Abstract
This experiment was to evaluate the effect of dietary resveratrol (Res) supplementation (0, 400 mg/kg) on growth performance, meat quality, and muscle anaerobic glycolysis and antioxidant capacity of transported broilers. A total of 360 21-day-old male Cobb broilers was randomly allotted to 2 dietary treatments (Res-free group and Res group) with 12 replicates of 15 birds each. On the morning of d 42, after a 9-hour fast, 24 birds (2 birds of each replicate) were selected from the Res-free group and then equally placed into 2 crates, and the other 12 birds (one bird of each replicate) were selected from the Res group and then placed into the other crate. All birds in the 3 crates were transported according to the following protocols: 0-hour transport of birds in the Res-free group (control group), 3-hour transport of birds in the Res-free group (T group), and 3-hour transport of birds in the Res group (T + Res group). The results showed that Res not only improved feed conversion ratio (P < 0.05) but also tended to improve birds’ final body weight (P < 0.10). In the Res-free group, a 3-hour transport increased serum corticosterone concentration, muscle malondialdehyde (MDA) and lactate contents, and muscle lactate dehydrogenase (LDH) activity, while it decreased muscle glycogen content, total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-PX) activities (P < 0.05), which induced decreased breast meat quality (lower pH24h and higher drip loss and L*24 h, P < 0.05). Nevertheless, compared with the T group, Res increased muscle glycogen content and T-SOD and GSH-PX activities (P < 0.05 or P < 0.10), while it decreased muscle MDA content and LDH activity (P < 0.05), which is beneficial to the meat quality maintenance of transported broilers (lower drip loss, L*24 h, and higher pH24h, P < 0.05 or P < 0.10). This study provides the first evidence that dietary resveratrol supplementation prevents transport-stress-impaired meat quality of broilers, possibly through decreasing the muscle anaerobic glycolysis metabolism and improving the muscle antioxidant capacity.
Keywords: broiler, meat quality, resveratrol, transport stress, antioxidant capacity
INTRODUCTION
Preslaughter transport, which exposes broilers to many stress factors and has been reported to induce various physiological and metabolic changes (Savenije et al., 2002; Yue et al., 2010; Zhang et al., 2010; Wang et al., 2015), is unavoidable for the poultry processing industry. It is commonly reported that long duration transport, especially in summer, is detrimental to animal welfare and meat quality, inducing great economic loss to animal production (Dadgar et al., 2010; Kittelsen et al., 2015; Xing et al., 2015). Therefore, how to reduce the transport stress is becoming a major concern for the poultry industry. Wang et al. (2016) reported that a water-misting spray combined with forced ventilation treatment is a potential non-chemical addition method for improving meat quality of broilers transported during summer. Moreover, there is increasing interest in mitigating the stress and improving meat quality of transported broilers via dietary supplementation with certain nutrients, for example creatine monohydrate (Zhang et al., 2014b; Wang et al., 2015), vitamin C, and chromium (Perai et al., 2014).
Resveratrol (Res) is a naturally occurring polyphenol compound found in various plants, including grape, Polygonum cuspidatum, and peanut. Its biological activity, including antioxidant activity (Liu et al., 2014; Zhang et al., 2015b) and energy metabolism regulation effect (Lagouge et al., 2006), has been widely studied. Muscle antioxidant capacity (Ma et al., 2010; Zhang et al., 2015b) and energy metabolism status (Zhang et al., 2014b; Xing et al., 2015; Zhang et al., 2015b) are related to many aspects of meat quality traits that include pH value, drip loss, and meat color, among others. It is widely recognized that transport-stress-impaired meat quality is related to transport-stress-induced negative changes in muscle energy metabolism and antioxidant capacity (Yu et al., 2009; Zhang et al., 2014b; Wang et al., 2015; Xing et al., 2015). A previous study in pigs reported that dietary Res supplementation increases muscle antioxidant capacity and decreases glycolytic potential, thus leading to improved pork quality (Zhang et al., 2015b). For poultry production, research has shown that dietary Res supplementation can enhance the antioxidant status of quail (Sahin et al., 2010) and attenuate the heat-stress-impaired antioxidant function of black-boned chickens (Liu et al., 2014). However, to the best of our knowledge, there has been no relevant study conducted to test whether dietary Res supplementation is beneficial for the maintenance of meat quality of transported broilers in summer and, if so, whether antioxidant capacity and energy metabolism changes induced by Res are involved. In view of the foregoing, it is plausible to hypothesize that dietary Res supplementation may protect against transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. This study was conducted to test the above hypothesis.
MATERIALS AND METHODS
Bird husbandry, diets, and experimental design
All experimental procedures were approved by the Animal Care and Use Committee of Anhui Agricultural University. A total of 850 one-day-old male Cobb broilers was purchased from a commercial hatchery (Anhui Five Star Foodstuff Co. Ltd, Anhui, China). All birds were fed the same starter diet from one to 21 d of age. On d 21, a total of 360 healthy broilers was selected by weight and then randomly allotted to 2 groups with 12 replicates of 15 birds each. The groups were then randomly assigned to each of the 2 dietary treatments that consisted of a basal grower diet group (Res-free group) and a basal grower diet supplemented with 400 mg/kg Res group (Res group). The added level of resveratrol to feed was conducted as previously described (Liu et al., 2014; Zhang et al., 2014a). Basal diets were formulated to meet or exceed the nutrient requirements of the National Research Council (1994), and the ingredient composition and nutrient levels are shown in Table 1. Res (purity, >98.1%) was provided by Ci Yuan Biotechnology Co. Ltd. (Xi’an, Shanxi, China) and was added to the basal grower diet at the expense of corn. Experimental diets were prepared weekly. Diets were fed in mash form, and birds were provided ad libitum access to water and feed.
Table 1.
Composition and nutrient levels of the basal grower diet.
| Ingredients, % | Content | Nutrient level (calculated values) | |
|---|---|---|---|
| Corn | 62.02 | Metabolizable energy, kcal/kg | 3125 |
| Soybean oil | 4.30 | Crude protein, % | 19.03 |
| Soybean meal | 30.00 | Crude fiber, % | 3.37 |
| CaCO3 | 1.09 | Phosphorus, % | 0.53 |
| CaHPO4·2H2O | 1.09 | Calcium, % | 0.76 |
| NaHCO3 | 0.22 | Lysine, % | 1.20 |
| Salt | 0.25 | Methionine, % | 0.53 |
| Choline chloride | 0.15 | Methionine + cysteine, % | 0.83 |
| L-Lysine | 0.27 | ||
| D, L-Methionine | 0.25 | ||
| L-threonine | 0.06 | ||
| Premix1 | 0.30 | ||
| Total | 100.00 |
1Premix provided per kg of diet: Cu, 8 mg; Fe, 65 mg; Zn, 80 mg; Mn, 105 mg; I, 1 mg; Se, 0.3 mg; vitamin A, 9800 IU; vitamin D3, 3100IU; vitamin E, 26 IU; vitamin B1, 2.5 mg; vitamin B2, 7 mg; vitamin B12, 0.018 mg; vitamin K, 2.2 mg; biotin, 0.09 mg; folic acid, 1 mg; pantothenic acid, 11 mg; nicotinic acid, 38 mg.
On the morning of d 42, after a 9-hour fast, body weight and feed intake were measured for each replicate and used to calculate average daily feed intake (ADFI), average daily gain (ADG), and feed:gain ratio (F:G) from 21 to 42 days. Then, 24 birds (2 birds of each replicate) were selected from the Res-free group and equally placed into 2 crates, and the other 12 birds (one bird of each replicate) were selected from the Res group and placed into the other crate. The size of the crates was 1.2 × 0.7 × 0.25 meter. All birds in the 3 crates were transported according to the following protocols: 0-hour transport of birds in the Res-free group (control group), 3-hour transport of birds in the Res-free group (T group), 3-hour transport of birds in the Res group (T+Res group). The T group and T+Res group crates (birds) were placed at the rear of the truck. The total load of the truck was about 1,800 birds. The average speed of the truck was 50 km/hour. Transport was on a ring country road (approximately 25 kg/lap) near the poultry farm. The average inside relative humidity and temperature of the truck were ∼71 to 80% and 30.2 to 34.8°C, respectively. Transport was conducted from 0900 to 1200 h in September. The control group crates (birds) were placed under normal environmental condition (temperature: ∼26.2 to 29.9°C; humidity: ∼65 to 72%) from 0900 to 1200 hours. Therefore, all birds had equal fast time (12 h) before slaughter.
Sample collection
All birds were euthanized by cervical dislocation, and then slaughtered immediately by exsanguination from the jugular vein. Approximately 5 mL of blood from each bird was collected in a glass tube, then centrifuged at 2,000 × g for 10 min at 4°C for serum collection. Serum samples were stored at −20 °C until analysis. Within 10 min postmortem, the entire left pectoralis major (PM) was collected for determination of pH, meat color, and drip loss. Meanwhile, the entire right PM was collected. Then, some of the samples were quickly frozen in liquid nitrogen and stored at −80°C until analysis, and others were used to measure cooking loss and shear force.
Blood corticosterone measurement
The concentration of serum corticosterone (CORT) was determined using a commercial ELISA kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) and the corresponding procedure. The intra-assay coefficient of variation of the kit is less than 12%, and the assay range is from 0.2 ng/mL to 60 ng/mL.
Meat quality analysis
Muscle pH values were measured in triplicate at 45 min and 24 h postmortem using an electronic pH meter (pH-STAR, SFK-Technology, Copenhagen, Denmark), which was calibrated with pH 4.6 and 7.0 buffers equilibrated at 37°C. Measurement was directly conducted by inserting the probe into the muscle samples. Meat color (L*, a*, and b*) was determined in triplicate at 45 min and 24 h postmortem using a Chroma meter (CR-300, Minolta Camera, Osaka, Japan), which was previously calibrated against a white tile according to the manufacturer's manual. Drip loss was determined according to Zhang et al. (2015b) with modification. Briefly, about 20 g of muscle were manually trimmed into a specific shape (2 × 2 × 5 cm), and then weighed and suspended on a hook surrounded by an inflated plastic bag. After 24 h at 4°C, the sample was blotted dry on filter paper and reweighed. Drip loss (%) was calculated as follows: 100 × (initial weight- final weight)/initial weight. Cooking loss and shear force were determined according to Zhang et al. (2015b) with modification. Briefly, about 90 g of muscle were weighed and then put into a zip-sealed plastic bag and cooked in a water bath at 85°C. The samples were taken out after 30 min of cooking and placed into a refrigerator with a temperature of 4°C. After cooling to 4°C, samples were blotted dry on filter paper and reweighed. Cooking loss (%) was calculated as follows: 100 × (initial weight- cooked final weight)/initial weight. Then, the cooked samples were used for shear force measurement using a Texture Analyzer (Food Technology Corporation Co., Sterling, VA) according to the procedure described by Zhang et al. (2014b).
Muscle metabolite content and enzyme activity measurement
About 700 mg of frozen muscle samples were cut and homogenized for one min in 7 mL of ice-cold 0.9% saline solution. The homogenate was centrifuged at 2,200 × g for 10 min at 4°C, and then the supernatant was collected for determination of the activities of total superoxide dismutase (T-SOD), catalase (CAT), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px), and lactate dehydrogenase (LDH), and the contents of malondialdehyde (MDA) and lactate using colorimetric methods with a spectrophotometer (Biomate 5, Thermo Electron Corporation, Rochester, NY). All of the above assays and muscle glycogen content were determined with commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) according to the manufacturer's instructions.
Statistical analysis
The statistical analysis of the data was performed using SPSS 18.0 for Windows statistical software package (SPSS Inc., Chicago, IL). The data of growth performance were analyzed by a simple t test with each replicate as the experimental unit. All other data were analyzed by a one-way ANOVA model followed by Duncan's multiple range analysis with each bird as the experimental unit. Results are were presented as the mean ± SE. P < 0.05 and P < 0.10 were considered statistical significance and trends among means, respectively.
RESULTS
Growth performance and serum CORT concentration
As shown in Table 2, dietary Res supplementation had no effect (P > 0.10) on ADG or ADFI but resulted in lower (P < 0.05) F:G. Moreover, dietary Res supplementation tended to increase (P < 0.10) BW an at 42 d of age (Table 2). Transport significantly increased (P < 0.05) serum CORT concentration compared with the control group (Figure 1). However, dietary Res supplementation had no significant effect (P > 0.10) on serum CORT concentration compared with the T group (Figure 1).
Table 2.
Effect of dietary resveratrol supplementation on growth performance of broilers from 21 to 42 d of age1.
| Resveratrol supplemental level (mg/kg) | |||
|---|---|---|---|
| Item | 0 | 400 | P-value |
| Body weight at 21 d, g | 669.4 ± 0.96 | 670.2 ± 1.36 | 0.621 |
| Body weight at 42 d, g | 1876 ± 32.2 | 1957 ± 33.7 | 0.096 |
| ADG, g | 56.96 ± 1.609 | 59.93 ± 1.731 | 0.222 |
| ADFI, g | 123.2 ± 2.47 | 123.3 ± 2.47 | 0.981 |
| Feed:gain | 2.17 ± 0.039a | 2.06 ± 0.031b | 0.043 |
1All measurements are presented by mean values ± SE (n = 12).
a,bMeans within a row with different superscripts differ significantly (P < 0.05).
BW = body weight; ADFI = average daily feed intake; ADG = average daily gain; F:G = feed:gain.
Figure 1.
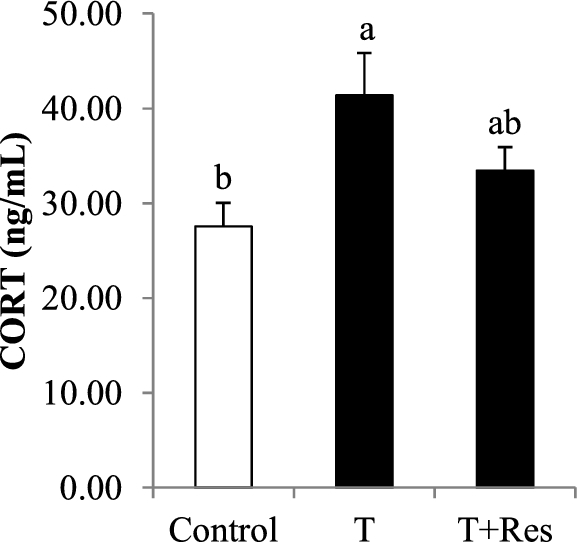
Effect of dietary resveratrol supplementation on serum corticosterone (CORT) concentration of transported broilers. All measurements are presented by mean values ± SE (n = 12). Within a panel bar, mean values labeled with different letters differ significantly (P < 0.05). T = transport group; T + Res = transport + resveratrol group.
Meat quality
Compared with the control group, transport had no effect (P > 0.10) on L*45min, pH45min, a*, b*, cooking loss, or shear force but resulted in greater (P < 0.05) L*24h and drip loss, and decreased (P < 0.05) pH24h (Table 3). Compared with the T group, dietary Res supplementation had no effect (P > 0.10) on L*45 min, pH45min, a*, b*, cooking loss, or shear force but resulted in decreased drip loss (P < 0.05) and L*24 h (P < 0.10), and greater pH24h (P < 0.10).
Table 3.
Effect of dietary resveratrol supplementation on meat quality of transported broilers1.
| Item | Control | T | T+Res | SEM | P-value |
|---|---|---|---|---|---|
| L*45min | 43.80 | 46.11 | 44.63 | 0.623 | 0.318 |
| a*45min | 4.43 | 4.59 | 4.34 | 0.216 | 0.903 |
| b*45min | 11.11 | 10.49 | 11.68 | 0.285 | 0.242 |
| pH 45min | 6.38 | 6.39 | 6.44 | 0.044 | 0.832 |
| L*24h | 48.18b | 52.69a | 49.14a,b | 0.771 | 0.037 |
| a*24h | 6.92 | 6.08 | 6.56 | 0.304 | 0.543 |
| b*24h | 15.24 | 17.60 | 15.59 | 0.481 | 0.094 |
| pH 24h | 5.88a | 5.54b | 5.76a,b | 0.049 | 0.012 |
| Drip loss (%) | 1.96b | 2.49a | 2.12b | 0.079 | 0.016 |
| Cooking loss (%) | 17.49 | 16.51 | 17.23 | 0.340 | 0.486 |
| Shear force (N) | 31.36 | 31.69 | 34.08 | 1.247 | 0.642 |
1All measurements are presented by mean values and SEM (n = 12).
a,bMeans within a row with different superscripts differ significantly (P < 0.05).
T = transport group; T+Res = transport + resveratrol group; L* = lightness; a* = redness; b* = yellowness.
Muscle glycogen, MDA and lactate contents, and LDH activity
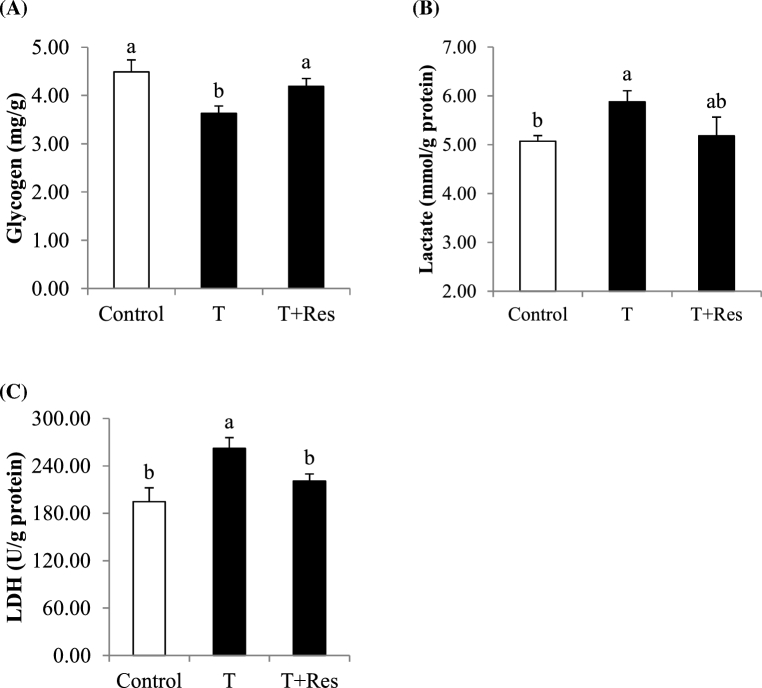
Compared with the control group, transport significantly increased (P < 0.05) muscle lactate content and LDH activity, as well as decreased (P < 0.05) the muscle glycogen content (Figure 2). Compared with the T group, dietary Res supplementation significantly increased (P < 0.05) muscle glycogen content and decreased (P < 0.05) muscle LDH activity (Figure 2).
Figure 2.
Effect of dietary resveratrol supplementation on muscle glycogen and lactate contents, and lactate dehydrogenase (LDH) activity of transported broilers. All measurements are presented by mean values ± SE (n = 12). Within a panel bar, mean values labeled with different letters differ significantly (P < 0.05). T = transport group; T + Res = transport + resveratrol group.
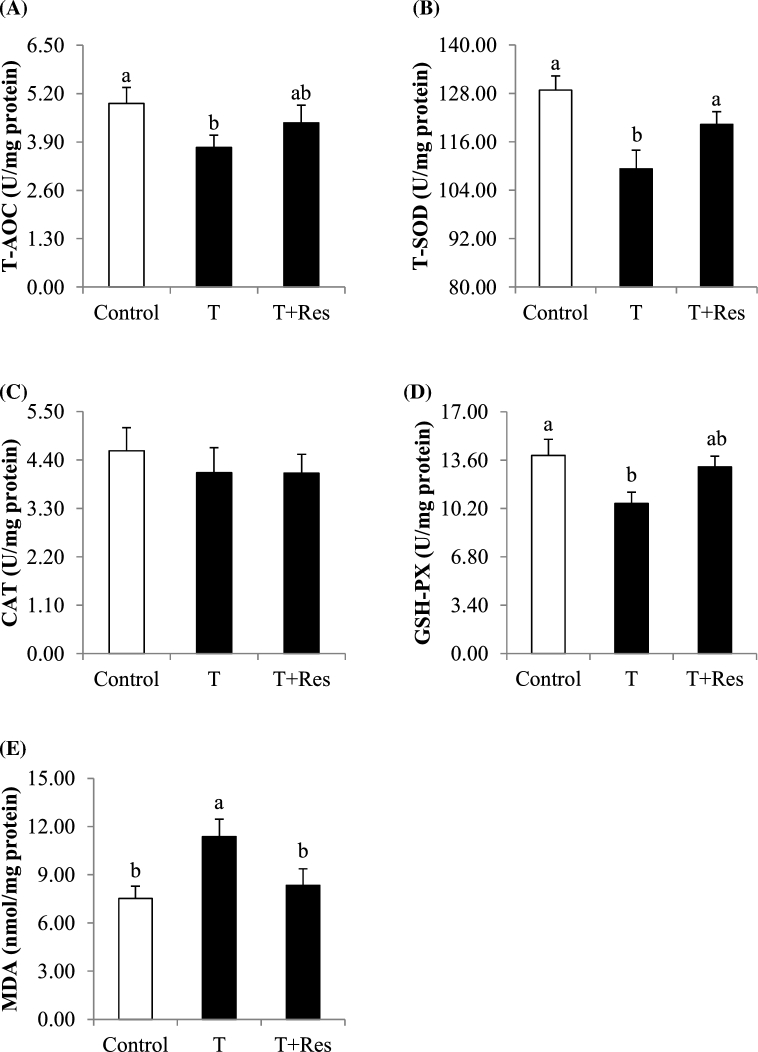
Muscle antioxidant enzyme activities and MDA content
Compared with the control group, transport had no effect (P > 0.10) on muscle CAT activity but resulted in greater muscle MDA content and decreased muscle T-AOC, T-SOD, and GSH-PX activity (Figure 3, P < 0.05). Compared with the T group, dietary Res supplementation significantly increased muscle T-SOD activity and decreased muscle MDA content (P < 0.05), and tended to increase (P < 0.10) muscle GSH-PX activity (Figure 3).
Figure 3.
Effect of dietary resveratrol supplementation on muscle antioxidant enzyme activities and MDA content of transported broilers. All measurements are presented by mean values ± SE (n = 12). Within a panel bar, mean values labeled with different letters differ significantly (P < 0.05). T = transport group; T + Res = transport + resveratrol group; T-AOC = total antioxidant capacity; T-SOD = total superoxide dismutase; CAT = catalase; GSH-PX = glutathione peroxidase; MDA = malonaldehyde.
DISCUSSION
Nowadays, numerous phytochemicals (e.g., isoflavones, carotenoids, and polyphenols) are attained and have been widely used in animal and poultry production to improve growth performance, product quality, and immune function, among others, because of their wider beneficial functions (Sahin et al., 2010; Kamboh and Zhu 2013; Goliomytis et al., 2014). Res is a naturally occurring polyphenol compound found in various plants, and its biological activities, including antimicrobial, antioxidant, anti-inflammatory, energy metabolism, and lipid metabolism regulating function, have been well investigated in vitro and in vivo (Paulo et al., 2010; Liu et al., 2014; Alagawany et al., 2015; Zhang et al., 2015a,b). Therefore, resveratrol has gained more and more attention in animal and poultry production.
A number of studies in poultry and pigs have been conducted previously to evaluate the functionality of Res and indicated that it is an effective feed additive to improve animal production and health (Alagawany et al., 2015; Zhang et al., 2015b). Although Zhang et al. (2015b) reported that dietary Res supplementation had no significant effect on growth, feed intakes, or feed efficiency in pigs, Zhang et al. (2014a) found that the ADG of chickens that receive conventional vaccinations was quadratically increased with increasing Res concentration (200, 400, and 800 mg/kg). Similarly, Liu et al. (2014) reported that dietary Res supplementation (200, 400, and 600 mg/kg) was able to improve the performance of black-boned chickens during heat stress. In the present study, we found that dietary Res supplementation (400 mg/kg) in broilers beneficially increased final body weight and decreased F:G, which indicates an improved growth performance.
Blood CORT concentration, which is a sensitive indicator in response to stress levels, can be increased by many stress factors (Zhang et al., 2009; Yue et al., 2010). Numerous previous studies showed that plasma CORT concentration in broilers was elevated by transport stress (Kannan et al., 1997; Zhang et al., 2009). Consistently, our present result indicated that the broilers in the T group exhibited higher CORT concentration than those in the control group, indicating that the broilers in the T group experienced more stress, which may induce various physiological and metabolic changes. Compared with the T group, there was a trend toward dietary Res supplementation inducing a lower serum CORT concentration, indicating that dietary Res supplementation is helpful in weakening transport stress.
Preslaughter transport is unavoidable for the poultry processing industry, but long duration transport, especially in summer, is detrimental to meat quality (Zhang et al., 2009; Yue et al., 2010; Xing et al., 2015), inducing great economic loss to poultry production. Consistently, our present study found that a 3-hour transport decreased pH24h and increased L*24 h and drip loss, indicating a lower meat quality. Dietary supplementation with certain nutrients, for example creatine monohydrate (Zhang et al., 2014b; Wang et al., 2015), vitamin C, and chromium (Perai et al., 2014), have been proven as effective ways to attenuate transport-stress-impaired meat quality of broilers. Resveratrol is a naturally occurring polyphenol compound and has been reported as a safe, effective feed additive to improve pork quality (Zhang et al., 2015b) and quail egg quality (Sahin et al., 2010). Excitingly, our present study provides the first evidence that Res can be served as an effective feed additive to improve meat quality (lower drip loss and L*24 h, and higher pH24h) of broilers subjected to transport stress.
Muscular energy metabolism and related enzymes affect many aspects of meat quality (Xing et al., 2015; Zhang et al., 2015b). Previous studies demonstrated that transport stress increased muscle glycogen anaerobic glycolysis, which leads to decreased muscle glycogen content and increased muscle lactate content, and thus results in muscle pH reduction and further meat quality deterioration (Zhang et al., 2014b; Xing et al., 2015). In the present study, we also found that a 3-hour transport decreased muscle glycogen content and increased muscle lactate content. Moreover, the present study found that the activity of muscle LDH, one of the key enzymes in muscle for anaerobic glycolysis metabolism, was improved by transport stress. Research also has shown that dietary resveratrol supplementation decreases the muscle glycolytic potential, lactate content, as well as LDH activity in pigs and therefore benefits pork quality (Zhang et al., 2015b). Similarly, this study is the first to demonstrate that the content of glycogen and the activity of LDH in muscle of broilers subjected to transport stress were increased and decreased, respectively. Therefore, the muscle glycolysis metabolism is changed by Res, which may be one of the reasons that Res attenuates transport-stress-impaired meat quality of broilers.
Muscle lipid peroxidation and antioxidant enzyme (e.g., SOD, CAT, and GSH-PX) defensive systems have significant effects on meat quality (Ma et al., 2010; Zhang et al., 2015b). Generally, decreasing lipid peroxidation and improving antioxidant status in muscle are beneficial to meat quality. Unfortunately, previous studies showed that long-term transport leads to free radical accumulation and decreased antioxidant enzyme activity, thus accelerating muscle lipid peroxidation and cell membrane damage (Zhang et al., 2010; Perai et al., 2014), and ultimately leading to meat quality deterioration of transported broilers (Kannan et al., 1997; Xing et al., 2015). Again, our present study found that muscle T-SOD and GSH-PX activities were decreased by transport stress, along with increased MDA content, which indicates a decreased body antioxidant capacity. Previous studies reported that dietary Res supplementation can enhance antioxidant status of quail (Sahin et al., 2010) and pigs (Zhang et al., 2015b). Moreover, according to Liu et al. (2014), dietary Res supplementation can attenuate the heat-stress-impaired antioxidant function of black-boned chickens. Fortunately, this study first demonstrates that dietary Res supplementation can beneficially increase antioxidant capacity (higher T-SOD and GSH-PX activities) and decrease lipid peroxidation (lower MDA content) in muscle of transported broilers, which is concordant with our hypothesis and may partly explain why Res can attenuate transport-stress-impaired meat quality of broilers as shown in the present study.
Taken together, to the best of our knowledge, this study provides the first evidence that resveratrol can serve as an effective and beneficial feed additive for protecting against transport-stress-impaired meat quality of broilers, and the mechanisms of action may be partly attributed to the decreased muscle anaerobic glycolysis metabolism and the improved muscle antioxidant capacity induced by resveratrol. However, the exact mechanism of action still needs further investigation.
Acknowledgments
This study was financed by the Anhui Higher Institutions Natural Science Foundation (KJ2016A240) and the Natural Science Foundation for Young Scholars of Anhui Agricultural University (2015ZD04).
REFERENCES
- Alagawany M. M., Farag M. R., Dhama K., Abd El-Hack M. E., Tiwari R., Alam G. M.. 2015. Mechanisms and beneficial applications of resveratrol as feed additive in animal and poultry nutrition: A review. Int. J. Pharmacol. 11:213–221. [Google Scholar]
- Dadgar S., Lee E. S., Leer T. L., Burlinguette N., Classen H. L., Crowe T. G., Shand P. J.. 2010. Effect of microclimate temperature during transportation of broiler chickens on quality of the pectoralis major muscle. Poult. Sci. 89:1033–1041. [DOI] [PubMed] [Google Scholar]
- Goliomytis M., Tsoureki D., Simitzis P. E., Charismiadou M. A., Hager-Theodorides A. L., Deligeorgis S. G.. 2014. The effects of quercetin dietary supplementation on broiler growth performance, meat quality, and oxidative stability. Poult. Sci. 93:1957–1962. [DOI] [PubMed] [Google Scholar]
- Kamboh A. A., Zhu W. Y.. 2013. Individual and combined effects of genistein and hesperidin supplementation on meat quality in meat-type broiler chickens. J. Sci. Food Agric. 93:3362–3367. [DOI] [PubMed] [Google Scholar]
- Kannan G., Heath J. L., Wabeck C. J., Souza M. C., Howe J. C., Mench J. A.. 1997. Effects of crating and transport on stress and meat quality characteristics in broilers. Poult. Sci. 76:523–529. [DOI] [PubMed] [Google Scholar]
- Kittelsen K. E., Granquist E. G., Kolbjornsen O., Nafstad O., Moe R. O.. 2015. A comparison of post-mortem findings in broilers dead-on-farm and broilers dead-on-arrival at the abattoir. Poult. Sci. 94:2622–2629. [DOI] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J.. 2006. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 127:1109–1122. [DOI] [PubMed] [Google Scholar]
- Liu L. L., He J. H., Xie H. B., Yang Y. S., Li J. C., Zou Y.. 2014. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 93:54–62. [DOI] [PubMed] [Google Scholar]
- Ma X., Lin Y., Jiang Z., Zheng C., Zhou G., Yu D., Cao T., Wang J., Chen F.. 2010. Dietary arginine supplementation enhances antioxidative capacity and improves meat quality of finishing pigs. Amino Acids. 38:95–102. [DOI] [PubMed] [Google Scholar]
- National Research Council 1994. Nutrient requirements of poultry. 9th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Paulo L., Ferreira S., Gallardo E., Queiroz J. A., Domingues F.. 2010. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World Journal of Microbiology and Biotechnology World J. Microb. Biot. 26:1533–1538. [Google Scholar]
- Perai A. H., Kermanshahi H., Nassiri Moghaddam H., Zarban A.. 2014. Effects of supplemental vitamin C and chromium on metabolic and hormonal responses, antioxidant status, and tonic immobility reactions of transported broiler chickens. Biol. Trace Elem. Res. 157:224–233. [DOI] [PubMed] [Google Scholar]
- Sahin K., Akdemir F., Orhan C., Tuzcu M., Hayirli A., Sahin N.. 2010. Effects of dietary resveratrol supplementation on egg production and antioxidant status. Poult. Sci. 89:1190–1198. [DOI] [PubMed] [Google Scholar]
- Savenije B., Lambooij E., Gerritzen M. A., Venema K., Korf J.. 2002. Effects of feed deprivation and transport on preslaughter blood metabolites, early postmortem muscle metabolites, and meat quality. Poult. Sci. 81:699–708. [DOI] [PubMed] [Google Scholar]
- Wang P., Zhao Y., Jiang N., Li K., Xing T., Chen L., Wang X., Tang Y., Xu X.. 2016. Effects of water-misting spray combined with forced ventilation on heat induced meat gelation in broiler after summer transport. Poult. Sci. 95:2441–2448. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Zhu X. D., Li Y. J., Liu Y., Li J. L., Gao F., Zhou G. H., Zhang L.. 2015. Effect of dietary creatine monohydrate supplementation on muscle lipid peroxidation and antioxidant capacity of transported broilers in summer. Poult. Sci. 94:2797–2804. [DOI] [PubMed] [Google Scholar]
- Xing T., Xu X. L., Zhou G. H., Wang P., Jiang N. N.. 2015. The effect of transportation of broilers during summer on the expression of heat shock protein 70, postmortem metabolism and meat quality. J. Anim. Sci. 93:62–70. [DOI] [PubMed] [Google Scholar]
- Yu J., Tang S., Bao E., Zhang M., Hao Q., Yue Z.. 2009. The effect of transportation on the expression of heat shock proteins and meat quality of M. longissimus dorsi in pigs. Meat Sci. 83:474–478. [DOI] [PubMed] [Google Scholar]
- Yue H. Y., Zhang L., Wu S. G., Xu L., Zhang H. J., Qi G. H.. 2010. Effects of transport stress on blood metabolism, glycolytic potential, and meat quality in meat-type yellow-feathered chickens. Poult. Sci. 89:413–419. [DOI] [PubMed] [Google Scholar]
- Zhang C., Luo J., Yu B., Chen J., Chen D.. 2015a. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J. Funct. Foods. 14:590–595. [Google Scholar]
- Zhang C., Luo J., Yu B., Zheng P., Huang Z., Mao X., He J., Yu J., Chen J., Chen D.. 2015b. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 102:15–21. [DOI] [PubMed] [Google Scholar]
- Zhang C., Tian Y., Yan F., Kang X., Han R., Sun G., Zhang H.. 2014a. Modulation of growth and immunity by dietary supplementation with resveratrol in young chickens receiving conventional vaccinations. Am. J. Vet. Res. 75:752–759. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li J. L., Gao T., Lin M., Wang X. F., Zhu X. D., Gao F., Zhou G. H.. 2014b. Effects of dietary supplementation with creatine monohydrate during the finishing period on growth performance, carcass traits, meat quality and muscle glycolytic potential of broilers subjected to transport stress. Animal. 8:1955–1962. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yue H. Y., Wu S. G., Xu L., Zhang H. J., Yan H. J., Cao Y. L., Gong Y. S., Qi G. H.. 2010. Transport stress in broilers. II. Superoxide production, adenosine phosphate concentrations, and mRNA levels of avian uncoupling protein, avian adenine nucleotide translocator, and avian peroxisome proliferator-activated receptor-gamma coactivator-1alpha in skeletal muscles. Poult. Sci. 89:393–400. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yue H. Y., Zhang H. J., Xu L., Wu S. G., Yan H. J., Gong Y. S., Qi G. H.. 2009. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult. Sci. 88:2033–2041. [DOI] [PubMed] [Google Scholar]