality discrimination, prediction model
INTRODUCTION
Shell eggs are perishable and can rapidly undergo quality deterioration during storage, causing a major economic loss to the poultry industry (Freeland-Graves and Peckman, 1987; Stadelman and Cotterill, 1995; Caner, 2005; No et al., 2005). This quality decay is associated with chemical, nutritional, functional, and hygienic changes. The rate at which these changes occur during storage depends on temperature, humidity (Tabidi, 2011), gaseous environment (Jo et al., 2011), storage time (Akyurek and Okur, 2009; Chung and Lee, 2014), hen age, and strain (Scott and Silversides, 2000; Scott and Silversides, 2001).
The modern poultry industry is interested in evaluating alternative methods that can be used to measure quality parameters more quickly. The main quality parameters of interest are: freshness, weight loss, size of air cell, albumen and yolk indices, Haugh units (HUs), and albumen and yolk pH (Karoui et al., 2006; Jin et al., 2011). Freshness, which is the characteristic most commonly related to egg quality, declines after laying mainly in a time- and temperature-dependent manner (Karoui et al., 2006; Tabidi, 2011; Yimenu et al., 2017). An alternative strategy for determining the level of egg freshness can potentially be achieved by sensing the organic volatiles emitted by eggs, by using electronic noses (e-noses).
Fresh shell eggs have a very low concentration of organic volatiles, and this concentration increases during storage (Adamiec et al., 2002). Wang et al. (2014) reported that the volatile components of egg yolk are esters, alcohols, alkenes, and nitrogenous compounds that change during the storage of shell eggs. Adamiec et al. (2002) reported a trend toward increasing palmitic acid and stearic acid released from the yolk of shell eggs stored at 35 °C for 12 d. These organic acids might have been the result of lipid hydrolysis. Yanagisawa et al. (2010) reported that an increase in hexanal during storage was observed via the identification of volatile compounds from yolk. Brown et al. (1986) reported the accumulation of compounds such as dimethyl sulfide, dimethyl disulfide, dimethyl trisulfide, methyl thioacetate, methanol, ethanol, 1-propanol, acetone, 2-butanone and ethyl acetate during storage.
An e-nose is used to detect volatile substances and consists of an array of sensors that collect chemical signals that are afterwards analyzed and interpreted in a way that imitates human nose sensory evaluation by using a pattern recognition method of multivariate statistical techniques (Wei et al., 2015). The e-nose has been used successfully in the food industry for quality detection (Antoce and Namolosanu, 2011). Many studies have been involved with assessment of fresh produce using e-nose methods in recent years. Some researchers have reported the assessment of egg freshness using e-nose methods in recent years. Studies carried out on eggs include the discrimination of eggs from 7 avian birds using GC–MS and e-nose (Wang et al., 2014), studies of egg freshness during storage (Dutta et al., 2003; Ming et al., 2010), and monitoring of egg storage time and quality attributes (Yongwei et al., 2009).
Fast gas chromatography (GC) electronic nose (e-nose) is a highly selective and sensitive specialty gas chromatograph, capable of performing very fast hydrocarbon measurements at low concentration in laboratory or field environments (ALPHA MOS, 2002). Its novel features include versatility and higher analysis speed, giving productivity advantages over the more traditional gas chromatographs (Marion et al., 2011). This type of e-nose was used for the study presented in this paper. In the literature, information can be found about application of fast GC e-nose for authenticity assessment of Polish homemade liqueurs (Śliwińska et al., 2016), for rapid and precise discrimination of wines (Antoce and Namolosanu, 2011), for discrimination of the geographic origin of extra virgin olive oil (Melucci et al., 2016), for rapid analysis of spirit beverages (Wiśniewska et al., 2016), and for age identification and brand classification of brandy (Yang et al., 2011).
There is no research that has reported the use of a fast GC-based e-nose for egg freshness determination. Moreover, most of the studies so far are focused solely on discrimination of samples rather than on prediction of sample quality. In most previous experiments, only an e-nose was used, with no other tests conducted using other methods. Therefore, even if we could predict the shell egg's storage time, we still cannot precisely determine its freshness degree since we do not have other indices as a reference.
Therefore, 2 objectives were emphasized using a fast GC e-nose: 1) to discriminate freshness of egg samples stored for different times using multivariate chemometric techniques and 2) to build freshness prediction models from correlation analysis between e-nose signals and sensory scores (Odor and Overall score), the HUs, and storage times of eggs using the partial least squares (PLS) model.
MATERIALS AND METHODS
Materials
Freshly laid special class unfertilized hen egg samples were obtained directly from an egg company in Seoul, and the storage experiments were conducted at Korea Food Research Institute. Samples were stored in their commercial packaging (cartons, each containing 10 egg specimens) in a temperature-controlled storage chamber set at 20 °C. This storage temperature was selected to mimic the sample storage in a room temperature. The storage relative humidity (RH) condition was 50 to 65% RH.
Sampling Procedure
In this study, 3 experiments were carried out: egg volatile detection, internal quality measurement, and sensory evaluation. The experiments were conducted on eggs for a total shelf life period based on sensory score (until an overall score of less than 2 is attained) to observe the variation trends of sensory scores, internal quality, and egg volatile changes. Egg sampling was conducted every 0, 3, 6, 9, 12, 16, and 20 d. During each sampling time, 4 egg cartons (each containing 10 eggs) were randomly selected: one carton for HUs, one carton for sensory evaluation and 2 cartons for the e-nose experiment.
Haugh Units
The weight (in grams) of eggs was established by individually weighing each egg using an analytical balance (Carter, 1975). Then, eggs were broken out on a flat, transparent glass surface using a spatula to obtain internal parameter measurements. The height of the thick albumen was measured using a digimatic indicator (ID-C1050XB, Mitutoyo Co., Japan). HUs were determined from the egg weight and albumen height of a broken egg spread on a horizontal plate using the expression (1) (Haugh, 1937).
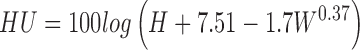 |
(1) |
where H = the height of albumen (mm), W = the egg weight when tested (g)
Sensory Evaluation
Eggs were ruptured and spread on a clean glass plate, and a 15-member sensory evaluation panel (10 females and 5 males) was used to evaluate the sensory characteristics. The panel consisted of research staff at Korea Food Research Institute. All panel members were familiar with egg freshness characters and descriptive analysis procedure. Each egg sample was assigned a 3-digit random code to ensure that the panelists were not biased regarding the samples. A previously prepared egg freshness chart was also provided for reference. The evaluated sensory characteristics were yolk and albumen color, yolk and albumen spread ratio, off odor, freshness, and overall acceptability using a 9-point scale ranging from very good (9 points), good (7 points), average (5 points), bad (3 points), to very bad (1 point). The mean of the 15 scores was considered the score of the egg sample for each descriptor.
Egg Volatile Determination
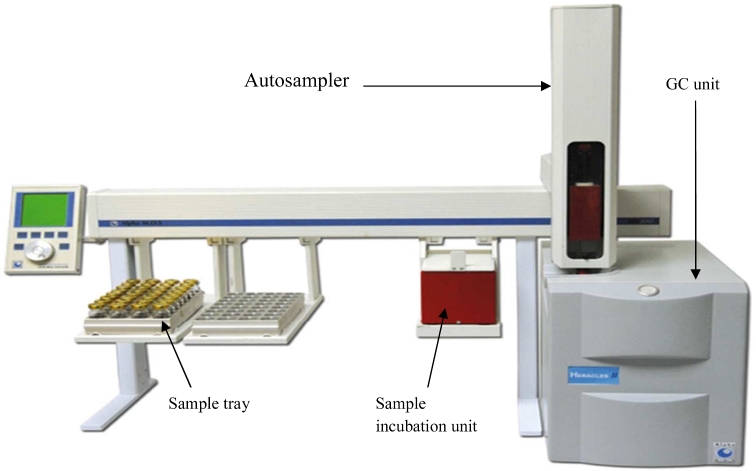
The fast GC e-nose called Heracles II (Alpha M.O.S., Toulouse, France) was used for the analysis. It consisted of a sampling system (HS100 autosampler), a detector system containing 2 short different polarity columns (MXT-5 a polar and MXT-1701 slightly polar) connected to 2 flame ionization detectors (FID) for a global fingerprint and a data acquisition and processing system (Alpha MOS proprietary software (Alpha Soft)).
For the calculation of Kovat's indices and the identification of volatile organic compounds, the alkane C6–C16 standard solution was used. The AroChemBase (Alpha MOS, France) library was used for confirming identification. The AroChembase is an add-on module that can be used within the HERACLES e-nose software, known as Alpha Soft. It allows one to pre-screen the chemical compounds and give sensory features from the HERACLES chromatograms (Marion et al., 2011). Figure 1 shows a general scheme of the HERACLES II Flash e-nose.
Figure 1.
Fast GC based HERACLES Electronic Nose with HS100 autosampler.
Two egg cartons (1 for calibration samples and 1 for validation samples), each containing 10 egg specimens, were used for e-nose analysis during every sampling time. After rupturing the eggs, the egg yolk was separated from the egg white, and only yolk was used for the following analysis. Egg yolk from 10 eggs (each cartoon) were collected in 100 mL beaker and homogenized (IKA T18 basic, Ulttra-Turrax, Germany) at a setting of 2× for 30 s. For the measurements, 4 g of homogenized sample was taken in a 20 mL glass vial provided with a pierce-able disk in the cap. All samples were sealed with 20-mm-thick polytetrafluoroethylene (PTFE)/silicone membrane caps.
A vial with a liquid sample was introduced into the autosampler (Odor Scanner HS 100, Gerstel, Mülheim, Germany) for headspace generation. Gas accumulated in the headspace of the sample was used for the analysis. The incubation time was 20 min at 60°C, and the agitation speed was maintained at 500 rpm. After the incubation process, a gas sample was taken from the headspace of the sample and transferred from the vial to the GC injector port at 200 °C. Totally 9 samples were analyzed during each sampling time; 5 replicates for calibration samples (set 1) and 4 replicates for validation samples (set 2).
To obtain maximum sensor response, the operating parameters for the e-nose were optimized for the egg samples as shown in Table 1.
Table 1.
The analysis parameters for electronic nose operation.
| Parameter | Conditions |
|---|---|
| Dispenser operation conditions | |
| Dispensing volume | 2,000 μL |
| Dispensing time | 21 s |
| Dispenser temperature | 200 ºC |
| Volumetric intensity of carrier gas flow | 30 mL/min |
| Parameters of sorption trap operation | |
| Trap temperature | 50 ºC |
| Retention time | 26 s |
| Conditions for chromatographic analysis | |
| Temperature program | 50 ºC (2 s) - |
| 3 ºC/s – 250 ºC | |
| (21 s) | |
| Carrier gas | Hydrogen |
| Dispenser temperature | 270 ºC |
| Detector operation conditions | |
| Detector temperature | 260 ºC |
| Acquisition time | 110 s |
Data Analysis
Alpha Soft (V14.2, Alpha M.O.S, Toulouse, France) software was used for instrument control and raw data processing. Three multivariate chemometric methods, principal component analysis (PCA), discriminant factor analysis (DFA), and partial least square (PLS), were used for data analysis of results obtained by the use of fast GC e-nose.
To discriminate different freshness degrees during storage time, 2 methods were used: PCA and DFA. PCA is a chemometric linear unsupervised pattern recognition technique used for analyzing and reducing the dimensionality of numerical datasets in a multivariate problem. It can observe the classification results of sample principal components through the PCA map. PCA is used to evaluate the detection of outliers and the discrimination and similarities between various samples or groups. DFA is another widely used multivariate statistical method. Similar to PCA, it also uses a linear combination of the original variable to construct a discriminant function (ALPHA MOS, 2016; Melucci et al., 2016).
The DFA is based on a search for directions along which the groups are as far apart as possible and the samples of the same group are as close together as possible. The DFA model also used to identify and classify unknown samples. Unlike PCA its aim is to maximize distances between groups, minimizing at the same time classification between samples in the same group; in this way, unknown samples may be projected onto this new scores map and assigned to one of the groups of the training set. In contrast to PCA, DFA is used to distinguish groups not to make the actual data visualization. In the case of the PCA the differences between the samples within the selected groups cannot be neglected (Buratti et al., 2004; ALPHA MOS, 2016; Wiśniewska et al., 2016).
A multivariate PLS analysis-based model was developed to predict the storage time, HUs, odor score, and the overall acceptability score of the eggs. PLS modeling is a common method used in quantitative multivariate analysis. The PLS algorithm is based on linear regression methods. Y is the matrix containing the quantitative measurements, Y’ is the matrix containing the productive values, and X is the matrix built with the detector measurements (gas sensors). To simplify, it can be said that the PLS model looks for a matrix B minimizing the distance between Y and Y’ with Y’ = X.B. After model building, the matrix B is used to predict the quantitative information contained in an unknown sample. The measurement matrix is then multiplied by B to obtain the prediction (ALPHA MOS, 2016).
To investigate the predictability of the e-nose regarding changes in quality descriptors during storage, projection of validation samples onto optimized calibration models derived from PLS regressions performed with e-nose data (X-matrix) and the storage time, the HUs, the odor score and the overall acceptability score changes during storage (Y-matrices) was carried out. The calibration models were based on data from calibration samples. These calibration models were used for prediction of validation samples. The models developed and used are shown in Table 2.
Table 2.
Overview of data analysis strategy for calibration and prediction models.
| Prediction | Training sample | X-data | Y-data | Calibration model | |
|---|---|---|---|---|---|
| Storage time | set 1 | E-nose | D of Storage | C-model (storage time) | |
| Haugh units | set 1 | E-nose | Haugh unit values | C-model (HU) | |
| Odor | set 1 | E-nose | Odor scores | C-model (odor) | |
| Overall acceptability | set 1 | E-nose | Overall acceptability scores | C-model (overall acceptability) | |
| Validation | Unknown sample | X-data | via Calibration model | Y predicted | |
| Storage time | set 2 | E-nose | C-model (storage time) | D of storage | |
| Haugh units | set 2 | E-nose | C-model (HU) | Haugh unit value | |
| Odor | set 2 | E-nose | C-model (odor) | Odor score | |
| Overall acceptability | set 2 | E-nose | C-model (overall acceptability) | Overall acceptability score |
Correlation coefficients (r) between reference and predicted values were used to determine predictions using PLS models. Training error, which is the mean of the difference between the predicted values and the calculated values of the samples declared as calibration samples, was also used to evaluate the PLS models. Using the statistical analysis system (SAS, 2008), analysis of variance (ANOVA) was performed. To examine whether there were significant differences between PLS model results and corresponding reference values, the Tukey's studentized range test (HSD) at the 95% confidence level was calculated.
RESULTS AND DISCUSSION
Haugh Units
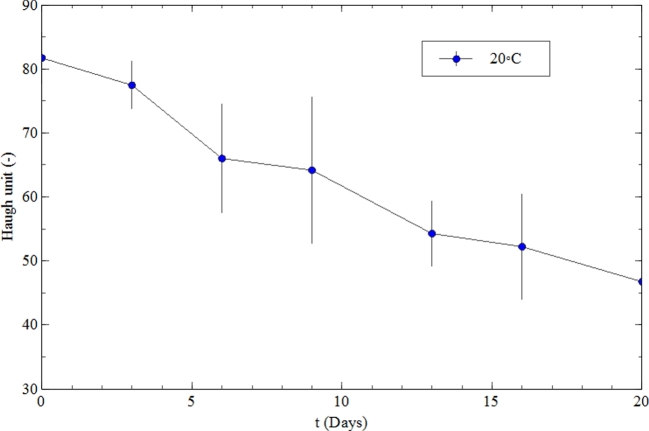
Haugh unit (HU) values decreased significantly (P < 0.05) with increased egg storage time (Figure 2). The HU reduction occurs due to a decrease in the thick albumen height, because during egg storage, albumen becomes thinner and loses CO2, which allows the electrostatic complex between lysozyme and ovomucin to rupture, which helps increase the pH of eggs (Scott and Silversides, 2000). This result is in agreement with those of Samli et al. (2005); Akyurek and Okur (2009); and Akter et al. (2014), who reported a significant (P ≤ 0.05) decrease in HU due to storage time and temperature.
Figure 2.
Changes in the Haugh units of eggs during storage at 20°C (mean ± SD, n = 10).
The HU decreased from 81.70 to 46.75 during 20 d of storage. This finding implies that the deterioration of egg quality increased in a storage time dependent manner. Therefore, one should bear in mind that the deterioration of internal egg quality is a function of storage time. Similar results were also demonstrated by other researchers: the HU was significantly (P ≤ 0.05) affected by storage time (Jin et al., 2011; Tayeb, 2012; Tebesi et al., 2012; Yimenu et al., 2017).
Sensory Quality
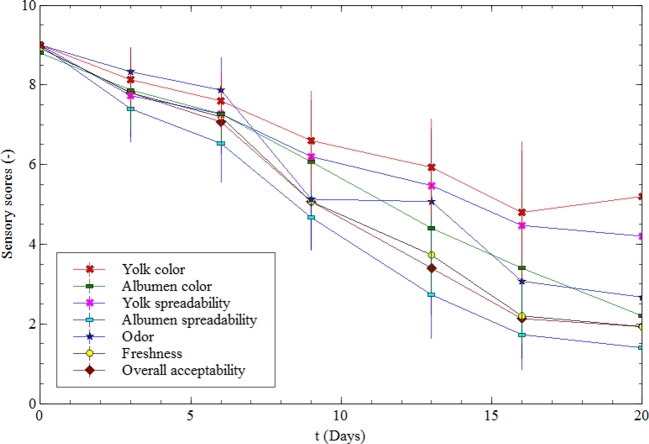
Various sensory quality attributes (yolk color, albumen color, yolk spreadability, albumen spreadability, off-odor, freshness, and overall acceptability) were evaluated, and it is apparent from Figure 3 that all sensory descriptors had a decreasing trend during the storage period.
Figure 3.
Changes in the sensory quality of eggs during storage at 20°C (mean ± SD, n = 15).
It is also noticeable that all of the curves (yolk color, albumen color, yolk spreadability, albumen spreadability, off-odor, freshness, and overall acceptability) decreased quickly after the d 6, while during the first 6 d, the overall acceptability score curve only declined slightly, and the odor score remained nearly the same. This indicated that the egg sample did not decrease in quality up to d 6. Therefore, in the first 6 d, it remained fresh, with its color (yolk and albumen) and odor changing little.
E-Nose Analysis Results
The egg samples stored for 0 to 20 d (2 egg cartons, each containing 10 egg specimens, were taken every sampling time; 5 replicates were prepared from one carton for calibration samples and 4 replicates were prepared from the remaining carton for validation samples) were analyzed by e-nose under the optimum experimental parameters, and the e-nose signals were processed by multivariate data analysis techniques. Using fast GC e-nose, the chromatographic peak area is treated as input data for multivariate chemometric analysis. All samples were classified and plotted in space according to the degree of similarity and difference in the data. These parameters are related to the number and size of chromatographic peaks recorded by the fast GC e-nose. For all analyses, sensors (peak areas) with the highest performance power were automatically selected using the sensors menu function of the Alpha Soft software.
Principal Component Analysis
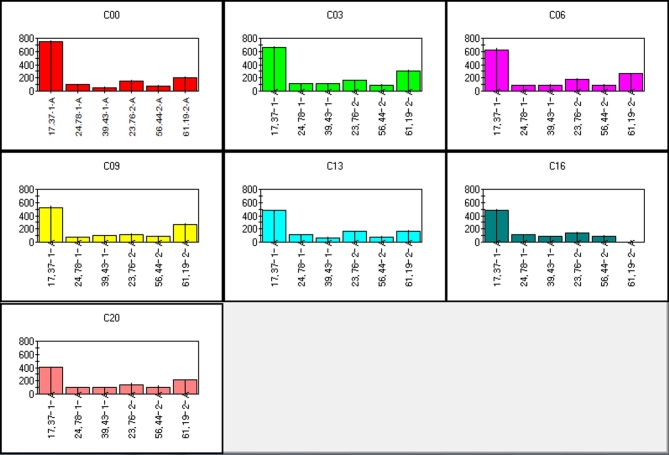
Calibration samples were used for PCA analysis. Qualitative sensor (peak) selection was performed to select only those sensors that provide the best discrimination possible. The variables chosen for PCA, namely, qualitative sensors (the most discriminant peak areas of specific compounds), were treated as an input dataset for chemometric analysis. This dataset is presented in Figure 4, and the names of selected substances are listed in Table 3. Due to satisfactory discrimination of sample groups after application of the PCA method, the chosen variables were also used as input data in the DFA method.
Figure 4.
Mean bar graphs of selected peak areas used as raw data in chemometrics representing key chemical compounds, which are important for discrimination of egg freshness (where C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20). Along the x-axis are variables (sensors) and values along the y-axis represent abundance.
Table 3.
Key chemical compounds (variables), which were important for sample discrimination (qualitative) and prediction (quantitative).
| Names of variables (sensors) from—retention times in column 1 (MXT-5) or in column 2 (MXT-1701) | Name of the compound | Formula |
|---|---|---|
| Qualitative sensors | ||
| 17.37–1 | Propan-2-one | C3H6O |
| 24.78–1 | Acetic acid | C2H4O2 |
| 39.43–1 | Cyclopentanone | C5H8O |
| 23.76–2 | Ethane, 1,1-dichloro- | C2H4Cl2 |
| 56.44–2 | Nonane 3-methyl | C10H22 |
| 61.19–2 | Psi-Cumene | C9H12 |
| Quantitative sensors | ||
| 14.53–1 | Trimethylamine | C3H9N |
| 17.37–1 | Propan-2-one | C3H6O |
| 20.08–1 | Ethane, 1,1-dichloro- | C2H4Cl2 |
| 24.78–1 | Acetic acid | C2H4O2 |
| 27.83–1 | 1-Hydroxy-2-propanone | C3H6O2 |
| 39.43–1 | Cyclopentanone | C5H8O |
| 16.75–2 | 2-Methylbutane | C5H12 |
| 17.38–2 | Pentane | C5H12 |
| 19.71–2 | 1,1-Dichloroethene | C2H2Cl2 |
| 23.76–2 | Ethane, 1,1-dichloro- | C2H4Cl2 |
| 27.06–2 | 2-Methylbutanal | C5H10O |
| 29.04–2 | Benzene | C6H6 |
| 41.19–2 | [E]-2-Octene | C8H16 |
| 50.08–2 | 2-mercaptoethanol | C2H6OS |
| 56.44–2 | Nonane 3-methyl | C10H22 |
| 61.19–2 | Psi-Cumene | C9H12 |
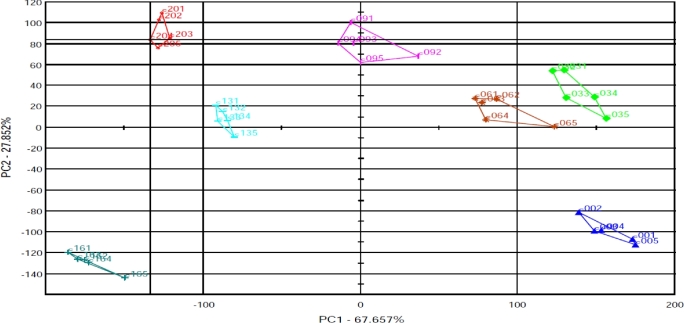
Figure 5 presents PCA score plots based on the peak area values for discrimination of egg sample freshness stored for different d. PC1 explains 67.65% of the total variance, and PC2 contributes 27.85% of the total variation. The first 2 PCs cumulatively represent 95.7% of the data variance, which appeared to provide sufficient information to explain the odor difference of egg samples. The discrimination index was 85%, which indicates how distant each cluster is from the other. Based on the distance between each sample cluster, the samples were divided into 7 groups in Figure 5.
Figure 5.
PCA score plots of eggs stored for 0 to 20 d. Individual symbols indicate replicate samples taken at different storage time. Where C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
The egg yolk samples appear classified into 2 groups: d 13, d 16, and d 20 samples are placed on the second and third quadrants of the PCA map, whereas the samples from d 0, d 3, and d 6 are situated on the first and fourth quadrants of the map. The d 9 samples appear on the first and second quadrants. This may indicate that in the first 0 to 6 d, fresh eggs stored at 20 °C in the storage chamber still remained fresh, and the change in their volatile gases was low. Thus, the data that the e-nose extracted were similar to each other. In general, all the samples can be clearly classified into 7 clusters according to their storage time.
The distribution of the samples in the PCA score plots shows good trends, which may indicate that the egg quality information contained in the e-nose signal has a strong correlation with the storage time. Moreover, the peak area values, may also have some correlation with the sensor values (the samples in the same group may have similar levels of volatile chemicals). These results are comparable with the sensory score results. In addition, it suggests good quality of the sensor response used as input (calibration set) for construction of further DFA and PLS prediction models.
Discriminant Factor Analysis
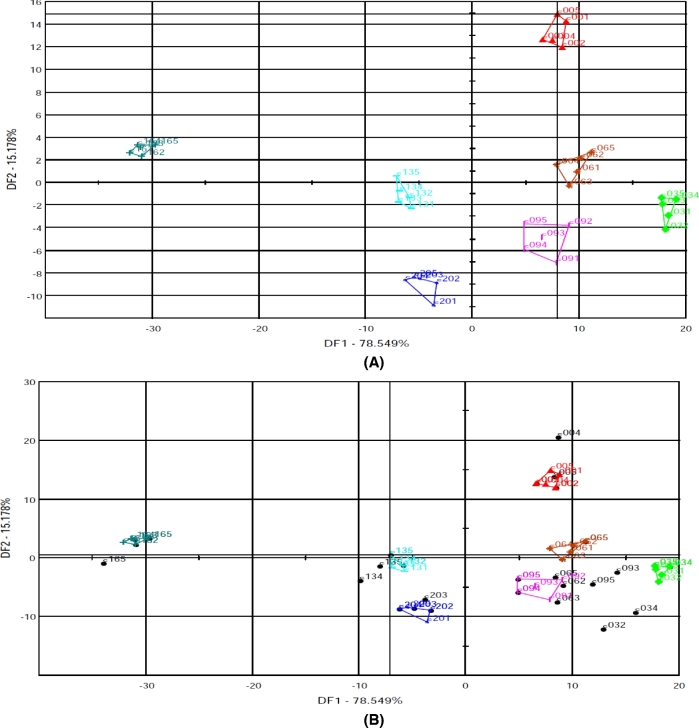
The DFA plot developed from the same data and sensors that provided the PCA plot is shown in Figure 6(A). It is a 2-dimensional spatial plot defined by 2 discriminant functions and shows clear discrimination of the different storage period samples. The first discriminant function (DF1) explains 78.54% of the total variation, and the second discriminant function (DF2) explains 15.17% of the total variation. The total variation contributed by the 2 discriminant functions is 93.71%, which means these 2 variation sources reflect 93.71% of the original information.
Figure 6.
DFA model analysis results based on electronic nose data obtained from egg yolk samples ((A), calibration plot; (B), validation plot) for the discrimination of eggs stored for 0 to 20 d. Individual symbols indicate replicate samples taken at different storage time. C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
As it is shown in Figure 6(A), similar to the PCA result, the discrimination was confirmed as all samples from the same d are grouped together and there was no intersection between the various groups analyzed. These discriminated groups correctly correspond to different storage d. In Figure 6(A), it can be seen that samples belonging to the same group of storage times are definitely closer than in the case of PCA. On the other hand, differences between individual samples belonging to the same storage times are disappearing.
To determine whether the unknown samples are identified by the e-nose as having a volatile chemical profile close to the calibration samples, the projection of the validation samples onto the DFA model was performed. As we can see in Figure 6(B), all the validation samples, except d 3 samples, were placed close to the most likely (samples of the same storage d) group created by the calibration samples, confirming that the DFA model can discriminate and classify the egg samples according to their freshness. The cross validation result had a validation score of 75, indicating that approximately 75% of the validation samples were fairly well recognized by the DFA model.
The Partial Least Squares Method
The PLS algorithm established a model that describes the relationship between the sensor signals and the egg quality indices (the odor score, the overall acceptability, and the HUs), as well as the storage time. The same samples used for DFA were used during PLS regression analysis for model development. Unlike in the case of PCA and DFA, quantitative sensors (peaks) were selected during PLS analysis. During the training phase, sensor responses from the e-nose were trained and matched with the egg quality index values determined by conventional methods and the storage time.
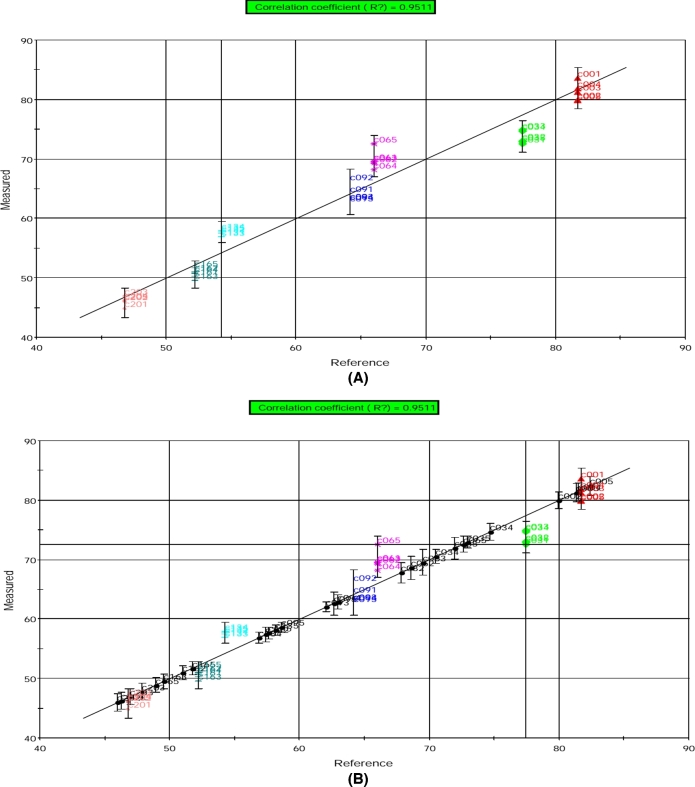
A model for HU evaluation from sensor responses was built. To determine the accuracy of the model, the training samples (only sensor responses from calibration samples) were introduced into PLS. For the training phase, PLS gave output data in terms of HUs (predicted value). Figure 7(A) shows predicted (measured) versus actual (reference) values of HUs. The correlation coefficient between the predicted and actual values is 0.9511. HU predictions were found to be fairly good with training error of 1.24. These results reveal a high correlation between e-nose results and typical HU analysis. It confirms the high performance of our e-nose as a rapid and alternative method for HU evaluation of hen eggs. To confirm the accuracy and validation of our model, the validation samples were projected onto the PLS model developed by the calibration samples. As we can see in Figure 7(B), all the validation samples were well correlated.
Figure 7.
PLS model results for the prediction of Haugh units (HU) based on electronic nose data obtained from (A) calibration and (B) validation egg yolk samples. The prediction is shown as measured (predicted by e-nose) vs. reference (measured by conventional method) HU values. Individual symbols indicate replicate samples taken at different storage time. C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
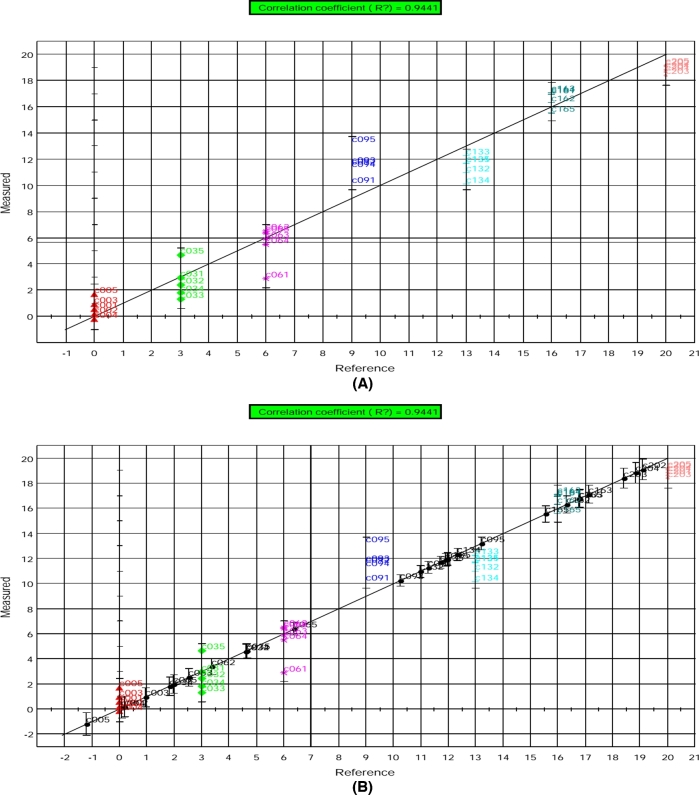
The e-nose data from calibration samples showed very good correlation (R2 = 0.9441) with d of storage as shown in Figure 8(A). This may have been due to the formation of deteriorative volatiles in the eggs as storage time increases. The training error of the prediction model was 0.887 that shows its high accuracy. Projection of the e-nose data from the validation sample for prediction of storage time onto the calibration model (Figure 8(B)) was successful, as all unknown samples lie close to their respective group on the correlation line.
Figure 8.
PLS model results for the prediction of storage time based on electronic nose data obtained from (A) calibration and (B) validation egg yolk samples. The prediction is shown as measured (predicted by e-nose) vs. reference (measured by conventional method) d of storage. Individual symbols indicate replicate samples taken at different storage time. C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
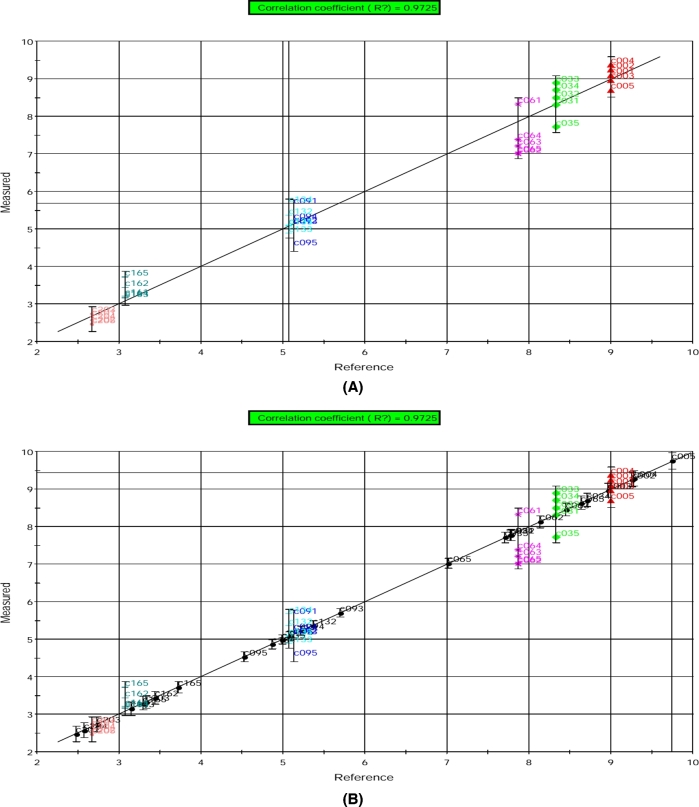
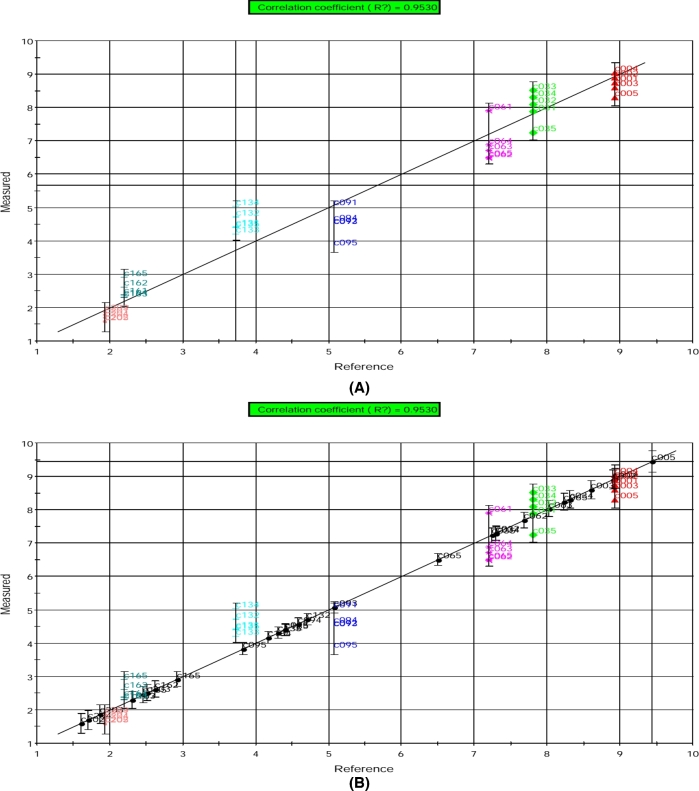
The modeled relationships between the e-nose signal data and the sensory score data are shown in Figures 9 and 10 for 2 sensory descriptors (odor and overall acceptability). The calibration of the models resulted in high correlation coefficient of R2 = 0.9725 (Figure 9(A)) and R2 = 0.9530 (Figure 10(A)) for the prediction of odor and overall acceptability scores, respectively. The training errors were 0.626 for odor scores and 0.629 for overall acceptability scores. The prediction of sensory descriptors for validation samples as projected onto their respective calibration model is shown in Figures 9(B) and 10(B). The prediction of the sensory quality changes during storage for validation samples was found to be very good that each sample was placed on the regression line close to its respective group.
Figure 9.
PLS model results for the prediction of odor scores based on electronic nose data obtained from (A) calibration and (B) validation egg yolk samples. The prediction is shown as measured (predicted by e-nose) vs. reference (measured by conventional method) odor scores. Individual symbols indicate replicate samples taken at different storage time. C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
Figure 10.
PLS model results for the prediction of overall acceptability scores based on electronic nose data obtained from (A) calibration and (B) validation egg yolk samples. The prediction is shown as measured (predicted by e-nose) vs. reference (measured by conventional method) overall acceptability scores. Individual symbols indicate replicate samples taken at different storage time. C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
It should be noted that both the calibration samples and the validation samples stored for 0–20 d could be correctly predicted. In addition, the signals determined by the e-nose from the egg yolk samples indicated that the changes in egg volatiles showed better correlation with the changes in sensory score values during the storage period. That the highest correlation coefficient was obtained for odor scores may be due to similar detection of odor by the sensory panelists and the e-nose.
As observed in Table 4, the PLS models resulted in e-nose predicted values comparable with the reference values measured by conventional methods. Overall, the training and unknown samples were predicted correctly. As a result of ANOVA, most of the PLS prediction results from the e-nose sensor signals of egg yolk samples were not significantly different from the corresponding reference values (Table 4). Significantly (P < 0.05) different results were seen only between: reference and validation odor scores on d 0, reference and validation HUs values on d 3, and reference and prediction as well as validation storage times on d 9, d 13, and d 20. These results suggest that it is possible to monitor and predict egg freshness by detecting egg yolk using the fast GC e-nose technique.
Table 4.
ANOVA of PLS model result mean values for quality indices of egg during storage.
| Storage period | Descriptor | Reference* | Prediction** | Validation*** |
|---|---|---|---|---|
| C00**** | Odor score | 9a, ***** | 9.076a,b | 9.31b |
| Overall score | 8.93 | 8.722 | 8.97 | |
| Haugh units | 81.7 | 81.302 | 81.28 | |
| Storage time (d) | 0 | 0.6438 | 0.008575 | |
| C03 | Odor score | 8.33 | 8.42 | 7.9875 |
| Overall score | 7.8 | 8.004 | 7.535 | |
| Haugh units | 77.45a | 73.616a,b | 71.4875b | |
| Storage time (d) | 3 | 2.628 | 3.915 | |
| C06 | Odor score | 7.87 | 7.386 | 8.0575 |
| Overall score | 7.2 | 6.894 | 7.615 | |
| Haugh units | 66 | 69.81 | 70.66 | |
| Storage time (d) | 6 | 5.428 | 3.565 | |
| C09 | Odor score | 5.13 | 5.124 | 5.1325 |
| Overall score | 5.07 | 4.476 | 4.4725 | |
| Haugh units | 64.17 | 63.792 | 61.53 | |
| Storage time (d) | 9a | 11.55b | 11.6475b | |
| C13 | Odor score | 5.07 | 5.22 | 5.0725 |
| Overall score | 3.73 | 4.554 | 4.3975 | |
| Haugh units | 54.27 | 57.372 | 57.51 | |
| Storage time (d) | 13a | 11.342b | 11.73b | |
| C16 | Odor score | 3.07 | 3.336 | 3.4 |
| Overall score | 2.2 | 2.51 | 2.575 | |
| Haugh units | 52.22 | 50.636 | 50.2775 | |
| Storage time (d) | 16 | 16.58 | 16.43 | |
| C20 | Odor score | 2.67 | 2.578 | 2.7775 |
| Overall score | 1.93 | 1.706 | 1.92 | |
| Haugh units | 46.75 | 46.03 | 46.7375 | |
| Storage time (d) | 20a | 18.828b | 18.275b |
*Measured by conventional methods.
**Predicted from training data.
***Predicted from unknown data.
****C00 = d 0, C03 = d 3, C06 = d 6, C09 = d 9, C13 = d 13, C16 = d 16, and C20 = d 20.
*****Means sharing the same superscript within a row are not significantly different from each other at P < 0.05.
CONCLUSIONS
With proper selection of the qualitative sensors (peaks) used for multivariate chemometric analysis, samples belonging to different storage times were well discriminated by the e-nose. The PCA and DFA analysis results indicated that it was possible for a fast GC e-nose to discriminate the freshness quality (different storage time) of eggs, and they provided a theoretical and experimental basis for monitoring the freshness of eggs.
The e-nose's ability to predict storage time, HUs, and sensory score values from eggs was verified in the research. After appropriate quantitative sensor (peak) selection, the PLS regression provided accurate quality index models between e-nose signals and the storage time, HUs, odor score, and overall acceptability score changes during storage with correlation coefficients of R2 = 0.9441, R2 = 0.9511, R2 = 0.9725, and R2 = 0.9530, respectively. The training errors were 0.887, 1.24, 0.626, and 0.629, respectively. By performing ANOVA for comparing reference and prediction mean values, evidence was obtained for the predictive power of the fast GC e-nose regarding the storage time and quality indices. PLS provided a clear indication of the GC based e-nose's ability—it could clearly detect a positive trend in the prediction of the sensory score, HU and storage time values based on its responses, and the results demonstrated its capability.
These results confirm that the fast GC e-nose, with good selection of the sensors (peaks) used for multivariate chemometric analysis, has the potential of being a reliable instrument for the assessment and prediction of egg freshness during its circulation in the supply chain.
Acknowledgements
The authors appreciate u-Food Project, Korea Food Research Institute for funding and granting us access to its facilities.
REFERENCES
- Adamiec J., Doležal M., Míková K., Davídek J.. 2002. Changes in egg volatiles during storage. Czech J. Food Sci. 20:79–82. [Google Scholar]
- Akter Y., Kasim A., Omar H., Sazili A.Q.. 2014. Effect of storage time and temperature on the quality characteristics of chicken eggs. J. Food Agric. Environ. 12:87–92. [Google Scholar]
- Akyurek H., Okur A. A.. 2009. Effect of storage time, temperature and hen age on egg quality in free-range layer hens. J. Anim. Vet. Adv. 8:1953–1958. [Google Scholar]
- ALPHA MOS 2002. Technical Note N-DT-01: Statistical Analysis, p. 1.
- ALPHA MOS 2016. No101274E-HERACLES II User Manual: Application development guide, p. 117.
- Antoce A. O., Namolosanu I.. 2011. Rapid and precise discrimination of wines by means of an electronic nose based on gas-chromatography. Rev. Chim. Bucharest. 62:593. [Google Scholar]
- Brown M. L., Holbrook D. M., Hoerning E. F., Legendre M. G., Angelo A. J. S.. 1986. Volatile indicators of deterioration in liquid egg products. Poult. Sci. 65:1925–1933. [Google Scholar]
- Buratti S., Benedetti S., Scampicchio M., Pangerod E.C.. 2004. Characterization and classification of Italian Barbera wines by using an electronic nose and an amperometric electronic tongue. Anal. Chim. Acta. 525:133–139. [Google Scholar]
- Caner C. 2005. The effect of edible eggshell coatings: On egg quality and consumer perception. J. Sci. Food Agric. 85:1897–1902. [Google Scholar]
- Carter J.C. 1975. The hen's egg estimation of shell superficial area and egg volume using measurements of fresh weight and shell length and breadth alone or in combination. Br. Poult. Sci. 16:541–543. [Google Scholar]
- Chung S. H., Lee K. W.. 2014. Effect of hen age, storage duration and temperature on egg quality in laying hens. Int. J. Poult. Sci. 13:634–636. [Google Scholar]
- Dutta R., Hines E. L., Gardner J. W., Udrea D. D., Boilot P.. 2003. Non-destructive egg freshness determination: an electronic nose based approach. Meas. Sci. Technol. 14:190–198. [Google Scholar]
- Freeland-Graves J. H., Peckman G. C.. 1987. Eggs. Pages 415–440 in Foundation of Food Preparation. Macmillan, New York, NY. [Google Scholar]
- Haugh R. R. 1937. A new method for determining the quality of an egg. U.S. Egg Poultry. 39:27–49. [Google Scholar]
- Jin Y. H., Lee K. T., Lee W. I., Han Y. K.. 2011. Effects of storage temperature and time on the quality of eggs from laying hens at peak production. Asian-Australas. J. Anim. Sci. 24:279–284. [Google Scholar]
- Jo C., Ahn D. U., Liu X. D., Kim K. H., Nam‖ K. C.. 2011. Effects of chitosan coating and storage with dry ice on the freshness and quality of eggs. Poult. Sci. 90:467–472. [DOI] [PubMed] [Google Scholar]
- Karoui R., Kemps B., Bamelis F., De Ketelaere B., Decuypere E., De Baerdemaeker J.. 2006. Methods to evaluate egg freshness in research and industry: A review. Eur. Food Res. Technol. 222:727–732. [Google Scholar]
- Marion B., Herve L., Fatma A.. 2011. Rancidity control of nut mixtures using an electronic nose. Focus Food Anal. 22:12–15. [Google Scholar]
- Melucci D., Bendini A., Tesini F., Barbieri S., Zappi A., Vichi S., Conte L., Toschi T. G.. 2016. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 204:263–273. [DOI] [PubMed] [Google Scholar]
- Ming L., Leiqing P., Kang T.. 2010. Determination of egg freshness during shelf life with electronic nose. Trans. CSAE. 26:317–321. [Google Scholar]
- No H. K., Prinyawiwatkul W., Meyers S. P., 2005. Comparison of shelf life of eggs coated with chitosans prepared under various deproteinization and demineralization times. J. Food Sci. 70:377–382. [Google Scholar]
- Samli H. E., Agna A., Senkoylu N.. 2005. Effects of storage time and temperature on egg quality in old laying hens. J. Appl. Poult. Res. 14:548–533. [Google Scholar]
- SAS 2008. SAS/STAT User's Guide. Version 9.2. SAS Institute Inc., Cary, NC. [Google Scholar]
- Scott T. A., Silversides F. G.. 2000. The effect of storage and strain of hen on egg quality. Poult. Sci. 79:1725–1729. [DOI] [PubMed] [Google Scholar]
- Scott T. A., Silversides F.G.. 2001. Effect of storage and layer age on quality of eggs from 2 lines of hens. Poult Sci. 80:1240–1245. [DOI] [PubMed] [Google Scholar]
- Śliwińska M., Wiśniewska P., Dymerski T., Wardencki W., Namieśnik J.. 2016. Application of electronic nose based on fast GC for authenticity assessment of Polish homemade liqueurs called nalewka. Food Anal. Methods. 9:2670–2681. [Google Scholar]
- Stadelman W.J., Cotterill O.J.. 1995. Egg Science and Technology. The Haworth Press, Inc, New York. [Google Scholar]
- Tabidi M. H. 2011. Impact of storage period and quality on composition of table egg. Adv. Environ. Biol. 5:856–861. [Google Scholar]
- Tayeb I.T. 2012. Effects of storage temperature and length on egg quality parameters of laying hen. Journal of Animal Scientist. 1:32–36. [Google Scholar]
- Tebesi T., Madibela O. R., Moreki J. C.. 2012. Effect of storage time on internal and external characteristics of guinea fowl (Numida meleagris) eggs. J. Anim. Sci Adv. 2:534–542. [Google Scholar]
- Wang Q., Jin G., Jin Y., Ma M., Wang N., Liu C., He L.. 2014. Discriminating eggs from different poultry species by fatty acids and volatiles profiling: Comparison of SPME-GC/MS, electronic nose, and principal component analysis method. Eur. J. Lipid Sci. Technol. 116:1044–1053. [Google Scholar]
- Wei Z., Wang J., Zhang W.. 2015. Detecting internal quality of peanuts during storage using electronic nose responses combined with physicochemical methods. Food Chem. 177:89–96. [DOI] [PubMed] [Google Scholar]
- Wiśniewska P., Nska M. U., Vnik J. N., Wardencki W., Dymerski T.. 2016. The verification of the usefulness of electronic nose based on ultra-fast gas chromatography and 4 different chemometric methods for rapid analysis of spirit beverages. J. Anal. Methods Chem. http://dx.doi.org/10.1155/2016/8763436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T., Ariizumi M., Shigematsu Y., Kobayashi H., Hasegawa M., Watanabe K.. 2010. Combination of super chilling and high carbon dioxide concentration techniques most effectively to preserve freshness of shell eggs during long-term storage. Food engineering and physical properties. J. Food Sci. 75:78–82. [DOI] [PubMed] [Google Scholar]
- Yang Y., Zhao Y., Zhang S., Ni Y., Zhan J.. 2011. Qualitative analysis of age and brand of unblended brandy by electronic nose. IFIP Adv. Inform. Commun. Technol. 369:619–628. [Google Scholar]
- Yimenu S. M., Kim J. Y., Koo J., Kim B. S.. 2017. Predictive modeling for monitoring egg freshness during variable temperature storage conditions. Poult. Sci. 96 DOI: https://doi.org/10.3382/ps/pex038. [DOI] [PubMed] [Google Scholar]
- Yongwei W., Wang J., Zhou B., Lu Q.. 2009. Monitoring storage time and quality attribute of egg based on electronic nose. Anal. Chim. Acta. 650:183–188. [DOI] [PubMed] [Google Scholar]